Abstract
In this research, Enterococcus species with probiotic properties were isolated from raw milk and some traditional dairy products and these species were identified by using biochemical, phenotypical and genotypic methods and their potential use in Izmir Tulum cheese production as adjunct culture were investigated. Among the isolated and identified Enterococcus species, three Enterococcus faecium and one Enterococcus durans strains with no vancomycin resistance, no decarboxylation activity, high antimicrobial activity and resistance to acidity were selected, and a mix culture was produced. This culture was mixed with commercial cheese culture containing Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris in different proportions and used in Izmir Tulum cheese production. Cheese samples were left to ripening for 180 days, and their physical, chemical, microbiological and sensory properties were evaluated with comparison to prior studies. Evaluations showed that using adjunct culture in Izmir Tulum cheese production has no negative effect on physical, chemical and microbiological properties and higher proportions of probiotic Enterococci in the culture have a positive effect on the sensory properties, especially on flavor.
Keywords: Enterococci, Probiotic properties, Lactic acid bacteria, Izmir Tulum cheese, Adjunct cultures
Introduction
Many traditional cheeses are produced in various areas of the world locally or industrially. More than 50 varieties of cheese can be found in different regions of Turkey. Among them, Beyaz (White cheese), Kaşar, and Tulum cheeses are the most popular. Izmir Tulum cheese is an important Turkish traditional cheese and it is called “Teneke Tulum cheese” or “Salamura Tulum cheese” according to the packaging material used (Hayaloglu et al. 2008; Kamber 2008). This cheese is generally creamy and in white color, however the type of milk used, the production method and the amount of salt in the cheese affect its color and other properties. Although ewe’s milk is preferred in the production of Izmir Tulum cheese, generally a mixture of ewe’s, goat’s and cow’s milk is used. Usually, milk isn’t pasteurized for production of Tulum cheese and heated to acidifying temperature and fermented immediately. However, in methods applied in some dairy plants after milk is heated to 50–60 °C, it is cooled to inoculation temperature and added rennin. Curd is kept brined in 16% brine overnight. Then, the curd is filled in cans with 12% brine for ripening at 4–6 °C for 90–180 days. This type of Tulum cheese has some differences like being kept in brine for ripening in cans (Akpinar et al. 2017; Durlu-Özkaya and Gün 2014; Kamber 2008).
The use of Enterococci in cheeses is highly controversial. Enterococci are considered essential for cheese flavor in most of the Southern European countries, while most Northern European countries have focused on the negative aspects. These divergent viewpoints may simply be due to cultural differences (Bulajić and Mijačević 2004). Several studies have suggested that dairy food strains of Enterococci have a positive influence on ripening of traditional cheeses. Enterococci counts in Mediterranean-type cheese curds range from 104 to 106 cfu/g, and in the fully ripened cheeses from 105 to 107 cfu/g. E. faecalis and E. faecium are the species most frequently isolated from dairy products (Giraffa et al. 1997; Foulquie Moreno et al. 2006). However, E. durans is now also frequently isolated and its presence in dairy products has often been underestimated, E. durans strains have probably been mis-identified in the past. The enterococci contribute to the ripening and aroma development of cheeses such as Cheddar, Feta, Water-buffalo Mozarella, Cebreiro, Venaco and Hispanico due to their proteolytic and esterolytic activities and their diacetyl production by citrate metabolism. Because of their role in ripening, flavor development and bacteriocin production in cheeses, it has been suggested that Enterococci with desirable technological and metabolic properties could be included in starter cultures for various cheeses (Sarantinopoulos et al. 2002; Ogier and Serror 2008).
In this research, Enterococcus species with probiotic properties were isolated from raw milk and some traditional dairy products by using biochemical, phenotypical and genotypical methods and their potential use in Izmir Tulum cheese production as adjunct culture were investigated. The aim of this study was to produce a high quality Izmir Tulum cheese with similar chemical and biochemical properties and microbiological and sensory characteristics to that of traditional type Izmir Tulum cheese. In addition, the effect of using Enterococci at different ratios was investigated.
Materials and methods
Raw cow’s milk was supplied from Semsi Egi Food Products Co. (Pinarbasi, Izmir, Turkey). The mean composition of raw cow’s milk used in making Izmir Tulum cheese was pH 6.55 ± 0.00, total solids 12.08 ± 0.05 g/100 ml, fat 3.48 ± 0.04 g/100 ml, lactose 3.35 ± 0.05 g/100 ml, total protein 3.14 ± 0.02 g/100 ml and acidity as lactic acid 0.158 ± 0.01 g/100 ml. Animal-origin rennet (calf) was obtained from Peyma Hansen (Naturen® Mandra 155) Industry and Trade A.S. (Istanbul, Turkey) and used to coagulate milk in liquid form (coagulating power 1:25,000). Calcium chloride was obtained commercially from Atilgan Tuz Factory (Çanakkale, Turkey). Cheese starter culture containing Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris (1:1) were obtained from Maysa® CM11 Mystarter (Tuzla-Istanbul, Turkey). The cheeses produced in the study were packaged in 4.5 L lacquered tin plate boxes obtained from Şimşek Packing A.Ş. (Izmir, Turkey).
Selection of Enterococcus species for Izmir Tulum cheese production and preparation of culture combinations
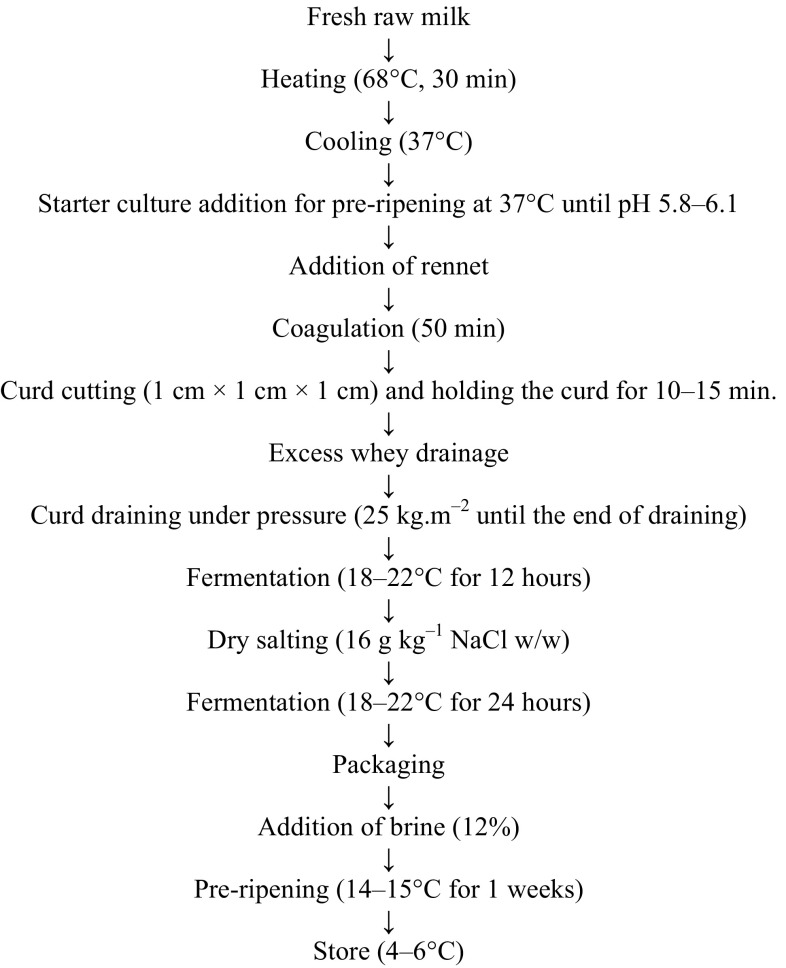
As a result of activity tests conducted on the cultures that will be used in the production of cheese, E. faecium K65E2, E. faecium K66E2, E. faecium K73E2 and E. durans K61E5 probiotic strains which showed the highest activity rates were used. The selected Enterococcus strains were transferred to M-17 medium twice and activated. Then, the cultures were inoculated in sterile media that were prepared with 12% fat-free skim milk powder (Oxoid, UK) in equal ratios and left to incubate at 37 °C. The incubation was ended at pH 4.8. Enterococcus ssp. content of the medium kept at 4 °C overnight was 11.23 ± 0.01 Log cfu/mL. In the same way, commercial cheese culture containing Lactococcus lactis and Lactococcus cremoris (Maystarter, Maysa, Turkey) were prepared according to the instructions of the producer. Lyophilized culture was activated using sterile reconstituted skim milk prepared with 12% fat-free skim milk powder (Oxoid, UK). Bacterial content of this prepared culture was 11.09 ± 0.02 Log cfu/mL when which are in the log phase. The two cultures were separated according to 0%, 5%, 10%, 20% Enterococcus ssp. contents and the combinations were formed. Lactococcus ssp. and Enterococcus ssp. contents of the cultures used in the production of Izmir Tulum cheese are given in Table 1. The cheese produced without using Enterococcus ssp. was coded as A, the cheese produced with 5% Enterococcus ssp. content was coded as B, the cheese produced with 10% Enterococcus ssp. content was coded as C and the cheese produced with 20% Enterococcus ssp. content was coded as D. The prepared starter cultures were added to milk cooled to 37 °C after heat treatment (Fig. 1).
