Abstract
This study developed a high performance liquid chromatography with diode array detection (HPLC–DAD) and tandem mass spectrometry (MS–MS) method for determination of prenylflavonoids and hop bitter acids in surplus yeast, a byproduct from beer brewing process. This method enabled the simultaneous separation of 4 prenylflavonoids and 20 hop bitter acids within 30 min by employing a Hypersil-Keystone HyPURITY C18 column and a gradient mobile phase composed of phosphoric acid aqueous solution at pH 1.6 and acetonitrile. For HPLC–DAD analysis, the limits of detection and limits of quantitation ranged from 0.04 to 0.15 µg/mL and from 0.12 to 0.45 µg/mL, respectively, and the recoveries ranged from 82.6 to 99.7%. The intra-day variability and inter-day variability ranged from 1.37 to 8.82% and from 0.68 to 9.74%, respectively. For qualitation by MS–MS, the positive mode was discovered to possess satisfactory collision capacity and high sensitivity for prenylflavonoids, while the negative mode was more suitable for the ionization of hop bitter acids. The content of hop bitter acids in surplus yeast were higher than that of prenylflavonoids, and isomers and oxidation products of hop bitter acids were found. This study has advantages in identifying more components, short separation time, satisfactory resolution, high accuracy and high precision.
Keywords: Prenylflavonoids, Hop bitter acids, Surplus yeast, Brewing byproducts, HPLC–DAD, Tandem mass spectrometry
Introduction
Beer is a common alcoholic beverage. Studies have shown that beer contains phenylflavonoids and hop bitter acids, which have several physiological activities (Yang et al. 2007). However, the contents of the two functional components in beer are not high, and the substantial calorie value and alcoholic content also limit health effects of beer. Conversely, brewing byproducts may contain more functional components that can be further applied. Conventional processing methods for food byproducts have been a burden on the environment, and numerous byproducts actually have nutritional value. Their further recycling not only achieves waste reduction but also increases their commercial value.
A variety of by-products are produced in the beer brewing process, such as spent grains (SG) produced after milling, mashing, and lautering; spent hops (SH) produced by boiling wort with hops; and surplus yeast (SY) produced by fermentation and aging after yeast inoculation (Mussatto 2007). These by-products are shown rich in nutrients, although most of them are used as animal feed and fertilizers (Mussatto 2007). Approximately 20 kg of byproducts are produced for every 100 Ls of beer brewed, of which SG, SH, and SY account for approximately 85%, 5%, and 10%, respectively (Fillaudeau et al. 2006). Because SH and SY are the by-products produced after the addition of hops, they theoretically contain more functional components. Moreover, the yield of SY is higher than that of SH. Thus, this study focused on SY.
Prenylflavonoids are flavonoids with a prenyl group bonded to their A-ring, and it is divided into prenylchalcones and prenylflavanones based on whether they exhibit a ring opening. The most typical prenylflavonoid is represented by xanthohumol. Its physiological activities include anti-inflammation (Lee et al. 2011), inhibition of cancer cell growth (Festa et al. 2011), and prevention of obesity (Mendes et al. 2008). Hop bitter acid is a secondary metabolite of hop. It is divided into α-acids (also known as humulone) and β-acids (also known as lupulone), both of which are prenylated phloroglucinol derivatives. The content of α-acids in hop is higher than that of β-acids, and α-acids form isomerization products in the boiling process of wort. Their physiological activities include the inhibition of cancer cell growth (Lamy et al. 2007), anti-angiogenesis (Siegel et al. 2008), improvement of metabolic syndrome (Ding et al. 2008), and improvement of osteoporosis (Yajima et al. 2004).
To our knowledge, current studies mainly focus on separation only prenylflavonoid or hop bitter acid, and samples used are chiefly hops and beer (Česlova et al. 2009). Data on identification of phenylflavonoids and hop bitter acids by mass spectrometry (MS) are lacking. The variety and content of phenylflavonoids and hop bitter acids in SY are also remain uncertain. Thus, in this study we provided an high performance liquid chromatography system with diode array detection and tandem mass spectrometry (HPLC–DAD–MS–MS) method that can identify more kinds of prenylflavonoids and hop bitter acids than the present studies. In addition, the composition of these two groups of functional components in SY was analyzed by to give additional application values.
Materials and methods
Materials
Surplus yeast was provided by local beer brewing company (Taipei, Tiawan). Surplus yeast was centrifuged at 6000 g at 25°C for 20 min to remove supernatant, and was subsequently stored at − 20°C in vacuum packs after it was freeze-dried (− 40°C, 60 millitorr).
Chemicals and reagents
α-acids and β-acids mixture standard (ICE-2) was from Labor Veritas Co. (Zürich, Switzerland), which contained α-acids with 14.45% of cohumulone and 34.94% of humulone + adhumulone and β-acids with 12.92% of colupulone and 12.02% of lupulone + adlupulone. ICS-I3 was an iso-α-acids mixture standard and was also purchased from Labor Veritas Co., which contains 32.7% of trans-isocohumulone, 54.5% of trans-isohumulone, and 12.8% of trans-isoadhumulone. Xanthohumol was from Extrasynthese Co. (Genay, France). Isoxanthohumol was from ChromaDex Co. (Irvine, CA, USA). 6-prenylnaringenin and 8-prenylnaringenin were from Sigma-Aldrich Co. (Billerica, MA, USA). 95% ethanol was purchased from Taiwan Tobacco and Liquor Co. (Taipei, Taiwan). Deionized water was obtained using the Milli-Q water purification system of Millipore Co. (MA, USA). HPLC-grade solvents were purchased from Merck (Darmstadt, Germany).
Instrumentation
HPLC-diode array detection (HPLC–DAD) system was from JASCO Co. (Tokyo, Japan), was composed of a PU-2089 plus pump and a MS-2010 plus diode array detector. The HPLC-tandem mass (HPLC–MS–MS) system was from Thermo Fisher Scientific Co. (San Jose, CA USA), which composed of Accela 600 HPLC system and LTQ Orbitrap XL tandem mass spectrometer with multiple ion source. The C18 columns HyPURITY (150 mm × 4.6 mm I.D., 5 µm) were from Thermo Hypersil-Keystone Co. (Bellefonte, PA, USA) and with a security guard C18 guard column from Phenomenex Co. (Torrance, CA, USA).
Extraction of prenylflavonoids and hop bitter acids from surplus yeast
Surplus yeast (0.2 g) was added to 95% ethanol (4 mL) and the mixture was subjected to ultrasonic extraction for 10 min followed by shaking extraction for 20 min. Subsequently, the mixture was centrifuged at 3320g for 10 min, and the supernatant was collected for concentration under vacuum followed by filtering using a 0.22-µm syringe filter. The extract was adjusted to 2 mL with ethanol for HPLC analysis.
Chromatographic conditions
A binary solvent system of deionized water at pH 1.6 adjusted with phosphoric acid (A) and acetonitrile (B) with the following gradient elution was developed: 40% B initially, maintained for 3 min, increased to 51% B at the 5th min, 58% B at the 7th min and maintained for 8 min, increased to 71% B at the 20th min, 76% B at the 21th min and 78% B at the 30th min. The column temperature was at 35 °C, quantity injected was 20 µL, flow rate at 1.0 mL/min and detection at 314 nm. The pH of water in mobile phase (A) was adjusted by formic acid instead of phosphoric acid when MS–MS was used for detection.
The peak purity of each peak was automatically determined by DAD. The retention factor (k) was calculated using the formula k = (tR − t0)/t0, where tR denotes retention time of sample components and t0 denotes retention time of sample solvent. The separation factor (α) was based on the formula α = k2/k1, where k1 and k2 represents retention factor of two neighboring peaks.
