Abstract
Fruit ripening induces changes that strongly affect their matrices, and consequently, the bioaccessibility/bioavailability of its phenolic compounds. Flesh from ‘slightly’ (SR), ‘moderately’ (MR) and ‘fully’ (FR) ripe ‘Ataulfo’ mangoes were physicochemically characterized, and digested in vitro to evaluate how ripening impacts the bioaccessibility/bioavailability of its phenolic compounds. Ripening increased the flesh’s pH and total soluble solids, while decreasing citric acid, malic acid and titratable acidity. MR and FR mango phenolics had higher bioaccessibility/bioavailability, which was related to a decreased starch and dietary fiber (soluble and insoluble) content. These results suggest that phenolics are strongly bound to the fruit’s matrix of SR mango, but ripening liberates them as the major polysaccharides are hydrolyzed, thus breaking covalent bonds and disrupting carbohydrate–phenolic complexes. There was also a higher release percentage in the gastric digestion phase, as compared to the intestinal. Our data showed that the bioaccessibility/bioavailability of mango phenolics depends on fruit ripening and on digestion phase.
Keywords: Mangifera indica L., Phenolic compounds, Gastrointestinal digestion, Bioaccessibility, Ripening, Food matrix
Introduction
Mango (Mangifera indica L.) cv. ‘Ataulfo’ is of Mexican origin; it is cultivated in the country’s southern region for national and international markets. It has excellent sensorial (color, aroma, flavor), nutritional (vitamins, minerals, dietary fiber) and functional (phytochemicals) attributes. Previous data indicates that this mango cultivar has good antioxidant capacity, due to its high content of phenolic compounds, which can easily donate electrons and/or hydrogen atoms (Palafox-Carlos et al. 2012b, c). These and other bioactive properties make phenolic compounds health-promoting micronutrients.
In order to exert any effects, phenolic compounds must first be released (become bioaccessible) from the food matrix, be absorbed by the intestinal epithelia and released into the bloodstream (bioavailability), or be metabolized by colonic bacteria into different metabolites. Several factors regulate the absorption efficiency of phenolic compounds or other phytochemicals, for example, their molecular structure, concentration, degree of fruit ripening, processing treatments and numerous others (Quirós-Sauceda et al. 2014a, b).
Regarding fruit ripening, many physiological, chemical and biochemical changes occur during this complex process that strongly affect fruits’ matrices, and consequently, the bioaccessibility/bioavailability of phenolic compounds contained therein (Domínguez-Rosas et al. 2018). For example, changes to the cell wall modify their microstructure and composition, resulting in loss of firmness, mostly related to enzymatic modification of polysaccharides like starch, cellulose and pectin. In this sense, previous studies have shown that the distinctive pattern of extractable phenolics of tropical fruits (including mango) and their antioxidant capacity, are related to several physicochemical (e.g. firmness, soluble solids, pH), physiological (e.g. respiration rate, ethylene production) and biochemical (e.g. enzyme expression and activity) changes (Domínguez-Avila et al. 2018; Palafox-Carlos et al. 2012b, c). These changes are likely to alter the bioaccessibility and antioxidant capacity of phenolic compounds from ‘Ataulfo’ mango during ripening, but this has not been conclusively demonstrated to date for this particular mango variety. In support of this, Ornelas-Paz et al. (2008a) examined how ripening of ‘Ataulfo’ mango affected micellization during digestion and intestinal cell uptake of β-carotene. They report that the amount of β-carotene transferred to the micelles during a simulated digestion, significantly increased as the fruit ripened. Therefore, the objective of this work was to evaluate the effect of ripening stage on in vitro bioaccessibility and antioxidant capacity of phenolic compounds from ‘Ataulfo’ mango.
Materials and methods
Fruit material
Mangoes (210–250 g) were purchased from a local market in Hermosillo, Sonora, Mexico, and immediately transported to the laboratory for evaluation. Fruits were sanitized with chlorinated water (200 ppm sodium hypochlorite) for 5 min, left to dry at room temperature for 15 min, and classified according to their peel color into the following groups: “slightly ripe” (SR), “moderately ripe” (MR) and “fully ripe” (FR). Fruit classification according to ripening stage was based on subjective evaluation of fruit intensity of the yellow-green pigmentation of the skin, since this parameter correlates well with four different ripening stages of mango (Palafox-Carlos et al. 2012b). Thirty-five mangoes of each group were peeled, and the flesh was immediately processed (physicochemical analyses) or freeze-dried (phenolic compounds and in vitro digestion), as further explained.
Physiological and chemical characterization
Color of mango flesh and peel was longitudinally determined on four points of each flat side (n = 3), using a Minolta CR-300 colorimeter (Konica Minolta Sensing, Inc., Ramsey, NJ, USA), using the Lab color space scale. L is luminosity on a scale of 0 (black) to 100 (white), a ranges from negative (green) to positive (red), and b ranges from negative (blue) to positive (yellow). To know the real color changes of the fruit, a and b values were used to calculate Hue angle (°Hue) value, according to Eq. 1:
| 1 |
Flesh firmness was measured by the puncture method, using a Chatillon Penetrometer (Model DFM50, Ametek Inc., Largo, FL, USA) with an 8 mm diameter flat-head stainless-steel cylindrical prove. Tissue’s opposition force against penetration was registered on 3 points in the equatorial region of a piece of fruit with skin removed, and results were reported in Newton (N).
pH, total soluble solids (TSS, °Brix) and titratable acidity (TA, g citric acid/L) were evaluated in a 10 g sample of homogenized fruit pulp, in 50 mL of distilled water. The mixture was filtered and used to quantify pH and TA (model DL28 titrator, Mettler Toledo, Columbus, OH, USA), while TSS was measured in the filtered residue, using an Abbe digital refractometer (American Optical Co., Buffalo, NY, USA) (Kim et al. 2010).
