Abstract
Nivolumab-induced multiple organ immune-related adverse events (irAEs) have been described in some case reports. The symptoms of endocrinological irAEs are especially nonspecific. A 63-year-old man with a postoperative recurrence of pulmonary adenocarcinoma who was treated with nivolumab presented fever, anorexia and fatigue after the 7th cycle. He underwent a rapid adrenocorticotrophic hormone test, four-hormone tolerance test and thyroid gland scintigraphy. The results were consistent with destructive thyroiditis, hypophysitis and secondary adrenal insufficiency. Nivolumab was restarted following glucocorticoid and thyroid hormone replacement treatment. When a patient presents nonspecific symptoms, the possibility of endocrinological irAEs should be considered as it may enable their early detection.
Keywords: nivolumab, hypophysitis, secondary adrenal insufficiency, destructive thyroiditis, lung adenocarcinoma
Introduction
Nivolumab is an anti-programmed death-1 specific monoclonal antibody and is an immune checkpoint inhibitor (ICPI) that is used for the treatment of non-small cell lung carcinoma. ICPIs cause immune-related adverse events (irAEs), which can occur in every organ of the patient (1). Some case reports have described patients with multiple irAEs induced by nivolumab (2,3). Furthermore, the symptoms of endocrinological irAEs are nonspecific; thus, the physician must be alert to their possible occurrence (4).
There have been a few reports of cases with a combination of hypothyroidism and hypophysitis with secondary adrenal insufficiency resulting from nivolumab (5,6); however, this is the first report of a case with both hyperthyroidism and adrenal insufficiency. The treatment of patients with adrenal insufficiency and thyroiditis requires attention. We need to consider the possibility that patients receiving ICPIs may have more than one concurrent irAE.
Case Report
A 63-year-old man with diabetes mellitus was diagnosed with pulmonary adenocarcinoma, clinical T1aN0M0 (stage IA). He underwent right lower lobectomy and lymph node dissection. As the pathological stage was T2aN2M0 (stage IIIA), he received 4 courses of adjuvant chemotherapy with cisplatin and vinorelbine. At two and half years after surgery, recurrent cancer was detected in a lower paratracheal lymph node and at the fourth lumbar vertebra. He had radiation therapy for vertebral metastasis and six courses of chemotherapy with carboplatin, pemetrexed and bevacizumab, followed by two courses of maintenance therapy. The maintenance therapy was stopped because of drug induced pneumonitis, which was attributed to pemetrexed. The pneumonitis resolved spontaneously, following the cessation of treatment. After a further year, positron emission tomography-computed tomography (PET-CT) revealed local tumor recurrence in the mediastinal lymph nodes and right diaphragm metastasis. He received two courses of chemotherapy with docetaxel, but the lung cancer showed progression. Nivolumab (3 mg/kg) treatment was initiated, after which the extent of the local recurrence and metastasis were reduced. The patient's thyroid function was normal before nivolumab treatment. He received thyroid function examinations routinely after the start of nivolumab treatment. On Day 12, the patient's thyroid-stimulating hormone (TSH) level decreased, but his free thyroxine (fT4) and free triiodothyronine (fT3) levels remained normal, indicating latent hyperthyroidism. His TSH level returned to the normal range on Day 77, but decreased again on Day 104, then his fT4 and fT3 levels increased on Day 117. Blood tests did not show eosinophilia or electrolyte abnormalities. Nivolumab had a therapeutic effect against his cancer, and a partial response (PR) was achieved after two months of nivolumab treatment.
The patient experienced anorexia and diarrhea at 1 week after the 7th cycle, and from Day 110 after the initiation of nivolumab treatment. After Day 120, the patient presented intermittent fever and pain in the right hypochondrium, and he was admitted to our hospital on Day 123 after the initiation of nivolumab treatment. On admission, the patient's temperature was 36.9℃, his blood pressure was 96/42 mmHg, his heart rate was 110 bpm, and oxygen saturation was 94% on room air. A physical examination revealed no abnormalities other than spontaneous right hypochondriac pain.
