Abstract
Endocrine disrupting chemicals (EDCs) are ubiquitous, and pregnancy is a sensitive window for toxicant exposure. EDCs may disrupt the maternal immune system, which may lead to poor pregnancy outcomes. Most studies investigate single EDCs, even though “real life” exposures do not occur in isolation. We tested the hypothesis that uniquely weighted mixtures of early pregnancy exposures are associated with distinct changes in the maternal and neonatal inflammasome. First trimester urine samples were tested for 12 phthalates, 12 phenols, and 17 metals in 56 women. Twelve cytokines were measured in first trimester and term maternal plasma, and in cord blood after delivery. Spearman correlations and linear regression were used to relate individual exposures with inflammatory cytokines. Linear regression was used to relate cytokine levels with gestational age and birth weight. Principal component analysis was used to assess the effect of weighted EDC mixtures on maternal and neonatal inflammation. Our results demonstrated that maternal and cord blood cytokines were differentially associated with (1) individual EDCs and (2) EDC mixtures. Several individual cytokines were positively associated with gestational age and birth weight. These observed associations between EDC mixtures and the pregnancy inflammasome may have clinical and public health implications for women of childbearing age.
Introduction
Environmental toxicants, which include endocrine disrupting chemicals (EDC) such as phenols, phthalates, metals, and organochlorines, are globally ubiquitous and represent an area of major public health concern1,2. Data from the National Health and Nutrition Examination Survey (NHANES) show that the vast majority of US adults have evidence of exposure to phthalates and phenols, such as bisphenol A (BPA) and polyfluoroalkyl chemicals, as measured in urine samples3–6. Children also demonstrate exposure to a wide variety of environmental chemicals7,8. EDC exposure may occur on a daily basis via packaged foods, plastics, cosmetics, and pharmaceuticals, and industrial applications may lead to widespread environmental exposure to heavy metals2,9,10.
EDC exposures are of particular significance to pregnant women, as fetal development is sensitive to maternal nutritional, chemical, and environmental stressors. In utero exposures may compromise early developmental processes and predispose the fetus to adverse health risks later in life, based on the Developmental Origins of Health and Disease (DOHaD) hypothesis11–13. Epidemiologic studies have demonstrated that EDC exposures are nearly universal in pregnant women14–18. Exposure to EDC classes including phenols, phthalates, parabens, flame retardants, and heavy metals may occur in pregnancy by way of personal hygiene products, cosmetics, household cleaning products, the use of electronic devices, and consumption of animal, plant, or processed foods19,20.
Pregnancy is a clinically relevant susceptibility period for investigating EDC exposures, due to the growing body of evidence that EDCs modulate physiologic processes in both exposed individuals and their offspring2. Epidemiologic studies have also demonstrated that prenatal exposure to BPA, phthalates, and polyfluoroalkyl chemicals may be associated with fetal growth restriction or small for gestational age babies21–27, both of which are risk factors for adult onset disease28–30. However, the literature is mixed, with some studies demonstrating no effect of EDC exposure on birth outcomes or anthropometric measures31,32.
Biologically, EDCs may mimic or interfere with estrogenic, androgenic, glucocorticoid, thyroid, and insulin signaling pathways, leading to a variety of downstream, tissue-specific effects33,34. EDCs may exert physiologic effects at low doses, and modulation of hormonal pathways occurs in a non-monotonic manner for substances such as BPA, dioxin, lead, and cadmium33,35,36. While most EDCs have been studied in isolation, there is increasing interest in the cumulative impact of EDC exposures, as humans are exposed to a multitude of EDCs at varying doses which may have additive, synergistic, or negative biologic effects37–39.
EDC exposures in pregnancy have been associated with changes in the gestational endocrine milieu, including altered levels of sex steroids40,41. Further, there is evidence that EDCs such as BPA and polycyclic aromatic hydrocarbons, and metals such as arsenic, cadmium, lead, and mercury, may modulate the immune system and alter inflammatory cytokine milieu to favor a pro-inflammatory state42–44. Meanwhile, findings from other pregnancy cohorts have demonstrated that urinary paraben and phenol measures may affect circulating markers of inflammation including IL-6, IL-10, CRP, and TNF-α45,46. Importantly, aberrant maternal inflammatory pathways have been linked to adverse pregnancy outcomes including pregnancy loss, preterm labor, preeclampsia, and fetal growth restriction47–50. Epidemiologic and animal studies indicate that such alterations induced in the maternal environment by EDCs may alter the developmental trajectory of the developing fetus via epigenetic modifications51.
The purpose of our pilot study is to use the Michigan Mother-Infant Pairs (MMIP) birth cohort to assess the impact of phenols, phthalates, and metals, both individually and in combination, on the maternal and neonatal inflammasome. Using maternal urinary measures of environmental toxicants during the first trimester, a sensitive period in fetal development, we correlated exposures to plasma levels of inflammatory cytokines in both maternal (at two time points) and neonatal umbilical cord blood. Exposures and inflammatory cytokine levels were then correlated with birth outcomes, namely infant birth weight and gestational age at delivery.
