Abstract
Background
This study aimed to investigate the association of pre-treatment inflammatory status with survival time and to develop a prognostic nomogram incorporating inflammatory cytokines in non-Hodgkin's lymphoma.
Methods
A total of 228 patients with diffuse large B-cell lymphoma (DLBCL) received R-CHOP-based regimens from a prospective randomized study (NCT01852435) were included as a training cohort. Other cohorts of 886 lymphoma patients were served as validation cohorts. Lymphocyte-monocyte ratio (LMR), serum levels of soluble interleukin s(IL)-2R, IL-6, IL-8, IL-10 and tumor necrosis factor-α (TNF-α), were assessed before treatment. Least absolute shrinkage and selection operator (LASSO) regression were used to select variables for nomogram of overall survival (OS). The predictive accuracy of the nomogram was determined by concordance index (C-index).
Findings
The nomogram included lactate dehydrogenase (LDH), sIL-2R, TNF-α and decreased LMR. The C-index of the nomogram for OS prediction were range from 0.61 to 0.86 for training cohort of DLBCL and validation cohorts of DLBCL, PTCL, NKTCL and ASCT, which were superior to the predictive power of International Prognostic Index (IPI, 0.67 to 0.84) or NCCN-IPI (0.59 to 0.78), but not in those of indolent lymphoma like FL and MALT.
Interpretations
The nomogram incorporating inflammatory cytokines provides a useful tool for risk stratification in aggressive non-Hodgkin's lymphomas.
Fund
National Natural Science Foundation of China, the Shanghai Commission of Science and Technology, Multicenter Clinical Research Project by Shanghai Jiao Tong University School of Medicine, Clinical Research Plan of SHDC, and Chang Jiang Scholars Program.
Keywords: Non-Hodgkin's lymphoma, Lymphocyte-monocyte ratio, Interleukin-2 receptor, Tumor necrosis factor-α, Prognosis
Research in context.
Multiple clinical prognostic indexes have been developed in non-Hodgkin's lymphoma, but predictive value differs from histological subtypes. It is thus important to establish a predictive model independent on histological subtypes. Inflammatory status plays an essential role on lymphoma progression. No large-scale study on the association of inflammatory status with clinical outcomes has been performed in non-Hodgkin's lymphoma, especially in Chinese patients. Here we used the test and validation cohorts of 1114 lymphoma patients and established a new prognostic nomogram, providing a useful tool of risk stratification for aggressive lymphomas.
Alt-text: Unlabelled Box
1. Introduction
Non-Hodgkin's lymphomas (NHLs) are the most common hematological malignancies worldwide and represent a heterogeneous entity. Multiple clinical prognostic indexes have been developed and proved helpful for risk stratification. However, predictive value of the clinical indexes may differ from histological subtypes of lymphomas. For example, International Prognostic Index (IPI) [1] and National Comprehensive Cancer Network-IPI (NCCN-IPI) [2] work more efficiently in B-cell lymphomas, while Prognostic Index for PTCL-U (PIT) [3] and Korean Prognostic Index (KPI) [4] in T-cell and natural-killer/T-cell lymphoma (NKTCL). Therefore, it remains great interests to establish a prognostic nomogram easily applicable and well adapted to various lymphomas subtypes.
In addition to lymphoma cells themselves, tumor microenvironment plays an important role on disease progression. Inflammatory status is essential in tumor microenvironment, inducing by antigen stimulation, autoimmune disorders, or environmental conditions during lymphomagenesis [5]. Inflammatory-IPI model [6] was proposed to predict prognosis in diffuse large B-cell lymphoma (DLBCL) patients treated with rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone, R-CHOP), based on inflammatory factors lactate dehydrogenase (LDH), absolute lymphocyte count (ALC), albumin (ALB), β2-microglobulin (β2-MG), C-reactive protein (CRP), and ferritin. Inflammation-based cumulative prognostic score system (ICPS) [7], also developed from DLBCL, includes lymphocyte-monocyte ratio (LMR), ALB and CRP. Besides inflammatory factors, serum cytokines stimulate lymphocyte proliferation and regulate balance of T-cell subsets. High pre-treatment serum level of soluble interleukin-2 receptor (sIL-2R) predicts inferior outcomes in DLBCL [8], PTCL [9], NKTCL [10] and follicular lymphoma (FL) [11], while interleukin-6 (IL-6) only in DLBCL [8]. Interleukin-8 (IL-8) produced by DLBCL cells recruits neutrophils [12] and elevates in patients with FL and mucosal-associated lymphoid tissue (MALT) lymphoma [13]. IL-10 is required for induction of CD4+ regulatory T cells [14], and increased IL-10 is associated with poor prognosis in NKTCL [15]. Similarly, dysregulation of tumor necrosis factor (TNF)/TNF receptor signaling contributes to adverse clinical features in DLBCL [8] and PTCL [16].