Identification of prenylflavonoids and hop bitter acids
The identification of various compounds was carried out by comparison of retention time, absorption spectra and mass spectra with reference standards, and the results with those reported in the literature. For improving the identification, a ion trap tandem mass coupled with electrospray ionization was used for providing the MS–MS data of each compound. The positive mode was used for determination of prenylflavonoids whereas negative mode was used for hop bitter acids analysis. The condition of MS–MS was scanning range 100–600 m/z, spray voltage 4.5 kV (for positive mode) or − 4.0 kV (for negative mode), heated temp 350°C, sheath gas flow rate 50 arb, aux gas flow rate 20 arb, capillary temp 275°C, capillary voltage 30 V and tube lens 150 V.
Method validation
Precision study
Surplus yeast extract was injected in HPLC–DAD and HPLC–MS–MS nine times on the same day, with the relative standard deviation (RSD%) being calculated to obtain the intra-day variability. Similarly, the extract was injected three times on three non–continuous days, and the inter-day variability was measured based on RSD%.
Detection and quantitation limits
Three concentrations of standards were prepared separately for detection and quantitation limits test for each component. 0.02, 0.05 and 0.08 µg/mL for isoxanthohumol and xanthohumol; 0.02, 0.04 and 0.08 µg/mL for 8-prenylnaringenin and 6-prenylnaringenin; 0.05, 0.08 and 0.10 µg/mL for cohumulone and humulone; 0.08, 0.10 and 0.12 µg/mL for trans-isohumulone and colupulone; 0.10, 0.12 and 0.15 µg/mL for trans-isocohumulone; 0.12, 0.15 and 0.18 µg/mL for trans-isoadhumulone, adhumulone, lupulone and adlupulone. These solutions were analyzed three times each using HPLC–DAD. The detection limit was determined based on S/N ≥ 3, whereas the quantitation limit measured was based on S/N ≥ 10.
Recovery
Two preparations of 0.2 g of surplus yeast powder were spiked with 20 and 50 µg of isoxanthohumol, 10 and 20 µg of xanthohumol, 2 and 5 µg of 8-prenylnaringenin and 6-prenylnaringenin, 62.7 and 125.4 µg of ICS-I3 as well as 182.3 and 364.6 µg of ICE2, respectively. Following extraction and HPLC analysis, the recovery of each prenylflavonoid and hop bitter acid was obtained based on the following formula:
Quantification of prenylflavonoids and hop bitter acids
All compounds were quantified by calibration curve. Prenylflavonoids and hop bitter acids that with commercial standards were quantified using their respective calibration curves prepared by HPLC–DAD system. Other hop bitter acid derivatives without standards were quantified using the calibration curve of compounds with similar maximum absorption wavelengths. For example, cohulupone and hulupone were quantified using the calibration curves of colupulone and lupulone, respectively. Adhulupone, postlupulone, prelupulone and adprelupulone were quantified using the calibration curve of adlupulone. Prehumulone and adprehumulone were quantified using the calibration curve of adhumulone. Since DAD limited in identifying cis- and trans-isomers, cis-iso-α-acids were quantified by trans-iso-α-acids using HPLC–MS–MS.
For preparation of calibration curves for DAD analysis, each standard was dissolved in ethanol. The concentrations for isoxanthohumol and xanthohumol were 2, 4, 5, 8, 10, 16 and 20 µg/mL; 8-prenylnaringenin and 6-prenylnaringenin were 0.2, 0.4, 0.5, 0.8, 1, 1.6 and 2 µg/mL; ICS-I3 were 10, 25, 50, 100, 125, 150 and 200 µg/mL; ICE2 were 25, 50, 100, 125, 150, 175 and 200 µg/mL. These standards were analyzed in triplicate using HPLC–DAD and the peak areas were collected at different wavelength for quantitation of iso-α-acids (276 nm), 8-prenylnaringenin, 6-prenylnaringenin and isoxanthohumol (292 nm), α-acids and β-acids (330 nm) and xanthohumol (368 nm), respectively. For quantitation of cis- and trans-iso-α-acids by HPLC–MS–MS, ICS-I3 was also prepared as five concentrations (8, 16, 32, 64 and 96 µg/mL) for determination. All calibration curves were obtained by plotting concentration ratio against its area ratio, with the regression equation and correlation coefficient (r2) being calculated automatically. The contents of prenylflavonoids and hop bitter acids in surplus yeast (µg/g) were quantified using the following formula:
A peak area of prenylflavonoid and hop bitter acid, a slope of calibration curve, b intercept of calibration curve, V volume of extract, f dilution factor, Ws weight of sample (g).
Statistical analysis
All the analyzes were done in triplicate unless otherwise stated, and the data were subjected to analysis of variance (ANOVA) and Duncan’s multiple range test for mean comparison (α = 0.05) by using SAS (2016).
Results and discussion
Improved method for simultaneous separation of prenylflavonoids and hop bitter acids
In our previous study (Kao and Wu 2013), we developed a method that can analyze 12 components. However, it required further improvement because typically more components need to be tested. In this study, we reassessed the column types (including Vydac 201TP54 C18 5 µm, HyPURITY C18 5 µm and 3 µm) as well as the gradient of mobile phase. The results revealed the following separation conditions: a Thermo Hypersil-Keystone HyPURITY C18 column (150 mm × 4.6 mm I.D., 5 µm) and a mobile phase containing a phosphoric acid aqueous solution at pH 1.6 (A) and acetonitrile (B), and with a gradient showed in the section of “Chromatographic conditions” in Materials and Methods.
Regarding wavelength selection, according to literature (Stevens et al. 2003; Intelmann et al. 2009; Wilhelm and Wessjohann 2006; Kao and Wu 2013) and actual detection results, iso-α-acids was quantified at a wavelength of 276 nm, 8-prenylnaringenin, 6-prenylnaringenin and isoxanthohumol were quantified at a wavelength of 292 nm, α-acids and β-acids were quantified at a wavelength of 330 nm, and xanthohumol was quantified at a wavelength of 368 nm. Simultaneous separation was performed at 292 nm because the test compounds showed appropriate absorption at this wavelength.