Total phenolic content and antioxidant capacity
Extractable phenolic compounds (free) were quantified after an organic-aqueous extraction was performed on 1 g mango samples, with 30 mL of methanol–water (80:20 v/v) solution (30 mL), and sonicated for 30 min. To extract non-extractable phenolic compounds (linked to food matrix), residues from the organic-aqueous extraction were dispersed in 20 mL of methanol, and 2 mL of sulfuric acid were then added. Extracts were incubated in a shaking water bath at 85 °C for 20 h, they were allowed to cool to room temperature, and centrifuged at 3000×g for 10 min. The residue was washed twice with 10 mL of distilled water and the supernatants were pooled in a 50 mL volumetric flask. Final extracts were stored at − 25 °C to determine phenolic compounds and antioxidant capacity of raw samples (before in vitro digestion) (Esparza-Martinez et al. 2016).
Total phenolic content is given by the sum of extractable and non-extractable phenolics, which were measured by the Folin–Ciocalteu reagent using a microplate reader. Results were expressed as mg of gallic acid equivalents (GAE)/g of dry weight (Palafox-Carlos et al. 2012a).
Antioxidant capacity was determined with the Ferric Reducing Antioxidant Power (FRAP) and Trolox Equivalent Antioxidant Capacity (TEAC) assays. The FRAP reagent (25 mL of acetate buffer 300 mM pH 3.6, mixed with 2.5 mL of 20 mM ferric chloride and 2.5 mL of 10 mM TPTZ in 40 mM HCl) and sample solutions (280 and 20 µL, respectively) were added to a microplate well and thoroughly mixed. Absorbance was read at 593 nm, after 30 min incubation in dark conditions (Domínguez-Avila et al. 2018).
For TEAC, a working solution of ABTS radical cation (ABTS•+) was generated by mixing 19.2 mg of ABTS, 5 mL of milli-Q water and 88 µL of a potassium persulfate solution (37.8 mg/mL), and incubating it in the dark, at room temperature for 16 h. Absorbance of the ABTS•+ solution was adjusted to 0.70 ± 0.02 at 734 nm, using ethanol. The reaction was initiated by mixing 245 µL of ABTS•+ and 5 µL of sample, and allowed to react for 5 min; absorbance was then measured at 734. Trolox [(±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid] was used as a standard in both assays, and results were expressed as µmol of Trolox equivalents (TE)/g of dry weight (Domínguez-Avila et al. 2018).
Carbohydrate composition
Soluble sugars (glucose, fructose and sucrose) and organic acids (citric and malic) were extracted three from a 0.1 g mango sample, using ethanol–water (80:20, v/v). Dissolved samples were centrifuged at 3000×g for 10 min, supernatants were combined and dried using a nitrogen stream, dry extracts were re-dispersed in 2 mL of distilled water (AOAC International 2016). Soluble sugars and organic acids were analyzed using enzymatic and spectrophotometric methods of analysis (Megazyme, International Ireland Ltd Wicklow, Ireland) in a UV–Vis spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA). In order to quantify starch, precipitates were incubated with thermostable α-amylase (150 U) and amyloglucosidase (40 U) at pH 4.5 in a boiling water bath for 15 min at 100 °C. Starch was quantified from the enzymatically-generated free d-glucose, using a GODOP format assay kit (Megazyme, International Ireland Ltd Wicklow, Ireland), following manufacturer’s instructions.
Insoluble and soluble dietary fibers were determined according to AOAC method 991.43 (AOAC International 2016), using a Megazyme kit. Neutral sugar composition of both soluble and insoluble fibers, were determined with the alditol acetates method (Samalova et al. 2016). Briefly, 2 mg samples were hydrolyzed with 500 μL of 2 N trifluoroacetic acid, containing 100 μL/mL of myo-inositol, at 121 °C for 1 h. The resulting hydrolysates were recovered in methanol and after evaporation they were converted to alditol acetates by reduction in NaBH4 and further acetylation with acetic anhydride and methylimidazole as a catalyst. Samples were injected into an Agilent 7890B gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled to an FID detector (250 °C), using a DB-23 capillary column 30 m × 0.25 mm (J&W Scientific, Folsom, CA, USA) kept at 210 °C, and with helium as carrier (3 mL/min). Results were calculated using external standards of rhamnose, fucose, arabinose, xylose, mannose, galactose and glucose, using myo-inositol as internal standard (Sigma-Aldrich, St. Louis, MO, USA).
Scanning electron microscopy (SEM)
Differences in microstructure between ripening stages (SR, MR and FR) were analyzed through SEM analyses. Samples were covered in gold/palladium, and examined at 250x and 1000x, using an accelerative voltage of 15 kV, in a JEOL 5410LV Scanning Electron Microscope (SEM) (JEOL, Tokyo, Japan) equipped with an INCA dispersive X-ray detector system (Oxford Instruments, Austin, TX, USA).
In vitro bioaccessibility
To analyze the effect of ripening stage on the release of phenolic compounds under simulated gastrointestinal conditions, their bioaccessibility was determined according to the methodology of Saura-Calixto et al. (2000) with slight modifications (Saura-Calixto 2018). Freeze-dried samples (300 mg) were dissolved in 10 mL of an HCl–KCl solution (pH 1.5), to which 0.2 mL of a pepsin solution (300 mg/mL) were added. Samples were then incubated in a water bath at 40 °C, with constant shaking, for 60 min, thereby simulating gastric conditions. After the incubation period, 4.5 mL of phosphate buffer were added, and pH was adjusted to 7.5. To initiate the intestinal stage, 1 mL of pancreatin (5 mg/mL) was added, and the mixture was incubated at 37 °C for 6 h. After the intestinal stage concluded, digested samples were centrifuged (2615×g for 15 min); precipitate corresponds to the indigestible part that reaches the colon, while supernatant contains bioaccessible phenolic compounds. Supernatants were immediately transferred into semipermeable cellulose dialysis bags (12–14 kDa, Sigma Aldrich), sealed with clips, immersed into tubes containing phosphate buffer (pH 7.5), and dialyzed at 37 °C for 3 h, in order to simulate absorption by passive diffusion. Phenolics were quantified with the Folin–Ciocalteu method, and antioxidant capacity with the FRAP and TEAC assays.
Individual experiments were conducted to measure bioaccessible phenolic compounds during each digestion stage, in order to not alter the reaction’s volume. Bioaccessibility was calculated according to Eq. 2 (Juaniz et al. 2017):
| 2 |
Statistical analyses
All measurements were made in triplicate (n = 3). Results are expressed as mean ± standard deviation of the mean (SD). Data were statistically analyzed by one-way ANOVA and Tukey–Kramer multiple comparison test, at 95 confidence level. Pearson’s product moment correlations (r) were also determined between different variables. All data was analyzed in the Number Cruncher Statistical System version 6.0 software (NCSS, LLC, Kaysville, UT, USA).
