Abstract
Background
Gut integrity is compromised in abdominal sepsis with increased cellular apoptosis and altered barrier permeability. Intestinal epithelial cells (IEC) form a physiochemical barrier that separates the intestinal lumen from the host's internal milieu and is strongly involved in the mucosal inflammatory response and immune response. Recent research indicates the involvement of the stimulator of interferons genes (STING) pathway in uncontrolled inflammation and gut mucosal immune response.
Methods
We investigated the role of STING signaling in sepsis and intestinal barrier function using intestinal biopsies from human patients with abdominal sepsis and with an established model of abdominal sepsis in mice.
Findings
In human abdominal sepsis, STING expression was elevated in peripheral blood mononuclear cells and intestinal biopsies compared with healthy controls, and the degree of STING expression in the human intestinal lamina propria correlated with the intestinal inflammation in septic patients. Moreover, elevated STING expression was associated with high levels of serum intestinal fatty acid binding protein that served as a marker of enterocyte damage. In mice, the intestinal STING signaling pathway was markedly activated following the induction of sepsis induced by cecal ligation perforation (CLP). STING knockout mice showed an alleviated inflammatory response, attenuated gut permeability, and decreased bacterial translocation. Whereas mice treated with a STING agonist (DMXAA) following CLP developed greater intestinal apoptosis and a more severe systemic inflammatory response. We demonstrated that mitochondrial DNA (mtDNA) was released during sepsis, inducing the intestinal inflammatory response through activating the STING pathway. We finally investigated DNase I administration at 5 hours post CLP surgery, showing that it reduced systemic mtDNA and inflammatory cytokines levels, organ damage, and bacterial translocation, suggesting that inhibition of mtDNA-STING signaling pathway protects against CLP-induced intestinal barrier dysfunction.
Interpretation
Our results indicate that the STING signaling pathway can contribute to lethal sepsis by promoting IEC apoptosis and through disrupting the intestinal barrier. Our findings suggest that regulation of the mtDNA-STING pathway may be a promising therapeutic strategy to promote mucosal healing and protect the intestinal barrier in septic patients.
Fund
National Natural Science Foundation of China.
Keywords: STING, Sepsis, IEC apoptosis, Intestinal inflammation
Research in context.
Evidence before the study
The gut has been suggested as the ‘central organ’ of organ failure. Gut integrity is compromised in abdominal sepsis with altered barrier permeability. Recent studies demonstrate the involvement of STING signaling in gut mucosal immune homeostasis. However, whether STING-mediated intestinal inflammation is responsible for gut barrier injury in sepsis remains to be clarified.
Added value of this study
In the present study, we demonstrated that intestinal STING was activated in human abdominal sepsis, and hyperactivation of intestinal STING signaling led to hyper-inflammation, IECs apoptosis, intestinal barrier dysfunction, and lethal sepsis.
Implication of all the available evidence
These data suggest that PAMPs and/or DAMPs could activate STING signaling pathway in sepsis. Activated STING signaling contributes to over-exuberant inflammatory and IFNs responses during sepsis, which may contribute to comprehensive epithelial damage, allowing viable bacteria to translocate. Therefore, STING signaling may be an effective therapeutic target in human sepsis.
Alt-text: Unlabelled Box
1. Introduction
Sepsis has been recently defined as a life-threating organ dysfunction secondary to a dysregulated host immune response to infection [1]. The gut has been described as the motor of sepsis and organ failure [2]. Although the pathogenesis of sepsis is complicated, numerous studies have demonstrated increased apoptosis in intestinal epithelial cells (IECs), resulting from the enhanced secretion of pro-inflammatory cytokines and interferon [3]. Disruption of the gut barrier may drive lethal sepsis and multiple organ failure.
The progression from infection to sepsis can be demonstrated by the detection of pathogen (PAMPs) and danger (DAMPs) associated molecular patterns that are derived from microbes and host tissues, respectively [4]. Specific pattern recognition receptors (PRRs) bind to PAMPs and/or DAMPs and stimulate the secretion of inflammatory mediators which amplify the local inflammatory response, leading to tissue and organ damage associated with sepsis. Our recent studies indicated that microbial components, such as CpG DNA and cyclic dinucleotides (CDNs), are recognized by PRRs to mount Th17 responses and induce the mucosal inflammatory response [5,6]. We also showed that released mitochondrial DAMPs (mitochondrial DNA) from IECs following infection are associated with an increased inflammatory response and subsequent gut barrier injury [7]. Collectively, the synergistic effects of PAMPs and DAMPs seem to induce intestinal mucosal inflammation. However, the precise function and mechanisms of PAMPs and/or DAMPs sensors in intestinal inflammation have yet to be clarified.
The stimulator of interferons genes (STING) is an adaptor protein involved in the innate immune response to the bacterial product CDNs [8]. Apart from microbial sensing, STING can also be activated by host self-DNA [9]. After nuclear DNA (nDNA) damage or mitochondrial disruption, nDNA or mitochondrial DNA (mtDNA) is released into the cytosol, stimulating STING signaling [10,11]. The activation of STING initiates interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), leading to enhanced expression of type I interferons (IFNs) and inflammatory cytokines [12,13]. Increasingly evidence suggests that aberrant activation of the STING pathway has been associated with the pathogenesis of metabolic disorders, infections and inflammatory disease [9,12,14].
The emerging role of STING in gut homeostasis has been revealed by several recent studies. Fisher et al. [15] investigated the role of STING signaling in tissue repair during acute intestinal damage, and found that modulation of STING may offer a strategy to reduce epithelial barrier dysfunction and promote epithelial integrity from intestinal damage by chemotherapy. Canesso et al. [16] confirmed that STING is an important signaling molecule involved in maintaining gut homeostasis. However, Jiang's group recently demonstrated that STING signaling may be related to sepsis-associated intestinal injury through induction of a significant inflammatory response [17]. Furthermore, Konrad et al. [18] suggested that STING signaling could promote TNF production and cell death in intestinal organoids. Whether STING-mediated intestinal inflammation is responsible for intestinal barrier dysfunction during sepsis therefore remains to be determined.
We hypothesised that STING is involved in the pathogenesis of sepsis by mediating IEC apoptosis induced by increased intestinal inflammation. We demonstrated that PAMPs and/or DAMPs could activate STING signaling pathway in abdominal-sepsis patients and CLP-induced sepsis mouse models. We further showed the activation of STING was associated with IEC damage, intestinal mucosal inflammation, and severity of sepsis. In addition, Deoxyribonuclease I (DNase I) administration post CLP surgery reduced systemic mtDNA and inflammatory cytokines levels, organ damage, and bacterial translocation.
2. Methods
2.1. Ethics statement
This study was carried out according to the Recommendations of Guidelines for Clinical Trials by the Ethics Committee of Jinling Hospital. All animal experiments in our study were carried out according to the principles of the Declaration of Helsinki, and were approved by the Animal Ethical Committee of Jinling Hospital. The protocol was approved by the Ethics Committee of Jinling Hospital. All patients provided written informed consent before any study-related procedure was performed.