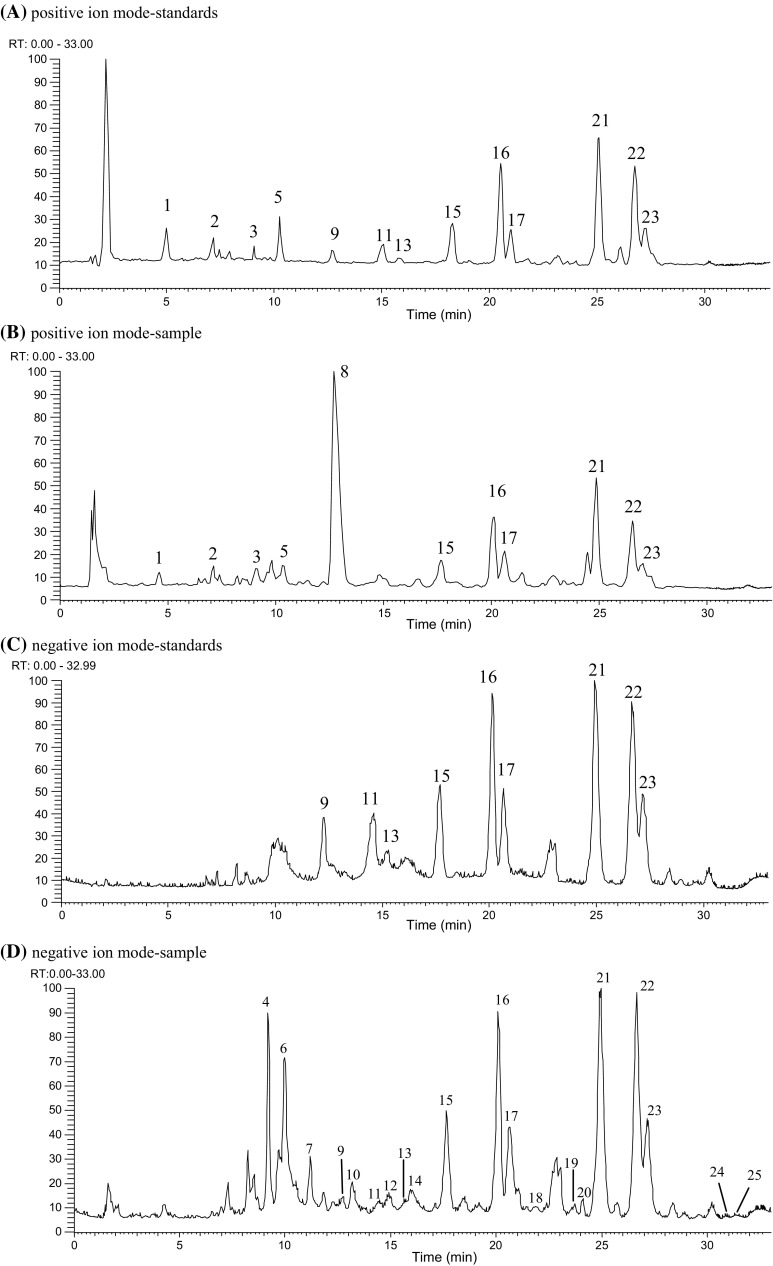
Figure 1 shows the HPLC–DAD chromatograms of standards and samples, and Table 1 shows the separation parameters of each peak. The results indicated that the α values of all peaks were higher than 1, and their k values were between 1.81 and 17.28, which showed the resolution and separation time were appropriate.
Fig. 1.
HPLC–DAD chromatograms of prenylflavonoid and hop bitter acid standards (A) and surplus yeast extract (B) by employing the method developed in this study. Chromatographic conditions were showed in the text. See Table 1 for peak identification. Detection wavelength was 292 nm
Table 1.
Retention time (tR), retention factor (k), separation factor (α), peak purity and analytical precision of prenylflavonoids and hop bitter acids in surplus yeast
| Peak No. | Compound | tR (min)a | Retention factor (k)a | Separation factor (α)ac | Peak purity (%)b | Intra-day variability RSD (%)d | Inter-day variability RSD (%)d |
|---|---|---|---|---|---|---|---|
| 1 | Isoxanthohumol | 4.64 | 1.81 | 1.73 (1, 2)b | 99.6 | 3.56b | 2.39b |
| 2 | 8-prenylnaringenin | 6.82 | 3.13 | 1.37 (2, 3) | 99.3 | 7.23b | 7.67b |
| 3 | 6-prenylnaringenin | 8.71 | 4.28 | 1.06 (3, 4) | 97.6 | 3.27b | 3.47b |
| 4 | Cohulupone | 9.16 | 4.55 | 1.09 (4, 5) | 98.5 | 3.16b | 1.61b |
| 5 | Xanthohumol | 9.82 | 4.95 | 1.02 (5, 6) | 88.0 | 1.52b | 0.68b |
| 6 | Hulupone | 10.00 | 5.06 | 1.14 (6, 7) | 99.3 | 2.26b | 0.78b |
| 7 | Adhulupone | 11.20 | 5.79 | 1.16 (7, 8) | 97.6 | 7.24b | 4.30b |
| 8 | Unknown | 12.55 | 6.60 | 1.14 (8, 9) | –e | – | – |
| 9 | Trans-isocohumulone | 12.71 | 6.70 | 1.06 (9, 10) | 97.6 | 8.00a | 8.49a |
| 10 | Cis-isocohumulone | 14.30 | 7.67 | 1.01 (10, 11) | – | 6.56a | 5.76a |
| 11 | Trans-isohumulone | 14.46 | 7.76 | 1.04 (11, 12) | 96.1 | 5.44a | 8.34a |
| 12 | Cis-isohumulone | 14.93 | 8.05 | 1.02 (12, 13) | – | 1.45a | 2.03a |
| 13 | Trans-isoadhumulone | 15.22 | 8.22 | 1.05 (13, 14) | 95.7 | 1.77a | 2.80a |
| 14 | Cis-isoadhumulone | 15.86 | 8.61 | 1.13 (14, 15) | – | 1.81a | 1.65a |
| 15 | Cohumulone | 17.67 | 9.71 | 1.15 (15, 16) | 99.0 | 6.63b | 3.22b |
| 16 | Humulone | 20.11 | 11.19 | 1.03 (16, 17) | 99.7 | 1.37b | 1.02b |
| 17 | Adhumulone | 20.63 | 11.50 | 1.11 (17, 18) | 94.8 | 2.47b | 2.09b |
| 18 | Prehumulone | 22.71 | 12.76 | 1.01 (18, 19) | 94.1 | 2.71b | 8.85b |
| 19 | Postlupulone | 22.87 | 12.86 | 1.01 (19, 20) | 99.7 | 8.82b | 9.74b |
| 20 | Adprehumulone | 23.02 | 12.95 | 1.09 (20, 21) | 97.1 | 3.38b | 6.67b |
| 21 | Colupulone | 24.90 | 14.09 | 1.07 (21, 22) | 98.1 | 3.15b | 2.51b |
| 22 | Lupulone | 26.64 | 15.15 | 1.02 (22, 23) | 97.1 | 3.77b | 3.45b |
| 23 | Adlupulone | 27.12 | 15.44 | 1.10 (23, 24) | 94.5 | 4.43b | 4.70b |
| 24 | Prelupulone | 29.62 | 16.95 | 1.02 (24, 25) | 99.4 | 8.23a | 8.55a |
| 25 | Adprelupulone | 30.16 | 17.28 | 1.02 (24, 25) | 93.5 | 4.69a | 6.75a |
aData collected from HPLC–MS–MS
bData collected from HPLC–DAD
cNumbers in parentheses represent values between two neighboring peaks
dRSD% = (SD/mean) × 100%
e“–” Data not available
Component identification
Although 21 types of components were discovered by DAD, there only 13 components (isoxanthohumol, 8-prenylnaringenin, 6-prenylnaringenin, xanthohumol, trans-isocohumulone, trans-isohumulone, trans-isoadhumulone, cohumulone, humulone, adhumulone, colupulone, lupulone and adlupulone) could be identified by comparing their absorption spectrum with that of the standards. The remaining components which without standards for comparison further identified by mass spectrometry.