Results and discussion
Physiological and chemical characterization
Physiological and chemical analyses of ‘Ataulfo’ mangoes at different ripening stages are shown in Table 1. Some parameters increased with ripening, namely, pH, TSS and peel’s L value, while organic acids, TA and flesh’s tone decreased. Flesh firmness significantly decreased (p < 0.05) during ripening, while peel color changed from green to yellow, reported as lightness (L). Flesh color also changed from green to yellow, reported as °Hue.
Table 1.
Physicochemical characterization of “slightly ripe” (SR), “moderately ripe” (MR) and “fully ripe” (FR) Ataulfo mangoes
| Ripening state | pH | TSS (°Brix) | Citric acid (g/100 g) | Malic acid (g/100 g) | TA (g citric acid/L) | Firmness (N) | Color | |
|---|---|---|---|---|---|---|---|---|
| Flesh (°Hue) | Peel (L*) | |||||||
| SR | 2.10a | 12.5a | 7.46a | 0.08a | 1.17a | 17.9a | 97.4a | 60.5a |
| MR | 3.21b | 13.0b | 3.62b | 0.05b | 0.74b | 14.8b | 88.9b | 67.8b |
| FR | 4.49c | 15.2c | 1.85c | 0.02c | 0.36c | 9.73c | 82.7c | 72.2c |
Mean values. Different letter between ripeness stages indicates significant difference (p < 0.05)
Our results are in agreement with previous studies (Palafox-Carlos et al. 2014), where the decline in organic acids concentration correlated with increased soluble sugars (r ≥ −0.73). The sugar-to-acid ratio has been related to consumer’s acceptance, which promotes sweet taste that is characteristic of mango. This change is associated with gluconeogenic metabolism of acids (mainly citric, ascorbic and malic) into sugars, which results in a net decline in TA, as observed in this study (r ≥ 0.98).
The observed increase in TSS during ripening is attributed to the accumulation of free sugars (r = 0.57), as a consequence of starch hydrolysis (r = −0.60) by ethylene-dependent amylases, as shown in Table 2. Loss of firmness is related to cell wall modification and degradation by lytic enzymes, whose activity commonly increase during the late stages of ripening. Moreover, it has been reported that color of mango peel and flesh changes from green to yellow during ripening, and is related to carotenoid concentration (Ornelas-Paz et al. 2008b).
Table 2.
Dietary fiber, starch, total soluble sugars and neutral sugars content in “slightly ripe” (SR), “moderately ripe” (MR) and “fully ripe” (FR) ‘Ataulfo’ mangoes (%)
| Parameter | SR | MR | FR |
|---|---|---|---|
| Total dietary fiber | 25.5a | 18.3b | 15.1c |
| Soluble fiber | 15.2a | 9.89b | 7.82c |
| Insoluble fiber | 10.3a | 8.43b | 7.37b |
| Starch | 5.70a | 0.96b | 0.34c |
| Total soluble sugars | 50.2a | 68.4b | 72.2b |
| Glucose | 6.83a | 5.46b | 2.59c |
| Fructose | 12.3a | 18.1b | 18.1b |
| Sucrose | 31.1a | 44.8b | 51.5c |
| Neutral sugars (soluble fiber) | 12.4a | 13.7a | 12.2a |
| Rhamnose | 0.45a | 0.42a | 0.37a |
| Fucose | 0.05a | 0.05a | 0.05a |
| Arabinose | 2.50a | 2.44a | 2.28a |
| Xylose | 0.12a | 0.09a | 0.12a |
| Mannose | 3.69a | 4.15a | 3.32a |
| Galactose | 3.41a | 3.31a | 3.07a |
| Glucose | 2.24a | 3.29b | 3.06b |
| Neutral sugars (insoluble fiber) | 12.0a | 8.53b | 6.83c |
| Rhamnose | 0.26a | 0.14b | 0.11b |
| Fucose | 0.63a | 0.49b | 0.38c |
| Arabinose | 1.64a | 1.14b | 0.88c |
| Xylose | 3.61a | 2.63b | 1.90c |
| Mannose | 1.10a | 0.74b | 0.62b |
| Galactose | 3.15a | 2.26b | 1.91c |
| Glucose | 1.68a | 1.13a | 1.04a |
Means values. Different letter between ripening stages indicates significant difference (p < 0.05)
Total phenolics and antioxidant capacity
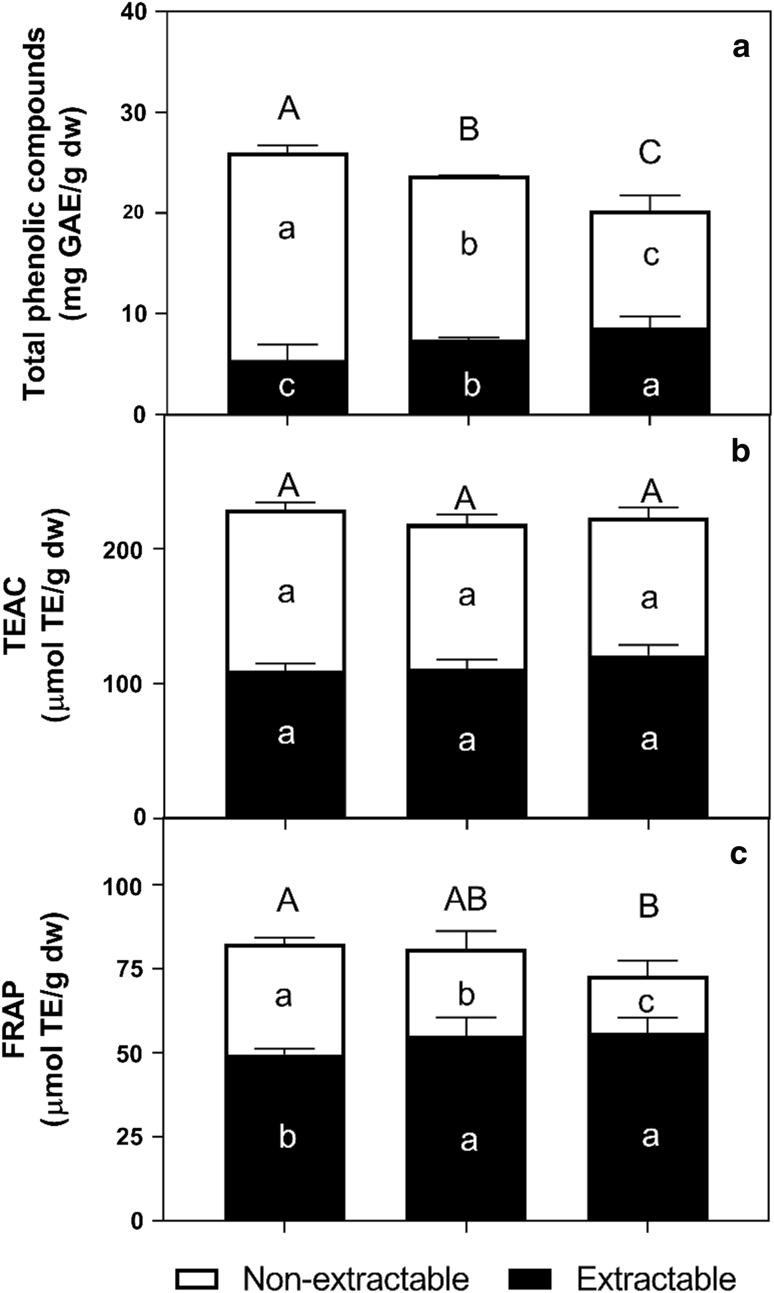
Concentration of total phenolics (extractable and non-extractable) and antioxidant capacity of mangoes of different ripening stages is presented in Fig. 1. Non-extractable phenolics are composed of hydrolyzable (phenolic acids polymers) and condensed (flavonoid polymers) tannins, but those of ‘Ataulfo’ mango appear to contain only hydrolyzable tannins (Velderrain-Rodriguez et al. 2016). Extractable phenolic compounds in ‘Ataulfo’ mango have been previously reported (Palafox-Carlos et al. 2012c), with gallic, chlorogenic, protocatechuic and vanillic acids being the major contributors to antioxidant capacity. Our results show that total and non-extractable phenolics decreased during ripening, while extractable phenolics increased (p < 0.05).