To further determine the clinical relevance of inflammatory status on disease progression of lymphoma patients, we detected serum inflammatory cytokines in Chinese cohorts of various lymphoma subtypes and established a prognostic nomogram incorporating inflammatory cytokines for the estimation of survival time in aggressive NHLs.
2. Methods and materials
2.1. Patients and treatments
A total of 1114 patients were enrolled in this study, including 990 de novo cases (DLBCL, n = 538, PTCL, n = 134, NKTCL, n = 105, FL, n = 151, MALT, n = 62) and 124 cases undergone autologous stem cell transplantation (ASCT) (DLBCL, n = 77, PTCL, n = 38, NKTCL, n = 9). The histological diagnosis was established according to World Health Organization (WHO) classification [17]. Among DLBCL patients, 228 patients were enrolled in a randomized controlled phase III trial (NCT01852435) who received six courses of R-CHOP, at either standard doses (doxorubicin 50 mg/m2, R-CHOP50 or epirubucin 70 mg/m2, R-CEOP70), or a high dose (epirubucin 90 mg/m2, R-CEOP90), followed by additional two cycles of rituximab and referred as the training cohort. The validation cohorts included 310 DLBCL, 151 FL and 62 MALT patients treated with R-CHOP-based regimens, 134 PTCL patients with CHOP-based regimens, 105 NKTCL patients with pegasparagase-based regimens, and 124 patients with ASCT. Patients were stratified according to IPI [1], NCCN-IPI [2], inflammatory-IPI [6], ICPS [7], PIT [3] or KPI [4] as necessary. The study was approved by the Institutional Review Board with informed consent obtained in accordance with the Declaration of Helsinki.
2.2. Inflammatory factors and cytokines assessment
Serum specimens were collected at diagnosis or before ASCT. β2-MG was detected using chemiluminescence immunoassay and CRP using rate nephelometry method. As for cytokines, sIL-2R, IL-8, IL-10 and TNF-α were assessed by IMMUNITE 1000 chemiluminescence analyzer (Siemens), and IL-6 by IMMUNITE 2000 chemiluminescence analyzer (Siemens). Lower limit of detection (LOD) for sIL-2R, IL-6, IL-8, IL-10 and TNF-α were 5 U/ml, 2 pg/ml, 5 pg/ml, 5 pg/ml and 4 pg/ml, respectively. Upper LOD for sIL-2R, IL-6, IL-8, IL-10 and TNF-α were 7500 U/ml, 1000 pg/ml, 7500 pg/ml, 1000 pg/ml and 1000 pg/ml, respectively. T-cell subsets containing CD3+, CD3 + CD4+, CD3 + CD8+, CD4 + CD28+, CD8 + CD28+, CD4 + CD45RA+, CD4 + CD45RO+, CD4 + CD25+, CD4 + CD25 + CD127(low), CD3 + HLA-DR+, CD3 + CD69+, NK (CD56 + CD16+) and absolute count of CD3+, CD4+, CD8+ T cells were detected in DLBCL patients of the training and validation cohorts by flow cytometry (BD FACSCanto II).
2.3. Construction and validation of the nomogram
In the design of the nomogram, we incorporated clinical features and inflammatory factors as prognostic features. These factors included age, gender, performance status (ECOG), Ann Arbor stage, extranodal involvement, LDH, LMR, ALB, β2-MG, CRP, sIL-2R, IL-6, IL-8, IL-10 and TNF-α. We used least absolute shrinkage and selection operator (LASSO) regression with 10-fold cross-validation to select the most useful predictive variables via minimum criteria for nomogram of overall survival (OS) from the training cohort. Nomogram validation comprised several stages. Internal validation was first undertaken, with a concordance index (C-index) being estimated. Next, calibration curves were plotted to determine whether the predicted and observed probabilities for survival time were in concordance. Bootstrap resampling (1000 resamples) was used for this plot. Finally, external validation was performed, in which the nomogram was used to assess each patient in the validation cohorts, and Cox regression analysis in total score of each patient as an independent factor. The regression analysis was then carried out to derive the C-index and the calibration curve. Comparisons between nomogram and other prognostic models were evaluated by C-index.
2.4. Statistical analysis
All statistical analysis was carried out using R software (version 3.4.3; http://www.R-project.org) and Statistical Package for the Social Sciences (SPSS) 20.0 software (SPSS Inc., Chicago, IL, USA). Association between clinical characteristics and inflammatory factors/cytokines, or between disease progression and T-cell subsets were assessed using independent-sample t-test. OS was measured from the date of diagnosis to the date of death or the last follow-up. Survival functions were estimated using the Kaplan-Meier method and compared by the log-rank test.
3. Results
3.1. Clinical characteristics
Clinical characteristics of the training and validation cohorts were shown in Table S1. Serum levels of inflammatory factors were well balanced among histological subtypes, including LMR, ALB, β2-MG, CRP, cytokines sIL-2R, IL-6, IL-8, IL-10 and TNF-α. All the patients were categorized according to IPI and NCCN-IPI, and patients with available data categorized according to inflammatory-IPI and ICPS.
