Abstract Abstract
Stag beetles (Coleoptera, Scarabaeoidea, Lucanidae) have received extensive attention from researchers in behavioral ecology and evolutionary biology. There have been no previous quantitative analyses, particularly using a geometric morphometric approach based on a large sample of data, to shed light on the morphological diversity and evolution of Lucanidae. Thoracic adaptation and ecological differentiation are intimately related, and the pronotum bears important muscles and supports the locomotion of prothoracic legs. The elytron is an autapomorphy of the Coleoptera. To reconstruct and visualize the patterns of evolutionary diversification and phylogenetic history of shape change, an ancestral groundplan can be reconstructed by mapping geometric morphometric data onto a phylogenetic tree. In this study, the morphologies of the pronotum and elytron in 1303 stag beetles (Lucanidae), including approximately 99.2% of all globally described species, were examined, thus revealing several aspects of morphological diversity and evolution. First, on the basis of geometric morphometric analysis, we found significant morphological differences in the pronotum or elytron between any two Lucanidae subfamilies. And we subsequently reconstructed the ancestral groundplans of the two structures in stag beetles and compared them with those of extant species (through cladistic and geometric morphometric methods). The ancestral groundplan of Lucanidae was found to be most similar to extant Nicagini in both the pronotum and elytron, according to Mahalanobis distances. Furthermore, we analyzed species richness and morphological diversity of stag beetles and the relationships between them and found that the two parameters were not always correlated. Aesalinae was found to be the most diverse subfamily in both the pronotum and elytron, despite its poor species richness, and the diversity of the pronotum or elytron was not superior in Lucaninae, despite its high species richness. Our study provides insights into the morphological variations and evolutionary history of the pronotum and elytron in four subfamilies of stag beetles, and it illuminates the relationship between morphological diversity and species richness. Intriguingly, our analysis indicates that morphological diversity and species richness are not always correlated. These findings may stimulate further studies in this field.
Keywords: Elytron, geometric morphometrics, morphological diversity, pronotum, species richness, stag beetle
Introduction
Stag beetles (Lucanidae) comprise more than 1300 described species, which are grouped into over 100 genera and exist in all zoogeographical regions except Antarctica (Fujita 2010; Kim and Farrell 2015). Owing to their sexual dimorphism, male polymorphism, and unique behaviors, stag beetles have received extensive attention from coleopterists and evolutionary biologists.
In recent years, multiple aspects of stag beetle morphology have been studied, and numerous evolutionary interpretations have been proposed. For instance, a study of the evolution of the Lucanidae has suggested that negative wing allometry may reflect a morphological cost of evolving oversized mandibles (Kawano 1997), and finite-element modeling has revealed force modulation of jaw adductors in stag beetles (Goyens et al. 2014). In addition, some studies have attempted to address evolutionary questions in specific subfamilies, such as Penichrolucaninae (Ratcliffe, 1984). Hosoya and Araya (2005, 2006) have inferred the phylogeny and evolution of Japanese stag beetles from morphological characters and 16S mtrRNA gene sequences, and the same team has investigated the phylogeny of the genus Dorcus and its allied genera by using allozyme or molecular data (Hosoya et al. 2002, 2003; Hosoya 2011). Furthermore, a new genus has been proposed, and a phylogenetic tree of Lucanidae based on two gene regions of ribosomal DNA has indicated the monophyly of four subfamilies (Paulsen 2013). Both morphological diversity and species richness are important in the study of diversity. The species richness of stag beetles has been revealed through various monographs (Fujita 2010; Maes and Pinratana 2003; Paulsen 2010), descriptions of new species (Okuda 2012b; Nguyen 2013) and reviews of certain taxa (Paulsen and Mondaca 2006; Huang and Chen 2012).
Among the morphological characters of stag beetles previous research was focused on the mandibles (Kawano 2003; Knell et al. 2004; Knight 2014), allometry (Kawano 2000; Tatsuta et al. 2001; Hardersen et al. 2011), sexual dimorphism or male polymorphism (Kawano 2006; Iguchi 2013), and genitalia (Tatsuta et al. 2001; Imura 2007). The pronotum and elytron have typically been used as indexes for body size (Tatsuta et al. 2004; Chiari et al. 2014), both of which contain important information about the evolution of the Lucanidae. Thoracic adaptation and ecological differentiation are intimately related and, differences in size, structure and function in the prothorax are readily perceived and correlated with physical demands of various environments (Hlavac 1972). As a part of the prothorax, the pronotum bears important muscles and supports the locomotion of the prothoracic legs (Evans 1977). In fact, the muscles in the prothorax of a stag beetle are hypertrophied to help raise the head while lifting opponents (Goyens et al. 2015). The elytron is an autapomorphy of the Coleoptera, which was being transformed into elytra in the Permian (Beutel and Leschen 2005; Ponomarenko 2004). These two traits are also correlated to mandibles in morphology. The mandibles of most males are highly developed, thus contributing to the morphological diversity of the stag beetles. The species with relatively large mandibles have proportionally enlarged prothorax and smaller wings, which may be developmental integration trade-offs generated by resource competition between characters (Kawano 1997; Okada and Miyatake 2009). As mandible shape has wide variation even within the same species between large and small males in stag beetles, this study focuses on the pronotum and elytron, which are relatively conservative within species and more feasible for a large sample size.
However, no quantitative analyses have been conducted, especially through a geometric morphometric approach (Bai et al. 2012, 2014b; Friedman 2010), to reveal the morphological diversity and evolution of Lucanidae on the basis of a large sample size. Additionally, the relationship remains unclear between the species richness and morphological diversity of the subfamilies of Lucanidae . To reconstruct and visualize the phylogenetic history of shape change, the phylogeny can be projected into the shape tangent space, and provides intuitive graphical displays that show, as far as it is possible to infer from the shape information of terminal taxa, how specific clades diversified and spread through the space of morphometric variables (Klingenberg and Marugán-Lobón 2013).
Three major aspects of pronotum and elytron morphology of stag beetles were investigated in this study. First, the morphological variations in the pronotum and elytron of 1303 stag beetles were analyzed through a geometric morphometric approach. Second, the ancestral groundplans of the pronotum and elytron of the subfamilies of Lucanidae were reconstructed, and the evolution of the two structures was inferred and discussed. Furthermore, the species richness and morphological diversity of four subfamilies were compared.
Methods
Taxa examined
This study analyzed 1447 species including 1303 lucanid species and 144 outgroup species. All four subfamilies (Aesalinae, Lampriminae, Lucaninae, and Syndesinae), all 105 lucanid genera, and 1303 lucanid species (approximately 99.2% of all described lucanid species) from around the world were included as the inner group, and the outgroups consisted of 4 Diphyllostomatidae species, 16 Hybosoridae species, 16 Geotrupidae (Geotrupinae+Bolboceratinae) species, 11 Passalidae (Passalinae+Aulacocyclinae) species, 6 Glaresidae species, 19 Ochodaeidae species, 43 Scarabaeidae species, 9 Trogidae species, 12 Silphidae species, and 8 Histeridae species. The measurements of all lucanid species and most outgroup species were based on published images (photographs or specimen drawings) (Dallwitz 1980; Dallwitz et al. 1993, 1995, 2000; Sakai and Nagai 1998; Bunalski 1999; Král 2001; Arnett et al. 2002; Ocampo 2006; Mondaca and Smith 2008; Schenk 2008; Nikolajev 2009; Bomans 2010; Fujita 2010; Imura 2010, 2011, 2012; Boilly 2011; Kobayashi and Matsumoto 2011; Okajima and Araya 2012; Okuda 2012a; Palestrini et al. 2012; Paulsen and Ocampo 2012; Ballerio 2013; Král et al. 2013; Paulsen 2013; Bezborodov and Koshkin 2014), and those of the Passalidae species were based on the specimens housed in the Institute of Zoology of the Chinese Academy of Sciences (Suppl. material 1: Table S1). In consideration of sexual dimorphism and male polymorphism in Lucanidae, images of the male specimens were selected in this study, as they are more accessible in publications, and medium-sized specimens were chosen if there were images of different body sizes available.
