Abstract
The central nervous system is a common site of relapse in patients receiving crizotinib, which is presumed to be associated with the low concentration of crizotinib in the cerebrospinal fluid (CSF). Our patient received surgical treatment for anaplastic lymphoma kinase-positive stage IIA lung adenocarcinoma. His cancer recurred with brain metastases and carcinomatous meningitis. We started whole-brain radiation therapy (WBRT) and subsequently administered crizotinib. The concentration of crizotinib on day 15 in the plasma was 158 ng/mL, and that in the spinal fluid was 4.32 ng/mL. WBRT may elevate the CSF/plasma crizotinib concentration ratio; clinicians may therefore consider performing WBRT prior to crizotinib initiation.
Keywords: anaplastic lymphoma kinase, central nervous system, cerebrospinal fluid, crizotinib, non-small cell lung cancer
Introduction
The echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion gene was first identified in 2007 by Soda et al., and they reported the gene rearrangement in 6.7% (5/75) of the examined patients who had non-small cell lung cancer (NSCLC) (1). Treatment with ALK-tyrosine kinase inhibitors (TKIs) showed superiority over chemotherapy, and ALK-TKIs are recommended for the first-line treatment of NSCLC patients positive for the ALK fusion protein.
Crizotinib was the first drug approved for treating advanced ALK-positive NSCLC. Although alectinib showed a superior survival benefit compared to crizotinib, crizotinib is still a key drug for the treatment of ALK-positive NSCLC. Furthermore, crizotinib was approved for the treatment of advanced NSCLC with a ROS1 mutation.
The central nervous system (CNS) is a common site of relapse in patients with progressive disease who are receiving crizotinib (2). One possible reason for this is the low concentration of crizotinib in the cerebrospinal fluid (CSF). However, to date, there have only been three cases reported in the literature of a low crizotinib concentration in the CSF (3,4).
We herein report the fourth case of ALK-positive advanced NSCLC and carcinomatous meningitis.
Case Report
A 61-year-old man visited our hospital complaining of diplopia and incontinence. He had a history of stage IIA lung adenocarcinoma with EML4-ALK fusion, which was confirmed by immunohistochemistry and fluorescence in situ hybridization, treated by right lower lobectomy and adjuvant chemotherapy (cisplatin and vinorelbine). He also had a history of type C hepatitis and cirrhosis.
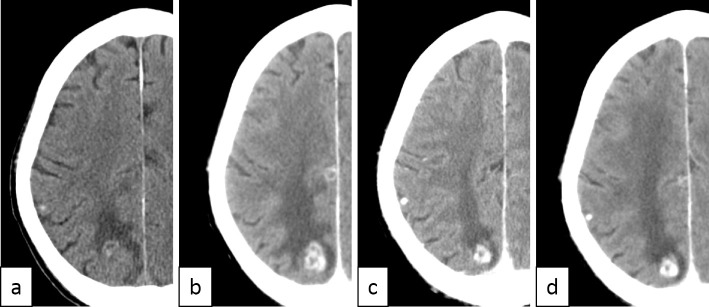
A physical examination revealed left oculomotor nerve palsy and perianal sensory impairment. Meningeal irritation was not apparent. Computed tomography (CT) of the head showed a nodular lesion in his right posterior lobe, suggesting recurrence of lung cancer with brain metastasis (Figure a). A further examination using brain magnetic resonance imaging could not be performed because of tattoos present on his entire body other than his face, hands, and feet. A cytological analysis of the CSF revealed adenocarcinoma positivity, and reverse transcription-polymerase chain reaction revealed that this adenocarcinoma was positive for the ALK fusion gene. We clinically diagnosed the patient with brain metastasis of lung cancer and carcinomatous meningitis.
Figure.
Computed tomography findings of the brain metastasis. a) Relapse of brain metastasis and carcinomatous meningitis. Whole-brain radiation therapy (WBRT) was initiated. b) One month after performing WBRT and just prior to crizotinib initiation, progression of the metastatic lesion was observed. A cytological analysis of the cerebrospinal fluid (CSF) was positive for malignant cells. c) One month after crizotinib initiation, the response of the metastatic lesion was observed. A cytological analysis of the CSF was negative for malignant cells. d) One month after withdrawal of crizotinib, no remarkable change was observed.
We started the patient on whole-brain radiation therapy (WBRT). Despite the radiation therapy, his symptoms worsened, and he developed aspiration pneumonia. Head CT showed tumor progression after 1 month of radiation therapy (Figure b). We started administration of 250 mg crizotinib twice daily after improvement in the pneumonia. One month after crizotinib initiation, his diplopia improved, and head CT showed shrinkage of the metastatic lesion (Figure c). A cytological analysis of the CSF was now negative for malignant cells. Crizotinib concentrations in the CSF and plasma on day 15 were 4.32 ng/mL and 158 ng/mL, respectively. Despite the efficacy of this drug, we had to withdraw crizotinib due to grade 3 AST/ALT elevation two months after crizotinib initiation. The diplopia worsened, and disturbance of the consciousness was again observed. Head CT revealed no remarkable changes (Figure d). After improvement in side effects, we restarted crizotinib at 200 mg twice daily (80% dose). However, the patient's condition worsened, and he died of carcinomatous meningitis one month after re-administration.
We were unable to administer other ALK-TKIs because crizotinib was the only drug available for ALK-positive NSCLC at the time.