Table 1.
Lactococcus ssp. and Enterococcus ssp. contents of the cultures used in the production of Izmir Tulum cheese (Log cfu/mL)
| Cultere code | Lactococcus ssp. | Enterococcus ssp. | Yeast and moulds | Total coliform |
|---|---|---|---|---|
| A | 11.09 ± 0.02 | < 10 | < 10 | < 10 |
| B | 11.05 ± 0.01 | 9.92 ± 0.01 | < 10 | < 10 |
| C | 11.00 ± 0.03 | 10.21 ± 0.04 | < 10 | < 10 |
| D | 10.95 ± 0.01 | 10.53 ± 0.02 | < 10 | < 10 |
A: The cheese produced without using Enterococcus ssp. B: the cheese produced with 5% Enterococcus ssp. C: the cheese produced with 10% Enterococcus ssp. D: the cheese produced with 20% Enterococcus ssp
Fig. 1.
Izmir Tulum cheese production using starter culture
Physical and chemical analysis
The dry matter content of the cheese samples was measured by the gravimetric method (ISO 2004); acidity was determined titrimetrically as lactic acid % (Anonymous 1996). Salinity was measured according to the method described in TS 11966 (Anonymous 1996) and fat was determined by the Gerber method using Van-Gulik butyrometer (ISO 2008). The pH of cheese samples was measured using a pH-meter with a combined electrode (Hanna pH 211 Microprocessor, Portugal). Total nitrogen (TN) of cheese was determined using Leco FP 528 Nitrogen analyzer. Total protein ratio was calculated by multiplying the Nitrogen value by 6.38 (Anonymous 1993; Renner 1993). The water-soluble fraction (WSN) was prepared essentially as described by Ardö and Polychroniadou (1999), using 20 g cheese with 100 mL pure water. The mixture was homogenized for 5 min using a SilentCrusher M Ultraturrax (Heidolph Instruments, Germany). Water-soluble N content of the cheese extract was determined by the Kjeldahl method, using 10 mL of cheese extracts (Gürsoy and Kınık 2010). The ripening index was calculated using the formula of WSN/TN × 100 (Venema et al. 1987). The acid degree value (ADV) is an indicator of fat hydrolysis and was determined according to the method described by Renner (1993).
Microbiologic analysis
Cheese samples (10 g) were homogenized with 90 mL sterile 0.1% peptone water solution using a Stomacher for at least 2 min. Sequential decimal dilutions of the homogenate in sterile peptone water were plated in duplicates on the specific media. Man Ragosa Sharpe Agar (MRS, Merck, Darmstadt, Germany) was used for the enumeration of Lactobacillus ssp., M17 Agar (Merck, Darmstadt, Germany) was used for the enumeration of Lactococcus ssp. MRS Agar plates were incubated at 42 °C for 72 h anaerobically using Anaerocult A (Merck, Darmstadt, Germany) for Lactobacillus, M-17 Agar was incubated at 37 °C for 72 h aerobically for Lactococcus ssp. (Terzaghi and Sandine 1975; Bracquart 1981). Enterococci was determined Kanamycin-Esculin Azide Agar (Merck, Darmstadt, Germany) at 37 °C for 24–48 h (Reuter and Klein 2003). Yeast and mould counts were enumerated on yeast extract glucose chloramphenicol agar (Merck, Darmstadt, Germany). The plates were incubated at 25 ± 1 °C for 5 days (ISO 1992). All microbiological analyses were performed in triplicates and the results are presented as mean and standard deviation. Results were expressed as cfu/g of cheese.
Sensory analysis
Organoleptic evaluation of the cheese samples during the ripening period was carried out by a seven-member panel formed from the expert staff of Ege University, Dairy Technology Department, selected based on their interest and experience in sensory evaluation of Izmir Tulum cheese products according to TS-11966. Izmir Tulum cheese samples were placed on white plates coded with three-digit random numbers.
Statistical analysis
Statistical analysis of the data was carried out using the analysis of variance in SPSS v.15.00 (Chicago, Illinois, USA). Means with a significant difference were compared by Duncan’s multiple range tests. P values < 0.05 were considered statistically significant. All analyses were performed in duplicate.
Results and discussion
Physical and chemical properties of Izmir Tulum cheese samples
Physical and chemical properties of Izmir Tulum cheese samples produced with the adjunct culture Enterococcus ssp. are given in Tables 2 and 3. The dry matter contents varied between 49.00% and 53.49%, and the highest average dry matter content was in sample A. The differences between the dry matter ratios were statistically significant (P < 0.05). The dry matter ratio generally decreases in cheeses ripened in brine. This is associated with water-soluble proteins and peptides passing to the brine. In addition, dry matter of cheese may decrease due to the decomposition of peptide bonds in αs1-casein and the newly formed ionic groups binding the water in the brine in addition to the loss in the water absorption abilities of proteins during storage at low temperatures (Kesenkaş 2005).
Table 2.