3.2. Inflammatory factors and cytokines
In the training cohort of DLBCL, serum levels of LDH, β2-MG, CRP, sIL-2R, IL-6, IL-8, IL-10 and TNF-α were significantly elevated, while LMR and ALB reduced, as compared to healthy volunteers (Table S2). Meanwhile, association of inflammatory factors with clinical characteristics was analyzed. Patients >60 years had higher levels of β2-MG than those of patients ≤60 years. Decreased levels of LMR, ALB, but increased levels of LDH, β2-MG, CRP, sIL-2R, IL-6, IL-10 and TNF-α were closely related to advanced Ann Arbor stage and poor performance status. Increased levels of sIL-2R were observed in patients with bone marrow or liver involvement, and increased levels of TNF-α in patients with lung involvement.
3.3. Nomogram development and internal validation
The median OS of the training cohort of DLBCL was 32.8 (2.6–57.6) months, with 4-year OS rate of 83.9%. The 2-year OS of the validation cohorts of DLBCL, PTCL, NKTCL, FL, MALT and ASCT were 79.6%, 42.7%, 76.9%, 96.5%, 98.2% and 78.1%, respectively.
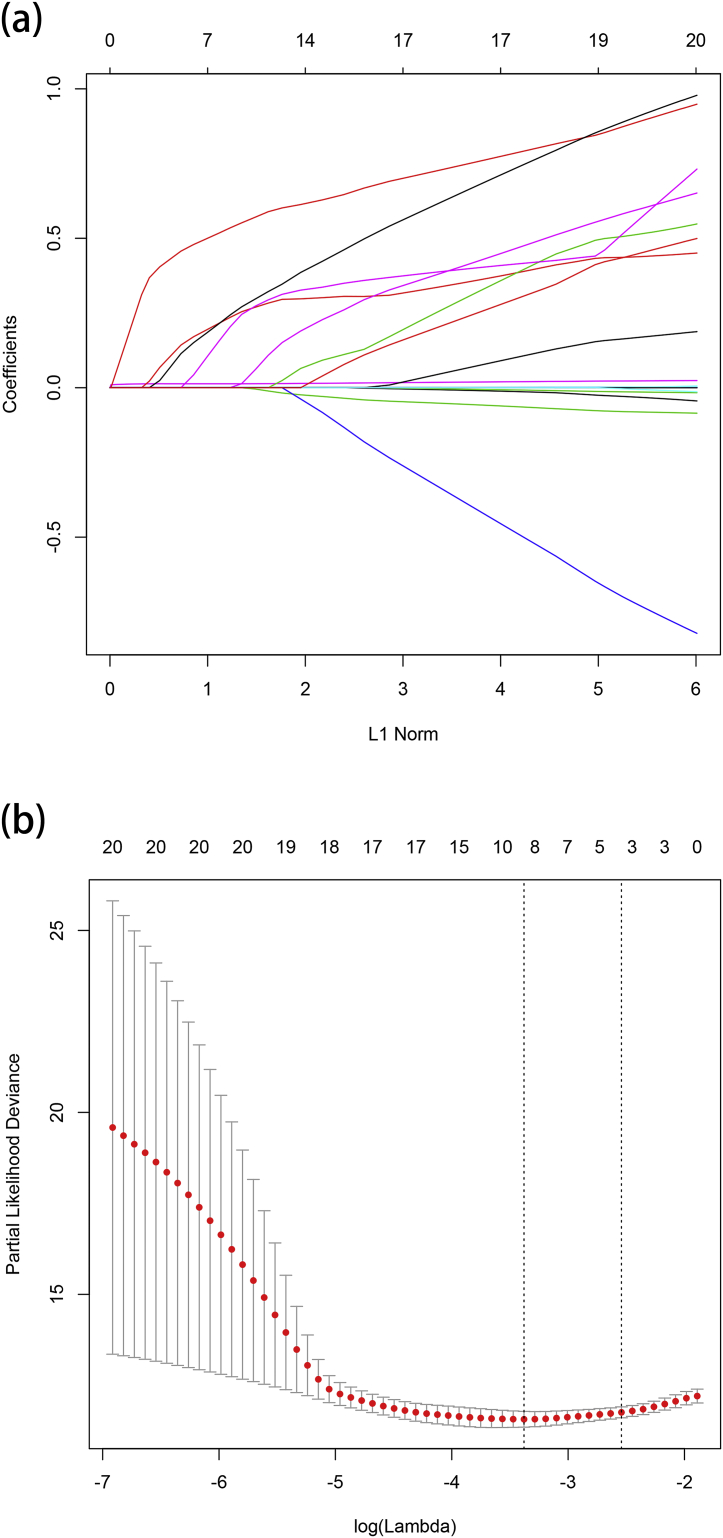
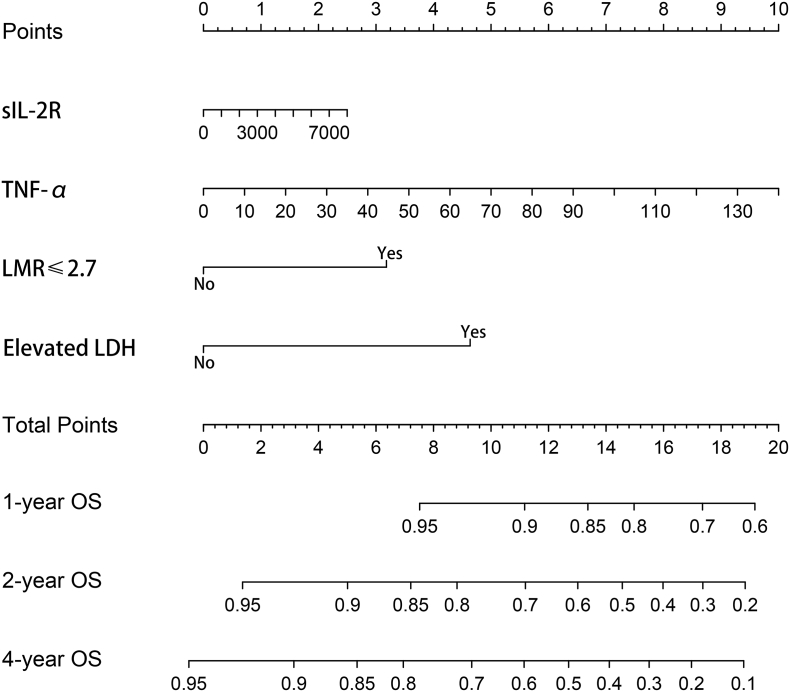
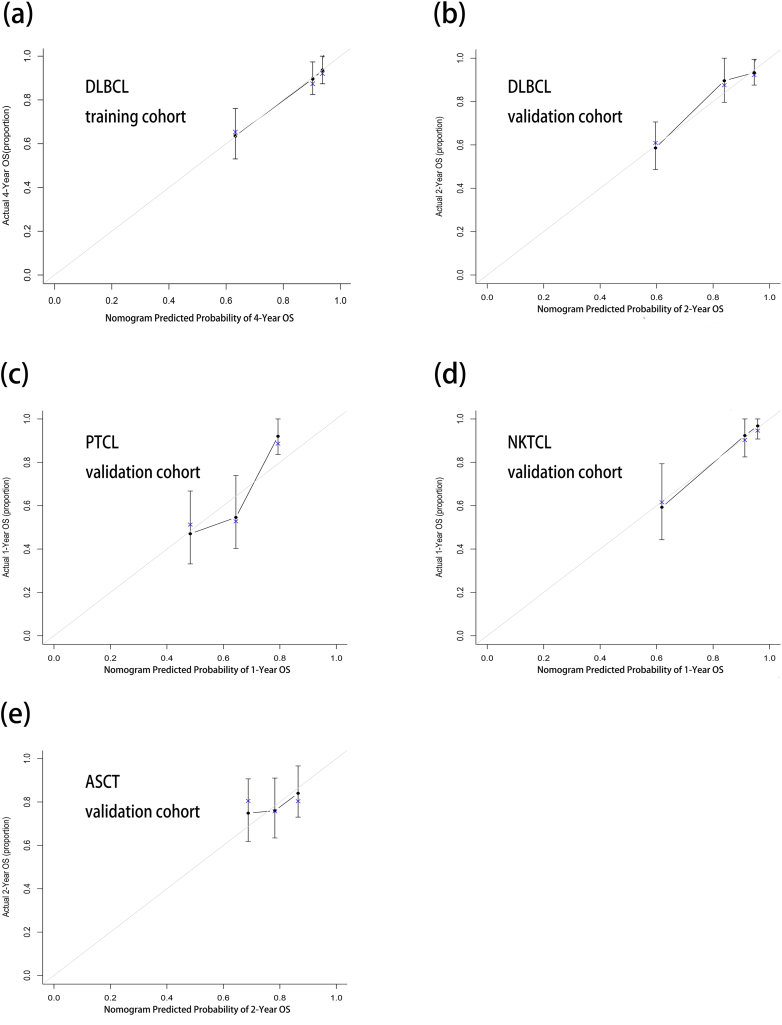
For the development of nomogram, we incorporated clinical features and inflammatory factors as prognostic features: age, gender, performance status, Ann Arbor stage, extranodal involvement, LDH, LMR, ALB, β2-MG, CRP, sIL-2R, IL-6, IL-8, IL-10 and TNF-α. All these parameters were reduced to the most useful 4 potential predictors for OS in the training cohort, with nonzero coefficients in the LASSO cox regression model (Fig. 1). Nomogram to predict 4-year OS was developed using the results from the LASSO cox regression model. sIL-2R, TNF-α, LMR ≤2.7 and elevated LDH were independent risk factors (Fig. 2 and Table 1). The predictive accuracy for OS as measured by C-index was 0.78 in the internal validation. The calibration plot for the probability of 4-year OS showed a good correlation between the actual observed outcome and the prediction by the nomogram (Fig. 3a).