Fig. 1.
Total phenolic compounds and antioxidant capacity in “slightly ripe” (SR), “moderately ripe” (MR) and “fully ripe” (FR) ‘Ataulfo’ mangoes flesh. Mean values. Different uppercase letters indicate significant difference (p < 0.05) for total amount, black lowercase letters indicate significant difference (p < 0.05) for non-extractable phenolics, and white lowercase letters indicate significant difference (p < 0.05) for extractable phenolics
The concentration of total phenolics reported herein was higher than those of other studies (Palafox-Carlos et al. 2012b), which could be attributed to various factors such as cultivar, growing conditions, among others. It should also be emphasized that this difference may be attributed to the fact that, contrary to this study, only extractable phenolics are usually reported.
The decrease in non-extractable phenolics during ripening is associated with their depolymerization, which changes the fruits taste and entices animal consumption (Yahia 2017). It has also been shown that the genetic expression of key enzymes of the phenylpropanoid pathway changes in ‘Ataulfo’ mango, in order to yield the previously-mentioned depolymerization reactions (Palafox-Carlos et al. 2012b). As hydrolyzable tannins are depolymerized, the end-products are free soluble phenolics, such as gallic acid and ellagic acid, which increase the content of extractable compounds. Similar results were reported by Palafox-Carlos et al. (2012b) reported a similar trend during the first three ripening stages of ‘Ataulfo’ mango. The decrease of total phenolics reported here could be related to fruit senescence, probably mediated by channeling carbohydrates from phase I to polymeric phenolic compounds (non-extractable) during phase II metabolism, as supported by the inverse correlation between TSS and total and non-extractable phenolics (r = −0.73).
Antioxidant capacity is usually evaluated through different methods, in order to consider the varying affinity for particular free radicals used in each assay. According to Fig. 1, total antioxidant capacity did not vary among ripening stages when analyzed with the TEAC assay, while also showing an almost equal contribution from its extractable and non-extractable phenolics. In contrast, the FRAP assay showed that the contribution of non-extractable phenolics decreases significantly (p < 0.05) during ripening, while an increased contribution from extractable phenolics is apparent from SR to MR, which remains unchanged until FR. As previously reported by Palafox-Carlos et al. (2012b), concentration of main mango phenolics (and possibly other antioxidants such as ascorbic acid) fluctuates during ripening, but without having a significant effect on its overall antioxidant capacity.
Dietary fiber, cell wall composition and structural analysis
As shown in Table 2, percentage of total and soluble dietary fibers, starch and free glucose decreased during ripening (p < 0.05). It is noteworthy that total dietary fiber content reported here, is higher than that obtained from commercially-ripe ‘Ataulfo’ mango (6% dietary fiber content, data not yet published). This can be attributed to differences in the analytical method used, for example, the AOAC method (991.43) used here requires filtration, instead of centrifugation, to separate the soluble and insoluble fractions. This method also precipitates the soluble fiber fraction with ethanol, instead of dialyzing against water.
From a biochemical standpoint, enzyme-catalyzed structural changes to the main cell wall polysaccharides (pectin, hemicellulose and cellulose), seems to be responsible for its softening during ripening. Pectin solubilization and depolymerization in particular, decrease the content of total soluble dietary fiber. The starch content of ‘Ataulfo’ mango was lower than that reported for mango cv. ‘Alphonso’, but its concentration had a similar decrease of approximately 95% during ripening (Singh et al. 2013b). As fruits ripen, their taste becomes sweeter due to increased polysaccharide hydrolysis, especially starch, and a concomitant accumulation of simple sugars.