Data analysis
Geometric morphometric analysis of the variations in the pronotum and elytron were based on one curve for each structure (Fig. 1), and the curves for the pronotum or elytron were resampled by length after 25 and 50 semi-landmarks (SLM), respectively. The curves were digitized with TPS-DIG 2.05 (Rohlf 2006), and the data file was modified as .txt file to convert the semi-landmarks to landmarks (MacLeod 2017). The two lines with the curve number and point number were deleted, and the landmark number was replaced by the point number. This approach was used earlier by Bai et al. (2014a) and Li et al. (2016). The landmark configurations were scaled, translated, and rotated against the consensus configuration by using the Procrustes superimposition method (Bookstein 1991). The differences in the shapes and diversity indexes of the pronotum and elytron were inferred on the basis of principal component analysis (PCA) in MORPHOJ 1.06a (Klingenberg 2011) (Figs 2, 3, 8, 9, Table 1, Suppl. material 1: Table S10). The diversity index was quantified as Procrustes variance, which measures the dispersion of all observations around the mean shape of the respective taxa (Zelditch et al. 2004; Sherratt et al. 2014). The association of morphological diversity with species richness at genus-level was measured using Pearsons correlation coefficient, r in PAST 3.01 software (Sherratt et al. 2014; Hammer et al. 2001) (Suppl. material 1: Table S10, S11).
Figure 1.
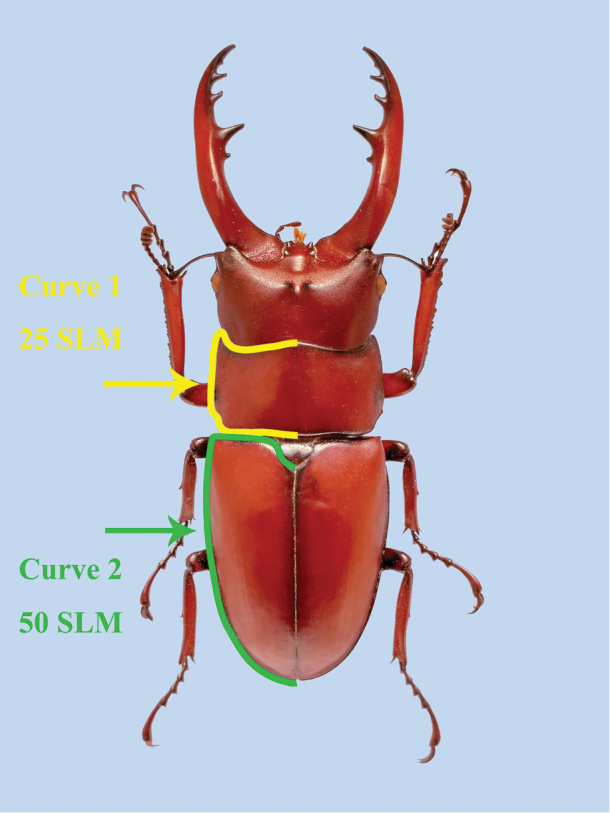
Description of the curves used in geometric morphometric analysis. The positions selected for the pronotum and elytron curves are represented by Prosopocoilus sp. in dorsal view. The curves were resampled in 25 or 50 semi-landmarks (SLM).
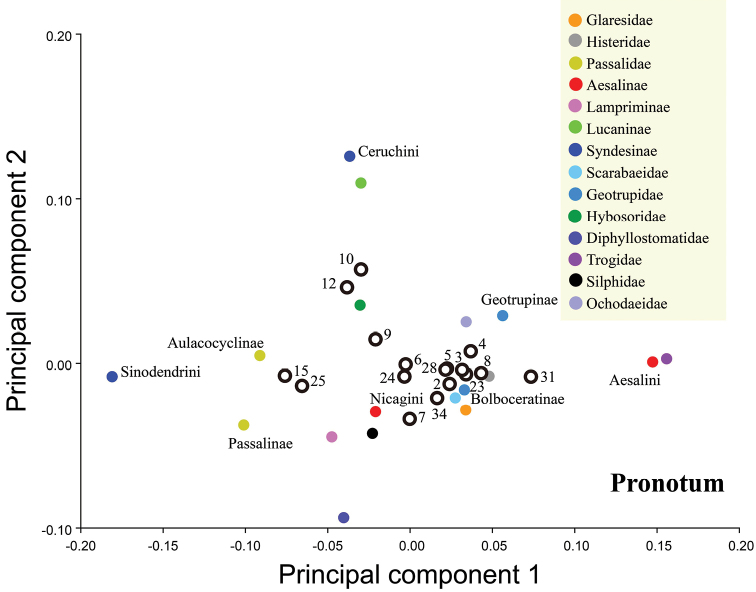
Figure 2.
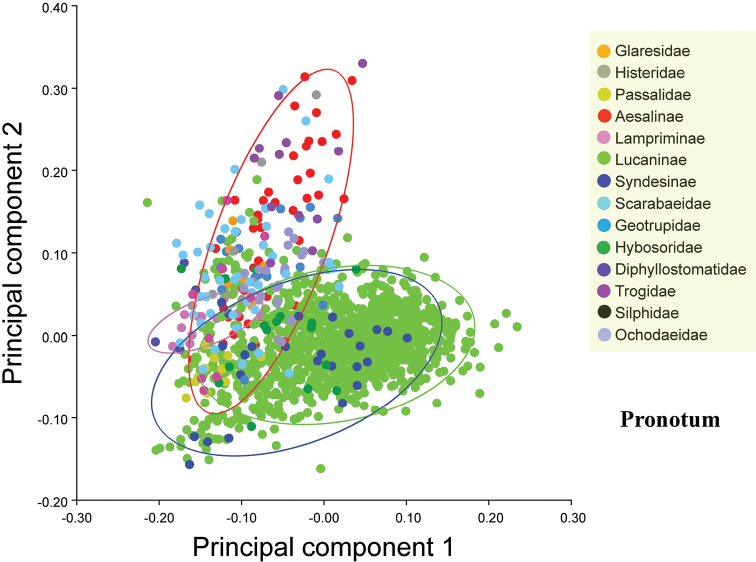
Differences in pronotum shape between outgroups and Lucanidae, on the basis of principal component analysis at the species level. The four circles are 90%-equal frequency ellipses of Lucanidae subfamilies.