Fig. 1.
Feature selection using the least absolute shrinkage and selection operator (LASSO) cox regression model. (a) LASSO coefficient profiles of the 15 features for OS. (b) Tuning parameter (lamda) selection in the LASSO model used 10-fold cross-validation via minimum criteria for OS.
Fig. 2.
Nomogram of OS in aggressive non-Hodgkin's lymphomas.
Table 1.
Risk factors for the nomogram of overall survival in DLBCL.
| β | Overall survival |
p value | |
|---|---|---|---|
| HR (95% CI) | |||
| sIL-2R | <0.001 | 1.000 | <0.001 |
| TNF-α | 0.029 | 1.030 (1.025 to 1.035) | <0.001 |
| LMR ≤ 2.7 | 1.530 | 4.610 (4.250 to 4.970) | <0.001 |
| Elevated LDH | 1.720 | 5.590 (5.230 to 5.950) | <0.001 |
Fig. 3.
Calibration curves of the nomogram in training cohort and validation cohorts of lymphoma. (a) Prediction of 4-year OS in training cohort of DLBCL. (b) Prediction of 2-year OS in validation cohort of DLBCL. (c) Prediction of 1-year OS in PTCL validation cohort. (d) Prediction of 1-year OS in NKTCL validation cohort. (e) Prediction of 2-year OS in ASCT validation cohort.
3.4. External validation of nomogram
The nomogram was externally validated in the DLBCL, PTCL, NKTCL, FL, MALT and ASCT validation cohorts. The C-index of the nomogram for the prediction of 2-year OS was 0.81 in DLBCL. The models also showed a good level of discriminative ability to predict 1-year OS in PTCL (0.68) and NKTCL (0.86). However, the nomogram was not effectively significant in FL and MALT. In the ASCT group, the nomogram significantly predicted prognosis, but C-index (0.61) was lower. The nomogram was well calibrated as revealed by the calibration curves (Fig. 3b to e) and performed well in predicting OS of patients with aggressive NHLs at diagnosis or after ASCT.
3.5. Comparison of nomogram with clinical prognostic indexes
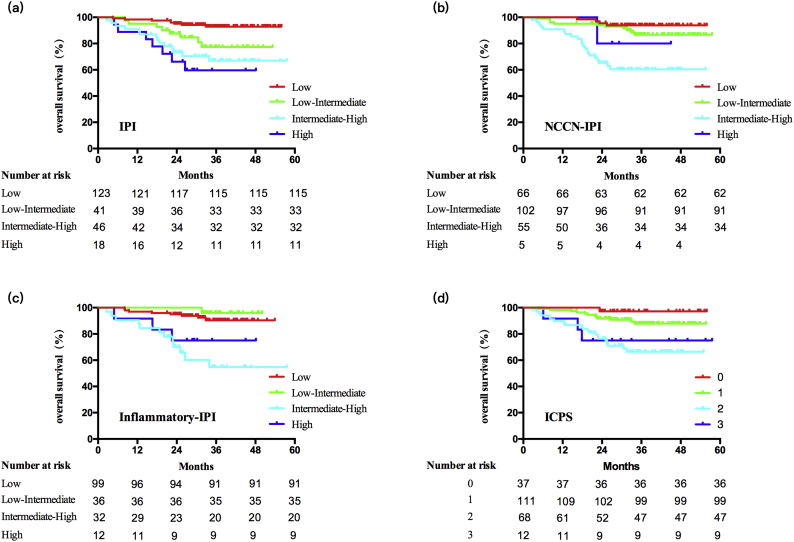
IPI, NCCN-IPI and inflammatory-IPI showed good levels of risk stratification both in training cohort (0.72, 0.72, 0.69) (Fig. 4) and validation cohorts of DLBCL (0.77, 0.76, 0.74), PTCL (0.67, 0.67, 0.68) and NKTCL (0.84, 0.78, 0.82), as well as ICPS in DLBCL (0.69 both in training and validation cohort), PIT (0.67) in PTCL and KPI (0.76) in NKTCL, NCCN-IPI (0.59) in ASCT (Table 2). The nomogram displayed the highest levels of accuracy to predict OS in all cohorts. Therefore, the prognostic nomogram works accurately and well adapted for patients with aggressive NHLs.
Fig. 4.
Kaplan-Meier survival curves of the training cohort of DLBCL. (a) International Prognostic Index (IPI). (b) National Comprehensive Cancer Network-International Prognostic Index (NCCN-IPI). (c) Inflammatory-IPI. (d) Inflammation-based cumulative prognostic score (ICPS).