Neutral sugars like fucose, arabinose, xylose and galactose associated to insoluble, but not soluble fiber, decreased during ripening, while other neutral sugars remained at relatively low concentrations (Table 2). A similar pattern was reported for mango cv. ‘Kensington Pride’, during abscisic acid-induced ripening (Zaharah et al. 2013). Bound neutral sugars in both dietary fibers were xylose/mannose (insoluble and soluble fiber, respectively) > galactose > glucose/arabinose which are key substrates for ascorbic acid synthesis (Wang et al. 2013). The presence of xylose and galactose in insoluble dietary fiber suggests the presence of hemicellulose, while the high concentration of specific neutral sugars could be the result of their bio-transformation from other sugars (e.g. sucrose) by enzymatic modification (Quirós-Sauceda et al. 2014b).
A high correlation was observed between loss of firmness and decrease in starch (r = 0.83) and total–soluble–insoluble dietary fiber content (r > 0.91). This suggests that starch is being metabolized to glucose during ripening of ‘Ataulfo’ mango, as has been demonstrated for other fruits. Subsequent sucrose metabolism yields glucose and fructose, which contribute to increased concentration of total soluble sugars during ripening, as observed during ripening of other mango varieties (Wongmetha et al. 2012) and apples (Núñez-Gastélum et al. 2015). Sucrose is synthesized by sucrose phosphate synthase, an enzyme which has been shown to increase this disaccharide’s concentration in mango, even when other enzymes concomitantly hydrolyze it (Wongmetha et al. 2015), similar to the behavior found in our samples.
It has been reported that starch content in food affects texture, viscosity and gel forming ability, which may consequently affect the absorption/release of phenolic compounds during digestion (Singh et al. 2013a). Furthermore, starch (and other nutrients) have been reported to alter each other’s release and absorption kinetics; this happens because phenolic–starch complexes sometimes occur within the food matrix or along the digestive tract (Domínguez-Avila et al. 2017; Zhu 2015). Differences in fiber content, cell wall constituents and morphological structure between samples, clearly indicates that the architecture of mango flesh differs during ripening. Scanning electron microscopy micrographs studies revealed notable structural differences during mango ripening, as shown in Fig. 2. The images show an amorphous structure in SR mango, which is changing into a softer structure in FR mango. This amorphous matrix of SR mango could be related to the high content of soluble dietary fiber and starch, since there are more granules (< 10 µm) at this stage, which disappear during ripening. This coincides with the negligible presence of starch in FR mango (Table 2), and the resulting microstructure rearrangement and metabolic adaptations that seem to be related to a higher content of free phenolics. In support of this, positive (TSS) and negative (firmness, citric acid, non-extractable phenols, starch and total, soluble and insoluble dietary fibers) correlations explain (r ≥ 0.92) the higher content of extractable phenolic compounds during ripening.
Fig. 2.
Microstructure of “slightly ripe” (SR), “moderately ripe” (MR) and “fully ripe” (FR) ‘Ataulfo’ mangoes flesh, by scanning electron microscopy
In vitro bioaccessibility
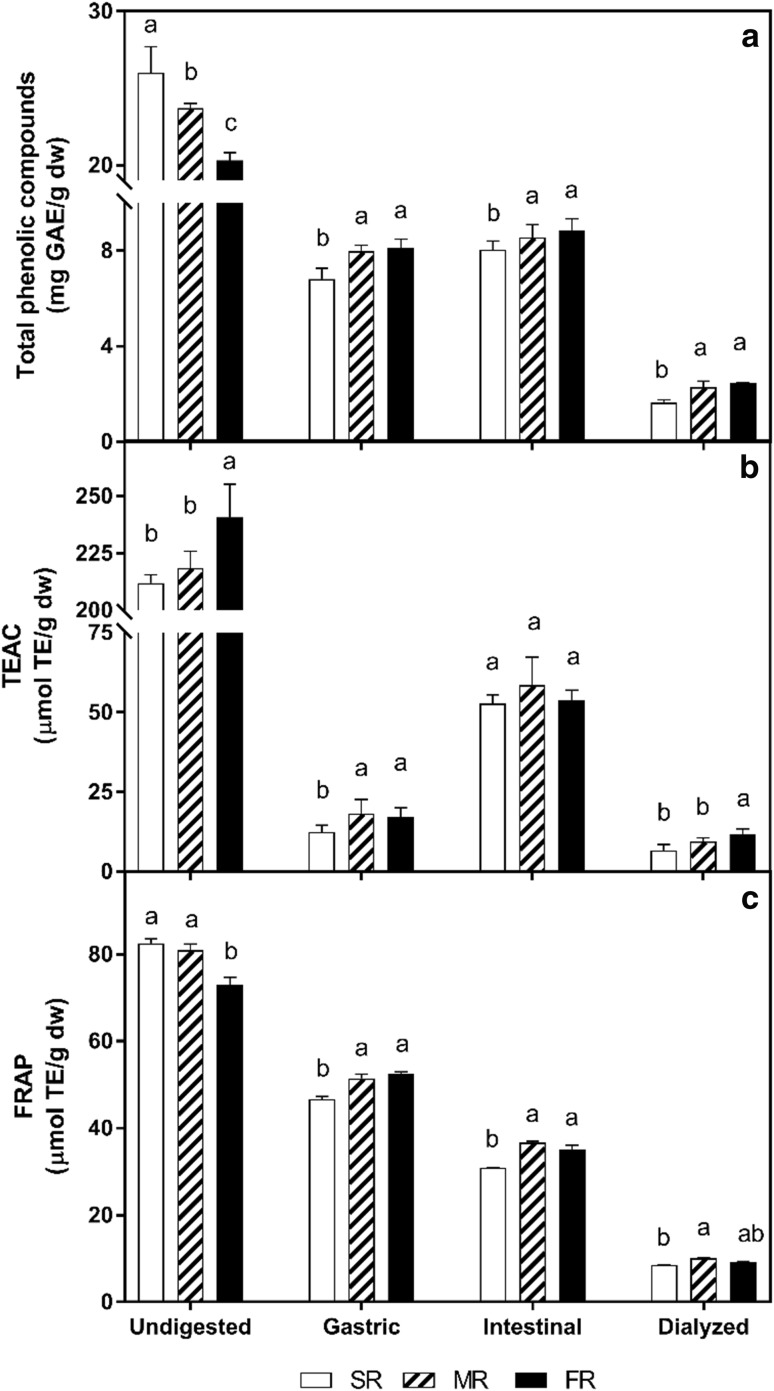
The bioaccessibility at different in vitro digestion phases of extractable phenolic compounds of different ripening stages of ‘Ataulfo’ mango is shown in Fig. 3, and cumulative data is shown in Table 3.