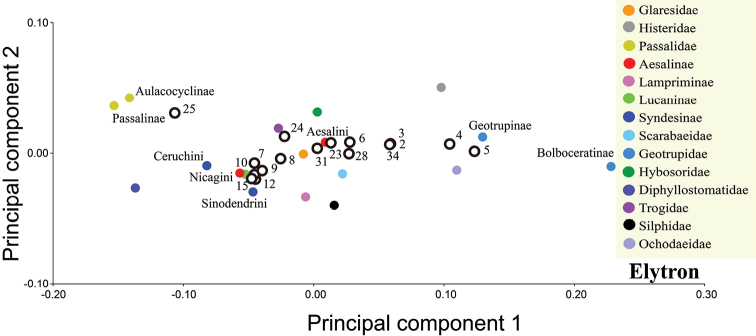
Figure 3.
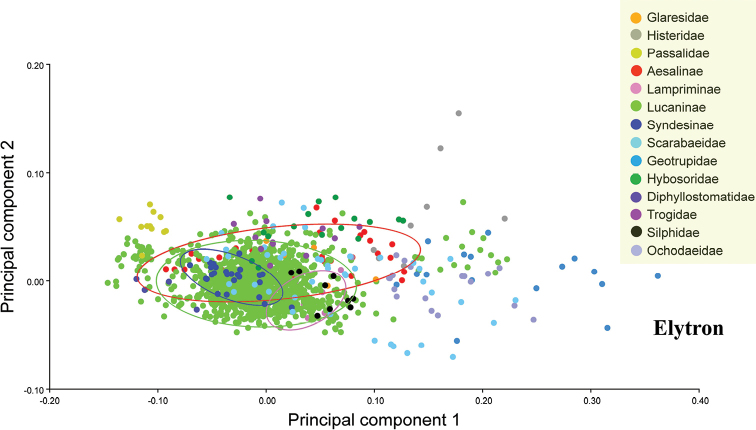
Differences in elytron shape between outgroups and Lucanidae, on the basis of principal component analysis at the species level. The four circles are 90%-equal frequency ellipses of Lucanidae subfamilies.
Figure 8.
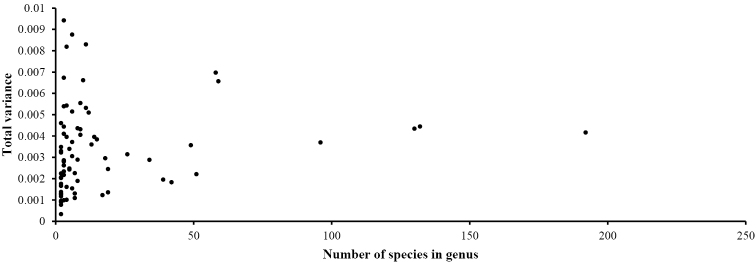
Species richness and morphological diversity of the pronotum at the genus level.
Figure 9.
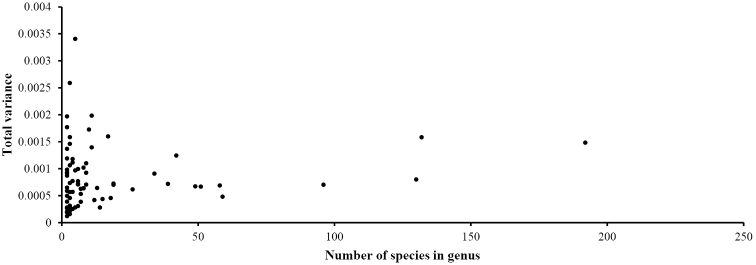
Species richness and morphological diversity of the elytron at the genus level.
Table 1.
Species richness and morphological diversity of the pronotum and elytron at the subfamily level.
| Subfamily | Species number | Total variance | |
|---|---|---|---|
| Pronotum | Elytron | ||
| Aesalinae | 47 | 0.0158 | 0.0044 |
| Lampriminae | 11 | 0.0030 | 0.0007 |
| Lucaninae | 1220 | 0.0111 | 0.0025 |
| Syndesinae | 25 | 0.0123 | 0.0008 |
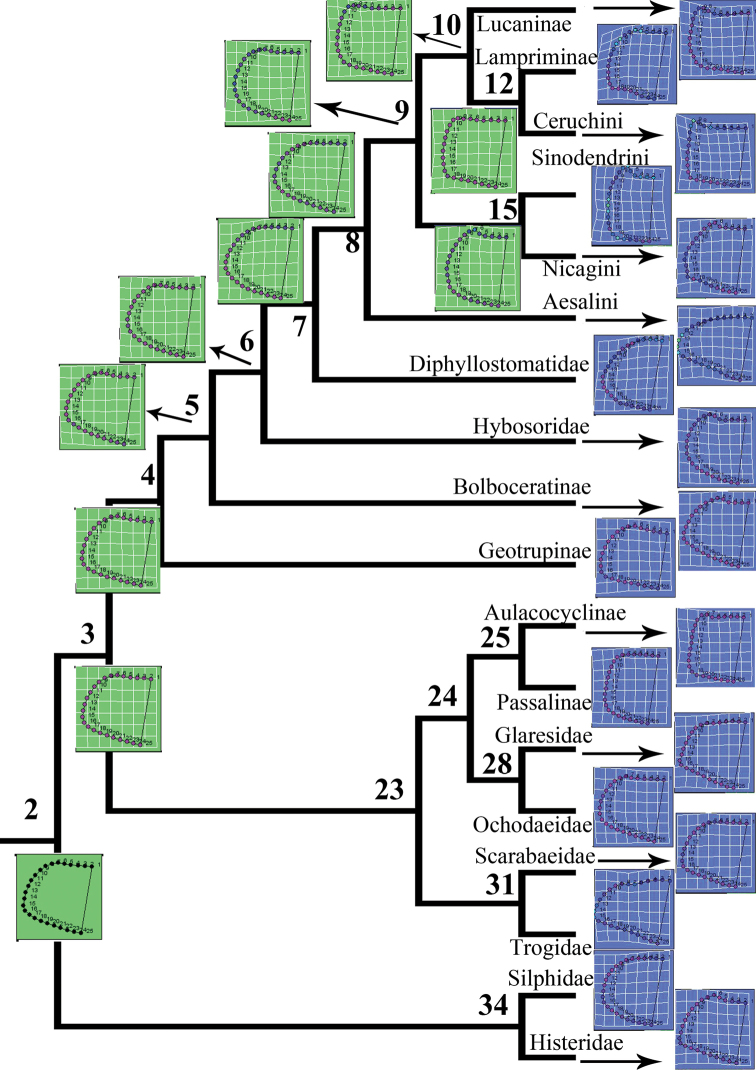
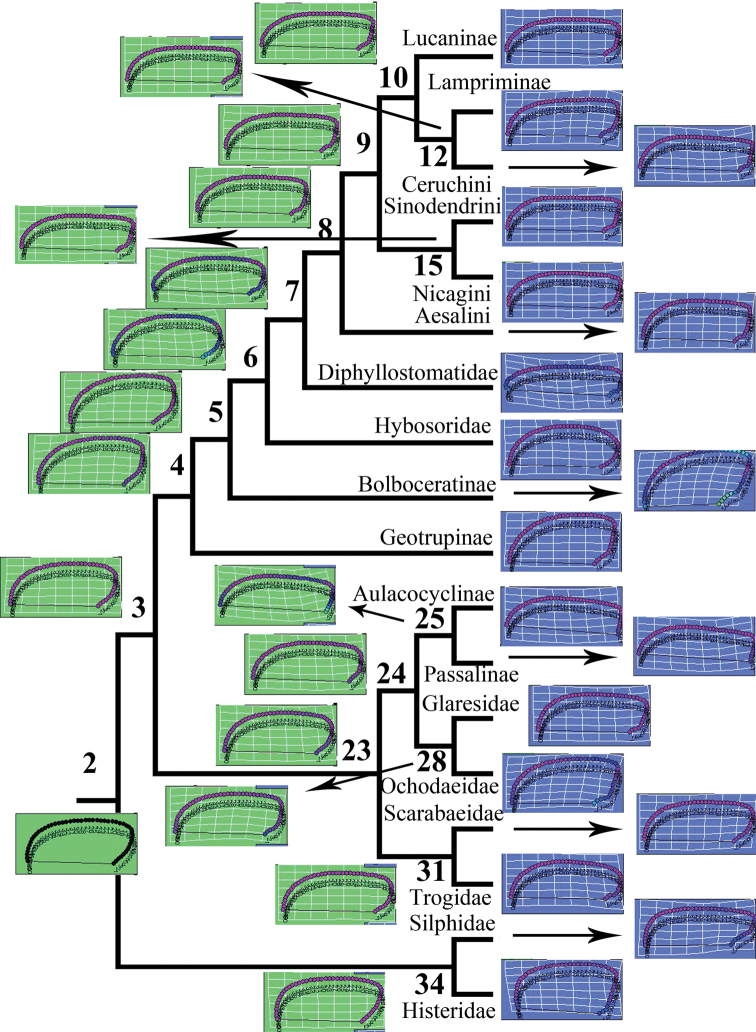
A phylogenetic tree was visualized in MESQUITE 2.72 (Maddison and Maddison 2011) on the basis of earlier molecular analysis (Kim and Farrell 2015), and the aligned landmark data were entered into MESQUITE 2.72 as a continuous matrix and linked to the tree (Figs 4, 5). Because the branch lengths (Grafen 1989) were missing, we followed the evaluation proposed by Klingenberg and Marugán-Lobón (Klingenberg and Marugán-Lobón 2013) and assigned an equal length to all branches (i.e., an evolutionary model with the same expected amount of morphological change on every branch was assumed).