The patient's serum sodium level was slightly low (134 mEq/L); his potassium level was within the normal range (4.1 mEq/L). His blood glucose level was normal. The results of liver and renal function tests were within the normal limits (Table).
Table.
Results of Blood Test on Admission.
| Hematology | ||
|---|---|---|
| White blood cells | 4,500 | /µL |
| Neutrophil | 57 | % |
| Lymphocyte | 22 | % |
| Basophil | 2 | % |
| Eosinophil | 5 | % |
| Monocyte | 14 | % |
| Hemoglobin | 12.8 | g/dL |
| Hematocrit | 38.2 | % |
| Platelets | 16.9×104 | /µL |
| Blood chemistry | ||
| Total protein | 5.6 | g/dL |
| Albumin | 3.1 | g/dL |
| Urea nitrogen | 12 | mg/dL |
| Creatinine | 0.71 | mg/dL |
| Sodium | 134 | mEq/L |
| Potassium | 4.1 | mEq/L |
| Chloride | 97 | mEq/L |
| Calcium | 8.2 | mg/dL |
| Lactate dehydrogenase | 184 | U/L |
| Aspartate transaminase | 28 | U/L |
| Alanine transaminase | 17 | U/L |
| Alkaline phosphatase | 98 | U/L |
| Total bilirubin | 0.8 | mg/dL |
| C-reactive protein | 6.01 | mg/dL |
| Procalcitonin | 0.07 | ng/mL |
| Casual plasma glucose | 227 | mg/dL |
| Glycated hemoglobin | 7.5 | % |
| Thiroid-stimulating hormone | <0.01 | µIU/mL |
| Free thyroxine | 3.2 | ng/dL |
| Free triiodothyronine | 7.8 | pg/mL |
| Adrenocorticotropic hormone | <1.0 | pg/mL |
| Cortisol | <0.2 | µg/dL |
| Anti-diuretic hormone | 5.1 | pg/mL |
| Aldosterone | 9.5 | ng/dL |
| Angiotensin converting enzyme | 15.3 | U/L |
| Immunoglobulin G4 | 18 | mg/dL |
| Anti-thyroid peroxydase antibody | 5.8 | U/mL |
| Thyroid stimulating hormone receptor antibody | 1.1 | IU/L |
| Anti-thyroglobulin antibody | 17.4 | U/mL |
| Pituitary cell antibody-1 | negative | |
The patient's C-reactive protein (CRP) level was elevated to 6.01 mg/dL, but his procalcitonin level was within the normal limits (0.07 mg/dL). Thyroid function tests showed that his TSH level was <0.01 μIU/mL (normal range 0.38-4.31 μIU/mL), his fT3 level was 7.8 pg/mL (2.1-3.8 pg/mL), and his fT4 level was 3.2 ng/mL (0.8-1.6 ng/mL), confirming hyperthyroidism. An electrocardiogram showed sinus tachycardia. A chest X-ray showed increased vascular markings in the right lower lung field and computed tomography (CT) revealed increased lower lobe bronchovascular markings bilaterally and right pleural effusion. The gallbladder wall showed a normal thickness on CT.
Despite antibacterial treatment, his fever continued. On Day 125, he experienced a loss of consciousness and the results of adrenocorticotrophic hormone (ACTH) and cortisol tests were available; the ACTH level was <1.0 pg/mL, and the serum cortisol level was <0.2 μg/dL. Based on the low ACTH and cortisol levels, the patient was diagnosed with an adrenal crisis. He was treated with hydrocortisone (200 mg/day). On Day 126, his fever resolved and his weakness improved. Diarrhea and pain in the right hypochondrium were relieved on Day 129 and the patient was diarrhea- and pain-free on Day 131.