Some physicochemical properties of Izmir Tulum cheese samples
| Storage days | Izmir Tulum cheese samples | ||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Dry matter (%) | 1 | 53.473 ± 0.09dE | 52.805 ± 0.01cD | 52.215 ± 0.13bD | 51.842 ± 0.07aD |
| 30 | 51.627 ± 0.10dA | 50.550 ± 0.10cB | 49.022 ± 0.12aA | 50.007 ± 0.16bB | |
| 60 | 52.889 ± 0.12dD | 49.660 ± 0.12aA | 50.508 ± 0.15cC | 49.996 ± 0.08bB | |
| 90 | 53.487 ± 0.03aE | 50.378 ± 0.14aB | 51.996 ± 0.12Cd | 51.136 ± 0.01bC | |
| 120 | 51.950 ± 0.16cB | 49.822 ± 0.12bA | 49.042 ± 0.15aA | 49.001 ± 0.16aA | |
| 150 | 52.420 ± 0.14bC | 51.998 ± 0.15bC | 49.202 ± 0.17aA | 49.029 ± 0.15aA | |
| 180 | 53.457 ± 0.03cE | 54.287 ± 0.02bE | 50.019 ± 0.03aB | 50.016 ± 0.02aB | |
| Fat (%) | 1 | 22.75 ± 0.35A | 23.50 ± 0.71AB | 23.50 ± 0.71BC | 24.00 ± 0.35C |
| 30 | 23.25 ± 0.35abAB | 22.75 ± 0.35aA | 22.25 ± 0.35aA | 24.50 ± 0.71bB | |
| 60 | 24.25 ± 0.35bBC | 24.75 ± 0.35bB | 22.25 ± 0.35aA | 22.75 ± 0.35aA | |
| 90 | 23.75 ± 0.35BC | 22.25 ± 0.35A | 22.25 ± 0.35A | 23.00 ± 0.71A | |
| 120 | 24.50 ± 0.70BC | 23.50 ± 0.71AB | 24.00 ± 0.00C | 23.50 ± 0.71AB | |
| 150 | 24.25 ± 0.35bBC | 24.50 ± 0.71bB | 23.00 ± 0.00aAB | 22.75 ± 0.35aA | |
| 180 | 24.25 ± 0.35bBC | 24.75 ± 0.35bB | 22.75 ± 0.35aAB | 22.25 ± 0.35aA | |
| Salt (%) | 1 | 2.93 ± 0.16aA | 3.40 ± 0.16bA | 3.45 ± 0.09bA | 4.33 ± 0.17cA |
| 30 | 4.33 ± 0.17B | 5.03 ± 0.17B | 4.21 ± 0.33B | 4.57 ± 0.16AB | |
| 60 | 4.68 ± 0.00aC | 5.21 ± 0.07bBC | 4.56 ± 0.13aC | 4.69 ± 0.01aB | |
| 90 | 5.23 ± 0.12abD | 5.38 ± 0.00bC | 5.15 ± 0.00aD | 5.62 ± 0.00cC | |
| 120 | 5.38 ± 0.00aDE | 5.38 ± 0.00aC | 5.38 ± 0.00aDE | 5.74 ± 0.16bC | |
| 150 | 5.53 ± 0.13aEF | 5.61 ± 0.00aD | 5.61 ± 0.00aE | 5.85 ± 0.00bC | |
| 180 | 5.71 ± 0.01aF | 5.67 ± 0.06aD | 5.64 ± 0.01aE | 5.86 ± 0.01bC | |
| pH (%) | 1 | 4.76 ± 0.01bBC | 4.92 ± 0.02cD | 4.97 ± 0.01dF | 4.71 ± 0.01aB |
| 30 | 4.73 ± 0.00bBC | 4.86 ± 0.01cC | 4.92 ± 0.01dE | 4.69 ± 0.00aB | |
| 60 | 4.69 ± 0.01aAB | 4.77 ± 0.01bB | 4.86 ± 0.01cC | 4.68 ± 0.01aB | |
| 90 | 4.64 ± 0.01aA | 4.72 ± 0.01bA | 4.78 ± 0.01cB | 4.61 ± 0.04aA | |
| 120 | 4.75 ± 0.01bBC | 4.77 ± 0.00cB | 4.68 ± 0.01aA | 4.83 ± 0.01dC | |
| 150 | 4.77 ± 0.06C | 4.83 ± 0.04C | 4.86 ± 0.01D | 4.78 ± 0.04C | |
| 180 | 4.84 ± 0.01aD | 4.91 ± 0.01bD | 4.96 ± 0.01bF | 5.05 ± 0.03cD | |
| Titratable acidity (lactic acid %) | 1 | 0.66 ± 0.01b | 0.52 ± 0.03aA | 0.45 ± 0.04aA | 0.55 ± 0.01aA |
| 30 | 0.69 ± 0.01c | 0.57 ± 0.04aA | 0.65 ± 0.00bcB | 0.60 ± 0.03abB | |
| 60 | 0.70 ± 0.00 | 0.68 ± 0.01B | 0.70 ± 0.03C | 0.68 ± 0.01C | |
| 90 | 0.72 ± 0.01 | 0.70 ± 0.02B | 0.72 ± 0.00C | 0.71 ± 0.01C | |
| 120 | 0.70 ± 0.01ab | 0.70 ± 0.03abB | 0.77 ± 0.01bD | 0.69 ± 0.04aAB | |
| 150 | 0.76 ± 0.01 | 0.76 ± 0.01B | 0.77 ± 0.01D | 0.78 ± 0.01D | |
| 180 | 0.71 ± 0.01a | 0.70 ± 0.02aC | 0.71 ± 0.01aC | 0.80 ± 0.01bD | |
A: The cheese produced without using Enterococcus ssp. B: the cheese produced with 5% Enterococcus ssp. C: the cheese produced with 10% Enterococcus ssp. D: the cheese produced with 20% Enterococcus ssp
X, Y, Z: Values with the different letters in the same row differ significantly (P < 0.05)
a, b, c, d, e: Values with the different letters in the same column differ significantly (P < 0.05)
Table 3.