Methods
Subject recruitment
The MMIP project is an ongoing birth cohort study (2010-present) at the University of Michigan (UM). Pregnant women are recruited into MMIP at their first prenatal appointment between 8 and 14 weeks of gestation. Those eligible for MMIP participation are between 18 and 42 years old, have a naturally conceived singleton pregnancy, and intend to deliver at UM. Pregnancy dating is determined by the obstetric provider, either based on last menstrual period or ultrasound. As of October 2018, 289 women in the MMIP cohort have delivered. Other studies involving subsets of MMIP participants have been published previously21,52,53.
A subset of fifty-six women, recruited between November 2012 and May 2015, were selected for the specific analyses described here. All 56 women met additional inclusion criteria as follows: 1. had available biospecimens from mother and infant, at all relevant time points, and 2. had provided complete demographic and health information at their initial study visit. Study procedures were approved by the UM Institutional Review Board, and all participants provided written informed consent. All research was performed in accordance with relevant guidelines and regulations.
Sample collection
Spot urine and venous blood samples were collected from participants at their first prenatal appointment between 8 and 14 weeks of gestation, and upon arrival to the hospital for delivery, prior to IV placement. Urine samples were collected in polypropylene containers before being transferred to glass vials. All samples were stored at −80 degrees Celsius until analysis.
Exposure assessments: EDCs, metals and metalloids
In total, 41 exposure measures were quantified per subject. These 41 exposures comprise a wide range of commonly encountered environmental toxicants and were chosen due to their heterogeneity.
Details on phthalate and phenol exposure assessment have been previously published by Montrose et al., 201853. Briefly, using maternal urine samples collected between 8 and 14 weeks’ gestation, 12 phthalate metabolites and 12 phenol metabolites were quantified via isotope dilution liquid chromatography and tandem mass spectrometry.
Phthalate analytes included metabolites of diethylhexyl phthalates (DEHP): mono (2-ethyl-5-carboxylpentyl) phthalate (MECPP), mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono (2-ethylhexyl) phthalate (MEHP), and mono (2-ethyl-5-oxohexyl) phthalate (MEOHP); and dibutyl phthalates (DBP): mono-isobutyl phthalate (MIBP) and mono n-butyl phthalate (MnBP). Concentrations of mono-benzyl phthalate (MBzP), mono-carboxy isononyl phthalate (mCINP), mono (3-carboxypropyl) phthalate (MCPP), mono (6-COOH-2-methylheptyl) phthalate (MCOMHP), monoethyl phthalate (MEP), and mono-isononyl phthalate (mINP) were also assessed.
Phenol analytes included parabens (butyl, ethyl, methyl, and propyl paraben [BuPB, EtPB, MePB, PrPB]), bisphenols (BPA, BPF, BPS), 2,4 and 2,5-dichlorophenol (DCP24, DCP25), benzophenone-3 (BP3), triclocarban (TCC), and triclosan (TCS).
In first trimester urine samples, 17 heavy metals and metalloids were also quantified via isotope chromatography plasma tandem mass spectrometry. These included arsenic (As), barium (Ba), beryllium (Be), cadmium (Cd), chromium (Cr), copper (Cu), mercury (Hg), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), selenium (Se), tin (Sn), thallium (Tl), uranium (U), tungsten (W), and zinc (Zn).
Concentrations below the limit of detection (LOD) were assigned a value of LOD/√2. Specific gravity of maternal urine samples to indicate urine dilution was measured via a digital handheld device (ATAGO Company, Ltd., Tokyo, Japan). Detection limit and coefficient of variation for the EDC measures has been previously addressed54.
Inflammatory biomarkers
Maternal venous blood samples at two time points (first trimester, and on admission to the hospital prior to delivery), and neonatal umbilical cord blood samples were obtained, divided into aliquots, and stored at −80 degrees Celsius. Cord blood samples were of mixed arterial and venous origin, and obtained and stored within thirty minutes of delivery. Maternal and neonatal plasma samples were then analyzed at the clinical chemistry laboratory at the Michigan Diabetes Research Center (Ann Arbor, MI) for the following inflammatory cytokines: granulocyte macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, monocyte chemotactic protein (MCP)-1, MCP-3, macrophage inflammatory protein (MIP)-1α and MIP-1β, tumor necrosis-factor (TNF)-α, vascular endothelial growth factor (VEGF), interleukin (IL)-1β, IL-6, IL-8, and IL-17α. These 12 cytokines were chosen due to their various roles in immune function. Cytokines were analyzed in duplicates and the average measurement recorded for data analysis. Cytokine levels below the limit of detection (LOD) were replaced by LOD/√2. LOD for individual cytokines was: GM-CSF 0.43 pg/ml, IFN-γ 0.12 pg/ml, MCP-1 1.10 pg/ml, MCP-3 0.24 pg/ml, MIP-1α 1.14 pg/ml, MIP-1β 0.22 pg/ml, TNFα 0.49 pg/ml, VEGF 0.48 pg/ml, IL-1β 0.06 pg/ml, IL-6 0.4 pg/ml, IL-8 0.17 pg/ml, and IL-17α 0.08 pg/ml. Coefficients of variation for individual cytokines were: GM-CSF 9.1%, IFN-γ 6.0%, MCP-1 5.1%, MCP-3 6.3%, MIP-1α 6.4%, MIP-1β 6.5%, TNFα 7.8%, VEGF 18.9%, IL-1β 7.4%, IL-6 10.4%, IL-8 6.5%, and IL-17α 10.5%.