Fig. 3.
Total phenolic compounds and antioxidant capacity in “slightly ripe” (SR), “moderately ripe” (MR) and “fully ripe” (FR) ‘Ataulfo’ mangoes during in vitro digestion phases. Mean values. Different letter at each ripeness stage indicates significant difference (p < 0.05) between digestion phases
Table 3.
Bioaccessibility (%) of phenolic compounds in “slightly ripe” (SR), “moderately ripe” (MR) and “fully ripe” (FR) ‘Ataulfo’ mangoes during gastric and intestinal in vitro digestion
| Ripening stage | Bioaccessibility (%) | Accumulated (%) | |
|---|---|---|---|
| Gastric | Intestinal | ||
| SR | 26.1a | 4.7a | 30.8a |
| MR | 33.5b | 2.4b | 35.9b |
| FR | 40.0b | 3.4b | 43.4c |
Mean values. Different letter between ripeness stage indicates significant difference (p < 0.05)
Digestion efficiency follows a noticeable trend according to ripening stage, becoming more digestible as it ripens. Release of phenolics was significantly higher (p < 0.05) at gastric level stage, not being statistically different at later stages, as it also happens at simulated intestinal conditions (p > 0.05). The effect of intestinal pH could also play a role in this response, because the stability of phenolic compounds tends to be higher in acid than in alkaline conditions (Velderrain-Rodríguez et al. 2014). Results obtained for FR mango are similar to data obtained in a previous work, where the bioaccessibility of phenolic compounds of commercially-ripe ‘Ataulfo’ mango flesh was evaluated (Velderrain-Rodriguez et al. 2016). These results also agree with the later study as to most of mangoes phenolic compounds are released during gastric phase, with a minor contribution of the intestinal phase. This behavior is attributed to the acidic hydrolysis of phenol glycosides to their corresponding aglycons during simulated gastric digestion (Pineda-Vadillo et al. 2016). The hydrolysis reactions during digestion may have an important role in breaking down cell walls and releasing phenolic compounds (Rodríguez-Roque et al. 2015). Moreover, the increased bioaccessibility of phenolic compounds after intestinal phase is attributed to the prolonged reaction time (6 h), and the effect of pancreatic digestive enzymes on the complex food matrix (mainly carbohydrates), facilitating the release of some phenolic compounds associated to the food matrix (Bouayed et al. 2011).
α-glucosidase and α-amylase are key enzymes involved in carbohydrate digestion. α-amylase degrades complex carbohydrates to oligosaccharides, and disaccharides that are ultimately converted to monosaccharides by α-glucosidase. This process takes places in the small intestine, where the enzymes are secreted. In addition, recent studies report that intestinal microbiota are involved in fast import and conversion of simple carbohydrates, contributing to a rapid adaptation to overall nutrient availability and other bioactivities (Cockburn and Koropatkin 2016; Salazar-Lopez et al. 2017). All of these factors can contribute to the release of phenolic compounds documented herein.
The amount of dialyzable (i.e. that could be passively absorbed) phenolic compounds significantly (p < 0.05) increased from SR to MR mango, and remained at similar values in FR mango. It should also be noted that the amount of dialyzable phenolics was lower than those quantified at the intestinal phase, suggesting a possible interaction between them and the food matrix components, which prevents their transport across the dialysis membrane. This is consistent with previous findings (data not yet published), where the diffusion behavior of digested phenolics from ‘Ataulfo’ mango was evaluated, and results showed interactions between phenolic compounds and food matrix components. Our data also coincides with a previous study of commercially-ripe ‘Ataulfo’ mango flesh (Velderrain-Rodriguez et al. 2016).
Although SR mango had the highest concentration of phenolics, they were less bioaccessible at the gastric phase than MR and FR mango, this could be attributed to the high concentration of non-extractable phenolic compounds, which are strongly linked to the food matrix and require extreme conditions to be released (Pérez-Jiménez et al. 2013). The characteristics of the food matrix found at this ripening stage, such as firmness, amorphous structure, higher dietary fiber and starch content, may also decrease the phenolics’ bioaccessibility. For example, phenolics from the non-extractable fraction are partially bioaccessible during gastrointestinal digestion, but most of them (> 95%) arrive nearly intact to the colon (Palafox-Carlos et al. 2011). Once there, non-extractable compounds become accessible after bacterial fermentation, or after hydrolysis by some intestinal esterases (Pérez-Jiménez et al. 2013).
In contrast to the gastric phase, SR mango had higher release of phenolics at the intestinal phase, likely due to digestive enzyme activity on the amorphous fruit matrix that releases embedded phenolic compounds (Palafox-Carlos et al. 2011). Furthermore, as fruits become softer during ripening, this facilitates the mechanical and enzymatic disruption of the flesh during digestion, which increases the bioaccessibility of various phytochemicals. Thus, ripening likely has a similar effect as homogenization and thermal treatments, which disrupt cell walls to provide access to digestive enzymes and facilitates release of phenolics (Ornelas-Paz et al. 2008a). These findings indicate that the bioaccessibility of mango phenolics is closely related to fruit ripening, and depends on digestion phase. Similar results by Ornelas-Paz et al. (2008a) show that the in vitro bioaccessibility of ‘Ataulfo’ mango carotenoids varies during ripening.
Significant correlations (p < 0.05) were found between bioaccessibility of extractable phenolics and other variables like TSS (r ≥ 0.79), starch and dietary fibers (r ≥ −0.88), which suggests that their bioaccessibility depends on carbohydrate metabolism. According to this data, we hypothesized that dietary fibers and starch are the main macromolecules that influence the release of phenolic compounds. It has also been reported that phenolic compounds have significant influence on starch digestion (Domínguez-Avila et al. 2017), for example, hydrolyzable tannins can decrease it, which in turn affects the release of phenolic compounds embedded in the starch matrix. Furthermore, starch and phenolic compounds interact to form inclusion complexes in the form of amylose single helices, which are facilitated by hydrophobic effects or hydrogen bonds. These interactions may have an impact on the bioaccessibility and bioavailability of phenolic compounds.