2.2. Humans
Intestinal biopsies from five adult patients diagnosed with abdominal sepsis complicated with enterocutaneous fistula were obtained using biopsy forceps through the fistula. Representative abdominal pictures for fistula patients are shown in Supplementary Fig. 1. Five patients with enterocutaneous fistulas and radiological evidence (CT scan and fistulography) of intraperitoneal infection leading to life-threatening organ dysfunction (Sepsis 3 definition) were enrolled in this study (Supplementary Fig. 2A). Intestinal biopsies were obtained from patients undergoing formation of served as controls. Informed consent was obtained from all participants.
2.3. Animals and the Cecal Ligation Perforation (CLP) model
STING-KO (Tmem173−/−) and WT C57BL/6J mice (8–12 weeks) obtained from Model Animals Research Center of Nanjing University were maintained under specific conditions in a temperature-controlled room. Experiments were performed with randomly chosen littermates of the same sex and body weight and matched age.
The CLP model was used to induce polymicrobial sepsis as previously discussed [19]. Briefly, anesthesia was induced using ketamine (80 to 100 mg/kg, ip) and xylazine (10 to 12.5 mg/kg, ip). A midline incision was made in the peritoneum and the cecum was exteriorized. Fifty percent of the cecum was ligated and punctured twice with a 21-G needle, and a small drop of cecal content was extruded. The cecum was then returned to the peritoneal cavity and the abdominal incision closed with sutures. Mice were injected subcutaneously with 1 ml of Ringer's solution including analgesia (buprenorphine, 0.05 mg/kg). Antibiotics (25 mg/kg imipenem and 25 mg/kg cilastatin) were administered 3 hours post-CLP. Following CLP treatment, mice received an intraperitoneal (i.p.) injection of 10 mg/kg STING agonists (DMXAA; MedChem Express).
2.4. Measurement of intestinal permeability and bacterial translocation
All mice were administered fluorescein isothiocyanate (FITC)-dextran (FD40) by gavage at a dose of 600 mg/kg. Serum FD40 levels were evaluated to test intestinal permeability in vivo using fluorometry. To test gut permeability in vitro, Ussing Chamber analysis was performed as previously described [20].
Mesenteric lymph nodes (MLN) were harvested from mice and were homogenized using aseptic techniques. Diluted homogenates were cultured on Mac-Con-key's agar at 37 °C for 24 h. Moreover, blood samples were also obtained for bacterial colony-forming counts (CFU). Bacterial growth on the plates was quantified by colony-forming units/g of tissue [21].
2.5. Histology and immunofluorescence
Pathological slides were fixed in 10% formalin, cut into 4-μm sections, and processed on H&E slides. A gastrointestinal pathologist expert, blinded to the experiments assessed the severity of intestinal injury using a pathological scoring system, as previously described [6,19].
For immunohistochemistry (IHC), slides were treated with anti-STING antibody (D2P2F; 13,647; Cell Signaling), anti-NF-κB antibody (ab16502; Abcam) according to the manufacturer's instructions. Image J software (US National Institutes of Health) was used to convert pixel intensities associated with staining into optical densities.
2.6. Quantitative PCR analysis
Quantification of serum mtDNA was detected as previously described [7]. In brief, circulating DNA from serum was isolated using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's recommendations. qPCR analysis was used to quantify selected mtDNA recognition sequences (COX3 and ND1).
Isolated total RNA from cells or tissues by TRIZOL reagent (Life Technologies Inc., Carlsbad, CA, USA) were reverse-transcribed using the oligo (dT) primed complementary DNA. Real-time PCR was performed by TaqMan Gene Expression Assay (Applied Biosystems) for genes of interest (STING, IRF3, IL-1β, TNF, IL-6). GAPDH was selected as the internal control. The primers for qPCR analyses of the relevant sequences are listed in the Supplementary Table 1. The relative messenger RNA level was quantified as 2-∆Ct (∆Ct, relative cycle threshold compared with GAPDH).
2.7. Western blot analysis
Proteins from the tissues or cells were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were then incubated overnight at 4 °C with antibodies against the protein of interest, including STING (D2P2F; 13,647; Cell signaling), IRF3 (D83B9; 4302; Cell signaling), p-IRF3 (Ser396; 29,047; Cell signaling), p-p65 (ab16502; Abcam), ZO-1 (ab96587; Abcam), Occludin (ab216327; Abcam) overnight at 4 °C. Protein quantification was measured in optical density units using Image Lab software (Bio-Rad, CA, USA) and was normalized to the corresponding sample expression of GAPDH.
2.8. Cell culture preparation
Dendritic cells (DCs) from small intestine lamina propria were isolated as previously described [6]. Briefly, we cut the small intestine into four pieces, and transferred them onto polyethylene tubes. Calcium- and magnesium-free PBS was used to wash three times, and 1 mM dithiothreitol and 30 mM EDTA were used to remove the mucus and epithelium, respectively. 333 μg/ml Liberase TL in DMEM containing 5% fetal bovine serum was used to digest the exposed lamina propria for 100 min at 37 °C in a 5% CO2 humidified incubator. All cells then were passed through a 70 μm nylon cell strainer and centrifuged (density gradient centrifugation) with OptiPrep (ρ = 1.055 g/ml) to yield DCs. Purified DCs were analyzed by FACSalibur flow cytometer (BD Bioscience).
Hind-limb femurs of 8–10 weeks old mice were obtained to isolate bone marrow-derived macrophages (BMDMs) as previously described [22]. In brief, femurs were flushed using a syringe containing RPMI 1640 and 10% FBS. BM cells were collected after centrifugation and exclusion of red blood cells and were cultured with 20% L929 conditioned medium.
2.9. Serum and tissue cytokines levels
The levels of TNF-α, IL-1β, IL-6, monocyte chemo-attractant protein-1 (MCP-1) and human intestinal fatty acid binding protein (I-FABP) in the serum were assessed using an enzyme-linked immunosorbent assay (ELISA) kit (P.R.C KeyGEN biotech). Lactic dehydrogenase (LDH) and alanine transaminase (ALT) were detected by commercial kits in accordance with the manufacture's protocol.
2.10. Analysis of apoptosis
Induction of apoptosis in the intestinal epithelium was detected using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) according to the manufacturer's instructions. The apoptotic index was quantified by the proportion of TUNEL-positive cells [23].
2.11. Statistical analysis
Continuous variables are shown as mean ± SD and a normal distribution test was performed for normality before using the t-test. Differences between groups were tested by the student's t-test or one-way analysis of variance. Categorical data are shown as median ± IQR. All data were analyzed by GraphPad Prism software 6.0 (La Jolla, CA, USA) and SPSS 19.0 (Chicago, IL, USA). The log-rank tests were used to compare the survival rates between groups. All p-values <.05 are considered significant.