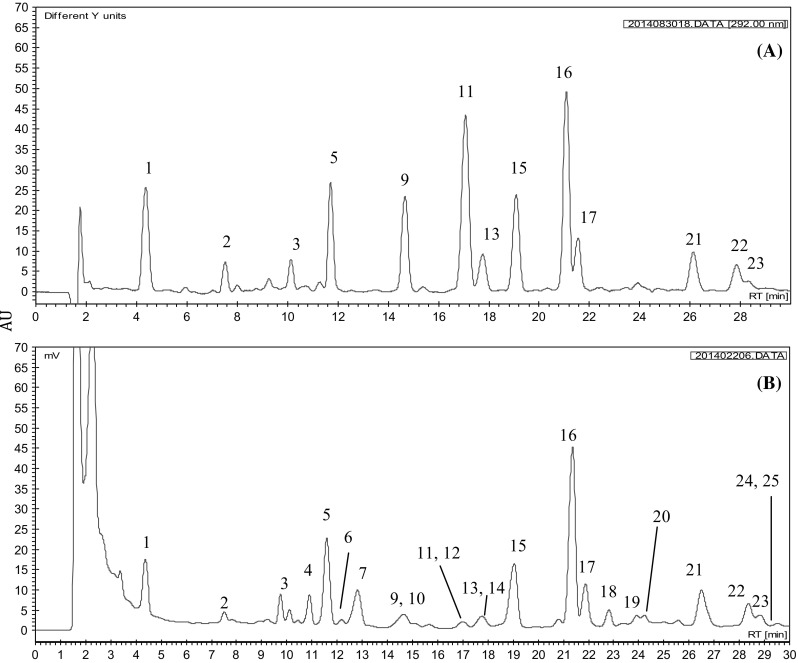
During the ionization process of mass spectrometry, we found that the positive mode was discovered to possess satisfactory collision capacity and high sensitivity for prenylflavonoids (peaks 1–3 and 5). Conversely, the negative mode was more suitable for the ionization of hop bitter acids (peaks 4 and 6–25) (Fig. 2). In the literature, the positive mode (Zhang et al. 2004) and the negative mode (Intelmann et al. 2009) were used for the ionization of hop bitter acids. Hofte and Hoeven (1998) noted that if the mobile phase contains an acidic aqueous solution, the negative mode has higher sensitivity for hop bitter acids. However, these studies have only focused on hop bitter acids, and their separation systems did not analyze prenylflavonoids. The advantage of this study is the use of a HPLC system that simultaneously separated prenylflavonoids and hop bitter acids. Subsequently, the identification of the two functional components was performed through distinct ion modes of MS, and the MS-MS spectra and fragmentation structures for each peak were showed in (Fig. 3).
Fig. 2.
Chromatograms of prenylflavonoids and hop bitter acids standards (A, C) and surplus yeast extract (B, D) by employing HPLC–ESI–MS–MS with positive and negative ion modes
Fig. 3.
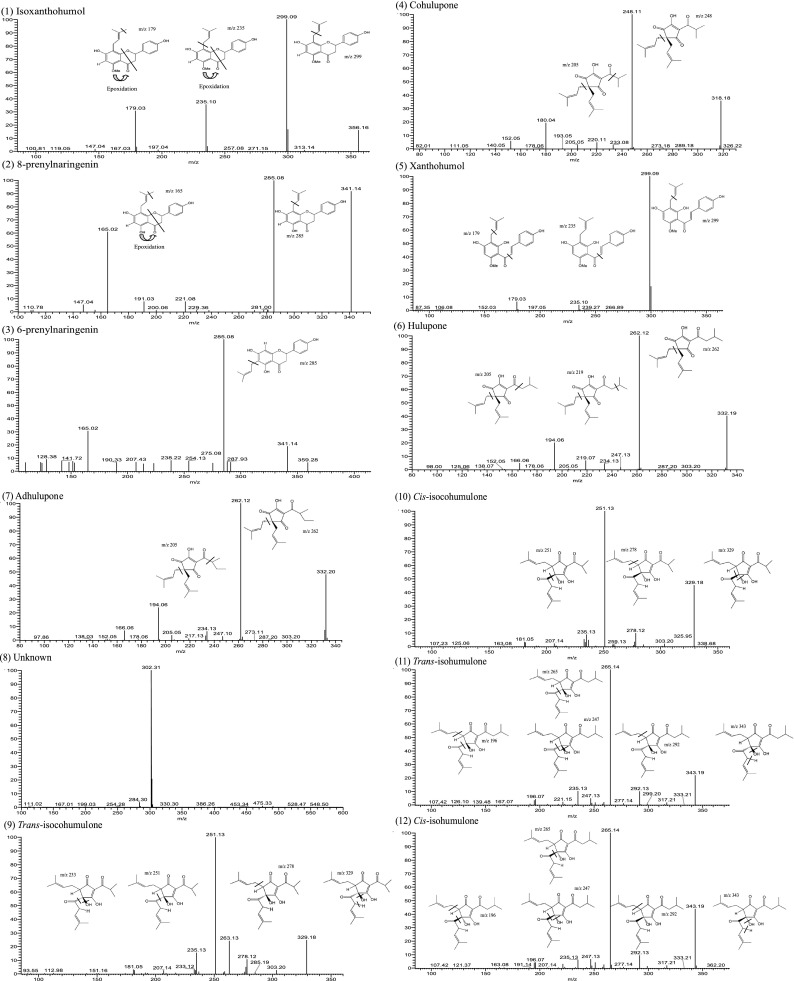
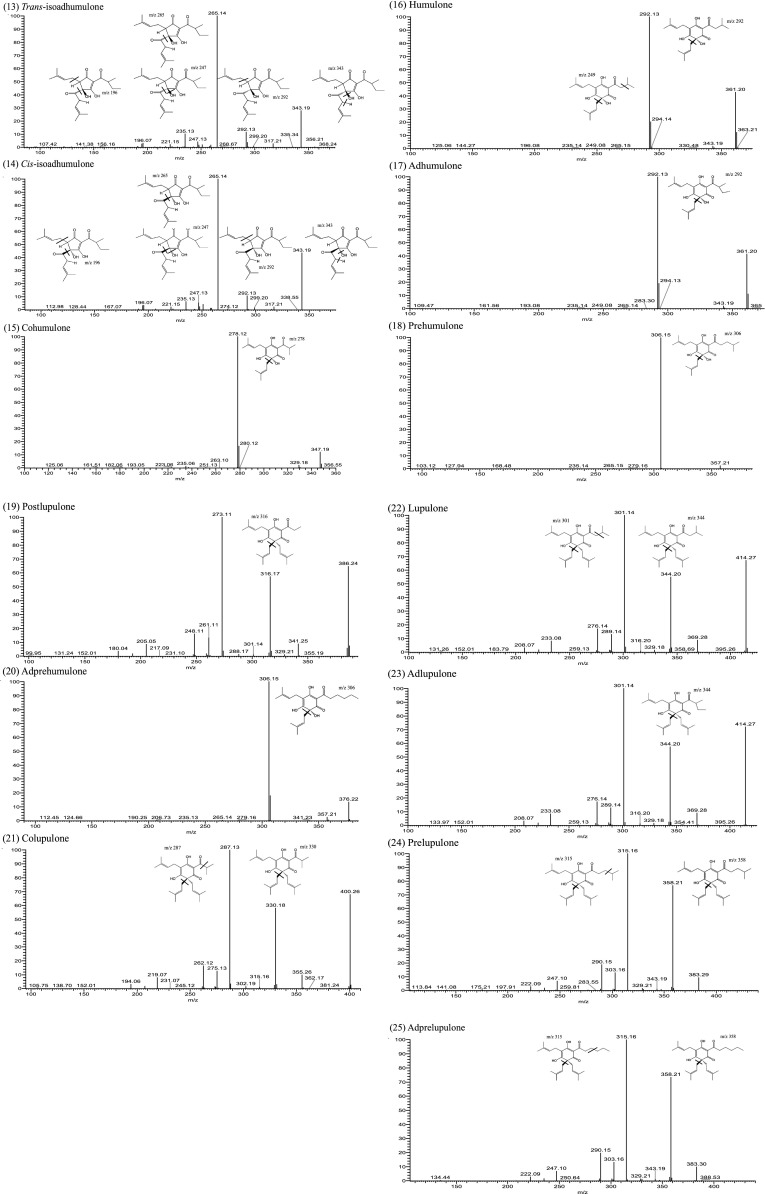
Tandem mass spectra and fragmentation structure of prenylflavonoids and hop bitter acids in surplus yeast extract
Table 2 shows the UV spectrum and mass spectrometry data for each peak, where in peaks 1–3, 5, 9, 11, 13, 15–17, and 21–23 were confirmed through comparison with the MS spectra and MS–MS spectra of the standards. In addition, the fragmentation outcomes provided by this research, which were not presented in the current studies, further enhanced component identification. The remaining components without standards for comparison were identified as follows:
Table 2.