Figure 4.
Reconstruction of ancestral groundplans of the pronotum in Lucanidae and the outgroups. The splines indicate deformation of the shapes relative to the reference configuration. The phylogenetic tree was summarized and reconstructed from earlier molecular results (Kim and Farrell 2015).
Figure 5.
Reconstruction of ancestral groundplans of the elytron in Lucanidae and the outgroups. The splines indicate the deformation of the shapes relative to the reference configuration. The phylogenetic tree was summarized and reconstructed from earlier molecular results (Kim and Farrell 2015).
The ancestral groundplans of the Lucanidae pronotum and elytron were reconstructed by combining the landmark data with the phylogenetic tree, and the ancestral groundplans of all nodes were reconstructed by using the trace-all-characters and/or landmark-drawing modules of the RHETENOR package in MESQUITE. The ancestral states of all nodes were calculated and exported, and the data computed for the nodes were integrated with the original landmark data for the two characteristics from the 1303 stag beetles in EXCEL and NTSYS-PC (Rohlf 2007), respectively. The thin-plate splines showing the deformation of the landmarks compared with the original computed by MESQUITE were mapped onto the phylogenetic tree (Figs 4, 5).
In this case, the differences in the shapes of the pronotum and elytron among extant and extinct Lucanidae were inferred on the basis of PCA in MORPHOJ 1.06a and PAST 3.01 (Figs 6, 7). The canonical variate analysis (CVA) and discriminant function analysis (DFA) of the landmark data were based on MORPHOJ 1.06a (Suppl. material 1: Tables S2–S9, S12, S13).
Figure 6.
Differences in pronotum shape among each branch and ancestor, on the basis of principal component analysis. Empty dots indicate the number of the node on the phylogenetic tree; solid dots indicate the average shape of the extant subfamily/tribe of each branch.
Figure 7.
Differences in elytron shape among each branch and ancestor, on the basis of principal component analysis. Empty dots indicate the number of the node on the phylogenetic tree; solid dots indicate the average shape of the extant subfamily/tribe of each branch.
Results
Comparison of pronotum/elytron morphology among lucanid subfamilies
The first two principal components of the pronotum and elytron from all 1447 species accounted for 77.37% and 88.40% of the variation among the species, respectively. The first two principal components were plotted to indicate variation along the two axes, which provided 90% equal frequency ellipses containing approximately 90% of the data points of each group (Figs 2, 3). The pronotum morphologies of the outgroups were mostly within the entire morphological variation of the Lucanidae (Fig. 2).
All the p-values obtained from the permutation tests (10000 permutation rounds) for both Mahalanobis distances and Procrustes distances between any two Lucanidae subfamilies were less than 0.05 for both the pronotum and elytron. Most of the p-values for the pronotum and elytron Mahalanobis or Procrustes distances between the Lucanidae subfamilies and outgroups were less than 0.05, except for some of the distances between the Aesalinae or Lampriminae and the outgroups.
There were significant differences in both the pronotum and elytron between any two of the Lucanidae subfamilies (Suppl. material 1: Table S3, S5, S7, S9). For the pronotum, there was a significant difference between Lampriminae, Lucaninae, or Syndesinae and the outgroups; the Lucanidae and the outgroups partly overlapped, because the pronotum morphology of Aesalinae could not be distinguished from that of Geotrupidae, Glaresidae, Ochodaeidae, and Histeridae (Suppl. material 1: Table S3, S5). However, the differences in the pronotum among these four pairs were not equivalent. On the basis of the Procrustes distance, a measure of the absolute magnitude of the deviation in shape that indicates the extent of the differences between the average group shapes, the differences in the pronotum between Aesalinae and Geotrupidae, Glaresidae, Ochodaeidae, and Histeridae were 0.0512, 0.0637, 0.0488 and 0.0659, respectively (Table S4). For the elytron, there was a significant difference between Lucaninae or Syndesinae and the outgroups, but the morphology of Aesalinae and Lampriminae could not be distinguished from the morphologies of Glaresidae, Scarabaeidae, or Trogidae (Table S7, 9). There was greater overlap in the pronotum morphology of the Lucanidae subfamilies and outgroups than the elytron morphology (Suppl. material 1: Table S2, S6).
Ancestral reconstruction of the pronotum and elytron
In a comparison of the ancestor of Lucanidae and all outgroups (node 2 in Fig. 4), the anterior angle of the pronotum of the ancestor of Lucanidae (node 8 in Fig. 4) is more obtuse (landmarks 4–12 at the splines in Fig. 4). It is shown in Figure 6 that a clear divergence between the two lineages of Lucanidae, the Aesalini lineage and lineage I (node 9 in Fig. 4), is primarily in the direction of the first principal component. Moreover, within lineage I, there is continued diversification in the direction of the first principal component (the horizontal direction in Fig. 6). The pronotum morphology of Lampriminae and Ceruchini showed clear changes in different directions, almost in the inverse direction of the second principal component (the vertical direction in Fig. 6), particularly in the anterior angle (landmarks 4–12 at the splines in Fig. 4).