3. Results
3.1. Intestinal apoptosis and STING induction in human abdominal sepsis
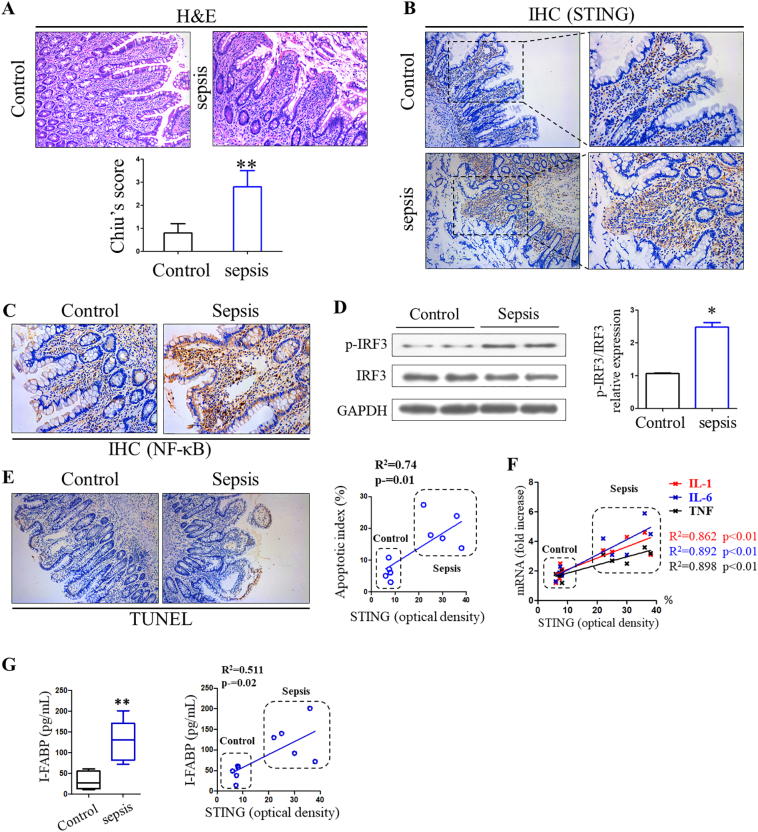
To investigate the role of STING signaling in sepsis and its associated IEC apoptosis, we first determined whether the STING pathway is altered in the primary peripheral blood mononuclear cells (PBMCs) of these patients. STING and IRF3 mRNA expressions were elevated in abdominal sepsis patients compared with healthy controls (Supplementary Fig. 2B). Furthermore, the protein expressions of STING and phosphorylated IRF3 were significantly increased in the sepsis group, suggesting overall activation of STING signaling pathways during human sepsis (Supplementary Fig. 2C). We then investigated the correlation between intestinal STING signaling and intestinal inflammation, IEC apoptosis and enterocyte damage. H&E staining of intestines showed increased intestinal injury, evidenced by tissue destruction and inflammatory infiltration in the sepsis group (Fig. 1A). Immunostaining analysis demonstrated that STING was mainly expressed in the intestinal lamina propria, and this expression was significantly elevated in abdominal sepsis patients. Additionally, STING-controlled IRF3 and NF-κB activation were also activated compared with controls (Fig. 1B, C, and D). TUNEL staining revealed ICE apoptosis in every biopsy obtained from human abdominal sepsis patients compared to healthy controls (Fig. 1E), and linear regression analysis demonstrated a positive correlation between apoptosis index and optical density of STING expression. Apoptosis induction was further confirmed via cleaved caspase-3 (c-c3) staining, a biomarker of apoptosis (Supplementary Fig. 3). Moreover, we found a significant correlation between IL-1, IL-6, and TNF mRNA expression in intestines to STING expression (Fig. 1F).
Fig. 1.
Stimulator of interferon genes (STINGs) was activated in sepsis and correlates with intestinal inflammation and gut barrier dysfunction. (A) Representative images of intestinal histology (H&E staining) in control group and sepsis patients. (B) Expression level of STING and (C) NF-κB in human intestines were analyzed through IHC staining. (D) Activation of IRF3 signaling in gut of sepsis patients. (E) Correlation between apoptosis indexes and expression of STING in human gut. (F, G) Correlation of inflammatory cytokines and I-FABP to the expression level of STING. Image J was used to detect STING optical density, and each symbol represents an individual patient. H&E, hematoxylin and eosin; IHC, immunohistochemistry, IRF3, interferon regulatory factor 3; I-FABP, intestinal fatty acid binding protein. ⁎P < .05, ⁎⁎P < .01 vs control group.
Enterocyte damage helps the intensivist to identify critically ill patients with intestinal damage, bacterial translocation, and SIRS [24]. I-FABP serves as a plasma biomarker for IEC damage, which is associated with sequential organ failure assessment score and mortality [24]. In our study, serum I-FABP levels were markedly increased in the sepsis group compared with controls, indicating evident IEC damage in abdominal-sepsis patients (Fig. 1G). Simultaneously, there was a significant correlation between serum I-FABP and STING density. Collectively, these findings support a potential role for STING in mucosal inflammation and intestinal damage during human sepsis.
3.2. Induction of STING and its downstream effectors by CLP in mouse intestinal mucosa
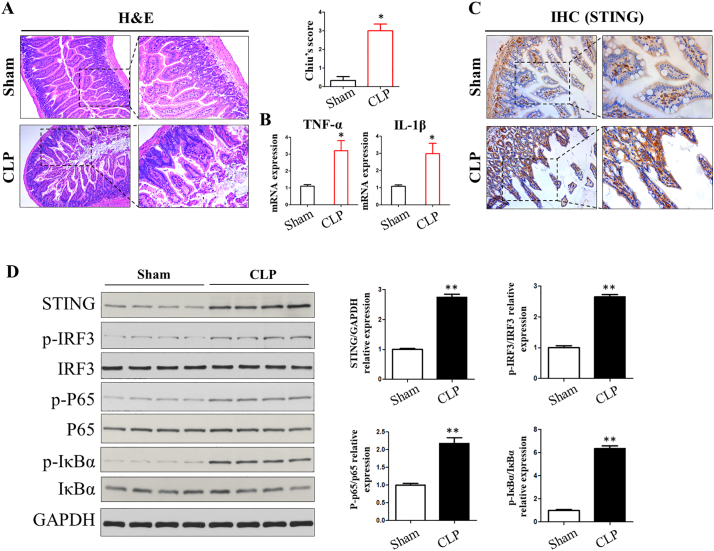
The above findings encouraged us to use a mouse model to further investigate the role of STING signaling in sepsis and intestinal injury. A model of severe polymicrobial sepsis (CLP) is used to produce high mortality, which is the most relevant to the clinical course of abdominal sepsis in humans. Histopathological evaluation of stained tissue sections showed that after severe CLP, WT mice exhibited damaged intestinal villi, inflammatory cell infiltration, and local apoptotic nuclei (Fig. 2A). Increased mRNA expression of inflammatory cytokines (TNF-α and IL-1β) at 24 h was seen in CLP-induced intestinal tissue (Fig. 2B). IHC analysis showed that STING signaling was significantly increased during CLP-induced sepsis (Fig. 2C). Moreover, STING protein expression was increased after CLP treatment, and p-IRF3, p-P65 as well as p-IκBα were activated by western blot (Fig. 2D). Thus, the induction of STING signaling pathway in the gut may suggest its pathological role in CLP-induced sepsis and the intestinal inflammatory response.