Table 2.
The C-index of the nomogram in DLBCL training cohort and DLBCL, PTCL, NKTCL, ASCT validation cohorts.
| Training cohort (n = 228) | DLBCL validation cohort (n = 310) | PTCL validation cohort (n = 134) | NKTCL validation cohort (n = 105) | ASCT validation cohort (n = 124) | |
|---|---|---|---|---|---|
| Nomogram | 0.78 | 0.81 | 0.68 | 0.86 | 0.61 |
| IPI | 0.72 | 0.77 | 0.67 | 0.84 | NA |
| NCCN-IPI | 0.72 | 0.76 | 0.67 | 0.78 | 0.59 |
| Inflammatory-IPI | 0.69 | 0.74 | 0.68 | 0.82 | |
| ICPS score | 0.69 | 0.69 | NA | NA | |
| PIT | 0.67 | ||||
| KPI | 0.76 |
3.6. Association of inflammatory cytokines with T-cell subsets
T-cell subsets were assessed in peripheral blood of 518 DLBCL patients. Patients were divided into the group with early death (OS ≤6 months) and with non-early death (OS >6 months). In early death group, absolute count of CD3 + T and CD4 + T cells, as well as percentage of functional subset of inductor/helper T cells (CD4 + CD28+) and cytotoxic T cells (CD8 + CD28+) were significantly decreased, while early-activated T cells (CD3 + CD69+) and late-activated T cells (CD3 + HLA-DR+) were significantly increased, as compared to non-early death group (Table S3).
4. Discussion
Inflammatory status provoked by tumor microenvironment plays an important role in disease progression of NHLs. Aiming to establish a prognostic system independent on histological subtypes of lymphoma, and possibly independent on therapeutic strategies (chemotherapy, ASCT, etc). We performed assessment on multiple inflammatory factors, as well as serum cytokines closely related to host immunity status. Meanwhile, both training and validation cohorts were applied to further improve the accuracy of the prognostic index. LMR, as a routinely available index which reflects host systemic immunity, predicts survival in DLBCL [18], NKTCL [19], and FL [20]. For cytokines, sIL-2R is critically involved in T-cell activation. Pre-treatment serum sIL-2R is associated with inferior outcomes of DLBCL both in pre-rituximab and rituximab era [8], PTCL [9], NKTCL [10] and FL [11]. TNF-α acts as a tumor-promoting factor and contributes to aggressive clinical behavior in DLBCL [8] and PTCL [16]. To our knowledge, this is the largest study on the association of inflammatory status with clinical outcomes in NHLs. Using a randomized study to define the possible role of serum cytokines in the prospective setting, a nomogram incorporating with inflammatory factors was set up and appeared more effective than clinical prognostic index. Therefore, in addition to tumor-associated LDH, immune response to tumor burden such as LMR, sIL-2R and TNF-α are valuable prognostic biomarkers, reflecting the combined effect of tumor and microenvironment on lymphoma progression.
Decreased LMR represents an imbalance between immunosurveillance and immunosuppression in lymphomas. Lymphopenia is considered as a poor prognostic factor in DLBCL [21], PTCL [22] and NKTCL [23], at diagnosis and after ASCT [24]. Monocytes promote tumor angiogenesis and metastasis via tumor-associated macrophages [25]. In our study, decreased LMR was resulted from lymphopenia and increased monocytes, which negatively regulated host immunity and contributed to lymphoma progression. IL-2R, a membrane receptor for IL-2, expressed on the surface of activated T-cells and shed into the circulation in a soluble form as sIL-2R [26]. sIL-2R was associated with increased late-activated T-cells [27] and decreased cytotoxic T-cells [28]. Here we showed that early-death group presented with significant elevation of late-activated T-cells and reduction of cytotoxic T-cells, which correspond to the immunosuppressive effect of sIL-2R on patients with extreme poor prognosis. TNF-α is a pro-inflammatory cytokine that has both anti-inflammatory and immunosuppressive effect. Positively correlated with late-activated T-cells [27], chronic stimulation with TNF-α may induce CD4 + T-cell exhaustion and impairs CD4 + T cell-mediated immunological control [29]. Meanwhile, TNF-R1-dependent TNF-α signaling triggered CD8 + T-cell death and inhibited accumulation of tumor-infiltrating CD8 + T cells [30]. Thus, in addition to sIL-2R, TNF-α induced suppression of CD4+ effector T-cells and cytotoxic T-cells, and served as another important component of prognostic nomogram incorporating with inflammatory status.