Hypothalamic hormone challenges were performed to assess the patient's adrenal failure. A rapid ACTH test showed that the peak blood concentration of cortisol was 1.4 μg/dL (<18 μg/dL), which indicated a lack of adrenal cortisol secretion (Fig. 1). He received a four-hormone (protirelin, 500 μg; gonadorelin acetate, 100 μg; corticorelin, 100 μg; somatorelin acetate, 100 μg) tolerance test, which showed that TSH, ACTH and cortisol were hyporeactive (Fig. 2). We concluded that the low TSH level resulted from negative feedback from the elevated thyroid hormone levels, but that the reduction in ACTH was caused by hypophysitis, and that cortisol was consequently reduced. Thus, the patient had isolated ACTH deficiency-type hypophysitis, and secondary adrenal insufficiency.
Figure 1.

The results of a rapid adrenocorticotropic hormone test. The peak blood concentration of cortisol was 1.4 µg/dL. A value of <18 µg/dL indicates that the adrenal cortisol secretion function was impaired.
Figure 2.

The results of tolerance tests for four hormones (protirelin, gonadorelin acetate, corticorelin, somatorelin acetate). TSH, ACTH and cortisol were hyporeactive. ACTH: adrenocorticotropic hormone, FSH: follicle stimulating hormone, GH: growth hormone, LH: luteinizing hormone, PRL: prolactin, TSH: thyroid-stimulating hormone
Brain magnetic resonance imaging (MRI) showed that that there were no brain metastasis and the pituitary body size was normal. Blood tests showed that that angiotensin converting enzyme and immunoglobulin G4 levels were within normal limits. The patient was negative for pituitary cell antibody-1, and he was diagnosed with nivolumab-induced hypophysitis. The steroid dosage was tapered and oral cortisol (20 mg/day) was administered continuously.
Scintigraphy of thyroid gland was performed for a detailed assessment of hyperthyroidism. It revealed that the uptake of technetium was reduced (Fig. 3). The patient was negative for anti-thyroglobulin antibodies. He was diagnosed with nivolumab-induced destructive thyroiditis. His fT4 and TSH levels were 0.7 ng/dL and 0.19 μIU/mL, respectively, on Day 166; thus, the patient was treated with levothyroxine. Since the PR of his lung cancer was maintained while nivolumab was stopped, nivolumab therapy was restarted from Day 166 in conjunction with hormone replacement treatment. The patient resumed his activities of daily living with no autoimmune-related symptoms.
Figure 3.
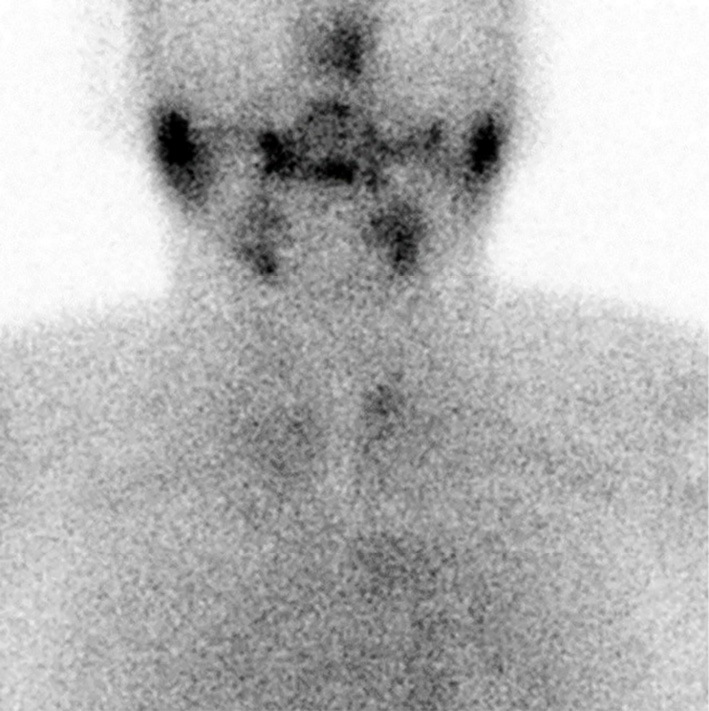
The results of scintigraphy of the thyroid gland. This revealed that the uptake of technetium was reduced.