Fig. 2.
Induction of STING signaling in CLP-induced sepsis. (A) H&E staining for intestinal histology after CLP treatment. (B) intestinal cytokines levels. (C, D) Activation of STING signaling in intestine following CLP was analyzed by IHC staining and western blot. CLP, cecum ligation perforation. Data are expressed as the mean ± SD. ⁎P < .05 vs sham group; n = 6 mice per group.
3.3. Suppression of CLP-induced sepsis in STING-KO mice
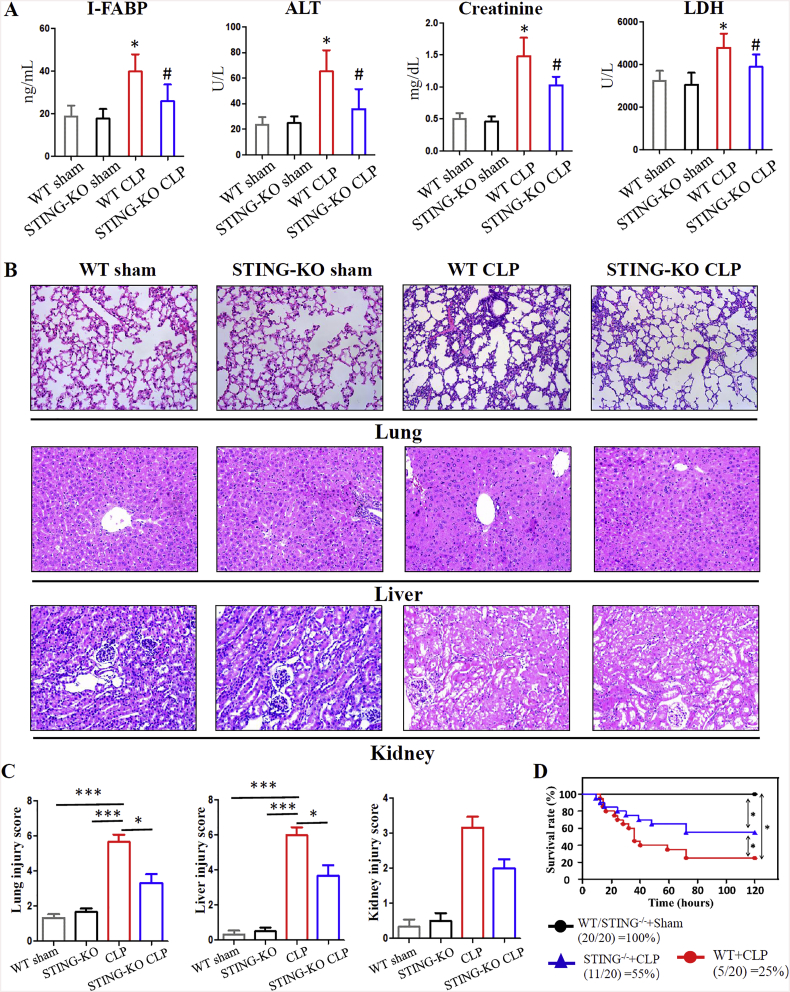
We next sought to investigate whether STING knockout mice are more resistant to polymicrobial sepsis. Organ damage is a hallmark of sepsis, and the concentration of serum I-FABP, ALT, creatine kinase (CK) and LDH were used to detect tissue injury. WT mice showed high levels of serum I-FABP, ALT, and LDH after CLP surgery, while the amount of these organ damage markers was significantly lower in STING KO mice (Fig. 3A). H&E staining confirmed that genetic STING depletion was associated with attenuated tissue injury (lung, liver and kidney) such as leukocyte infiltration and tissue destruction (Fig. 3B). Treatment with a STING agonist was suggested to induce sterile shock [25], therefore we next sought to test the role of STING signaling in systemic inflammation during sepsis. The levels of serum pro-inflammatory cytokines (including IL-6 and TNF-α) and the chemokine MCP-1 significantly increased after CLP. In comparison, STING depletion substantially reduced these serum cytokines levels (Supplementary Fig. 4). These findings indicated that STING signaling may contribute to organ damage and an increased pro-inflammatory response during severe polymicrobial sepsis.
Fig. 3.
STING-KO mice are protected from severe CLP-induced polymicrobial sepsis. (A) levels of serum I-FABP, ALT, Creatinine, and LDH. (B, C) Representative images of histology (H&E staining) and injury scores for lung, liver and kidney. (D) The Kaplan-Meier survival analysis 120 h after CLP in WT and STING-KO mice. The Kaplan-Meier survival curves were compared by the log-rank test. Line-graphs show mean and standard error. ALT, alanine transaminase; LDH, lactic dehydrogenase. Data are expressed as the mean ± SD. ⁎P < .05 vs sham group; #P < .05 vs CLP group. n = 6 mice per group.
Since the above data suggested the pathogenic effect of STING signaling in sepsis, survival analyses were performed. WT mice showed increased mortality after severe CLP, and 15 of 20 WT mice were dead 120 h post-CLP. In comparison, only 9 of 20 STING KO mice were dead following severe CLP (Fig. 3D). Collectively, these data indicate that STING signaling may contribute to disease pathogenesis in severe polymicrobial sepsis.
3.4. Effect of STING knockout on intestinal inflammation, IEC apoptosis and intestinal barrier dysfunction
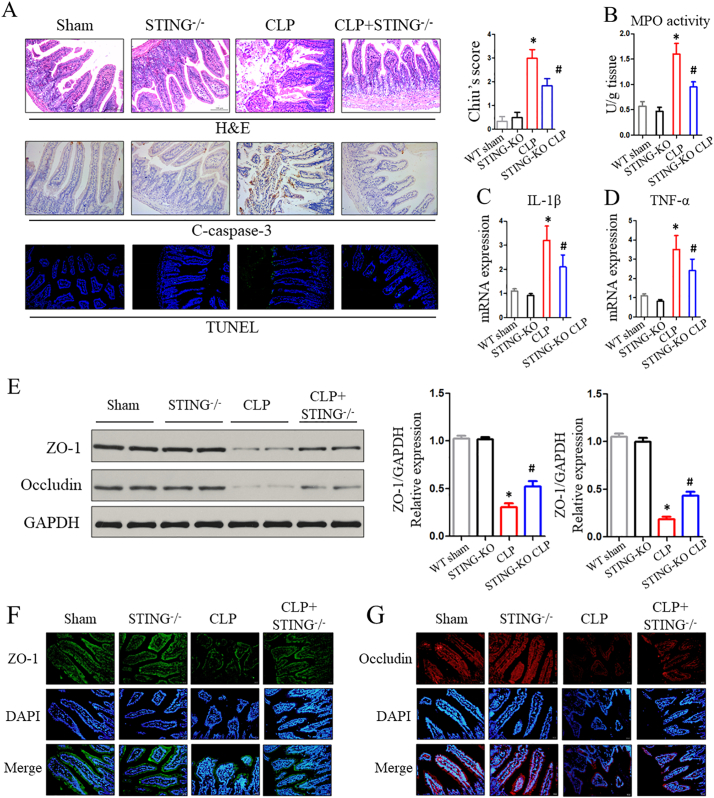
The histological inflammatory scores of mice that underwent severe CLP were markedly higher than those of the sham group by H&E staining evaluation. However, there was a significant reduction in intestinal damage scores in STING KO mice compared to WT mice following CLP (Fig. 4A). The MPO activity in intestinal mucosa was markedly increased after CLP, while there was a decrease in STING-KO sepsis mice compared with CLP-treated WT mice (Fig. 4B). Next, we sought to test whether STING depletion in sepsis could affect the secretion of inflammatory cytokines involved in intestinal inflammation. Our studies showed that severe CLP induced significantly increased production of inflammatory cytokines (including IL-1β and TNF-α) in intestinal mucosa (Fig. 4C and D).