Total N, protein, WSN, ripening index and free fatty acid values of Izmir Tulum cheese samples
| Izmir Tulum cheese samples | |||||
|---|---|---|---|---|---|
| Storage days | A | B | C | D | |
| Total N (%) | 1 | 3.41 ± 0.26bB | 3.86 ± 0.06cC | 3.24 ± 0.09aB | 3.34 ± 0.30abB |
| 30 | 3.88 ± 0.13cD | 3.18 ± 0.02aA | 3.72 ± 0.01cD | 3.50 ± 0.06bB | |
| 60 | 3.54 ± 0.09cB | 3.18 ± 0.02abA | 3.30 ± 0.05bB | 3.09 ± 0.03aA | |
| 90 | 3.10 ± 0.04aA | 3.14 ± 0.04aA | 3.69 ± 0.01cD | 3.44 ± 0.05bB | |
| 120 | 3.07 ± 0.04aA | 3.21 ± 0.11bA | 3.29 ± 0.06bB | 3.17 ± 0.01bA | |
| 150 | 3.70 ± 0.01cC | 3.06 ± 0.06aA | 3.15 ± 0.15aA | 3.35 ± 0.07bB | |
| 180 | 3.14 ± 0.04aA | 3.44 ± 0.03bB | 3.57 ± 0.40bcC | 3.78 ± 0.04cC | |
| Protein (%) | 1 | 21.75 ± 1.60bB | 24.60 ± 0.38cD | 20.65 ± 0.59aB | 21.28 ± 0.19abB |
| 30 | 24.73 ± 0.84dD | 20.26 ± 0.16aB | 23.74 ± 0.10cE | 22.33 ± 0.38bC | |
| 60 | 22.54 ± 0.56cBC | 20.31 ± 0.01abB | 21.04 ± 0.30bC | 19.73 ± 0.18aA | |
| 90 | 19.77 ± 0.28aA | 20.01 ± 0.21abA | 23.50 ± 0.08cD | 21.90 ± 0.33bC | |
| 120 | 19.60 ± 0.29aA | 20.45 ± 0.64cC | 20.97 ± 0.39cB | 20.23 ± 0.11bAB | |
| 150 | 23.61 ± 0.07cC | 19.51 ± 0.39aA | 19.83 ± 0.96aA | 21.38 ± 0.17bB | |
| 180 | 20.01 ± 0.21aA | 21.95 ± 0.18bC | 22.72 ± 0.50cD | 24.08 ± 0.21dD | |
| WSN (%) | 1 | 0.163 ± 0.00bA | 0.136 ± 0.00aA | 0.186 ± 0.01aA | 0.162 ± 0.00aA |
| 30 | 0.253 ± 0.00bB | 0.271 ± 0.00bB | 0.191 ± 0.00aA | 0.185 ± 0.00aA | |
| 60 | 0.356 ± 0.00C | 0.284 ± 0.00B | 0.265 ± 0.00B | 0.259 ± 0.01B | |
| 90 | 0.370 ± 0.01C | 0.349 ± 0.00C | 0.289 ± 0.00B | 0.307 ± 0.00AB | |
| 120 | 0.376 ± 0.00C | 0.459 ± 0.01D | 0.382 ± 0.00C | 0.347 ± 0.00B | |
| 150 | 0.538 ± 0.00bD | 0.465 ± 0.01aD | 0.514 ± 0.00bD | 0.393 ± 0.00aB | |
| 180 | 0.602 ± 0.00cE | 0.530 ± 0.01bE | 0.585 ± 0.00cE | 0.464 ± 0.01aC | |
| Ripening Index | 1 | 4.87 ± 0.49bA | 3.59 ± 0.15aA | 5.83 ± 0.27cA | 4.82 ± 0.70bA |
| 30 | 6.61 ± 0.10bB | 8.66 ± 0.11cB | 5.91 ± 0.08abA | 5.36 ± 0.16aAB | |
| 60 | 10.20 ± 0.38bC | 9.06 ± 0.16bBC | 8.14 ± 0.00aB | 8.49 ± 0.27aB | |
| 90 | 12.28 ± 0.25cD | 11.28 ± 0.24bC | 8.93 ± 0.09aBC | 9.07 ± 0.04aBC | |
| 120 | 12.30 ± 0.37bD | 14.52 ± 0.29cD | 11.79 ± 0.11abC | 11.08 ± 0.21aC | |
| 150 | 14.72 ± 0.17bE | 15.21 ± 0.54bcE | 16.80 ± 0.92cD | 11.88 ± 0.10aC | |
| 180 | 19.46 ± 0.09cF | 15.61 ± 0.39bE | 16.92 ± 0.18bcD | 12.46 ± 0.30aD | |
| Free fatty acid (mg KOH 100 g−1) | 1 | 1.37 ± 0.05aA | 1.47 ± 0.01bA | 1.51 ± 0.04bA | 1.57 ± 0.05cA |
| 30 | 1.78 ± 0.05aB | 1.89 ± 0.08aB | 2.44 ± 0.11bB | 2.22 ± 0.05aB | |
| 60 | 2.26 ± 0.05C | 2.31 ± 0.01C | 2.28 ± 0.04C | 2.29 ± 0.02B | |
| 90 | 2.26 ± 0.06aC | 2.38 ± 0.02abC | 2.50 ± 0.07bC | 2.54 ± 0.01bC | |
| 120 | 2.69 ± 0.05D | 2.55 ± 0.06D | 2.67 ± 0.08D | 2.68 ± 0.07D | |
| 150 | 2.73 ± 0.23D | 2.75 ± 0.01E | 2.76 ± 0.03D | 2.78 ± 0.06D | |
| 180 | 3.53 ± 0.14E | 3.48 ± 0.04F | 3.56 ± 0.06E | 3.57 ± 0.07E | |
A: The cheese produced without using Enterococcus ssp. B: the cheese produced with 5% Enterococcus ssp. C: the cheese produced with 10% Enterococcus ssp. D: the cheese produced with 20% Enterococcus ssp
X, Y, Z: Values with the different letters in the same row differ significantly (P < 0.05)
a, b, c, d, e: Values with the different letters in the same column differ significantly (P < 0.05)
The fat contents of A, B and C samples were close to each other on the 1st day of the storage (22.75%–23.50%), while the fat content of the D sample was higher (24%). Dry matter contents of the samples varied between 42.55 and 50.54% on the 1st day, while it changed between 44.49% and 45.59% at the 180th day of the storage. The effect of using probiotic Enterococcus ssp. in the starter cultures on the fat contents of the samples was statistically significant on the 90th and the 120th days (P < 0.05), and statistically not significant on the other analysis days of the storage (P > 0.05). Biochemical changes in cheese composition are especially associated with the changes in the dry matter content of cheeses ripened in brine during the ripening period.
The highest salinity was in D sample on the 1st day of the storage (4.33%), this was followed by C (3.45%), B (3.40%) and A (2.43) samples, respectively. The highest salinity on the 180th day of the storage was again in D sample (5.86%), and followed by A (5.71%), B 5.70%) and C (5.64%) samples, respectively. Additionally, the effect of using probiotic Enterococcus ssp. in the starter cultures at different ratios on the salinity was not significant only on the 30th day (P > 0.05), while it was significant on the other days of the storage (P < 0.05). Salt contents % in the dry matter of cheese samples showed a similar change to salinity values and showed a rapid increase in the first days while the decrease was slow in the further days. The highest values at the beginning and the end of the storage was determined in D sample, this was followed by C, B and A samples, respectively. The sample with the most appropriate content in accordance with Briny Type Cheese Standard was the C sample. The gradual increase in the salt content at the end of the ripening period can be associated with the decrease in the moisture in cheese, ambient temperature, pH and the changes in protein ratio (Ateş and Patır 2001).
At the beginning of storage, pH values were sorted from high to low as C, B, A and D while the sorting at the end of the storage period was D, C, A and B. pH values in Tulum cheese samples decreased in A, B and D samples until the 90th day and in C sample until the 120th day. Following these days, pH values began to increase. As a result of the statistical analysis, it was found that the effect of using probiotic Enterococcus ssp. in the starter cultures at different ratios on the pH values of the samples was not significant only on the 150th day (P > 0.05), whereas significant on the other days of the storage (P < 0.05). Lactic acid % content of the samples at the beginning of storage was sorted from low to high as C, D, B and A, and as B, A, C and D at the end of the storage. The effect of using effect of using probiotic Enterococcus ssp. in the starter cultures at different ratios on the lactic acid % contents were significant on the 1st, 30th, 120th and 180th days, whereas not significant on the other days of the storage (P < 0.05). In A and D samples, lactic acid values increased until the 90th day, then decreased on the 120th day, increased again on the 150th day and finally showed a decrease on the 180th day. Lactic acid contents detected in cheese samples did not cause any problems. The increase in pH values at the later days of the ripening period was associated with the formation of different organic acids as a result of the decomposition of lactic acid by the yeasts and the formation of ammonia and other similar compounds as a result the deamination of free amino acids (Schlesser et al. 1992; Azarnia et al. 1997).