Identification of prenylflavonoids and hop bitter acids in surplus yeast
| Peak No. | Compound | λmaxA | MW | RMD (ppm)C | MS (m/z)B | MS–MS fragment ions (m/z)B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Found | Standard | Reported | Found | Std | Reported | Found | Standard | Reported/found | ||||
| 1 | Isoxanthohumol | 288 D | 288 | 238/287a, 288b, 286c | 354 | 3.4 | 355 | 355 | 355abcf | 299, 235, 179 | 355, 299, 235, 179 | 299 [M + H–C4H8], 235 [M + H–C8H8O] 179 [M + H–C4H8–C8H8O] + f |
| 2 | 8-prenylnaringenin | 292 | 292 | 293/335d, 292/334c | 340 | 3.5 | 341 | 341 | 341acg | 341, 285, 165 | 341, 285, 165 | 285 [M + H–C4H8], 165 [M + H–C4H8–C8H8O]f |
| 3 | 6-prenylnaringenin | 292 | 292 | 292/334cd | 340 | 3.5 | 341 | 341 | 341acg | 341, 285, 165 | 341, 285, 165 | 285 [M + H–C4H8], 165 [M + H–C4H8–C8H8O]a |
| 4 | Cohulupone | 256/324 | –E | 255/327e | 318 | 0.9 | 317 | –c | 317eh | 248, 233, 220, 205, 180 | – | 317 [M–H], 248 [M–H–C5H9], 205 [M–H–C5H9–C3H7], 180e |
| 5 | Xanthohumol | 368 | 368 | 235/364a, 368bc, 272c | 354 | 3.9 | 355 | 355 | 355abcf | 299, 235, 179 | 355, 299, 235, 179 | 299 [M + H–C4H8], 235 [M + H–C8H8O], 179 [M + H–C4H8–C8H8O]bf |
| 6 | Hulupone | 256/328 | – | 332 | 1.5 | 331 | – | 331eh | 262, 247, 219, 205, 194, 166 | – | 219 [M–H–C5H9–C3H7]e, 262 [M–H–C5H9], 205 [M–H–C5H9–C4H9] | |
| 7 | Adhulupone | 256/324 | – | – | 332 | 1.2 | 331 | – | 331eh | 262, 247, 205, 194, 166 | – | 219 [M–H–C5H9–C3H7]e, 262 [M–H–C5H9], 205 [M–H–C5H9–C4H9] |
| 8 | Unknown | – | – | – | – | – | 302 | – | – | 302, 284 | – | 302 |
| 9 | Trans-isocohumulone | 228/272 | 228/272 | 257/271a | 348 | 4.6 | 347 | 347 | 347af | 329, 278, 251, 235, 233, 207, 181 | 347, 329, 278, 251, 233, 207, 182 | 347 [M–H], 329 [M–H–H2O], 278 [M–H–C5H9], 251 [M–H–C6H8O], 233 [M–H–C6H8O–H2O], 207 [M–H–C6H8O–C2H3O]af |
| 9–1 | Cis-isocohumulone | 228/272 | – | – | 348 | 4.6 | 347 | – | 347af | 329, 278, 251, 235, 207,181 | – | 347 [M–H], 329 [M–H–H2O], 278 [M–H–C5H9], 251 [M–H–C6H8O], 233 [M–H–C6H8O–H2O], 207 [M–H–C6H8O–C2H3O]af |
| 11 | Trans-isohumulone | 228/280 | 228/280 | 257/277a | 362 | 5.5 | 361 | 361 | 361af | 343, 292, 265, 247, 235, 221, 196 | 361, 343, 292, 265, 247, 235, 221, 196 | 361 [M–H], 343 [M–H–H2O], 265 [M–H–C6H8O] 247 [M–H–C6H8O–H2O]af, 292 [M–H–C5H9], 196 [M–H–C6H8O–C5H9] |
| 11–1 | Cis-isohumulone | 228/280 | – | – | 362 | 5.5 | 361 | – | 361af | 343, 292, 265, 247, 235, 196 | – | 361 [M–H], 343 [M–H–H2O], 265 [M–H–C6H8O] 247 [M–H–C6H8O–H2O]af, 292 [M–H–C5H9], 196 [M–H–C6H8O–C5H9] |
| 13 | Trans-isoadhumulone | 228/276 | 224/276 | 257/274a | 362 | 5.5 | 361 | 361 | 361af | 343, 292, 265, 247, 235, 196 | 361, 343, 292, 265, 247, 235, 196 | 361 [M–H], 343 [M–H–H2O], 265 [M–H–C6H8O], 247 [M–H– C6H8O–H2O]af, 292 [M–H–C5H9], 196 [M–H–C6H8O–C5H9] |
| 13–1 | Cis-isoadhumulone | 228/276 | – | – | 362 | 5.5 | 361 | – | 361af | 343, 292, 265, 247, 235, 196 | – | 361 [M–H], 343 [M–H–H2O],265 [M–H–C6H8O], 247 [M–H–C6H8O–H2O]af, 292 [M–H–C5H9], 196 [M–H–C6H8O–C5H9] |
| 15 | Cohumulone | 236/284/ 320 | 236/284/ 320 | 283/320a, 236/282/322c | 348 | 4.6 | 347 | 347 | 347af | 347, 329, 278 | 347, 329, 278 | 347 [M–H], 329 [M–H–H2O], 278 [M–H–C5H9]afi |
| 16 | Humulone | 236/284/320 | 236/284/320 | 283/320a, 236/282/322c | 362 | 5.5 | 361 | 361 | 361af | 361, 292, 249 | 361, 292, 249 | 361 [M–H], 292 [M–H–C5H9], 249 [M–H–C5H9–C3H7]af |
| 17 | Adhumulone | 236/282/320 | 236/282/320 | 283/320a, 236/282/322c | 362 | 5.5 | 361 | 361 | 361af | 361, 292, 249 | 361, 292, 249 | 361 [M–H], 292 [M–H–C5H9], 249 [M–H–C5H9–C3H7]af |
| 18 | Prehumulone | 228/288 | – | – | 376 | 2.7 | 375, 363 | – | 375a | 357, 306 | – | 306 [M–H–C5H9]a |
| 19 | Postlupulone | 288 | – | – | 386 | 1.0 | 385 | – | 385af | 316, 273, 261, 248, 205 | – | 273 [M–H–C5H9–C3H7], 316 [M–H–C5H9]af, 261, 248, 205f |
| 20 | Adprehumulone | 228/288 | – | – | 376 | 3.5 | 375 | – | 375a | 357, 306 | – | 306 [M–H–C5H9]a |
| 21 | Colupulone | 276/332 | 276/332 | 275/332ae, 278/336c | 400 | 3.3 | 399 | 399 | 399aefi | 330, 287, 262, 219 | 399, 330, 287, 262, 219 | 399 [M–H], 287 [M–H–C5H9–C3H7], 330 [M–H–C5H9]aefi |
| 22 | Lupulone | 276/332 | 276/332 | 274/331ae, 274/332c | 414 | 5.6 | 413 | 413 | 413aef | 344, 301, 233 | 413, 344, 301, 233 | 413 [M–H], 301 [M–H–C5H9–C3H7], 233 [M–H–C5H9–C3H7–C5H8]aef, 344 [M–H–C5H9] |
| 23 | Adlupulone | 280/332 | 276/332 | 280/332ae, 276/328c | 414 | 5.6 | 413 | 413 | 413aef | 344, 301, 233 | 413, 344, 301, 233 | 413 [M–H], 301 [M–H–C5H9–C3H7], 233 [M–H–C5H9–C3H7–C5H8]aef, 344 [M–H–C5H9] |
| 24 | Prelupulone | 288 | – | – | 428 | 1.6 | 427 | – | 427a | 383, 358, 315, 303, 290, 247, 222 | – | 315 [M–H–C5H9–C3H7]a, 358 [M–H–C5H9] |
| 25 | Adprelupulone | 288 | – | – | 428 | 1.6 | 427 | – | 427a | 383, 358, 315, 303, 290, 247, 222 | – | 315 [M–H–C5H9–C3H7]a, 358 [M–H–C5H9] |
AA gradient mobile phase of acetonitrile and phosphoric acid in water (pH 1.6) was used
BDeterminated by HPLC–ESI–MS–MS in the positive ion mode. A gradient mobile phase of acetonitrile and formic acid in water (pH 1.6) was used
CRelative mass difference = 106 × (mtrue-mmeasured)/mtrue
DMaximum absorption wavelength was marked as underline
E“-”: Data not available
aBased on a reference by Intelmann et al.15
bBased on a reference by Stevens et al. (2003)
cBased on a reference by Kao and Wu (2013)
dBased on a reference by Wilhelm and Wessjohann (2006)
eBased on a reference by Haseleu et al. (2009)
fBased on a reference by Česlova et al. (2009)
gBased on a reference by Rong et al. (2012)