Fig. 4.
Inhibition of the STING signaling protects against CLP-induced intestinal injury. (A) Representative images of H&E, c-caspase3, and TUNEL staining in WT and STING-KO mice after CLP treatment. (B, C, D) MPO activity and inflammatory cytokines within intestinal mucosa. (E) Proteins levels of ZO-1 and occludin in intestinal tissue were assessed by western blot. (F, G) Localization of ZO-1 and occludin within intestinal mucosa evaluated by immunofluorescence after CLP. Data are expressed as the mean ± SD. ⁎P < .05 vs sham group; #P < .05 vs CLP group. n = 6 mice per group.
To determine whether STING signaling is involved in sepsis-induced apoptosis, we compared apoptosis induction in CLP-treated WT and STING KO mice. TUNEL and c-caspase-3 staining analyses were used to test IEC apoptosis. IEC apoptosis was induced following CLP and was blocked in STING KO mice (Fig. 4A). IEC apoptosis is suggested to be one of the critical accelerants of intestinal barrier dysfunction, and tight junction proteins play a vital role in regulating epithelial permeability. In our study, western blot (WB) and immunofluorescence analysis were used to test the tight junction proteins. WB analyses suggested that, following CLP, the levels of ZO-1 and occludin were substantially increased in STING-KO group compare with WT mice (Fig. 4E). Simultaneously, tight junction proteins (ZO-1 and occludin) exhibited decreased surface staining within IECs and some villi of the sepsis-injured mucosa, whereas an improved appearance of tight junction proteins were found in STING KO mice that underwent CLP (Fig. 4F and G).
Both IEC apoptosis and decreased tight junction proteins lead to an increase in intestinal permeability. In this study, after CLP, WT mice showed increased permeability, as evidenced by high levels of serum FD-40 and lower TEER. In comparison, there was significant reduction of intestinal permeability in STING-KO sepsis mice (Supplementary Fig. 5A and B). Increased intestinal permeability may induce bacterial translocation, which is known to lead to lethal sepsis. CLP mice showed significant bacterial translocation to MLN and blood compared with the sham group. However, STING depletion obviously reduced bacterial translocation following severe CLP (Supplementary Fig. 5C and D). Collectively, these data indicate that the STING signaling pathway may lead to an intestinal inflammatory response, IEC apoptosis, and bacterial translocation during severe polymicrobial sepsis.
3.5. Treatment of STING agonist aggravates abdominal sepsis and sepsis-induced IEC apoptosis
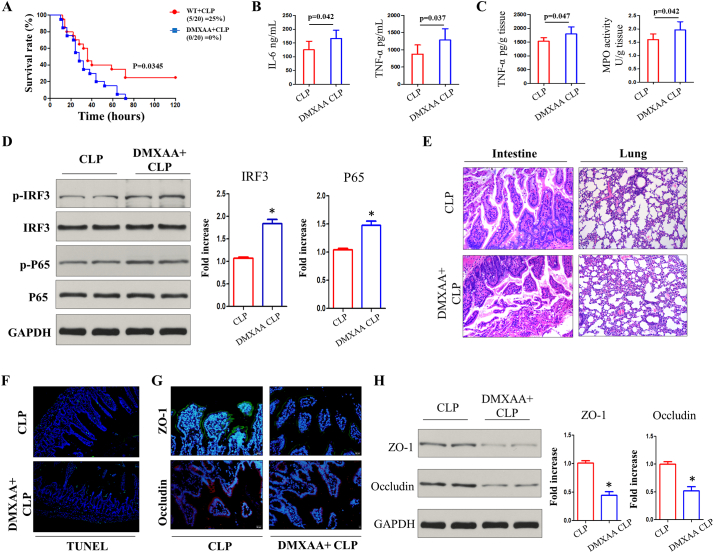
To further confirm the role of STING in sepsis, a pharmacologic agent was used to activate the STING pathway. In comparison with STING depletion, STING activation with DMXAA treatment amplified disease progression and led to higher mortality after CLP (Fig. 5A). The levels of serum pro-inflammatory cytokines (IL-6 and TNF-α) were also increased by DMXAA treatment (Fig. 5B), and intestinal inflammatory levels detected by TNF-α, and MPO activity were further increased by DMXAA treatment (Fig. 5C). Activation of STING signaling with DMXAA treatment (10 mg/kg) is further exhibited by its downstream activation of p-IRF3 and NF-kB (p-P65) in the intestine of severely septic mice (Fig. 5D). Simultaneously. Activation of STING by DMXAA treatment worsened intestinal mucosal and pulmonary injury after severe CLP (Fig. 5E), and DMXAA treatment drove heavier IECs apoptosis by TUNEL staining (Fig. 5F). The levels of ZO-1 and occludin expression were significantly decreased after DMXAA treatment (Fig. 5G and H). Consistent with the above results where STING deficiency led to the alleviation of disease, activation of STING signaling worsened CLP-induced sepsis, intestinal damage and disturbance of the intestinal barrier.
Fig. 5.
Treatment with STING agonist worsens polymicrobial sepsis. (A, B) Survival analysis and serum inflammatory cytokines after i.p. injection of 10 mg/kg DMXAA following CLP. (C) Intestinal TNF-α and MPO activity in CLP group after DMXAA treatment. (D) Activation of STING signaling pathway by i.p. injection of DMXAA following CLP. (E) Representative lung and gut H&E images. (F) Intestinal apoptosis by TUNEL analysis. (G, H) ZO-1 and occluding in intestinal sections were analyzed by immunofluorescence and western blot. MPO, Myeloperoxidase. Data are expressed as the mean ± SD. ⁎P < .05 vs CLP group. n = 6 mice per group.