The elytron of the ancestor of Lucanidae (node 8 in Fig. 5) is more slender than that of the outgroups (node 2 in Fig. 5) (the ratios of the length to the greatest width are 1.571 and 1.235, respectively) and narrower at the end (landmarks 35–50 at the splines in Fig. 5), but it is much wider than that of Diphyllostomatidae (the ratios of the length to the greatest width are 1.571 and 2.170, respectively). The elytron of Aesalini is wider than in lineage I (node 9 in Fig. 5) (the ratios of the length to the greatest width are 1.313 and 1.571, respectively), thus illustrating the major differences in the elytron between the two Lucanidae lineages. The humeral angle is narrower in the ancestor of Sinodendrini and Nicagini (node 15 in Fig. 5) than in the ancestor of Lucaninae, Lampriminae and Ceruchini (node 10 in Fig. 5) (landmarks 10–18 at the splines in Fig. 5).
Species richness and pronotum and elytron morphology
In terms of the species richness, Lucaninae is the largest subfamily of Lucanidae, comprising more than 90% (1220) of the species of stag beetles, followed by Aesalinae, Syndesinae, and Lampriminae, which have fewer than 50 species each (47, 25, 11). However, morphological diversity of the pronotum and elytra shape does not correspond with species richness (Table 1). Aesalinae, with fairly low species richness, was found to be the most diverse subfamily in both pronotum and elytron morphology, whereas Lucaninae exhibited low morphological diversity (both in the pronotum and elytron). This is an unexpected result considering its extremely high species richness.
In 73 genera (all Lucanidae genera with more than one species), similarly to the subfamily data, the morphological diversity of neither the pronotum nor the elytron was consistent with species richness. There is no significant correlation between morphological diversity and species richness in pronotum at genus-level (Procrustes variance r = 0.15, P =0.21), nor a relationship between morphological diversity and species richness in elytron at genus-level (Procrustes variance r = 0.11, P = 0.33). The total morphological variances in extremely species-rich genera, such as Aegus, Dorcus, Lucanus, and Prosopocoilus, were not predominant.
Discussion
Evolution of the pronotum and elytron in Lucanidae
According to the Mahalanobis distances from the DFA (Suppl. material 1: Table S12, S13), all lucanid ancestors (nodes 8–10, 12, and 15) most resemble Nicagini in their pronotum and elytron morphology. Procrustes distances indicated that the pronotum of the ancestor of Lucanidae (node 8) is closest to that of Scarabaeidae, and the elytron is closest to that of Glaresidae. The common ancestor of Lucaninae, Lampriminae, Syndesinae, and Nicagini (node 9) most resembles Hybosoridae in the pronotum and Lucaninae in the elytron, and the ancestor of Lampriminae and Ceruchini (node 12) most resembles Hybosoridae in the pronotum and Sinodendrini in the elytron. The ancestor of Lucaninae, Lampriminae, and Ceruchini (node 10) is most similar to Lucaninae in both the pronotum and elytron, and as shown by both Mahalanobis and Procrustes distances, the ancestor of Sinodendrini and Nicagini (node 15) most resembles Nicagini in the pronotum as well as the elytron.
The ancestral pronotum and elytra were reconstructed, which could be combined with fossil materials to uncover the ancestors’ habitat as well as evolution procedure. There is still a lack of sufficient data as well as studies of the functional morphology of the pronotum and the elytron in Lucanidae, but the functional morphology of other insect clades may allow for certain interpretations. Broad pronotum and elytra of stag beetles may provide advantages during locomotion and hunting prey, like ground beetles (Evans 1977; Forsythe 1981). The diversity of pronotum may reflect occupancy of diverse habitats and niches, as in the case of grylloblattids (Bai et al. 2010).
The inconsistency between morphological diversity and species richness
Morphological and taxonomic diversity provide insight into the expansion and contraction of major taxa, and the nature of the relationship between these two aspects of diversity has important implications in evolutionary mechanisms (Foote 1993). Lampriminae has the lowest pronotum and elytron diversity as well as species richness, but species richness and morphological diversity do not always vary consistently in Lucanidae subfamilies. Despite having many more species than the other three subfamilies combined, the subfamily Lucaninae has little morphological diversity. Furthermore, the results from the genus-level data reveal the same pattern in which some of the largest genera are not as diverse as the small groups in terms of either pronotum or elytron morphology.
The theory that species richness generates a variety of forms has been tested and supported in various studies (Williams and Humphries 1996; Roy and Foote 1997; Bell and Barnes 2001), thus suggesting that the relationship between morphological and species diversity should be monotonic or at least positive.
In contrast, numerous studies have indicated that richness and morphology do not always follow a common trend. Foote (1993) has found that morphological variety and taxonomic richness often increase together during the initial diversification of a clade, but two major patterns have been observed as clades decline. In Blastoidea, Trilobita, Libristoma, and Asaphina, morphological diversity continued to increase even in the face of striking decreases in taxonomic richness, but in Phacopida, Scutelluina, and to some extent in Proetida, morphological diversity decreased along with taxonomic diversity. Roy et al. (2001), using data from a large group of IndoPacific gastropods (family Strombidae), have shown that the species richness of a region is a poor predictor of morphological diversity. Areas with only a few species may harbor an impressive array of morphologies, and in contrast, morphological diversity in the most species-rich regions is no higher than that in regions with half the taxonomic diversity. Bell and Barnes (2002) have found no significant correlation between species richness and morphological diversity in cave or boulder habitats, although these variables are significantly correlated in coral reef and soft substratum habitats. In another scenario (Mohedano-Navarrete et al. 2008), the morphological diversity and species richness of Porites corals has been found to vary independently; some regions with few species had remarkably high morphological diversity, including peripheral areas such as Polynesia and East Africa. In a study of 107 families of passerine birds, morphological space was weakly related to the number of species in a family, i.e., the higher species richness of the order Passeriformes in the tropics compared with temperate regions was not matched by increased morphological diversity (Ricklefs 2012).
Conclusion
Our results showed significant differences in both pronotum and elytron morphology between any two lucanid subfamilies; in other words, the four subfamilies could be statistically separated and determined based on the two characters. On the basis of cladistic and geometric morphometric methods, the ancestral groundplans of the pronotum and elytron of extant Lucanidae were reconstructed and compared with those of extant species. The ancestor of Lucanidae is most similar to extant Nicagini in both pronotum and elytron morphology, according to Mahalanobis distances, but Procrustes distances indicated that the pronotum of the ancestor of Lucanidae is most similar to that of extant Scarabaeidae and that the elytron is most similar to that of extant Glaresidae. On the basis of a comparison of the four subfamilies as well as an analysis of Lucanidae genera, species richness and morphological diversity do not generally correlate. Lampriminae has the poorest morphological diversity in the pronotum and elytron as well as the poorest species richness, whereas Aesalinae is the most diverse subfamily with respect to both the pronotum and elytron, despite its small number of species.
However, the analyses are relatively limited, as there is morphological variation within species especially in male polymorphic stag beetles, and only one image per species was sampled during the procedure. In addition, as our results were limited to the morphological characters of the pronotum and elytron from the dorsal view, the investigation of more traits and groups should improve the understanding of the relationship between morphological diversity and species richness in beetles.
Acknowledgements
We thank the Group of Morphology and Evolution of Beetles of IZCAS for providing the platform of this research. The staff from the Beetle Collection of IZCAS is acknowledged for supplying materials for this study. We extend our sincere appreciation to all of the scholars and colleagues who encouraged and supported the first authors to accomplish this paper. We are also grateful to Mrs Xiaoyan Hu, Dr Menglei Zhang and Dr Sha Li for assisting with a portion of the previous stage of this research.