Total nitrogen % content at the beginning of the storage varied between 3.24 and 3.86%, while it changed between 3.14 and 3.78% at the end of the storage. In an evaluation based on the average values the highest total nitrogen % content was in C sample (3.42%), this was followed by A (3.41%), D (3.38%) and B (3.30%) samples, respectively. Protein % content at the beginning of the storage varied between 20.65% and 24.60%, while it changed between 20.21% and 24.088% at the end of the storage. As a result of the statistical analysis, it was found that the effect of using probiotic Enterococcus ssp. in the starter cultures at different ratios on protein content (%) was significant (P < 0.05). In an evaluation based on the average values the highest protein (%) content, parallel to the total nitrogen content, was in C sample (21.78%), this was followed by A (21.72%), D (21.56%) and B (21.01%) samples, respectively. Comparing the values at the beginning and the end of the storage period, A and B samples decreased, whereas C and D values increased.
Water soluble Nitrogen content is the indicator of the level of ripening in cheese and it includes whey proteins, protease peptones, and small and medium molecular-weight peptides resulting from the degradation of casein (McSweeney and Fox 1997). At the 1st day of the ripening period, the highest ratio of soluble nitrogen detected in the cheese samples was in C sample with 1.86%, while the lowest was in B sample with 0.136%. At the 180th day of the ripening period, the highest ratio of soluble nitrogen detected in the cheese samples was in A sample with 0.602%, while the lowest was in D sample with 0.464%. Considering the average soluble nitrogen contents, the highest was in A sample, and this was followed by B, C and D samples, respectively.
Ripening index values of Izmir Tulum cheese samples showed a regular increase, parallel to the increase in soluble nitrogen contents. At the first day of the ripening period, ripening index values detected in cheese samples varied between 3.59 and 4.87, while they changed between 12.46 and 19.46 at the end of the storage. The average ripening index was in A sample (11.49) and this was followed by B (11.13), C (10.63) and D (9.02) samples, respectively. Göncüoğlu et al. (2009) have reported that using E. faecium FAIR-E 198 may reduce the ripening period in White cheese and this strain can be used in the production of White cheese as a starter culture. De Vuyst et al. (2003) have reported that, when used as a preservative culture or mixed culture in cheese production, Enterococcus faecium strains had an antibacterial effect on Listeria and Clostiridium and that these strains might contribute to the ripening.
Free fatty acid contents at the 1st day of the storage varied between 1.37% and 1.57%. A regular increase was observed at the further days of the ripening and the values were close to each other, the effect of using probiotic Enterococcus ssp. in the starter cultures at different ratios were significant on the 1st, 30th and the 90th days (P < 0.05), whereas not significant on the other days of the storage. The effect of ripening period on the free fatty acid contents were also significant (P < 0.05). Litopoulou-Tzanetaki et al. (1993), in their study on Feta cheese, have reported that, among the culture combinations, the culture with the highest lypolytic activity was the group containing Lc. lactis + Lb. casei + E. durans + Leu. cremoris. Jensen et al. (1975), have reported that using E. faecium at high lypolytic activity did not affect lypolysis.
Microbiological properties of Izmir Tulum cheese samples
Microbiological properties of Izmir Tulum cheese samples and the change in microorganisms during storage is given in Table 4. Lactobacillus ssp. counts decreased in B sample during storage, while fluctuations were observed in A, C and D samples. Lactobacillus ssp. counts at the beginning of storage varied between 6.21 and 11.31 Log cfu/g, the highest count was in B sample (11.31) and this was followed by D (11.16), C (7.43) and A (6.21). At the end of the storage, the highest count was determined in C sample (7.85), and this was followed by D (7.82), B (7.14) and A (6.97) samples. As a result of the statistical evaluation, the effect of using different ratios of probiotic Enterococcus ssp. in the starter cultures on Lactobacillus ssp. counts in the production of Izmir Tulum cheese and the changes in Lactobacillus ssp. during storage were found to be significant (P < 0.05). Lactococcus ssp. counts at the 1st day of storage varied between 12.98 and 11.53 Log cfu/g, the highest count was in A sample (12.98) and this was followed by D (11.91), B (11.83) and C (11.53) samples. Lactococcus ssp. counts at the 180th day of storage varied between 8.27 and 8.92 Log cfu/g, the highest count was in C sample (8.92) and this was followed by D (8.83), B (8.72) and C (8.27) samples. Statistical analyses showed that the effect of using different ratios of probiotic Enterococcus ssp. in the starter cultures on Lactobacillus ssp. counts in the production of Izmir Tulum cheese was not significant (P > 0.05) on the 150th day of the storage whereas it was found to be significant (P < 0.05) on the other storage days. The changes in Lactococcus ssp. counts during the storage period was statistically significant (P < 0.05). Nunez and Medina (1979), Öner et al. (2004), and Büyükyörük and Soyutemiz (2010) have reported that Lc. lactis and Lc. cremoris were the dominant species in the cheese samples during ripening period. The highest Enterococcus ssp. count was determined in D sample (11.95), and this was followed by C (8.76), B (8.02) and A (3.81) samples. The highest values were detected on the 30th day of the storage, the following days the counts decreased. The sorting of Enterococcus ssp. counts at the end of storage did not change and varied between 3.70 and 7.90 Log cfu/g. Statistical evaluations showed that, the effect of using Enterococcus ssp. in starter cultures at different ratios had a significant effect on the Enterococcus ssp. counts and the changes in Enterococcus ssp. counts during storage period (P < 0.05). Yeast-mould counts varied between < 10 and 5.46 Log cfu/g at the beginning of storage, whereas changed between 4.22 and 4.77 Log cfu/g at the end of the storage. Considering the values at the end of the storage, the lowest yeast-mould count was determined in D sample (4.22), this was followed by A (4.45), C (4.47) and B (4.77) samples. Öner et al. (2005) in tulum cheese samples produced with conventional method and using starter culture, have reported that, with small fluctuations, yeast-mold counts did not exhibit any significant changes and varied between 4.22 ile 5.49 Log cfu/g. Gürses and Erdoğan (2006), in Tulum cheese samples ripened for 3 months, have reported that Lactobacilli were the dominant flora and Enterococci, lactococci and Leuconostocs were in lower levels while Çakmakçı et al. (2008) have reported that lactobacilli and enterococci levels might be high in Tulum cheese ripened for 9 months.
Table 4.