hBased on a reference by García-villalba et al. (2006)
iBased on a reference byVanhoenacker et al. (2004)
Peak 4 had maximum absorption wavelengths of 256 nm and 324 nm, which were similar to the main absorption wavelengths of 255 nm and 327 nm of cohulupone, as measured by Haseleu et al. (2009). Its molecular ion was m/z 317 [MH]−, which was consistent with the results of García-Villalba et al. (2006) and Haseleu et al. (2009) MS–MS spectrum revealed the presence of m/z 248, 205, and 180, which was similar to the results of Haseleu et al. (2009). Thus, Peak 4 was determined to be cohulupone. In addition, it was further speculated that the ions at m/z 248 were [MH-C5H9]−, whereas those at m/z 205 were [MH-C5H9-C3H7]− ions.
Peaks 6 and 7 had maximum absorption wavelengths of 256/328 nm and 256/324 nm, respectively, and no literature is currently available for comparison. Both had a molecular ion of m/z 331 [MH]− and were thus speculated to be the isomers hulupone and adhulupone (Haseleu et al. 2009; García-Villalba et al. 2006). However, hulupone has a higher polarity than adhulupone, hence their elution orders differ in reverse phase chromatography. Thus, Peaks 6 and 7 were hulupone and adhulupone, respectively. It was further speculated that the ions at m/z 262 were [MH-C5H9]−, whereas those at m/z 210 and m/z 205 were [MH-C5H9-C3H7]− and [M-H-C5H9-C4H9]− fragment ions, respectively.
Peak 8 did not appear in the DAD chromatogram. However, MS analysis indicated that its molecular ion was m/z 302 [M + H]+, and MS/MS showed the presence of m/z 302 and 284. Literature comparison yielded no definite results, thus, Peak 8 was still an unknown component.
Peaks 10, 12, and 14 did not appear in the DAD chromatogram (Fig. 1), and their molecular ions and MS/MS fragments were identical to those of peaks 9, 11 and 13. Thus, they were speculated to be isomers of each other. Isomer analysis revealed that their fragments were similar to those discovered in the literature (Česlova et al. 2009; Intelmann et al. 2009). Intelmann et al. (2009) discovered that the response of specific fragments exhibited distinct between isomers. For example, trans-isocohumulone showed more m/z 329 fragment than cis-isocohumulone, trans-isohumulone showed fewer m/z 343 fragment but more m/z 235 fragment compared with cis-isohumulone, and trans-isoadhumulone showed fewer m/z 343 and 247 fragment compared with cis-isoadhumolone. Based on the aforementioned characteristics, peak 10 was identified to be cis-isocohumulone, whereas peaks 12 and 14 were identified to be cis-isohumulone and cis-isoadhumulone, respectively.
The spectrum, molecular ions, and MS/MS fragments were the same between peaks 18 and 20 as well as peaks 24 and 25, which is consistent with the identification data for the prehumulone/adprehumulone and prelupulone/adprelupulone described in the literature(Česlova et al. 2009; Intelmann et al. 2009). However, prehumulone and prelupulone have higher polarity than adprehumulone and adprelupulone. Thus, peaks 18, 20, 24 and 25 were identified to be prehumulone, adprehumulone, prelupulone and adprelupulone, respectively. In addition, it was further inferred that the ions at m/z 306 and 315 were [M-H-C5H9]− fragment ions.
The molecular ion of Peak 19 was the same as that of postlupulone (Česlova et al. 2009; Intelmann et al. 2009), whereas the MS/MS fragment of Peak 19 was the same as that described Česlova et al. (2009). Thus, Peak 19 was determined to be postlupulone. In addition, it was further speculated that the ions at m/z 316 were [MH-C5H9]− fragment ion.
The results of the relative mass difference (RMD) further demonstrated that the RMD of all the components was less than 10 ppm (Kaufmann and Walker 2012), which was within the acceptable range, showing a high conformity in peak identification.
Method validation
Precision
Table 1 shows the RSD (%) for intra-day and inter-day variability of prenylflavonoids were 1.52%–7.23% and 0.68%–7.67%, respectively, whereas those of hop bitter acids were 1.37%–8.82% and 1.02–9.74%, respectively. The precision results from current studies were focused on beer or hop samples. Stevens et al. (1999) analyzed prenylflavonoids in beer and hop and showed that the RSD (%) for intra-day and inter-day variability of prenylflavonoids were 3.8%–7.9% and 3.9%–11.4%, respectively. Vanhoenacker et al. (2004) showed that the intra-day variability of hop bitter acids in beer was 4.5%. Jaskula et al. (2007) discovered that the intra-day variability of α-acids and iso-α-acids in beer and hop were 1.4%–4.3% and 0.7%–2.0%, respectively. The outcomes from our study demonstrated that this method possessed satisfactory precision in beer brewing byproduct sample.
Detection limit, quantitation limit, and recovery
Table 3 shows the detection limit (DL), quantitation limit (QL), and recovery of each component detected by DAD. Similar to precision data, the results from current studies were focused on beer or hop samples. Česlova et al. (2009) showed that the DL of isoxanthohumol, xanthohumol and hop bitter acids was 0.02, 0.02 and 0.1 µg/mL, respectively, and their QL in hop was 0.06, 0.06, and 0.3 µg/mL. Jaskula et al. (2007) showed that the recovery of iso-α-acids on beer was 82.8%–88.9%. Intelmann et al. (2009) indicated that the recovery of isoxanthohumol, xanthohumol, cohumulone and colupulone in beer was 95%, 94%, 94% and 100%, respectively. The results of this study are similar to the aforementioned results, and a high recovery (82.6–99.7%) was found for all detected components.