This study was approved by the Institutional Review Board of Shimane University and National Cancer Center Hospital. The crizotinib concentration was measured at the National Cancer Center Institute (UMIN000015840).
Discussion
This case had two important clinical findings. First, a low crizotinib concentration in the CSF was observed in our patient, consistent with the findings of the three previous ALK-positive NSCLC cases reported in the literature. Second, WBRT may slightly elevate the crizotinib concentration in the CSF.
With regard to the first finding, we noted in the present case that crizotinib concentrations in the CSF and plasma on day 15 were 4.32 ng/mL and 158 ng/mL, respectively; hence, the CSF-to-plasma concentration ratio was 0.026. Similarly, Costa et al. reported crizotinib concentrations in the CSF and plasma of 0.616 ng/mL and 237 ng/mL, respectively (3), and Metro et al. reported 2 patients with CSF crizotinib concentrations of 0.35 ng/mL and 0.80 ng/mL in the plasma and 587 ng/mL and 800 ng/mL in the plasma, respectively (4) (Table). In contrast, it has been reported that alectinib penetrates the CNS, and there is a linear relationship between alectinib concentrations in the CSF and plasma (5). Both crizotinib and alectinib are oil-soluble drugs; however, with regard to the oil/water coefficient, alectinib has a higher oil solubility than crizotinib (6,7). Although crizotinib is a substrate of the P-glycoprotein efflux transporter, alectinib is not (8). These characteristics contribute to the differences between crizotinib and alectinib in the CSF concentration and treatment outcome (9,10).
Table.
Crizotinib and Alectinib Concentrations in the CSF and Plasma.
| Reference | CSF | Plasma | CSF/plasma | WBRT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crizotinib (ng/mL) | 3 | 0.616 | 237 | 0.003 | + | |||||
| 4 | 0.35 | 587 | 0.0006 | - | ||||||
| 0.80 | 800 | 0.001 | + | |||||||
| Our case | 4.32 | 158 | 0.026 | + | ||||||
| Alectinib (nmol/L) | 5 | 2.69 | 3.12 | 0.86 |
CSF: cerebrospinal fluid, WBRT: whole-brain radiation therapy
Metro et al. also suggested the possibility that WBRT elevates the crizotinib concentration in the CSF (4). The CSF and plasma crizotinib concentration ratios in the 4 published cases, including the present case, ranged between 0.0006 and 0.026. The patient who showed the smallest ratio had not received WBRT prior to crizotinib administration. This tendency was also observed in the present case, and similar trends were reported for HER2-positive breast cancer patients with brain metastases receiving the anti-HER2 monoclonal antibody trastuzumab (11). Stemmler et al. presented clinical evidence that the trastuzumab levels in the CSF are increased under conditions that impair the blood-brain barrier, such as radiotherapy. Although the median progression-free survival (PFS) after crizotinib treatment reported in a previous study was 10.9 months (12), we found that the re-administration of crizotinib did not show efficacy and resulted in a PFS of only approximately 2 months. It is presumed that the poor efficacy of re-administration is associated with an inadequate crizotinib concentration in the CSF rather than tolerance to crizotinib.
With regard to the ALK-TKIs approved by the Food and Drug Administration (FDA) to date, crizotinib and ceritinib are substrates of the P-glycoprotein efflux transporter, and alectinib is the only ALK-TKI that is not its substrate (13). Crizotinib is the only drug approved by the FDA for the treatment of advanced NSCLC with a ROS1 mutation. The half-maximum inhibitory concentration of crizotinib in vitro is reported to be 36-108 ng/mL (3,13), which is higher than that reported in the CSF. Although Costa et al. suggested that the CNS benefits even from low concentrations of crizotinib in the CSF (3), performing WBRT prior to crizotinib to elevate the drug concentration should be considered.
Conclusion
Crizotinib remains a key drug for the treatment of NSCLC patients positive for ALK fusion protein or a ROS1 mutation. However, in the present case, the treatment attempt failed because the crizotinib concentration in the CSF was low, similar to the observations in the three previously reported cases. As WBRT may increase the crizotinib concentration in the CSF, clinicians should consider performing WBRT prior to crizotinib initiation.
The study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments. The study was approved by the institutional review board of Shimane University and National Cancer Center. Informed consent was obtained from the patient described in the study.
Author's disclosure of potential Conflicts of Interest (COI).
Takeshi Isobe: Honoraria, Boehringer-Ingelheim, AstraZeneca and Pfizer.
References
- 1. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561-566, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Ou SH, Janne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 25: 415-422, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 29: e443-e445, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Metro G, Lunardi G, Floridi P, et al. CSF Concentration of crizotinib in two ALK-positive non-small-cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol 10: e26-e27, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 15: 1119-1128, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Xalkori interview form. 9th ed. Pfizer, Tokyo, 2017 (in Japanese).
- 7.Alecensa interview form. 7th ed. Chugai Pharmaceutical, Tokyo, 2017 (in Japanese).
- 8. Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 74: 1023-1028, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377: 829-838, 2017. [DOI] [PubMed] [Google Scholar]
- 10. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390: 29-39, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti-cancer Drugs 18: 23-28, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371: 2167-2177, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Katayama R, Sakashita T, Yanagitani N, et al. P-glycoprotein mediates ceritinib resistance in anaplastic lymphoma kinase-rearranged non-small cell lung cancer. EBioMedicine 3: 54-66, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]