Microbiological properties of Izmir Tulum cheese samples (Log cfu/g)
| Izmir Tulum cheese samples | |||||
|---|---|---|---|---|---|
| Storage days | A | B | C | D | |
|
Lactobacillus ssp. (Log cfu/g) |
1 | 6.21 ± 0.04aB | 11.31 ± 0.13cE | 7.43 ± 0.16bA | 11.16 ± 0.07cE |
| 30 | 6.01 ± 0.15aA | 10.66 ± 0.64cD | 9.11 ± 0.06bD | 10.66 ± 0.10cD | |
| 60 | 7.51 ± 0.01aE | 9.15 ± 0.21cC | 8.29 ± 0.12bC | 8.45 ± 0.21bBC | |
| 90 | 6.13 ± 0.03aAB | 9.00 ± 0.14dC | 8.16 ± 0.06cC | 7.88 ± 0.04bA | |
| 120 | 6.28 ± 0.06aB | 7.91 ± 0.02bB | 8.94 ± 0.05cD | 8.61 ± 0.01dC | |
| 150 | 7.16 ± 0.06aD | 7.55 ± 0.02bAB | 7.56 ± 0.06bA | 8.29 ± 0.04cB | |
| 180 | 6.97 ± 0.01aC | 7.14 ± 0.03bA | 7.85 ± 0.02cB | 7.82 ± 0.06cA | |
|
Lactococcus ssp. (Log cfu/g) |
1 | 12.98 ± 0.14cF | 11.88 ± 0.04bD | 11.53 ± 0.09aE | 11.91 ± 0.03bC |
| 30 | 12.65 ± 0.07cE | 11.91 ± 0.07bD | 10.54 ± 0.09aD | 12.48 ± 0.07cD | |
| 60 | 9.70 ± 0.14aC | 9.96 ± 0.13aB | 9.88 ± 0.04aC | 11.84 ± 0.09bC | |
| 90 | 8.65 ± 0.07aB | 8.40 ± 0.13aA | 9.21 ± 0.08bB | 11.12 ± 0.26cB | |
| 120 | 10.25 ± 0.03aD | 10.48 ± 0.02bC | 10.52 ± 0.03bD | 10.89 ± 0.21cB | |
| 150 | 8.76 ± 0.05B | 8.58 ± 0.39A | 8.82 ± 0.05A | 8.84 ± 0.09A | |
| 180 | 8.27 ± 0.01aA | 8.72 ± 0.06bA | 8.92 ± 0.07cA | 8.83 ± 0.08bcA | |
|
Enterococcus ssp. (Log cfu/g) |
1 | 3.81 ± 0.04aAB | 8.02 ± 0.05bCD | 8.76 ± 0.15cC | 11.95 ± 0.03dE |
| 30 | 4.59 ± 0.16aC | 8.58 ± 0.39bE | 9.39 ± 0.13cD | 12.13 ± 0.07dE | |
| 60 | 4.40 ± 0.03aC | 8.26 ± 0.05bDE | 8.65 ± 0.07cC | 10.15 ± 0.21dD | |
| 90 | 3.97 ± 0.16aB | 7.19 ± 0.06bA | 7.91 ± 0.08cB | 8.78 ± 0.11dC | |
| 120 | 4.50 ± 0.02aC | 7.72 ± 0.06bBC | 7.90 ± 0.07cB | 8.23 ± 0.07 dB | |
| 150 | 3.95 ± 0.01aB | 7.49 ± 0.01bAB | 7.65 ± 0.03cA | 8.14 ± 0.85dAB | |
| 180 | 3.70 ± 0.08aA | 7.38 ± 0.03bAB | 7.78 ± 0.04cAB | 7.95 ± 0.06dA | |
| Yeast–mould (Log cfu/g) |
1 | 5.46 ± 0.21dE | 3.25 ± 0.07bA | 3.95 ± 0.08cA | < 10aA |
| 30 | 3.65 ± 0.08aB | 4.54 ± 0.09bBC | 5.11 ± 0.01cCD | 3.54 ± 0.08aB | |
| 60 | 4.75 ± 0.14bCD | 4.70 ± 0.07bC | 5.33 ± 0.04cDE | 4.25 ± 0.14aC | |
| 90 | 4.90 ± 0.07aD | 5.47 ± 0.03cD | 5.54 ± 0.06cE | 5.28 ± 0.06bD | |
| 120 | 3.92 ± 0.04aB | 5.35 ± 0.07cD | 4.90 ± 0.21bC | 5.15 ± 0.07bcD | |
| 150 | 3.24 ± 0.34aA | 4.33 ± 0.21bB | 4.25 ± 0.71bB | 4.25 ± 0.13bC | |
| 180 | 4.45 ± 0.06bC | 4.77 ± 0.03cC | 4.47 ± 0.03bB | 4.22 ± 0.06aC | |
A: The cheese produced without using Enterococcus ssp. B: the cheese produced with 5% Enterococcus ssp. C: the cheese produced with 10% Enterococcus ssp. D: the cheese produced with 20% Enterococcus ssp
X, Y, Z: Values with the different letters in the same row differ significantly (P < 0.05)
a, b, c, d, e: Values with the different letters in the same column differ significantly (P < 0.05)
Sensory properties
Sensorial characteristics such as surface appearance, color, texture, taste–aroma, overall acceptability of the Izmir Tulum cheese samples during storage are given in Table 5. The effect of using probiotic Enterococcus ssp. in the starter cultures at different ratios on the surface appearance and color scores and the changes in surface appearance scores during storage was not significant (P > 0.05). However, the panelists have reported that the structure unique to Izmir Tulum cheese was not fully formed. Texture scores of cheese samples on the 1st day varied between 4.70 and 4.95 and the C sample received the highest score. This was followed by B, A and D samples, respectively. Texture scores at the end of the storage period varied between 4.33 and 4.68 and D sample received the highest score. This was followed by C, B and A samples, respectively. Taste scores at the 1st day of the storage varied between 4.45 and 4.60 and the C sample received the highest score. This was followed by D, A and B samples, respectively. Taste scores at the end of the storage period varied between 4.35 and 4.83 and D sample received the highest score. This was followed by C, A and B samples, respectively. Overall scores at the end of the storage period varied between 4.45 and 4.68 and B sample received the highest score. This was followed by C, D and A samples, respectively. Overall scores at the end of the storage period varied between 4.78 and 4.85 and D sample received the highest score. This was followed by B, A and C samples, respectively.
Table 5.