Table 3.
Detection of limit, detection of quantation and recoveries of four prenylflavonoids and nine hop bitter acids
| Peak No. | Compound | LOD (µg/mL) | LOQ (µg/mL) | Recovery (%)a | ||
|---|---|---|---|---|---|---|
| Lowb | Highc | Means (RSD%)d | ||||
| 1 | Isoxanthohumol | 0.05 | 0.15 | 96.4 | 98.8 | 97.6 (1.7) |
| 2 | 8-prenylnaringenin | 0.04 | 0.12 | 98.1 | 93.5 | 95.8 (3.4) |
| 3 | 6-prenylnaringenin | 0.04 | 0.12 | 95.2 | 91.4 | 93.3 (4.6) |
| 5 | Xanthohumol | 0.05 | 0.15 | 94.1 | 90.1 | 92.1 (0.6) |
| 9 | Trans-isocohumulone | 0.12 | 0.36 | 91.1 | 84.7 | 87.9 (2.3) |
| 11 | Trans-isohumulone | 0.10 | 0.30 | 83.0 | 82.1 | 82.6 (1.0) |
| 13 | Trans-isoadhumulone | 0.15 | 0.45 | 93.3 | 94.6 | 93.9 (2.8) |
| 15 | Cohumulone | 0.08 | 0.24 | 98.5 | 97.7 | 98.1 (1.7) |
| 16 | Humulone | 0.08 | 0.24 | 86.4 | 84.2 | 85.3 (1.3) |
| 17 | Adhumulone | 0.15 | 0.45 | 93.0 | 87.9 | 90.4 (2.3) |
| 21 | Colupulone | 0.10 | 0.30 | 95.1 | 91.8 | 93.5 (1.2) |
| 22 | Lupulone | 0.15 | 0.45 | 90.00 | 91.7 | 90.9 (2.4) |
| 23 | Adlupulone | 0.15 | 0.45 | 104.0 | 95.3 | 99.7 (3.5) |
aRecovery (%) = (amount found – original amount)/amount spiked × 100%
bLow: surplus yeast was spiked with standards at low concentration level
cHigh: surplus yeast was spiked with standards at high concentration level
dRSD%: (SD/mean) × 100%
Variety and content of preylflavonoids and hop bitter acids in surplus yeast
Most studies have analyzed prenylflavonoids and hop bitter acids in beer and hop, and have discovered that the content of hop bitter acids in beer and hop is higher than that of prenylflavonoids (Stevens et al. 1999). The main types of prenylflavonoids in hop and beer are xanthohumol and isoxanthohumol, respectively (Intelmann et al. 2009). The main type of hop bitter acids in hop is α-acids and their derivatives, whereas the main types in beer are iso-α-acids and their derivatives, where in the cis-isomer content is higher than the trans-isomer content (Česlova et al. 2009; Intelmann et al. 2009; Haseleu et al. 2009; García-Villalba et al. 2006; Stevens et al. 1999). This study also found the same result for SY, wherein the total content of hop bitter acids was higher than that of prenylflavonoids, with a difference of up to 7.1 times between both components (Table 4).
Table 4.
Contents of prenylflavonoids and hop bitter acids in surplus yeast
| Peak No. | Compound | Contents (µg/g)A | Percentage (%) |
|---|---|---|---|
| 1 | Isoxanthohumol | 118.4 ± 1.1a | 8.25 |
| 2 | 8-prenylnaringenin | 3.8 ± 0.5a | 0.26 |
| 3 | 6-prenylnaringenin | 10.5 ± 0.4a | 0.73 |
| 4 | Cohulupone | 131.8 ± 3.7a | 9.19 |
| 5 | Xanthohumol | 44.2 ± 0.4a | 3.08 |
| 6 | Hulupone | 227.9 ± 6.6a | 15.88 |
| 7 | Adhulupone | 27.5 ± 1.4a | 1.92 |
| 8 | Unknown | – | – |
| 9 | Trans-isocohumulone | 7.8 ± 0.9b | 0.54 |
| 10 | Cis-isocohumulone | 18.9 ± 1.2b | 1.32 |
| 11 | Trans-isohumulone | 12.0 ± 0.9b | 0.84 |
| 12 | Cis-isohumulone | 59.2 ± 0.9b | 4.13 |
| 13 | Trans-isoadhumulone | 30.2 ± 0.9b | 2.10 |
| 14 | Cis-isoadhumulone | 40.7 ± 0.7b | 2.84 |
| 15 | Cohumulone | 105.4 ± 2.4a | 7.35 |
| 16 | Humulone | 279.2 ± 1.2a | 19.46 |
| 17 | Adhumulone | 59.3 ± 1.2a | 4.13 |
| 18 | Prehumulone | 9.2 ± 0.4a | 0.64 |
| 19 | Postlupulone | 5.0 ± 0.2a | 0.35 |
| 20 | Adprehumulone | 6.6 ± 0.1a | 0.46 |
| 21 | Colupulone | 109.8 ± 2.2a | 7.65 |
| 22 | Lupulone | 102.5 ± 3.3a | 7.14 |
| 23 | Adlupulone | 24.1 ± 1.3a | 1.68 |
| 24 | Prelupulone | 0.5 ± 0.0b | 0.03 |
| 25 | Adprelupulone | 0.4 ± 0.0b | 0.03 |
| Total | 1434.9 ± 31.9 | 100 |
AMean of triplicate analysis ± standard deviation
aData collected from HPLC–DAD
bData collected from HPLC–MS–MS
Isoxanthohumol was found to be the main prenylflavonoid, whereas the composition of hop bitter acids was relatively complex, wherein the difference between the total amount of α-acids (trans-isocohumulone, cis-isocohumulone, trans-isohumulone, cis-isohumulone, trans-isoadhumulone, cis-isoadhumulone, cohumulone, humulone, adhumulone, prehumulone and adprehumulone) and β-acids (cohulupone, hulupone, adhulupone, poslupulone, colupulone, lupulone, adlupulone, prelupulone and adprelupulone) in SY was insignificant. Humulone and hulupone were the α-acid and β-acid, respectively, with the highest content. Isomers accounted for approximately 26.9% of α-acids. Cis-isomer content was higher than trans-isomer content, which was similar to that in hop and beer, and approximately 61.5% of β-acids were oxidized to hulupone derivatives (cohulupone, hulupone and adhulupone).
Related studies have noted that during the brewing process, cohumulone, humulone and adhumulone are extremely susceptible to temperature, an increase in pH value, and UV irradiation, which result in the formation of cis- and trans-isomers (Höltzel et al. 1996). In the presence of oxygen, β-acids are transformed into a large number of hulupone derivatives (Van Cleemput et al. 2009). SY is a byproduct of fermentation, thus, xanthohumol and α-acids that originate from hop may isomerize into isoxanthohumol and iso-α-acids, respectively. Although alcohol fermentation occurs under anaerobic conditions, SY may be exposed to air after its separation from beer, thus producing a large number of hulupone derivatives.
Conclusion
In this study, our developed method was used to simultaneously separate 4 types of prenylflavonoids and 20 types of hop bitter acids and their derivatives, although one component was still unknown. This system possessed satisfactory resolution, accuracy, and precision, and can simultaneously separate two major categories of components within a short time. In addition, SY was further verified to be mainly composed of hop bitter acids, and isomers and oxidation products were found in SY. This study not only strengthens the currently inadequate mass spectrometry data for prenylflavonoids and hop bitter acids, but also provides the basis for the development of brewery byproducts as functional products.