The nomogram is useful to conduct risk-adapted therapeutic strategies. Efficient in aggressive subtype DLBCL, PTCL and NKTCL, the nomogram is not suitable for indolent subtype FL and MALT, probably due to the very good prognosis achieved upon R-CHOP-based chemotherapy. ASCT is considered as an important therapeutic strategy in high-risk patients with age adjusted-IPI (aa-IPI) score 2–3 [31,32]. However, the indication for front-line ASCT is controversial. Accordingly, using our prognostic nomogram, we also identified a subset of the patients with aa-IPI 2–3, but presented with a long-term survival without ASCT. For these patients, ASCT may not be necessary as front-line therapy. Meanwhile, there were still patients suffered from relapse or progression after ASCT. For these patients, instead of ASCT, novel therapeutic strategies should be applied, for example, ibrutinib targeting MYD88 L265P/CD79B mutant [33], lenalidomide targeting MYC-translocation [34], and chimeric antigen receptor therapy (Car-T) directly targeting immune dysregulation. Further studies should be conducted to validate the potential application of nomogram in patients treated with Car-T.
In conclusion, the nomogram proposed in this study incorporates inflammatory factors and cytokines, reflecting tumor burden and immunosuppressive microenvironment in lymphoma. It predicted the overall survival time more accurately than clinical prognostic index, providing a useful tool for risk stratification in aggressive NHLs.
Acknowledgments
Acknowledgements
We appreciate the effort of the physicians for enrolling patients and thank all the patients involved for allowing us to analyze their clinical data.
Funding
This study was supported, in part, by research funding from the National Natural Science Foundation of China (81520108003 and 81830007), the Shanghai Commission of Science and Technology (16JC1405800), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152206 and 20152208), Multicenter Clinical Research Project by Shanghai Jiao Tong University School of Medicine (DLY201601), Clinical Research Plan of SHDC (16CR2017A), and Chang Jiang Scholars Program.
Conflicts of interests
All authors declare no potential conflict of interest
Author contributions
HJZ, JC and SC performed the project, collected and analyzed the data, and wrote the article. SNC provided material support. RS, QS and MCZ collected the data. PPX, HYH and LW provided statistical analyses and support. DPW and WLZ designed and supervised the study, and wrote the article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.02.048.
Contributor Information
Depei Wu, Email: drwudepei@163.com.
Weili Zhao, Email: zhao.weili@yahoo.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.International Non-Hodgkin's Lymphoma Prognostic Factors P A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Z., Sehn L.H., Rademaker A.W. An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallamini A., Stelitano C., Calvi R. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 4.Lee J., Suh C., Park Y.H. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612–618. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 5.Baecklund E., Smedby K.E., Sutton L.A., Askling J., Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders--what are the driving forces? Semin Cancer Biol. 2014;24:61–70. doi: 10.1016/j.semcancer.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Kim C., Lee H., Jo J. Clinical usefulness of inflammatory factors based modified international prognostic index in diffuse large B cell lymphoma treated with rituximab combined chemotherapy. Blood. 2016;128:4220. [Google Scholar]
- 7.Sun F., Zhu J., Lu S. An inflammation-based cumulative prognostic score system in patients with diffuse large B cell lymphoma in rituximab era. BMC Cancer. 2018;18(1):5. doi: 10.1186/s12885-017-3931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dlouhy I., Filella X., Rovira J. High serum levels of soluble interleukin-2 receptor (sIL2-R), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF) are associated with adverse clinical features and predict poor outcome in diffuse large B-cell lymphoma. Leuk Res. 2017;59:20–25. doi: 10.1016/j.leukres.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Gupta M., Stenson M., O'Byrne M. Comprehensive serum cytokine analysis identifies IL-1RA and soluble IL-2Ralpha as predictors of event-free survival in T-cell lymphoma. Ann Oncol. 2016;27(1):165–172. doi: 10.1093/annonc/mdv486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Liao D.Z., Zhang J., Xia Z.J., Peng X.W., Lu Y. Clinical significance of serum soluble interleukin-2 receptor-alpha in extranodal natural killer/T-cell lymphoma (ENKTL): a predictive biomarker for treatment efficacy and valuable prognostic factor. Med Oncol. 2013;30(4):723. doi: 10.1007/s12032-013-0723-4. [DOI] [PubMed] [Google Scholar]
- 11.Kusano Y., Yokoyama M., Terui Y. High pretreatment level of soluble interleukin-2 receptor is a robust prognostic factor in patients with follicular lymphoma treated with R-CHOP-like therapy. Blood Cancer J. 2017;7(9):e614. doi: 10.1038/bcj.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfroi B., McKee T., Mayol J.F. CXCL-8/IL8 produced by diffuse large B-cell lymphomas recruits neutrophils expressing a proliferation-inducing ligand APRIL. Cancer Res. 2017;77(5):1097–1107. doi: 10.1158/0008-5472.CAN-16-0786. [DOI] [PubMed] [Google Scholar]