Sensorial properties of Izmir Tulum cheese samples
| Izmir Tulum cheese samples | |||||
|---|---|---|---|---|---|
| Storage days | A | B | C | D | |
| Surface appearance (5P) | 1 | 4.75 ± 0.35 | 4.88 ± 0.18 | 4.88 ± 0.18 | 4.93 ± 0.11 |
| 30 | 4.78 ± 0.11 | 4.93 ± 0.11 | 4.60 ± 0.14 | 4.65 ± 0.07 | |
| 60 | 4.73 ± 0.04 | 4.67 ± 0.06 | 4.73 ± 0.03 | 4.60 ± 0.00 | |
| 90 | 4.70 ± 0.14 | 4.60 ± 0.00 | 4.80 ± 0.00 | 4.65 ± 0.21 | |
| 120 | 4.93 ± 0.10 | 4.93 ± 0.11 | 4.86 ± 0.01 | 4.88 ± 0.00 | |
| 150 | 4.88 ± 0.00 | 4.88 ± 0.00 | 4.88 ± 0.00 | 4.92 ± 0.10 | |
| 180 | 4.92 ± 0.02 | 4.92 ± 0.03 | 4.93 ± 0.01 | 4.95 ± 0.00 | |
| Color (5P) | 1 | 4.50 ± 0.00 | 4.78 ± 0.04 | 4.83 ± 0.11 | 4.83 ± 0.11 |
| 30 | 4.83 ± 0.04 | 4.73 ± 0.18 | 4.70 ± 0.00 | 4.83 ± 0.04 | |
| 60 | 4.81 ± 008 | 4.75 ± 0.00 | 4.83 ± 0.11 | 4.74 ± 0.06 | |
| 90 | 4.70 ± 0.14 | 4.85 ± 0.21 | 4.75 ± 0.00 | 4.75 ± 0.07 | |
| 120 | 4.86 ± 0.21 | 4.75 ± 0.14 | 4.65 ± 0.07 | 4.75 ± 0.07 | |
| 150 | 4.75 ± 0.00 | 4.75 ± 0.00 | 4.75 ± 0.00 | 4.75 ± 0.00 | |
| 180 | 4.83 ± 0.04 | 4.80 ± 0.00 | 4.83 ± 0.04 | 4.83 ± 0.04 | |
| Texture (5P) | 1 | 4.78 ± 0.04 | 4.95 ± 0.07D | 4.95 ± 0.07 | 4.70 ± 0.28C |
| 30 | 4.75 ± 0.07 | 4.50 ± 0.28CD | 4.75 ± 0.07 | 4.60 ± 0.28C | |
| 60 | 4.49 ± 0.12 | 4.60 ± 0.04AB | 4.43 ± 0.11 | 4.53 ± 0.18BC | |
| 90 | 4.50 ± 0.14 | 4.40 ± 0.28CD | 4.73 ± 0.18 | 4.55 ± 0.07BC | |
| 120 | 4.50 ± 0.28 | 4.55 ± 0.07A | 4.25 ± 0.07 | 4.75 ± 0.14BC | |
| 150 | 4.10 ± 0.14 | 4.25 ± 0.35AB | 4.45 ± 0.07 | 4.69 ± 0.27A | |
| 180 | 4.33 ± 0.04a | 4.58 ± 0.04bBC | 4.63 ± 0.04b | 4.68 ± 0.04bAB | |
| Taste–aroma (5P) | 1 | 4.50 ± 0.00CD | 4.45 ± 0.07C | 4.60 ± 0.21 | 4.50 ± 0.00C |
| 30 | 4.20 ± 0.00B | 4.50 ± 0.00C | 4.33 ± 0.18 | 4.40 ± 0.00BC | |
| 60 | 4.54 ± 0.05CD | 4.54 ± 0.05C | 4.30 ± 0.14 | 4.28 ± 0.04B | |
| 90 | 4.00 ± 0.00bA | 4.10 ± 0.14bA | 4.40 ± 0.00b | 4.00 ± 0.14aA | |
| 120 | 4.40 ± 0.14Cbc | 4.53 ± 0.11aC | 4.68 ± 0.11ab | 4.31 ± 0.08Bc | |
| 150 | 4.55 ± 0.07bCD | 4.23 ± 0.04aAB | 4.38 ± 0.18a | 4.80 ± 0.05cD | |
| 180 | 4.63 ± 0.04bD | 4.35 ± 0.07aBC | 4.65 ± 0.07b | 4.83 ± 0.00cD | |
| Overall acceptability (5P) | 1 | 4.45 ± 0.07AB | 4.68 ± 0.11 | 4.63 ± 0.18 | 4.55 ± 0.07B |
| 30 | 4.35 ± 0.21AB | 4.18 ± 0.25 | 4.30 ± 0.14 | 4.45 ± 0.00B | |
| 60 | 4.60 ± 0.04BC | 4.61 ± 0.15 | 4.60 ± 0.14 | 4.28 ± 0.04A | |
| 90 | 4.20 ± 0.00A | 4.30 ± 0.28 | 4.28 ± 0.04 | 4.15 ± 0.07A | |
| 120 | 4.41 ± 0.28AB | 4.60 ± 0.14 | 4.63 ± 0.11 | 4.55 ± 0.07B | |
| 150 | 4.70 ± 0.07BC | 4.48 ± 0.39 | 4.50 ± 0.35 | 4.90 ± 0.14C | |
| 180 | 4.83 ± 0.04C | 4.83 ± 0.04 | 4.78 ± 0.04 | 4.85 ± 0.00C | |
A: The cheese produced without using Enterococcus ssp. B: the cheese produced with 5% Enterococcus ssp. C: the cheese produced with 10% Enterococcus ssp. D: the cheese produced with 20% Enterococcus ssp
X, Y, Z: Values with the different letters in the same row differ significantly (P < 0.05)
a, b, c, d, e: Values with the different letters in the same column differ significantly (P < 0.05)
Kılıç et al. (1997) produced two different cheese types by the traditional method and using culture containing Lc. cremoris, Lc. lactis and E. faecium. As a result, it was determined that dry matter, fat, salinity, ash, protein, ripening index, pH and free fatty acid values were different between the samples and the cheese produced using the culture received higher sensory evaluation scores. Dağdemir (2006) have reported that, in White Cheese production, E. fecalis culture improved the taste and aroma and positively affected the sensory evaluations and enhanced the textural properties of the cheese. Gürsoy and Kınık (2010), in their study in which they used the adjunct culture containing E. faecium, Lb. paracasei subsp. paracasei and B. bifidum probiotic bacteria, have reported that the White cheese samples preserved their probiotic properties for the 90-day storage period and that the sensory properties improved.
Conclusion
In conclusion, using probiotic Enterococcus species and strains in Izmir Tulum cheese production as adjunct culture did not negatively affect the physicochemical and microbiological properties and improved the sensory properties. In addition, probiotic properties were provided to the traditional cheese type Izmir Tulum cheese. Different strains of Enterococcus species in different ratios should be used combined with L. lactis subsp. lactis and L. lactis subsp. cremoris in Izmir Tulum cheese production and new process method studies should be conducted. Also, strains with high lipolytic characters and bacterial exopolysaccharide production abilities should be used and the results should be examined.