This research was supported by the National Natural Science Foundation of China (No. 31672345; 31572311), Research Equipment Development Project of Chinese Academy of Sciences (YZ201509), and by a Humboldt Fellowship (M.B.) from Alexander von Humboldt Foundation.
Citation
Zhang M, Ruan Y, Wan X, Tong Y, Yang X, Bai M (2019) Geometric morphometric analysis of the pronotum and elytron in stag beetles: insight into its diversity and evolution. ZooKeys 833: 21–40. https://doi.org/10.3897/zookeys.833.26164
Contributor Information
Xingke Yang, Email: yangxk@ioz.ac.cn.
Ming Bai, Email: baim@ioz.ac.cn.
Supplementary materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Mengna Zhang, Yongying Ruan, Xia Wan, Yijie Tong, Xingke Yang, Ming Bai
Geometric morphometric data
Data type: morphometric data
Explanation note: Tables S1–S12.
References
- Arnett RH Jr, Thomas MC, Skelley PE, Frank JH. (2002) American Beetles. Volume 2. Polyphaga: Scarabaeoidea through Curculionoidea. CRC Press LLC, Boca Raton, 861 pp. [Google Scholar]
- Bai M, Beutel RG, Liu WG, Li S, Zhang MN, Lu YY, Song KQ, Ren D, Yang XK. (2014a) Description of a new species of Glaresidae (Coleoptera: Scarabaeoidea) from the Jehol Biota of China with a geometric morphometric evaluation. Arthropod Systematics & Phylogeny 72: 223–236. [Google Scholar]
- Bai M, Beutel RG, Song KQ, Liu WG, Malqin H, Li S, Hu XY, Yang XK. (2012) Evolutionary patterns of hind wing morphology in dung beetles (Coleoptera: Scarabaeinae). Arthropod Structure & Development 41: 505–513. 10.1016/j.asd.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Bai M, Jarvis K, Wang SY, Song KQ, Wang YP, Wang ZL, Li WZ, Wang W, Yang XK. (2010) A second new species of ice crawlers from China (Insecta: Grylloblattodea), with thorax evolution and the prediction of potential distribution. PLoS ONE 5(9): e12850. 10.1371/journal.pone.0012850 [DOI] [PMC free article] [PubMed]
- Bai M, Yang XK, Li J, Wang WC. (2014b) Geometric Morphometrics, a super scientific computing tool in morphology comparison. Chinese Science Bulletin 59: 887–894. 10.1360/972012-1561 [DOI] [Google Scholar]
- Ballerio A. (2013) Revision of the Australian Ceratocanthinae (Coleoptera, Scarabaeoidea, Hybosoridae). ZooKeys 339: 67–91. 10.3897/zookeys.339.6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezborodov VG, Koshkin ES. (2014) A review of Bolboceratidae (Coleoptera, Scarabaeoidea) species from the Russian Far East. Entomological Review 94: 1313–1319. 10.1134/s0013873814090127 [DOI] [Google Scholar]
- Bell JJ, Barnes DKA. (2001) Sponge morphological diversity: a qualitative predictor of species diversity. Aquatic Conservation: Marine and Freshwater Ecosystems 11(2): 109–121. 10.1002/aqc.436 [DOI] [Google Scholar]
- Bell JJ, Barnes DKA. (2002) Modelling sponge species diversity using a morphological predictor: a tropical test of a temperate model. Journal for Nature Conservation 10(1): 41–50. 10.1078/1617-1381-00005 [DOI] [Google Scholar]
- Benesh B. (1960) Coleopterorum Catalogus. Pars 8. Lucanidea. Junk, The Hague, 178 pp. [Google Scholar]
- Beutel RG, Leschen RAB. (2005) Handbook of Zoology, Vol. IV, Part 38, Coleoptera, Beetles. Vol. 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). Walter de Gruyter, Berlin, New York, 567 pp. [Google Scholar]
- Boilly O. (2011) Les Bolboceratidae de Guyane. ACOREP, Coléoptères de Guyane 4: 19–31. [Google Scholar]
- Bomans HE. (2010) Description of a new species of Figulus Macleay, 1819 from Micronesia. (108th contribution on Lucanidae, Coleoptera). Lambillionea 110(3): 338–339. [Google Scholar]
- Bookstein FL. (1991) Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge University Press, Cambridge, 435 pp. [Google Scholar]
- Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CH, Newton AF, Reid CA, Schmitt M, Slipiński SA, Smith AB. (2011) Family-group names in Coleoptera (Insecta). ZooKeys 88: 1–972. 10.3897/zookeys.88.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunalski M. (1999) Die Blatthornkäfer Mitteleuropas. Coleoptera, Scarabaeoidea. Bestimmung - Verbreitung - Ökologie. Slamka, Bratislava, 80 pp. [Google Scholar]
- Chiari S, Zauli A, Audisio P, Campanaro A, Donzelli PF, Romiti F, Svensson GP, Tini M, Carpaneto GM. (2014) Monitoring presence, abundance and survival probability of the stag beetle, Lucanuscervus, using visual and odour-based capture methods: implications for conservation. Journal of Insect Conservation 18: 99–109. 10.1007/s10841-014-9618-8 [DOI] [Google Scholar]
- Dallwitz MJ. (1980) A general system for coding taxonomic descriptions. Taxon 29: 6–41. 10.2307/1219595 [DOI] [Google Scholar]
- Dallwitz MJ, Paine TA, Zurcher EJ. (1993) User’s Guide to the DELTA System: a General System for Processing Taxonomic Descriptions. 4th edition. http://delta-intkey.com
- Dallwitz MJ, Paine TA, Zurcher EJ. (1995) User’s Guide to Intkey: a Program for Interactive Identification and Information Retrieval. http://delta-intkey.com
- Dallwitz MJ, Paine TA, Zurcher EJ. (2000) Principles of interactive keys. http://delta-intkey.com
- Didier R, Seguy E. (1953) Catalogue illustré des Lucanides du globe. Texte. Encyclopédie Entomologique (A) 27: 1–223. [Google Scholar]
- Evans MEG. (1977) Locomotion in the ColeopteraAdephaga especially Carabidae. Journal of Zoology 181: 189–226. 10.1111/j.1469-7998.1977.tb03237.x [DOI] [Google Scholar]
- Foote M. (1993) Discordance and concordance between morphological and taxonomic diversity. Paleobiology 19(02): 185–204. 10.1017/s0094837300015864 [DOI] [Google Scholar]
- Forsythe TG. (1981) Running and pushing in relationship to hind leg structure in some Carabidae (Coleoptera). Coleoptera Bulletin 35: 353–378. [Google Scholar]
- Friedman M. (2010) Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proceedings of the Royal Society B: Biological Sciences 277(1688): 1675–1683. 10.1098/rspb.2009.2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H. (2010) The Lucanid beetles of the world. Mushi-Sha’s Iconographic Series of Insects. Mushisha, Tokyo, 472 pp. [Google Scholar]