Dietary fiber can physically trap phenolic compounds and prevent their release during digestion. For example, dietary fiber increases the viscosity of the bolus (particularly soluble fiber), which restricts the peristaltic mixing that promotes transport of enzymes to their substrates, as well as delaying or impeding release and absorption of various nutrients to the intestinal wall, including phenolic compounds.
The antioxidant capacity of SR, MR and FR ‘Ataulfo’ mangoes is shown in Fig. 3. A significant decrease (p < 0.05) in antioxidant capacity at the gastric phase is evident, as compared to undigested samples. This decrease is noticeable on both assays, but is more pronounced on the TEAC assay. Similarly, the FRAP test showed a significantly (p < 0.05) decreased value at the intestinal phase, and after dialysis, as compared to undigested samples. In addition to their concentration, pH also plays a role in the antioxidant activity of phenolic compounds. Some phenolics (quercetin and resveratrol from grape extracts) exert higher antioxidant activity during intestinal phase (neutral pH conditions) as measured by TEAC assay (Bouayed et al. 2011). It is thought that the transition from acid to alkaline environment enhances the antioxidant power of phenolic compounds by causing deprotonation of the hydroxyl moieties present on their aromatic rings. In contrast to this hypothesis, Bouayed et al. (2011) suggested that due to the chemical conditions of the FRAP assay (pH of 3.6), this test shows higher response in gastric phase, which could make it more appropriate to evaluate the antioxidant capacity during gastric digestion than intestinal digestion.
Conclusion
Our results showed that the physiological and ripening processes of ‘Ataulfo’ mango modify the carbohydrates present, particularly starch and fibers. Ripening also changes the concentration of phenolics, which also increases the bioaccessible portion of phenolic compounds, as well as their resulting antioxidant capacity. Future studies should evaluate the effect of colonic fermentation of phenolics bound to dietary fiber, as well as the possible physicochemical interactions between starch and phenolic compounds.
Acknowledgements
This work was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT), through Project No. 563: “Un Enfoque Multidisciplinario de la Farmacocinética de Polifenoles de Mango Ataulfo: Interacciones Moleculares, Estudios Preclínicos y Clínicos”.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC International . Official methods of analysis of AOAC International. 20. Rockville: AOAC; 2016. [Google Scholar]
- Bouayed J, Hoffmann L, Bohn T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: bioaccessibility and potential uptake. Food Chem. 2011;128:14–21. doi: 10.1016/j.foodchem.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Cockburn DW, Koropatkin NM. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J Mol Biol. 2016;428:3230–3252. doi: 10.1016/j.jmb.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Domínguez-Avila JA, Wall-Medrano A, Velderrain-Rodriguez GR, Chen CYO, Salazar-Lopez NJ, Robles-Sanchez M, Gonzalez-Aguilar GA. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017;8:15–38. doi: 10.1039/C6FO01475E. [DOI] [PubMed] [Google Scholar]
- Domínguez-Avila JA, Villegas-Ochoa MA, Alvarez-Parrilla E, Montalvo-Gonzalez E, González-Aguilar GA. Interactions between four common plant-derived phenolic acids and pectin, and its effect on antioxidant capacity. J Food Meas Charact. 2018;12:992–1004. doi: 10.1007/s11694-017-9714-z. [DOI] [Google Scholar]
- Domínguez-Rosas C, Domínguez-Avila JA, Pareek S, Villegas-Ochoa MA, Ayala-Zavala JF, Yahia E, Gonzalez-Aguilar GA. Content of bioactive compounds and their contribution to antioxidant capacity during ripening of pineapple (Ananas comosus L.) cv. Esmeralda. J Appl Bot Food Qual. 2018;91:61–68. [Google Scholar]
- Esparza-Martinez FJ, Miranda-Lopez R, Guzman-Maldonado SH. Effect of air-drying temperature on extractable and non-extractable phenolics and antioxidant capacity of lime wastes. Ind Crop Prod. 2016;84:1–6. doi: 10.1016/j.indcrop.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Juaniz I, et al. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota (vol 32, pg 195, 2017) J Funct Foods. 2017;34:480. doi: 10.1016/j.jff.2017.05.031. [DOI] [Google Scholar]
- Kim H, et al. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010;121:429–436. doi: 10.1016/j.foodchem.2009.12.060. [DOI] [Google Scholar]
- Núñez-Gastélum J, Alvarez-Parrilla E, de la Rosa L, Martínez-Ruíz N, González-Aguilar G, Rodrigo-García J. Effect of harvest date and storage duration on chemical composition, sugar and phenolic profile of ‘Golden Delicious’ apples from northwest Mexico. N Z J Crop Hortic Sci. 2015;43:214–221. doi: 10.1080/01140671.2015.1026358. [DOI] [Google Scholar]
- Ornelas-Paz JDJ, Failla ML, Yahia EM, Gardea-Bejar A. Impact of the stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in ‘Ataulfo’ mango. J Agric Food Chem. 2008;56:1511–1516. doi: 10.1021/jf072751r. [DOI] [PubMed] [Google Scholar]
- Ornelas-Paz JDJ, Yahia EM, Gardea AA. Changes in external and internal color during postharvest ripening of ‘Manila’ and ‘Ataulfo’ mango fruit and relationship with carotenoid content determined by liquid chromatography–APcI+-time-of-flight mass spectrometry. Postharvest Biol Technol. 2008;50:145–152. doi: 10.1016/j.postharvbio.2008.05.001. [DOI] [Google Scholar]