Discussion
Nivolumab is one of the ICPIs used to treat non-small cell lung carcinoma. ICPIs, which differ from cytotoxic anticancer drugs, may cause irAEs. A phase III trial of nivolumab reported the drug-induced hypothyroidism and hyperthyroidism occurred at varying incidence rates of 4-7% and 1-2%, respectively, but that hypophysitis was rare (1,7,8). Faje reported that the incidence of nivolumab-induced hypophysitis was <1% (9). IrAEs may affect any organ system. Some cases in which nivolumab treatment was associated with several concomitant adverse events have been reported (2,3), and there are a few reports of cases involving a combination of hypothyroidism and hypophysitis with secondary adrenal insufficiency resulting from nivolumab treatment (5,6); however, this is the first report of a patient with both hyperthyroidism and adrenal failure.
Another ICPI, ipilimumab (an anti-cytotoxic T-lymphocyte-associated antigen (CTLA-4) monoclonal antibody) is reported to cause hypophysitis in 10-15% of patients (7). The characteristics of ICPI-induced hypophysitis on contrast MRI are mild swelling of the pituitary gland and thickening of the stalk with homogenous enhancement (9,10). In one case report, MRI showed a normal pituitary gland and stalk in a patient with nivolumab-induced hypophysitis (11), which is similar to the present case. Based on the lack of response to ACTH, we concluded that the patient had hypophysitis leading to secondary adrenal insufficiency. Hypophysitis may cause deficiency of several hormones and consequent endocrinological disturbance (12). ACTH deficiency is most likely to be fatal because of adrenal insufficiency.
Thyroid gland malfunction may also be anticipated, since ICPIs increase the risk of both hypothyroidism and hyperthyroidism. Tanaka et al. hypothesized that among Japanese patients, nivolumab has a stronger propensity to cause thyroid related irAEs than was reported in a phase III study of nivolumab (13). In this case, we diagnosed destructive thyroiditis, a painless thyroiditis, based on technetium scintigraphy. Although some painless thyroiditis patients are only in the hypothyroidism phase, others are in the hypothyroidism phase after the hyperthyroidism phase, as was observed in the present case (14). The patients need to receive replacement treatment of thyroid hormone.
IrAEs often present as a set of nonspecific symptoms, for example fever and anorexia, which complicates their diagnosis. In patients with adrenal insufficiency in particular, differentiation from syndrome of inappropriate secretion of antidiuretic hormone (SIADH) and sepsis is necessary (4). In our case, the only blood test abnormalities were mild hyponatremia and hyper-CRP without hypoglycemia. Thus, it was difficult to detect the endocrinological abnormalities during the early stage with routine blood tests. As the patient had anorexia and a strong sense of fatigue in this case, addressing the patient's subjective symptoms was important for the early detection of endocrinological irAEs. It is necessary for a doctor prescribing ICPIs to consider that a patient may develop irAEs, and providing the patient with education about irAEs may be useful facilitating their early recognition (4).
Although hormone replacement is used to treat adrenal gland hypofunction, the proper replacement dose is still unknown. Ipilimumab-induced hypophysitis is often treated with high-dose corticosteroids (prednisolone, 1 mg/kg) (9). Okano et al. reported that an advanced malignant melanoma patient with adrenal failure due to nivolumab-induced hypophysitis was treated with hydrocortisone (100 mg/day) (15). In our case, the patient not only had adrenal failure but also hyperthyroidism, which suggested that the clearance of adrenocortical hormone may have been enhanced, and that the replacement dose that was required would be more than that required for adrenal failure alone. His condition improved immediately after the start of hydrocortisone (200 mg/day) treatment. When patients have hypothyroidism with adrenal insufficiency, steroid therapy should be initiated before thyroid hormone replacement because the administration of thyroid hormone without steroids may be life-threatening to such patients.