Acknowledgements
This study was supported by a Grant (MOST 103–2221-E-030–014) from the Ministry of Science and Technology, Taiwan.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Česlova L, Holčapek M, Fidler M, Drštičková J, Lisa M. Characterization of prenylflavonoids and hop bitter acids in various classes of Czech beers and hop extracts using high-performance liquid chromatography–mass spectrometry. J Chromatogr A. 2009;1216:7249–7257. doi: 10.1016/j.chroma.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Ding C, Parameswaran V, Udayan R, Burgess J, Jones GJ. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab. 2008;93:1952–1958. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- Festa M, Capsso A, D’Acunto CW, Masullo M, Rossi AG, Pizza C, Piacente S. Xanthohumol induces apoptosis in human malignant glioblastoma cells by increasing reactive oxygen species and activating MAPK pathways. J Nat Prod. 2011;74:2505–2513. doi: 10.1021/np200390x. [DOI] [PubMed] [Google Scholar]
- Fillaudeau L, Blanpain-Avet P, Daufin G. Water, wastewater and waste management in brewing industries. J Clean Prod. 2006;14:463–471. doi: 10.1016/j.jclepro.2005.01.002. [DOI] [Google Scholar]
- García-villalba R, Cortacero-ramírez S, Segura-carretero A, Martín-lagos JA, Fernnández-gutiérrez A. Analysis of hop acids and their oxidized derivatives and iso-α-acids in beer by capillary electrophoresis-electrospray ionization mass spectrometry. J Agric Food Chem. 2006;54:5400–5409. doi: 10.1021/jf060207x. [DOI] [PubMed] [Google Scholar]
- Haseleu G, Intelmann D, Hofmann T. Identification and RP-HPLC-ESI-MS/MS quantitation of bitter-tasting β-acid transformation products in beer. J Agric Food Chem. 2009;57:7480–7489. doi: 10.1021/jf901759y. [DOI] [PubMed] [Google Scholar]
- Hofte AJP, Hoeven RAM. Characterization of hop acids by liquid chromatography with negative electrospray ionization mass spectrometry. J Am Soc Brew Chem. 1998;56:118–122. [Google Scholar]
- Höltzel A, Schlotterbeck G, Albert K, Bayer E. Separation and characterization of hop bitter acids acids by HPLC-1H NMR coupling. Chromatographia. 1996;42:499–505. doi: 10.1007/BF02290283. [DOI] [Google Scholar]
- Intelmann D, Haseleu G, Hofmann T. LC–MS/MS quantitation of hop-derived bitter compounds in beer using the ECHO technique. J Agric Food Chem. 2009;57:1172–1182. doi: 10.1021/jf803040g. [DOI] [PubMed] [Google Scholar]
- Jaskula B, Goiris K, Roucl GD, Aerts G, Cooman LD. Enhanced quantitative extraction and HPLC determination of hop and beer bitter acids. J Inst Brew. 2007;113:381–390. doi: 10.1002/j.2050-0416.2007.tb00765.x. [DOI] [Google Scholar]
- Kao TH, Wu GY. Simultaneous determination of prenylflavonoidand hop bitter acid in beer lee by HPLC-DAD-MS. Food Chem. 2013;141:1218–1226. doi: 10.1016/j.foodchem.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Kaufmann A, Walker S. Accuracy of relative isotopic abundance and mass measurements in a single-stage orbitrap mass spectrometer. Rapid Commun. Mass Spectrometry. 2012;26:1081–1090. doi: 10.1002/rcm.6195. [DOI] [PubMed] [Google Scholar]
- Lamy V, Roussi S, Chaabi M, Gossé F, Schall N, Lobstein A, Raul F. Chemopreventive effects of lupulone, a hop β-acid, on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2007;28:1575–1581. doi: 10.1093/carcin/bgm080. [DOI] [PubMed] [Google Scholar]
- Lee IS, Lim J, Gal J, Kang JC, Kim HJ, Kang BY, Choi HJ. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem Int. 2011;58:153–160. doi: 10.1016/j.neuint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Mendes V, Monteiro R, Pestana D, Teixeira D, Chlhau C, Azevedo I. Xanthohumol influences preadipocyte differentiation: implication of antiproliferative and apoptotic effects. J Agric Food Chem. 2008;56:11631–11637. doi: 10.1021/jf802233q. [DOI] [PubMed] [Google Scholar]
- Mussatto SI. Biotechnological potential of brewing industry by-products. In: Nigam PS, Pandey A, editors. Biotechnology for agro-industrialresidues utilization. Cham: Springer; 2007. pp. 313–326. [Google Scholar]
- Rong H, Zhao Y, Lazou K, De Keukeleire D, Milligan SR, Sandra P. Quantitation of 8-prenylnaringenin, a novel phytoestrogen in hops (Humulus lupulus L.), hop products, and beers, by benchtop HPLC–MS using electrospray ionization. Chromatographia. 2012;51:545–551. doi: 10.1007/BF02490811. [DOI] [Google Scholar]
- SAS (2016) SAS Procedures and SAS/Graph User’s Guide, version 9.4. SAS Institute, Inc., Cary
- Siegel L, Miternique-Grosse A, Griffon C, Klein-Soyer C, Lobstein A, Raul F, Stephan D. Antiangiogenic properties of lupulone, a Bitter acid of hop cones. Anticancer Res. 2008;28:289–294. [PubMed] [Google Scholar]
- Stevens JF, Taylor AW, Deinzer ML. Quanitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 1999;832:97–107. doi: 10.1016/S0021-9673(98)01001-2. [DOI] [PubMed] [Google Scholar]
- Stevens JF, TaylMirandaor CL, Frei B, Buhler DR. Inhibition of peroxynitrite-mediated LDL oxidation by prenylated flavonoids: the α, β-unsaturated keto functionality of 2′-hydroxychalcones as a novel antioxidant pharmacophore. Chem Res Toxicol. 2003;16:1277–1286. doi: 10.1021/tx020100d. [DOI] [PubMed] [Google Scholar]
- Van Cleemput M, Cattoor K, Bosscher KD, Haegeman G, De Keukeleire D, Heyerick A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J Nat Prod. 2009;72:1220–1230. doi: 10.1021/np800740m. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker G, De Keukeleire D, Sandra P. Analysis of iso-α-acids and reduced iso-α-acids in beer by direct injection and liquid chromatography with ultraviolet absorbance detection or with mass spectrometry. J Chromatogr A. 2004;1035:53–61. doi: 10.1016/j.chroma.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Wilhelm H, Wessjohann LA. An efficient synthesis of the phytoestrogen 8-prenylnaringenin from xanthohumol by a novel demethylation process. Tetrahedron. 2006;3:213–232. [Google Scholar]
- Yajima H, Ikeshima E, Shiraki M, Kanaya T, Fujiwara D, Odai H, Tsuboyama-Kasaoka N, Ezaki O, Oikawa S, Kondo K. Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor α and γ and reduce insulin resistance. J Biol Chem. 2004;279:33456–33462. doi: 10.1074/jbc.M403456200. [DOI] [PubMed] [Google Scholar]
- Yang JY, Della-Fera MA, Rayalam S, Baile CA. Effect of xanthohumol andisoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. Apoptosis. 2007;12:1953–1964. doi: 10.1007/s10495-007-0130-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liang X, Xiao H, Xu Q. Direct characterization of bitter acids in a crude hop extract by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J Am Soc Mass Spectrom. 2004;15:180–187. doi: 10.1016/j.jasms.2003.09.014. [DOI] [PubMed] [Google Scholar]