- Palafox-Carlos H, Ayala-Zavala JF, González-Aguilar GA. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J Food Sci. 2011;76:R6–R15. doi: 10.1111/j.1750-3841.2010.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palafox-Carlos H, Gil-Chavez J, Sotelo-Mundo RR, Namiesnik J, Gorinstein S, Gonzalez-Aguilar GA. Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’ mango pulp: chlorogenic, gallic, protocatechuic and vanillic acids. Molecules. 2012;17:12657–12664. doi: 10.3390/molecules171112657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palafox-Carlos H, Yahia E, Islas-Osuna MA, Gutierrez-Martinez P, Robles-Sánchez M, González-Aguilar G. Effect of ripeness stage of mango fruit (Mangifera indica L. cv. Ataulfo) on physiological parameters and antioxidant activity. Sci Hortic. 2012;135:7–13. doi: 10.1016/j.scienta.2011.11.027. [DOI] [Google Scholar]
- Palafox-Carlos H, Yahia EM, Gonzalez-Aguilar GA. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC-DAD-MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012;135:105–111. doi: 10.1016/j.foodchem.2012.04.103. [DOI] [Google Scholar]
- Palafox-Carlos H, Contreras-Vergara C, Muhlia-Almazan A, Islas-Osuna M, Gonzalez-Aguilar G. Expression and enzymatic activity of phenylalanine ammonia-lyase and p-coumarate 3-hydroxylase in mango (Mangifera indica ‘Ataulfo’) during ripening. Genet Mol Res. 2014;13:3850–3858. doi: 10.4238/2014.May.16.10. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J, Díaz-Rubio ME, Saura-Calixto F. Non-extractable polyphenols, a major dietary antioxidant: occurrence, metabolic fate and health effects. Nutr Res Rev. 2013;26:118–129. doi: 10.1017/S0954422413000097. [DOI] [PubMed] [Google Scholar]
- Pineda-Vadillo C, et al. vitro digestion of dairy and egg products enriched with grape extracts: effect of the food matrix on polyphenol bioaccessibility and antioxidant activity. Food Res Int. 2016;88:284–292. doi: 10.1016/j.foodres.2016.01.029. [DOI] [Google Scholar]
- Quirós-Sauceda A, et al. Dietary fiber and phenolic compounds as functional ingredients: interaction and possible effect after ingestion. Food Funct. 2014;5:1063–1072. doi: 10.1039/C4FO00073K. [DOI] [PubMed] [Google Scholar]
- Quirós-Sauceda AE, Ayala-Zavala JF, Sáyago-Ayerdi SG, Vélez-de La Rocha R, Sañudo-Barajas A, González-Aguilar GA. Added dietary fiber reduces the antioxidant capacity of phenolic compounds extracted from tropical fruit. J Appl Bot Food Qual. 2014;87:227–233. [Google Scholar]
- Rodríguez-Roque MJ, de Ancos B, Sánchez-Moreno C, Cano MP, Elez-Martínez P, Martín-Belloso O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J Funct Foods. 2015;14:33–43. doi: 10.1016/j.jff.2015.01.020. [DOI] [Google Scholar]
- Salazar-Lopez NJ, Astiazaran-Garcia H, Gonzalez-Aguilar GA, Loarca-Pina G, Ezquerra-Brauer JM, Avila JAD, Robles-Sanchez M. Ferulic acid on glucose dysregulation, dyslipidemia, and inflammation in diet-induced obese rats: an integrated study. Nutrients. 2017;9:675. doi: 10.3390/nu9070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samalova M, Mélida H, Vilaplana F, Bulone V, Soanes DM, Talbot NJ, Gurr SJ. The β-1,3-glucanosyltransferases (Gels) affect the structure of the rice blast fungal cell wall during appressorium-mediated plant infection. Cell Microbiol. 2016;19:e12659. doi: 10.1111/cmi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura-Calixto F. The story of the introduction of non-extractable polyphenols into polyphenol research: origin, development and perspectives, chap 1. In: Saura-Calixto F, Pérez-Jiménez J, editors. Non-extractable polyphenols and carotenoids: importance in human nutrition and health. UK: Royal Society of Chemistry; 2018. pp. 1–16. [Google Scholar]
- Saura-Calixto F, García-Alonso A, Goni I, Bravo L. In vitro determination of the indigestible fraction in foods: an alternative to dietary fiber analysis. J Agric Food Chem. 2000;48:3342–3347. doi: 10.1021/jf0000373. [DOI] [PubMed] [Google Scholar]
- Singh J, Kaur L, Singh H. Food microstructure and starch digestion. Adv Food Nutr Res. 2013;70:137–179. doi: 10.1016/B978-0-12-416555-7.00004-7. [DOI] [PubMed] [Google Scholar]
- Singh Z, Singh RK, Sane VA, Nath P. Mango: postharvest biology and biotechnology. Crit Rev Plant Sci. 2013;32:217–236. doi: 10.1080/07352689.2012.743399. [DOI] [Google Scholar]
- Velderrain-Rodríguez G, et al. Phenolic compounds: their journey after intake. Food Funct. 2014;5:189–197. doi: 10.1039/C3FO60361J. [DOI] [PubMed] [Google Scholar]
- Velderrain-Rodriguez G, et al. Effect of dietary fiber on the bioaccessibility of phenolic compounds of mango, papaya and pineapple fruits by an in vitro digestion model. Food Sci Technol. 2016;36:188–194. doi: 10.1590/1678-457X.6729. [DOI] [Google Scholar]
- Wang J, Zhang Z, Huang R. Regulation of ascorbic acid synthesis in plants. Plant Signal Behav. 2013;8:e24536. doi: 10.4161/psb.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongmetha O, Ke L-S, Liang Y-S. Sucrose metabolism and physiological changes during mango cv. Irwin growth and development. Hortic Environ Biotechnol. 2012;53:373–377. doi: 10.1007/s13580-012-0078-3. [DOI] [Google Scholar]
- Wongmetha O, Ke LS, Liang YS. The changes in physical, bio-chemical, physiological characteristics and enzyme activities of mango cv. Jinhwang during fruit growth and development. NJAS-Wagening J Life Sci. 2015;72–73:7–12. doi: 10.1016/j.njas.2014.10.001. [DOI] [Google Scholar]
- Yahia EM. Fruit and vegetable phytochemicals: chemistry and human health. New York: Wiley; 2017. [Google Scholar]
- Zaharah SS, Singh Z, Symons GM, Reid JB. Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit. Postharvest Biol Technol. 2013;75:37–44. doi: 10.1016/j.postharvbio.2012.07.009. [DOI] [Google Scholar]
- Zhu F. Interactions between starch and phenolic compound. Trend Food Sci Technol. 2015;43:129–143. doi: 10.1016/j.tifs.2015.02.003. [DOI] [Google Scholar]