Acknowledgements
This study was presented at the 3rd International Symposium on Traditional Foods from Adriatic to Caucasus, Bosnia and Herzegovina on 01–04th October 2015 (Poster Presentation). The authors thank the Ege University Scientific Research Fund Council (Project No: 2011-ZRF-010) for financial support to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akpinar A, Yerlikaya O, Kinik O, Korel F, Kahraman C, Uysal HR. Some physicochemical characteristics and aroma compounds of Izmir tulum cheese produced with different milk types. Ege J Agric Res. 2017;54(1):27–35. [Google Scholar]
- Anonymous (1993) Milk determination of nitrogen content, protein-nitrogen content, and non-protein-nitrogen content, Kjeldahl method, FIL-IDF Standard 20B
- Anonymous (1996) Pickled Tulum cheese (Izmir Tulum Cheese), Turkish Standard TS ICS 67.100.30 TS 11966, March 1996
- Ardö Y, Polychroniadou A. Laboratory Manual for Chemical Analysis of Cheese, COST 95—Improvement of the quality of the production of raw milk cheeses. Brussels: Office for Official Publications of the European Communities; 1999. [Google Scholar]
- Ateş G, Patır B. Investigations on sensorial, chemical and microbiological features of tulum cheese with starter culture during maturation. Fırat Univ Vet J Health Sci. 2001;15(1):45–56. [Google Scholar]
- Azarnia S, Ehsani MR, Mirhadi SA. Evaluation of the physico-chemical characteristics of the curd curing the ripening of Iranian brine cheese. Int Dairy J. 1997;7:473–478. doi: 10.1016/S0958-6946(97)00034-4. [DOI] [Google Scholar]
- Bracquart P. An agar medium for the differential enumeration of Streptococcus thermophilus and Lactobacillus bulgaricus in yoghurt. J Appl Bacteriol. 1981;51:303–305. doi: 10.1111/j.1365-2672.1981.tb01246.x. [DOI] [Google Scholar]
- Bulajić S, Mijačević Z. Enterococci in cheese- phenotypization and antibiotic resistance. Acta Agric Slov. 2004;84(1):25–30. [Google Scholar]
- Büyükyörük S, Soyutemiz GE. Isolation of Lactococcus lactis (Lactococcus lactis subspecies lactis ve subspecies cremoris) strains from traditionally manufactured Izmir tulum cheese and identification by phenotypical and molecular technics. J Fac Vet Med Erciyes Univ. 2010;7(2):81–87. [Google Scholar]
- Çakmakçı S, Dağdemir E, Hayaloğlu AA, Gürses M, Gündoğdu E. Influence of ripening container on the lactic acid bacteria population in Tulum cheese. World J Microbiol Biotechnol. 2008;24:293–299. doi: 10.1007/s11274-007-9470-z. [DOI] [Google Scholar]
- Dağdemir E (2006) Identification of lactic acid bacteria isolated from white pickled cheeses and possibilities of using some selected isolates as culture, Graduate School of Natural and Applied Science (Ph.D. Thesis in Dairy Technology), 190p, Erzurum, Turkey
- De Vuyst L, Foulquie Moreno MR, Revets H. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int J Food Microbiol. 2003;84:299–318. doi: 10.1016/S0168-1605(02)00425-7. [DOI] [PubMed] [Google Scholar]
- Durlu-Özkaya F, Gün İ. Aroma compounds of some traditional turkish cheeses and their importance for Turkish cuisine. Food Nutr Sci. 2014;5:425–434. [Google Scholar]
- Foulquie Moreno MR, Sarantinopoulos P, Tsakalidou E, De Vuyst L. The role and application of enterococci in food and health. Int J Food Microbiol. 2006;106:1–24. doi: 10.1016/j.ijfoodmicro.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Giraffa G, Carminati D, Neviani E. Enterococci isolated from dairy products: a review of risks and potential technological use. J Food Protec. 1997;60:732–738. doi: 10.4315/0362-028X-60.6.732. [DOI] [PubMed] [Google Scholar]
- Göncüoğlu M, Bilir Ormancı FS, Kasımlıoğlu Doğru A. Usage of Enterococcus faecium as starter culture in white cheese production. Vet J Ank Univ. 2009;56:249–254. [Google Scholar]
- Gürses M, Erdoğan A. Identification of lactic acid bacteria isolated from Tulum cheese during ripening period. Int J Food Prop. 2006;9:551–557. doi: 10.1080/10942910600596126. [DOI] [Google Scholar]
- Gürsoy O, Kınık Ö. Incorporation of adjunct cultures of Enterococcus faecium, Lactobacillus paracasei subsp paracasei and Bifidobacterium bifidum into white pickled cheese. J Food Agric Environ. 2010;8(2):107–112. [Google Scholar]
- Hayaloglu AA, Özer BH, Fox PF. Cheeses of Turkey: 2. Varieties ripened under brine. Dairy Sci Tech. 2008;88:225–244. doi: 10.1051/dst:2007014. [DOI] [Google Scholar]
- International Organization for Standardization (ISO) (1992) Milk and milk products—Enumeration of yeast and moulds—Colony count technique at 25°C. International Standard ISO/DIS 6611 (1992)
- International Standard Organization (ISO) Cheese: determination of fat content: Van Gulik method (ISO 3433) Genava: ISO; 2008. [Google Scholar]
- International Standard Organization (ISO) Cheese and processed cheese: determination of the total solids content (ISO 5534) Genava: ISO; 2004. [Google Scholar]
- Jensen JP, Reinbold GW, Washam CJ, Vedamuthu ER. Role of enterococci in Cheddar cheese: proteolytic activity and lactic acid development. J Milk Food Technol. 1975;38:3–7. doi: 10.4315/0022-2747-38.1.3. [DOI] [Google Scholar]
- Kamber U. The traditional cheeses of Turkey: the Aegean region. Food Rev Int. 2008;24(1):39–61. doi: 10.1080/87559120701762195. [DOI] [Google Scholar]
- Kesenkaş H (2005) The using possibilities of some yeasts as starter culture in the production of white cheese, Ege University, Graduate School of Natural and Applied Science (Ph.D. Thesis in Dairy Technology), Izmir, Turkey
- Kılıç S, Gönç S, Uysal HR, Karagözlü C (1997) Comparison of the changes in the maturation process of Izmir Tulum Cheese made by traditional method and culture. Ege University Scientific Research Fund Council, Project Report Number: 96-ZRF-005, Izmir, Turkey
- Litopoulou-Tzanetaki E, Tzanetakis N, Vafopoulou-Mastrojiannaki A. Effect of type of lactic starter on microbiological, chemical and sensory characteristics of Feta cheese. Food Microbiol. 1993;10:31–41. doi: 10.1006/fmic.1993.1004. [DOI] [Google Scholar]
- McSweeney PLH, Fox PF. Chemical methods for the characterization of proteolysis in cheese during ripening. Lait. 1997;77:41–76. doi: 10.1051/lait:199713. [DOI] [Google Scholar]
- Nunez M, Medina M. La flore lactique du fromage blue de Cabrales. Le Lait INRA Ed. 1979;59(588):497–513. doi: 10.1051/lait:197958825. [DOI] [Google Scholar]
- Ogier JG, Serror P. Safety assessment of dairy microorganisms—the Enterococcus genus. J Food Microbiol. 2008;126:291–301. doi: 10.1016/j.ijfoodmicro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Öner Z, Sağdıç O, Şimşek B. Lactic acid bacteria profiles and tyramine and tryptamine contents of Turkish Tulum cheeses. Eur Food Res Technol. 2004;219:455–459. doi: 10.1007/s00217-004-0962-x. [DOI] [Google Scholar]
- Öner Z, Karahan A, Aloğlu H. Some properties of Tulum cheese produced by using starter culture. J Food. 2005;30(1):57–62. [Google Scholar]
- Renner E. Milchpraktikum Skriptum zu den übüngen. Giesen: Justus Liebig Universitat; 1993. [Google Scholar]
- Reuter G, Klein G. Culture media for enterococci and group D-streptococci. In: Corry JEL, Curtis GDW, Baird RM, editors. Handbook of culture media for food microbiology. Amsterdam: Elsevier; 2003. pp. 111–125. [Google Scholar]
- Sarantinopoulos P, Kalantzopoulos G, Tsakalidou E. Effect of Enterococcus faecium on microbiological, physicochemical and sensory characteristics of Greek Feta cheese. Int J Food Microbiol. 2002;76:93–105. doi: 10.1016/S0168-1605(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Schlesser JE, Schmidt SJ, Speckman RJ. Characterization of chemical and physical changes in Camembert cheese during ripening. J Dairy Sci. 1992;75:1753–1760. doi: 10.3168/jds.S0022-0302(92)77934-X. [DOI] [Google Scholar]
- Terzaghi BE, Sandine WE. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema DP, Herstel H, Elenbaas HL. Determination of the ripening time of Edam and Gouda Cheese by chemical analysis. Neth Milk Dairy J. 1987;41:215–216. [Google Scholar]