3.6. Bacteria and mitochondrial DNA are elevated in CLP mice, and activate STING signaling
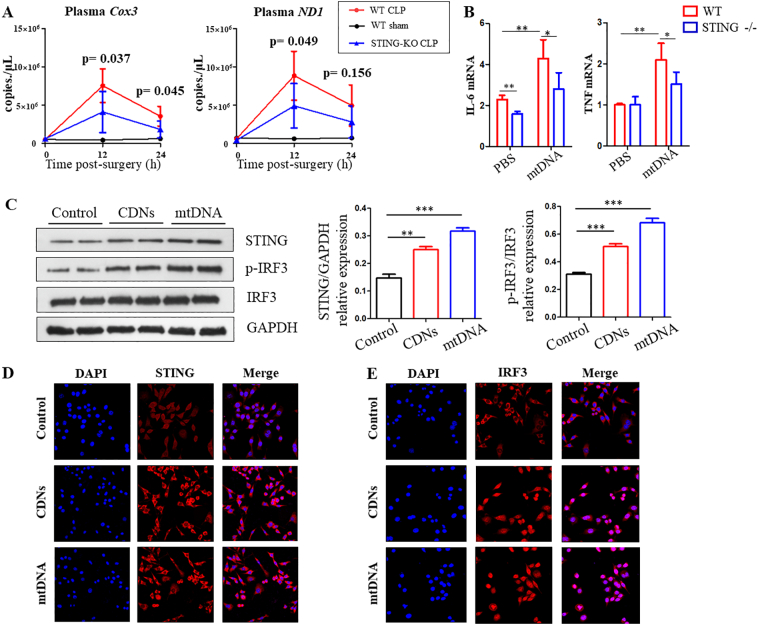
Both bacterial PAMPs and host DAMPs have been implicated in sepsis, and we have previously showed that the synergistic effect of PAMPs and DAMPs could increase the pro-inflammatory response and aggravate the severity of infection or sepsis. In our study bacteraemia and serum mitochondrial DNA levels were substantially elevated in CLP-treated mice (Supplementary Fig. 5 and Fig. 6A). Injection of foreign cyclic dinucleotides (CDNs) into mice induced an obvious elevation in IL-1β, TNF, IL-6 and IFN-β mRNA expression in the intestinal lamina propria classical DCs [6]. To further test the obligatory role of STING in response to DAMPs (mtDNA) in gut, i.p. injection of mtDNA into WT and STING-KO mice was performed. IL-6 and TNF mRNA expression were significantly increased in DCs isolated from the lamina propria; however, STING-deficient mucosal DCs failed to express higher mRNA levels of these mediators following treatment with mtDNA in vivo (Fig. 6B). Moreover, i.p. injection of mtDNA induced acute lung injury (ALI) and systemic inflammation. However, the STING knockout mice demonstrated reduced mtDNA-induced ALI and the associated inflammatory response (Supplementary Fig. 6).
Fig. 6.
Mitochondrial DNA are elevated in CLP-induced sepsis mice and activate IRF3 via STING in cultured BMDMs. (A) Serum mtDNA (COX3 and ND1) levels at 12 h and 24 h after CLP of WT and STING-KO mice. (B) Fold change of IL-6 and TNF gene expression in DCs of WT and STING-KO mice after i.p. injection of mtDNA. (C, D, E) Activation of STING and IRF3 signaling in BMDMs that cultured with CDNs and mtDNA.
To determine the activation of STING pathway, BMDMs were prepared from WT mice. These BMDMs were treated with foreign CDNs and mtDNA complexed with lipofectamine 3000, and the expression and subcellular location of STING signaling in BMDMs were measured by WB and immunostaining. WB data demonstrated that the protein levels of STING and p-IRF3 were increased following CDNs and mtDNA treatment in BMDMs (Fig. 6C). Immunostaining showed that STING was widely distributed in the cytosol in the control group within BMDMs, but treatment with CDNs or mtDNA could induce STING perinuclear translocation (Fig. 6D). In addition, immunostaining suggests mtDNA and CDNs induce IRF3 translocation from the cytoplasm to the nucleus (Fig. 6E). Collectively, these data indicate that increased bacteria and mtDNA can induce activation of the STING signaling pathway involved in increased systemic intestinal inflammatory response, leading to the aggravation of sepsis.
3.7. DNase I prevents sepsis-induced intestinal injury and bacterial translocation
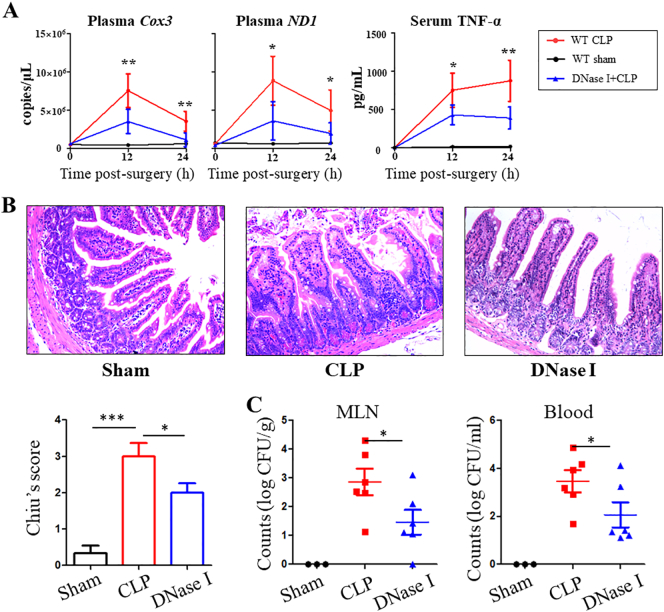
High circulating mtDNA level have been associated with organ injury [26,27], and our data suggest the mtDNA-STING signaling pathway contributes to the exacerbation of sepsis. DNase I has been used to treat patients with various conditions associated with increased cell-free DNA levels [13]. We investigated the therapeutic value of DNase I (i.p. infections 5 h after CLP) on gut following CLP treatment. Expectedly, administration of DNase I after CLP surgery resulted in significant decreases in mtDNA and TNF-α levels (Fig. 7A). Histology scores of intestinal injury were lower in septic mice given DNase I (Fig. 7B). Additionally, a decreased bacterial load was observed in the MLN and blood of septic mice given DNase I (Fig. 7C).
Fig. 7.
DNase I protects against CLP-induced intestinal injury by decreasing mtDNA levels. Mice were subjected to CLP surgery and administrated either DNase or saline 5 hours post-operatively. Blood was collected 12 h and 24 h in CLP-induced sepsis mice and (A) levels of serum mtDNA and TNF-α were quantified. (B) Representative intestinal H&E staining, and histology scores. (C) Bacterial CFU was quantified in MLN and blood. mtDNA, mitochondrial DNA. Data are expressed as the mean ± SD. ⁎P < .05, ⁎⁎P < .01; ⁎⁎⁎P < .001. n = 6 mice per group.
4. Conclusion
Clinically, human septic patients may demonstrate bacterial translocation or circulating DNA from injured host cells, and this is usually associated with poor outcomes. Although the potential mechanisms are yet to be clarified, it has been suggested that elevated PAMPs and/or DAMPs contribute to dysregulated systemic inflammation and intestinal inflammatory response [4].
Almost two decades ago, it was suggested that STING was an important protein for CDNs and a DNA-sensing signaling pathway. Our study has demonstrated the critical involvement of STING-induced excessive inflammation and IEC apoptosis sensing by CDNs and mtDNA during sepsis, leading intestinal barrier damage, increased intestinal permeability. Therefore, blocking the STING signaling may be a promising therapy in modulating excessive inflammatory response and intestinal barrier dysfunction in sepsis.