- 13.Miyata-Takata T., Takata K., Toji T. Elevation of serum interleukins 8, 4, and 1beta levels in patients with gastrointestinal low-grade B-cell lymphoma. Sci Rep. 2015;5 doi: 10.1038/srep18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncarolo M.G., Gregori S., Battaglia M., Bacchetta R., Fleischhauer K., Levings M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Wang L., Wuxiao Z. Increased serum levels of interleukin-10 predict poor prognosis in extranodal natural killer/T-cell lymphoma patients receiving asparaginase-based chemotherapy. Onco Targets Ther. 2015;8:2589–2599. doi: 10.2147/OTT.S91077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heemann C., Kreuz M., Stoller I. Circulating levels of TNF receptor II are prognostic for patients with peripheral T-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18(13):3637–3647. doi: 10.1158/1078-0432.CCR-11-3299. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow S.H., Campo E., Pileri S.A. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox R.A., Ristow K., Habermann T.M. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25(9):1502–1509. doi: 10.1038/leu.2011.112. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q.X., Li S.H., Ji B.Y. Lymphocyte/monocyte ratio is a novel predictor for early stage extranodal natural killer/T-cell lymphoma, nasal type. J Cancer. 2017;8(6):1030–1037. doi: 10.7150/jca.17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.F., Luque-Fernandez M.A. Prognostic value of lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio in follicular lymphoma: a retrospective cohort study. BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-017904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox M.C., Nofroni I., Ruco L. Low absolute lymphocyte count is a poor prognostic factor in diffuse-large-B-cell-lymphoma. Leuk Lymphoma. 2008;49(9):1745–1751. doi: 10.1080/10428190802226425. [DOI] [PubMed] [Google Scholar]
- 22.Mitrovic Z., Perry A.M., Suzumiya J. The prognostic significance of lymphopenia in peripheral T-cell and natural killer/T-cell lymphomas: a study of 826 cases from the international peripheral T-cell lymphoma project. Am J Hematol. 2012;87(8):790–794. doi: 10.1002/ajh.23205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J.J., Jiang W.Q., Lin T.Y. Absolute lymphocyte count is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. Ann Oncol. 2011;22(1):149–155. doi: 10.1093/annonc/mdq314. [DOI] [PubMed] [Google Scholar]
- 24.Porrata L.F., Inwards D.J., Ansell S.M. New-onset lymphopenia assessed during routine follow-up is a risk factor for relapse postautologous peripheral blood hematopoietic stem cell transplantation in patients with diffuse large B-cell lymphoma. Biol Blood Marrow Transplant. 2010;16(3):376–383. doi: 10.1016/j.bbmt.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Dirkx A.E., Oude Egbrink M.G., Wagstaff J., Griffioen A.W. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80(6):1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 26.Rubin L.A., Kurman C.C., Fritz M.E. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135(5):3172–3177. [PubMed] [Google Scholar]
- 27.Rea I.M., McNerlan S.E., Alexander H.D. CD69, CD25, and HLA-DR activation antigen expression on CD3+ lymphocytes and relationship to serum TNF-alpha, IFN-gamma, and sIL-2R levels in aging. Exp Gerontol. 1999;34(1):79–93. doi: 10.1016/s0531-5565(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 28.Gooding R., Riches P., Dadian G., Moore J., Gore M. Increased soluble interleukin-2 receptor concentration in plasma predicts a decreased cellular response to IL-2. Br J Cancer. 1995;72(2):452–455. doi: 10.1038/bjc.1995.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyer M., Abdullah Z., Chemnitz J.M. Tumor-necrosis factor impairs CD4(+) T cell-mediated immunological control in chronic viral infection. Nat Immunol. 2016;17(5):593–603. doi: 10.1038/ni.3399. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand F., Rochotte J., Colacios C. Blocking tumor necrosis factor alpha enhances CD8 T-cell-dependent immunity in experimental melanoma. Cancer Res. 2015;75(13):2619–2628. doi: 10.1158/0008-5472.CAN-14-2524. [DOI] [PubMed] [Google Scholar]
- 31.Cortelazzo S., Tarella C., Gianni A.M. Randomized trial comparing R-CHOP versus high-dose sequential chemotherapy in high-risk patients with diffuse large B-cell lymphomas. J Clin Oncol. 2016;34(33):4015–4022. doi: 10.1200/JCO.2016.67.2980. [DOI] [PubMed] [Google Scholar]
- 32.Chiappella A., Martelli M., Angelucci E. Rituximab-dose-dense chemotherapy with or without high-dose chemotherapy plus autologous stem-cell transplantation in high-risk diffuse large B-cell lymphoma (DLCL04): final results of a multicentre, open-label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18(8):1076–1088. doi: 10.1016/S1470-2045(17)30444-8. [DOI] [PubMed] [Google Scholar]
- 33.Wilson W.H., Young R.M., Schmitz R. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamuleau M., Nijland M., Zijlstra J. Successful treatment of MYC rearrangement positive large B cell lymphoma patients with R-CHOP21 plus lenalidomide: results of a multicenter phase II HOVON trial. Blood. 2018;132:876. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material