- Goyens J, Dirckx J, Aerts P. (2015) Stag beetle battle behavior and its associated anatomical adaptations. Journal of Insect Behavior 28: 227–244. 10.1007/s10905-015-9495-3 [DOI] [Google Scholar]
- Goyens J, Dirckx J, Dierick M, Van Hoorebeke L, Aerts P. (2014) Biomechanical determinants of bite force dimorphism in Cyclommatusmetallifer stag beetles. Journal of Experimental Biology 217(7): 1065–1071. 10.1242/jeb.091744 [DOI] [PubMed] [Google Scholar]
- Grafen A. (1989) The phylogenetic regression. Philosophical transactions of the Royal Society B 326: 119–157. 10.1098/rstb.1989.0106 [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9. [Google Scholar]
- Hardersen S, Macagno ALM, Sacchi R, Toni I. (2011) Seasonal constraints on the mandible allometry of Lucanuscervus (Coleoptera: Lucanidae). European Journal of Entomology 108(3): 461–468. 10.14411/eje.2011.059 [DOI] [Google Scholar]
- Hlavac TF. (1972) The prothorax of Coleoptera: origin, major features of variation. Psyche 79(3): 123–149. 10.1155/1972/31579 [DOI] [Google Scholar]
- Hosoya T. (2011) Phylogenetic relationships of the genus Dorcus and its related genera (Coleoptera, Lucanidae): a reanalysis of allozyme data. Elytra 1(2): 199–209. [Google Scholar]
- Hosoya T, Araya K. (2005) Phylogeny of Japanese stag beetles (Coleoptera: Lucanidae) inferred from 16S mtrRNA gene sequences, with reference to the evolution of sexual dimorphism of mandibles. Zoological Science 22(12): 1305–1318. 10.2108/zsj.22.1305 [DOI] [PubMed] [Google Scholar]
- Hosoya T, Araya K. (2006) Phylogeny and evolution of sexual dimorphism of mandibles in Japanese stag beetles (Coleoptera, Lucanidae). Gekkan-Mushi 426: 41–49. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Araya K, Shirota Y. (2002) Molecular phylogeny of Japanese and Taiwanese stag beetles of the genus Dorcus and allied genera (Coleoptera, Lucanidae) based on random amplified polymorphic DNA. Giornale Italiano di Entomologia 10(50): 133–140. [Google Scholar]
- Hosoya T, Araya K, Shirota Y. (2003) Molecular phylogeny of Japanese stag beetles of the genus Dorcus (Coleoptera, Lucanidae) and its allied genera inferred from mitochondrial COI gene sequences. Elytra 31(1): 127–142. [Google Scholar]
- Huang H, Chen CC. (2012) A review of the genera Prismognathus Motschulsky and Cladophyllus Houlbert (Coleoptera: Scarabaeoidea: Lucanidae) from China, with the description of two new species. Zootaxa 3255: 1–36. [Google Scholar]
- Iguchi Y. (2013) Male mandible trimorphism in the stag beetle Dorcusrectus (Coleoptera: Lucanidae). European Journal of Entomology 110(1): 159–163. 10.14411/eje.2013.022 [DOI] [Google Scholar]
- Imura Y. (2007) Endophallic structure of the genus Platycerus (Coleoptera, Lucanidae) of Japan, with descriptions of two new species. Elytra 35(2): 471–489. [Google Scholar]
- Imura Y. (2010) A new Platycerus (Coleoptera, Lucanidae) from the Baotianman Nature Reserve in western Henan, Central China. Elytra 38(2): 227–232. [Google Scholar]
- Imura Y. (2011) Two new taxa of the genus Platycerus (Coleoptera, Lucanidae) from China. In: Kawai S. (Ed.) Masumushi: entomological papers dedicated to Dr.Kimio Masumoto on the occasion of his retirement 1: 131–141.
- Imura Y. (2012) Description of a new Platycerus (Coleoptera, Lucanidae) from Sichuan, southwest China, with records of two other species belonging of the same genus. Elytra 2(1): 61–68. [Google Scholar]
- Kawano K. (1997) Cost of evolving exaggerated mandibles in stag beetles (Coleoptera: Lucanidae). Annals of the Entomological Society of America 90(4): 453–461. 10.1093/aesa/90.4.453 [DOI] [Google Scholar]
- Kawano K. (2000) Genera and allometry in the stag beetle family Lucanidae, Coleoptera Annals of the Entomological Society of America 93(2): 198–207. 10.1603/0013-8746(2000)093[0198:gaaits]2.0.co;2 [DOI]
- Kawano K. (2003) Character displacement in stag beetles (Coleoptera: Lucanidae). Annals of the Entomological Society of America 96(4): 503–511. 10.1603/0013-8746(2003)096[0503:cdisbc]2.0.co;2 [DOI]
- Kawano K. (2006) Sexual dimorphism and the making of oversized male characters in beetles (Coleoptera). Annals of the Entomological Society of America 99(2): 327–341. 10.1603/0013-8746(2006)099[0327:sdatmo]2.0.co;2 [DOI]
- Kim SI, Farrell BD. (2015) Phylogeny of world stag beetles (Coleoptera: Lucanidae) reveals a Gondwanan origin of Darwin’s stag beetle. Molecular Phylogenetics and Evolution 86: 35–48. 10.1016/j.ympev.2015.02.015 [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. (2011) MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources 11: 353–357. 10.1111/j.1755-0998.2010.02924.x [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Marugán-Lobón J. (2013) Evolutionary covariation in geometric morphometric data: analyzing integration, modularity, and allometry in a phylogenetic context. Systematic Biology 62: 591–610. 10.1093/sysbio/syt025 [DOI] [PubMed] [Google Scholar]
- Knell RJ, Pomfret JC, Tomkins JL. (2004) The limits of elaboration: curved allometries reveal the constraints on mandible size in stag beetles. Proceedings of the Royal Society B: Biological Sciences 271(1538): 523–528. 10.1098/rspb.2003.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. (2014) Stag beetle males compensate for massive mandibles. Journal of Experimental Biology 217(7): 1009 10.1242/jeb.105213 [DOI] [Google Scholar]
- Kobayashi H, Matsumoto T. (2011) Atlas of Japanese Scarabaeoidea, Volume 3: Phytophagous Group II. Roppon-Ashi Entomological Books, Tokyo, 178 pp. [Google Scholar]
- Král D. (2001) Revision of the genera Odontotrypes and Phelotrupes (Coleoptera: Geotrupidae). Folia Heyrovskyana Supplementum 8: 1–178. [Google Scholar]
- Král D, Hillert O, Drožová D, Šípek P. (2013) Lethrus (Lethrus) schneideri sp n. (Coleoptera, Geotrupidae) from Greece. ZooKeys 339: 93–106. 10.3897/zookeys.339.6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JF, Newton Jr AF. (1995) Families and subfamilies of Coleoptera. In: Pakaluk J, Slipinski SA. (Eds) Biology, Phylogeny, and Classification of Coleoptera: Papers Celebrating the 80th Birthday of Roy A.Crowson. Muzeum I Instytut Zoologii PAN, Warszawa, 559–1092.