Consistent with previous studies that the STING signaling pathway plays a central role in CDNs and DNA sensing to induce secretion of IFNs and pro-inflammatory mediators [22,28]. Our data demonstrate that STING depletion diminishes sepsis associated inflammation and intestinal barrier dysfunction, whereas the excessive activation of STING led to the worsening of disease. Therefore, pharmacologic inhibitors of STING signaling will be crucial in targeting IFN and the pro-inflammatory response in sepsis.
The gut has been suggested as the driver of organ failure and our previous results showed that DCs are the predominant antigen presenting cell in the lamina propria that express STING [6]. C-di-GMP stimulation induced strong inflammatory and immune responses depending on the STING signaling in classical DCs from the small intestinal lamina propria in vivo [6]. Our data show that the similar results where repeated injection of mtDNA into mice induced a substantial increase in IL-6 and TNF mRNA expression within DCs in lamina propria. IEC apoptotic cell death has been implicated as a vital homeostatic and pathogenic mechanism of the intestinal epithelium during sepsis. We showed that increased STING signaling is associated with elevated intestinal inflammation and induction of IEC apoptosis in human gut. STING genetic depletion attenuated CLP-induced IEC apoptosis and increased inflammatory response, suggesting the hypothesis that STING signaling function as an important mediator of IECs apoptosis, intestinal homeostasis and an important regulator of sepsis. Collectively, our findings highlight a novel mechanism involving crosstalk between dying IECs and DCs in the gut, providing evidence that the gut plays a central role in the progression of sepsis and MODS [2].
STING regulates gene expression by promoting the phosphorylation of several transcription factors, such as STAT3, STAT6, IRF3, and NF-κB [29,30]. Among these factors, IRF3 is a well-studied target, and it plays a detrimental role in CLP-induced sepsis [31]. Wendy et al. [31] showed that IRF3-KO mice have reduced disease scores, mortality, hypothermia, and bacterial load following CLP compared with the control group. IRF3 also promotes NF-κB transactivation by interacting with NF-κB [32]. In our study, IRF3 signaling was significantly activated in septic patients, and the elevation of IRF3 expression was associated with the severity of sepsis and intestinal injury. An overwhelming inflammatory response during sepsis impairs bacterial clearance, leading to the high bacterial load; however, the STING KO mice following CLP exhibit the lower bacterial load, indicating that the inhibition of the STING-IRF3 signaling pathway has the ability to clear bacteria during severe polymicrobial sepsis.
An amount of CDNs have been detected in the gut, which could induce local activation of STING at steady state. Additionally, continuous sources of free DNA, derived from dying cells and bacteria from microbiota, can also activate STING in the intestinal lamina propria [16]. However, the precise mechanisms and function of DNA sensors in intestinal physiology and inflammation have yet to be defined. Recent studies have also underscored the role of STING signaling in intestinal inflammation and gut homeostasis. Canesso et al. [16] suggested that DNA derived from faeces can activate STING, and they showed that STING KO mice exerts higher susceptibility to DSS-induced colitis. Barber's group demonstrated a key role for STING in interacting with commercial bacteria and influencing gut immune response homeostasis, and they suggested that loss of STING signaling reverse colitis exhibited in the absence of IL-10 in mice [28]. However, Jiang's laboratory recently showed that STING depletion was associated with attenuated tissue injury such as tissue destruction, necrosis, and leukocyte infiltration [15]. Consistent with Jiang's findings, we have showed that STING signaling may contribute to the pathogenesis of sepsis and intestinal pro-inflammatory response, and loss of STING reduces intestinal inflammation and sepsis. The discrepancy between our results and the dataset of Faria and Barber's group is likely due to the different disease and inflammation model. Severe systemic inflammation and organ pro-inflammatory responses are responsible for the high mortality during sepsis. It is well known that the compensatory anti-inflammatory response is an important contribution to the pathophysiology of sepsis. Therefore, the protective effect of sepsis by blocking STING signaling may lead to the decreased pro-inflammatory response.
Increasing plasma mtDNA levels was associated with the occurrence of sepsis and organs damage in ICU patients with trauma and/or infections [26,27]. We recently investigated that released mtDNA following intestinal ischemia reperfusion induced inflammatory response and intestinal barrier dysfunction [7]. Mounting evidence have suggested that mtDNA has ability to activate cGAS-STING-IRF3 pathway, leading various disease and infectious progession [12,13]. Our data suggested that i.p. injection of mtDNA induced tissue injuries and production of inflammatory cytokines within intestinal DCs via STING signaling. Additionally, decreasing systemic mtDNA by DNase I administration significant helps to attenuate the sepsis-induced pro-inflammatory response and intestinal injury. These data indicate that suppression of mtDNA-cGAS-STING signaling pathway may be protective in sepsis-induced organ injury and gut barrier dysfunction.
The host responds to both PAMPs and DAMPs by specific PRRs during infection, resulting in increased production of mediators and inflammatory response [33]. excessive inflammatory response induces organ injury. Sepsis syndrome is such a response that is initiated by both danger and microbial invasion. Modulation of this response to quosh unnecessary local inflammation at the focus of infection is a promising therapeutic goal [4]. Hyper-activation of intestinal inflammation induces IECs apoptosis, contributing to increased bacterial translocation and exacerbation of sepsis. Effective control of the intestinal over-exuberant inflammatory response has the potential to be a therapeutic target [4]. In our study, Decreased intestinal inflammation by STING depletion helps to inhibit the induction of IEC apoptosis during sepsis. Therefore, we propose a theory that stress and infections induced local IEC injury, provides a route for microbial components (CpG DNA/ CDNs) and/or mtDNA derived from IEC to activate STING signaling within classical DCs within the lamina propria. The activation of the STING signaling pathway induces increased production of pro-inflammatory cytokines and IFNs. Intestinal inflammatory response contribute to comprehensive epithelial damage, leading to a number of viable bacteria to translocate. These process not only amplify the activation of the STING signaling pathway, but also can induce the severe systemic inflammatory response, both of which could lead to a cytokines storm and induce lethal sepsis (Supplementary Fig. 7).
Numerous single-target therapies in phase 3 clinical trials have failed to reveal efficacy for the management of human sepsis. In our study, activation of the STING pathway is a crucial step contributing to the pathogenesis of sepsis by promoting intestinal inflammation and damaging gut barrier. Therefore, STING signaling has the potential to be an effective therapeutic target in human sepsis.
Conflict of interests
We declare no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Author contributions
Hu QY, Wu XW, Slade DA, and Ren JA participated in the design of this study, and Huang JJ performed the statistical analysis. Ren HJ carried out the study and collected important background information. Hu QY and Slade DA drafted the manuscript. Hu QY, Zhou Q, Wang DY, Zheng JS, and Chen J carried out the concepts, design, definition of intellectual content, literature search, and data analysis. All authors read and approved the final manuscript.