Whether patients can continue or resume nivolumab after they have irAEs will depend on the type of irAE. The cases of hypophysitis induced by ICPIs, ipilimumab and nivolumab, could be managed with hydrocortisone replacement therapy (15,16). It was reported that patients with ipilimumab-induced hypothyroidism could continue ipilimumab treatment with thyroid hormone replacement (17). In this case, the patient was able to resume nivolumab treatment with glucocorticoid hormone and thyroid hormone replacement treatment. One study reported that patients with ipilimumab-induced hypophysitis required prolonged hormone replenishment treatment (18); thus, long-term follow-up is required in the present case.
It is hypothesized that irAEs are related to the efficacy of nivolumab for lung cancer. One report showed that the objective response rate (ORR) and progression-free survival (PFS) of patients with irAEs was significantly higher and longer in comparison to patients without irAEs (19). In this case, nivolumab was effective against lung cancer.
In conclusion, we reported a case of hypophysitis, secondary adrenal insufficiency and destructive thyroiditis induced by nivolumab. We used a higher dose glucocorticoid hormone in comparison to other reports describing the treatment of irAEs. One should keep in mind the possibility that nivolumab can induce multiple irAEs, and the possibility of endocrinological irAEs should be considered when patients present nonspecific symptoms. When a patient has hypophysitis, one should evaluate whether they also have adrenal insufficiency. Nivolumab treatment may be continued safely with appropriate replacement treatment if patients have destructive thyroiditis and adrenal insufficiency.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Dr. James G. Martin (McGill University, Montreal, Canada) for his helpful advice and for checking the English of this paper.
References
- 1. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44: 51-60, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Kimura T, Fukushima S, Miyashita A, et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci 107: 1055-1058, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shirai T, Sano T, Kamijo F, et al. Acetylcholine receptor binding antibody-associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient with melanoma. Jpn J Clin Oncol 46: 86-88, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Lemech C, Arkenau HT. Novel treatments for metastatic cutaneous melanoma and the management of emergent toxicities. Clin Med Insights Oncol 6: 53-66, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeng MF, Chen L, Ye HY, et al. Primary hypothyroidism and isolated ACTH deficiency induced by nivolumab therapy. Medicine (Baltimore) 96: e8426, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kastrisiou M, Kostadima FL, Kefas A, et al. Nivolumab-induced hypothyroidism and selective pituitary insufficiency in a patient with lung adenocarcinoma: a case report and review of the literature. ESMO Open 2: e000217, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123-135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627-1639, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary 19: 82-92, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Corsello SM, Barnabel A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 98: 1361-1375, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Ishikawa M, Oashi K. Case of hypophysitis caused by nivolumab. J Dermatol 44: 109-110, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Honegger J, Schlaffer S, Menzel C, et al. Diagnosis of primary hypophysitis in Germany. J Clin Endocrinol Metab 100: 3841-3849, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka R, Fujisawa Y, Maruyama H, et al. Nivolumab-induced thyroid dysfunction. Jpn J Clin Oncol 46: 575-579, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab 100: 1738-1741, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Okano Y, Satoh T, Horiguchi K, et al. Nivolumab-induced hypophysitis in a patient with advanced malignant melanoma. Endocr J 63: 905-912, 2016. [DOI] [PubMed] [Google Scholar]
- 16. Marlier J, Cocquyt V, Brochez L, Van Belle S, Kruse V. Ipilimumab, not just another anti-cancer therapy: hypophysitis as side effect illustrated by four case-reports. Endocrine 47: 878-883, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30: 2691-2697, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Johnson DB, Friedman DL, Berry E, et al. Survivorship in immune therapy: assessing chronic immune toxicities, health outcomes, and functional status among long-term ipilimumab survivors at a single referral center. Cancer Immnol Res 3: 464-469, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115: 71-74, 2018. [DOI] [PubMed] [Google Scholar]