- Li S, Ricchiardi E, Bai M, Yang X. (2016) A taxonomy review of Oreoderus Burmeister, 1842 from China with a geometric morphometric evaluation (Coleoptera, Scarabaeidae, Valgini). Zookeys 552: 67–89. 10.3897/zookeys.552.6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod N. (2017) Morphometrics: history, development methods and prospects. Zoological Systematics 42: 4–33. 10.11865/zs.201702 [DOI] [Google Scholar]
- Maddison WP, Maddison DR. (2011) Mesquite: a Modular System for Evolutionary Analysis Version 2.75. http://mesquiteproject.org
- Maes JM, Pinratana A. (2003) Lucanidae of Thailand. Sunrise Printing, Bangkok, 447 pp. [Google Scholar]
- Mohedano-Navarrete A, Reyes-Bonilla H, López-Pérez RA. (2008) Species richness and morphological diversity of the Genus Porites in the Pacific Ocean. Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, Florida, 7–11.
- Mondaca J, Smith ABT. (2008) A revision of the southern South American genus Bolborhinum Boucomont (Coleoptera: Geotrupidae: Bolboceratinae). Zootaxa. 1–48. 10.5281/zenodo.182603 [DOI]
- Nguyen TQ. (2013) Description of a new species of the genus Neolucanus Thomson, 1862 (Coleoptera: Lucanidae) from central Vietnam. Zootaxa 3741(3): 377–384. 10.11646/zootaxa.3741.3.6 [DOI] [PubMed] [Google Scholar]
- Nikolajev GV. (1999) On the polyphyly of the subfamily Penichrolucaninae (Coleoptera, Lucanidae) with the erection of the new monotypic tribe Brasilucanini. Tethys Entomological Research 1: 171–172. [Google Scholar]
- Nikolajev GV. (2009) Ochodaeidae species of the Palaearctic’s Asia. Euroasian Entomological Journal 8(2): 205–211. [Google Scholar]
- Ocampo FC. (2006) Phylogenetic analysis of the scarab family Hybosoridae and monographic revision of the New World subfamily Anaidinae (Coleoptera: Scarabaeidae). Bulletin of the University of Nebraska State Museum 19: 13–177. [Google Scholar]
- Okada K, Miyatake T. (2009) Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatoceruscornutus. Animal Behaviour 77(5): 1057–1065. 10.1016/j.anbehav.2009.01.008 [DOI] [Google Scholar]
- Okajima S, Araya K. (2012) The Standard of Scarabaeoid Beetles in Japan. Gakken Publishing Co. Ltd, Tokyo, 445 pp. [Google Scholar]
- Okuda N. (2012a) A new species of the genus Nigidius MacLeay from Kon Tum Province, Central Vietnam. Kogane 13: 143–146. [Google Scholar]
- Okuda N. (2012b) Two new species of Aegus MacLeay (Coleoptera, Lucanidae) from south Sulawesi province, Indonesia. Gekkan-Mushi 493: 25–28. [Google Scholar]
- Palestrini C, Roggero A, Hernandez Nova LK, Giachino PM, Rolando A. (2012) On the evolution of shape and size divergence in Nebria (Nebriola) ground beetles (Coleoptera, Carabidae). Systematics and Biodiversity 10: 147–157. 10.1080/14772000.2012.685775 [DOI] [Google Scholar]
- Paulsen MJ. (2010) The stag beetles of southern South America (Coleoptera: Lucanidae). Bulletin of the University of Nebraska State Museum 24: 1–147. [Google Scholar]
- Paulsen MJ. (2013) A new genus for the Neotropical species of Aesalus Fabricius, with descriptions of eight new species (Coleoptera: Lucanidae: Aesalinae). Insecta Mundi 25: 1–25. [Google Scholar]
- Paulsen MJ, Mondaca EJ. (2006) Revision of the South American Ceratognathini (Coleoptera: Lucanidae: Aesalinae) with the description of a new genus and a new species. Zootaxa 1191: 1–19. [Google Scholar]
- Paulsen MJ, Ocampo FC. (2012) The Ochodaeidae of Argentina (Coleoptera, Scarabaeoidea). ZooKeys 174: 7–30. 10.3897/zookeys.174.2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarenko AG. (2004) Beetles (Insecta, Coleoptera) of the late Permian and early Triassic. Paleontological Journal 38(Supplement 2): S185–S196.
- Ratcliffe BC. (1984) A review of the Penichrolucaninae with analyses of phylogeny and biogeography, and description of a second New World species from the Amazon Basin (Coleoptera: Lucanidae). Quaestiones Entomologicae 20(2): 60–87. [Google Scholar]
- Ricklefs RE. (2012) Species richness and morphological diversity of passerine birds. Proceedings of the National Academy of Sciences 109(36): 14482–14487. 10.1073/pnas.1212079109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf FJ. (2006) tps-DIG, Digitize Landmarks and Outlines, Version 2.05. [Software and Manual]. Department of Ecology and Evolution, State University of New York at Stony Brook, New York. http://life.bio.sunysb.edu/morph/
- Rohlf FJ. (2007) . NTSYS-pc Numerical Taxonomy and Multivariate Analysis System, Version 2.20 for Windows [Software and Manual]. Exeter Software, New York.
- Roy K, Balch DP, Hellberg ME. (2001) Spatial patterns of morphological diversity across the Indo-Pacific: analyses using strombid gastropods. Proceedings of the Royal Society B: Biological Sciences 268(1485): 2503–2508. 10.1098/rspb.2000.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Foote M. (1997) Morphological approaches to measuring biodiversity. Trends in Ecology & Evolution 12(7): 277–281. 10.1016/s0169-5347(97)81026-9 [DOI] [PubMed] [Google Scholar]
- Sakai K, Nagai S. (1998) The Cetoniine Beetles of the World. Mushi-Sha, Tokyo, 421 pp. [Google Scholar]
- Schenk KD. (2008) Lucanidae vom Arunachal Pradesh, Indien und beschreibung von zwei neuen Arten. Entomologische Zeitschrift 118(4): 175–178. [Google Scholar]
- Sherratt E, Gower DJ, Klingenberg CP, Wilkinson M. (2014) Evolution of cranial shape in caecilians (Amphibia: Gymnophiona). Evolutionary Biology 41: 528–545. 10.1007/s11692-014-9287-2 [DOI] [Google Scholar]
- Tatsuta H, Mizota K, Akimoto SI. (2001) Allometric patterns of heads and genitalia in the stag beetle Lucanusmaculifemoratus (Coleoptera: Lucanidae). Annals of the Entomological Society of America 94(3): 462–466. 10.1603/0013-8746(2001)094[0462:apohag]2.0.co;2 [DOI]
- Tatsuta H, Mizota K, Akimoto SI. (2004) Relationship between size and shape in the sexually dimorphic beetle Prosopocoilusinclinatus (Coleoptera: Lucanidae). Biological Journal of the Linnean Society 81: 219–233. 10.1111/j.1095-8312.2003.00279.x [DOI] [Google Scholar]
- Van Roon G. (1910) Lucanidae. In: Schenkling S. (Ed.) Coleopterorum Catalogus.W. Junk, Berlin, 7–8.
- Williams PH, Humphries CJ. (1996) Comparing character diversity among biotas. In: Gaston KJ. (Ed.) Biodiversity: a Biology of Numbers and Differences.Blackwell Science, Oxford, 54–76.
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. (2004) Geometric Morphometrics for Biologists: a Primer. Elsevier, San Diego, 428 pp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Mengna Zhang, Yongying Ruan, Xia Wan, Yijie Tong, Xingke Yang, Ming Bai
Geometric morphometric data
Data type: morphometric data
Explanation note: Tables S1–S12.