Data availability
The authors provide detailed description of methods and original data upon request.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81571881, 81772052 and 81801971), and Medical Research Program of Jiangsu Commission of Health (H2018058).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.02.055.
Contributor Information
Dominic A. Slade, Email: dom.slade@talktalk.net.
Xiuwen Wu, Email: lygwxw@163.com.
Jianan Ren, Email: JiananR@gmail.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for Sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittal R., Coopersmith C.M. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–223. doi: 10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claudia G., Helmut N., Neurath M.F., Christoph B. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62(7):1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 4.Rajaee A., Barnett R., Cheadle W.G. Pathogen- and danger-associated molecular patterns and the cytokine response in Sepsis. Surg Infect (Larchmt) 2018;19(2):107–116. doi: 10.1089/sur.2017.264. [DOI] [PubMed] [Google Scholar]
- 5.Liu S., Zhang Y., Ren J., Li J. Microbial DNA recognition by cGAS-STING and other sensors in dendritic cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21(4):901–911. doi: 10.1097/MIB.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 6.Liu S., Xia Q., Wu X., Sun F., Hu Q., Wu J. Stimulator of interferon genes in classical dendritic cells controls mucosal Th17 responses to cyclic Dinucleotides for host Defenses against microbial infections in gut. Front Immunol. 2018;9:1085. doi: 10.3389/fimmu.2018.01085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Hu Q., Ren H., Ren J., Liu Q., Wu J., Wu X. Released mitochondrial DNA following intestinal ischemia reperfusion induces the inflammatory response and gut barrier dysfunction. Sci Rep. 2018;8(1):7350. doi: 10.1038/s41598-018-25387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinho F.V., Benmerzoug S., Oliveira S.C., Ryffel B., Quesniaux V.F.J. The emerging roles of STING in bacterial infections. Trends Microbiol. 2017;25(11):906–918. doi: 10.1016/j.tim.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q., Sun L., Chen Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 10.Mao Y., Luo W., Zhang L., Wu W., Yuan L., Xu H. STING-IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2017;37(5):920–929. doi: 10.1161/ATVBAHA.117.309017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T., Chen Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med. 2018;215(5):1287–1299. doi: 10.1084/jem.20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber G.N. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Q., Knight P.H., Ren Y., Ren H., Zheng J., Wu X. The emerging role of stimulator of interferons genes signaling in sepsis: inflammation, autophagy, and cell death. Acta physiol (Oxford, England) 2018;225(3) doi: 10.1111/apha.13194. [DOI] [PubMed] [Google Scholar]
- 14.Liu X., Wang C. The emerging roles of the STING adaptor protein in immunity and diseases. Immunology. 2016;147(3):285–291. doi: 10.1111/imm.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer J.C., Bscheider M., Eisenkolb G., Lin C.C., Wintges A., Otten V. RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury. Sci Transl Med. 2017;9(386):eaag2513. doi: 10.1126/scitranslmed.aag2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mcc C., Lemos L., Neves T.C., Marim F.M., Tbr C., Veloso É.S. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation. Mucosal Immunol. 2017;11(3) doi: 10.1038/mi.2017.88. [DOI] [PubMed] [Google Scholar]
- 17.Zeng L., Kang R., Zhu S., Wang X., Cao L., Wang H. ALK is a therapeutic target for lethal sepsis. Sci Transl Med. 2017;9(412):eaan5689. doi: 10.1126/scitranslmed.aan5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aden K., Tran F., Ito G., Sheibani-Tezerji R., Lipinski S., Kuiper J.W. Rosenstiel P: ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med. 2018;215(11):2868–2886. doi: 10.1084/jem.20171029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X., Ren J., Chen G., Wu L., Song X., Li G. Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci Rep. 2017;7(1):4364. doi: 10.1038/s41598-017-04231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Q., Ren J., Li G., Wu J., Wu X., Wang G. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018;9(3):403. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo L., Li Y., Wang H., Wu R., Zhu W., Zhang W. Cigarette smoking is associated with intestinal barrier dysfunction in the small intestine but not in the large intestine of mice. J Crohns Colitis. 2014;8(12):1710–1722. doi: 10.1016/j.crohns.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Q., Wei Y., Pandol S.J., Li L. Habtezion a: STING Signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology. 2018;154(6):1822–1835. doi: 10.1053/j.gastro.2018.01.065. (e1822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Q.Y., Ren J.N., Li G.W., Wu J., Wu X.W., Wang G.F. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018;9(3):403. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piton G., Belon F., Cypriani B., Regnard J., Puyraveau M., Manzon C. Enterocyte damage in critically ill patients is associated with shock condition and 28-day mortality. Crit Care Med. 2013;41(9):2169–2176. doi: 10.1097/CCM.0b013e31828c26b5. [DOI] [PubMed] [Google Scholar]
- 25.Brault M., Olsen T.M., Martinez J., Stetson D.B., Oberst A. Intracellular nucleic acid sensing triggers Necroptosis through synergistic type I IFN and TNF Signaling. J Immunol. 2018;200(8) doi: 10.4049/jimmunol.1701492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jon D., Simmon Y., Sujata Mulekar L., Jamie L., Kuck L., Sidney B. Richards: elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591–598. doi: 10.1097/SLA.0b013e3182a4ea46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Q., Ren J., Wu J., Li G., Wu X., Liu S. Elevated levels of plasma mitochondrial DNA are associated with clinical outcome in intra-abdominal infections caused by severe trauma. Surg Infect (Larchmt) 2017;18(5) doi: 10.1089/sur.2016.276. [DOI] [PubMed] [Google Scholar]
- 28.Curran E., Chen X., Corrales L., Kline D.E., Dubensky T.W., Jr., Duttagupta P. STING pathway activation stimulates potent immunity against acute myeloid Leukemia. Cell Rep. 2016;15(11):2357–2366. doi: 10.1016/j.celrep.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsia H.C., Hutti J.E., Baldwin A.S. Cytosolic DNA promotes signal transducer and activator of transcription 3 (STAT3) phosphorylation by TANK-binding kinase 1 (TBK1) to restrain STAT3 activity. J Biol Chem. 2017;292(13):5405–5417. doi: 10.1074/jbc.M116.771964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H., Sun H., You F., Sun W., Zhou X., Chen L. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147(2):436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Walker W.E., Bozzi A.T., Goldstein D.R. IRF3 contributes to sepsis pathogenesis in the mouse cecal ligation and puncture model. J Leukoc Biol. 2012;92(6):1261–1268. doi: 10.1189/jlb.0312138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiao J.T., Cui C., Qing L., Wang L.S., He T.Y., Yan F. Activation of the STING-IRF3 pathway promotes hepatocyte inflammation, apoptosis and induces metabolic disorders in nonalcoholic fatty liver disease. Metabolism. 2018;81:13–24. doi: 10.1016/j.metabol.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Watts E.R., Walmsley S.R. Getting DAMP(s) wets the whistle for neutrophil recruitment. Immunity. 2018;48(5):846–848. doi: 10.1016/j.immuni.2018.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The authors provide detailed description of methods and original data upon request.