Birth outcomes
Details on birth outcomes were abstracted from the medical record. Clinical outcomes assessed included estimated gestational age at delivery (calculated by last menstrual period or ultrasound dating), mode of delivery, infant sex, and birth weight. Details on possible confounding variables were provided by subjects at their initial study visit and confirmed in the medical record. Possible confounders included pre-pregnancy body mass index (BMI), maternal age, and history of smoking. Because only two women in our cohort developed gestational hypertension, and only one developed gestational diabetes, pregnancy conditions and complications were not included as confounders.
Statistical Analysis
To address the issue of measurements falling below the LOD, rank-based methods with ties were applied for correlation and regression analysis that is robust to either fixing truncated measurements at their upper bound or replacing them with LOD/√2.
Spearman correlation coefficients were calculated to determine the association between first trimester exposures and inflammatory cytokine markers at two time points (first trimester and delivery) in mothers and at delivery for newborns. EDC measurements were first adjusted for urine specific gravity and then log-transformed prior to correlation analysis. Each of the 12 inflammatory cytokines was tested for correlation with each of the 41 exposures, and FDR Benjamini–Hochberg (BH) correction was employed to account for multiplicity in the hypothesis testing, using FDR adjusted p-value 0.1 as the cutoff of signal calling. To account for confounding variables, including pre-pregnancy BMI, maternal age, history of smoking, mode of delivery (vaginal versus Cesarean), infant sex, and gestational age at delivery, linear regression was used to examine the association between log-transformed exposures and inflammatory markers. Linear regression was also used to test the association between inflammatory cytokine markers and two birth outcomes, infant gestational age and birthweight. For regression analyses, cytokine values were transformed using inverse normal transformation and birth outcomes were transformed using standard normal transformation. Birthweight z-score was calculated from birthweight in grams, and used as the outcome variable.
To investigate the effect of exposure mixtures on maternal and fetal inflammatory status, we conducted principal component analysis (PCA) on the 41 natural-log-transformed exposure variables. PCA was used in order to simplify the exposure data into fewer dimensions, and to summarize the data into a smaller number of principal components55. This resulted in 11 principal components (PC), each representing one type of linear combination of EDC exposure measures, collectively explaining 80% of the total variation in the data. Linear regression was used to evaluate the association between the PCA-weighted EDC exposures with maternal and fetal inflammatory cytokines, adjusted for confounding variables. PCA was also used to investigate the effect of EDC exposure mixtures on infant birth weight and gestational age at delivery. In the models with PC variables, the same inverse normal transformed cytokines and z-scores of birthweight were used, and p < 0.05 was considered significant. Statistical analysis was completed in R Software, version 3.0.
In addition to the cross-sectional data analysis of inflammatory cytokine measurements at each time point, we conducted a longitudinal analysis using a Linear Mixed Model (LMM) with random intercepts to account for temporal association between repeatedly measured concentrations of maternal cytokine levels in first trimester and at term. The LMM model, with interaction terms between time and PCA-weighted EDC exposures, analyzed both main effects of the PC-weighted EDC exposure and time, and more importantly how the association effect between the exposures and cytokines may have shifted from baseline to term.
Ethics approval and consent to participate
This study was approved by the University of Michigan IRB Committee.
Results
Subjects in the current analysis represent 56 mother-infant dyads in the MMIP cohort. Sample characteristics and infant birth outcomes are outlined in Table 1. First trimester urinary concentrations of metals, phenol and phthalate metabolites for the 56 women in this cohort have been previously detailed54. In general, women displayed an average of 30 detected EDC exposures during the first trimester, including a mean of 12.8 metals, 10.5 phthalate metabolites, and 7.7 phenols54.
Table 1.
Subject characteristics and birth outcomes (n = 56).
| N | % | Mean | Standard Deviation | Range | |
|---|---|---|---|---|---|
| Maternal age (years) | 31.9 | 3.7 | 26–40 | ||
| Maternal pre-pregnancy weight (kg) | 70.1 | 15.8 | 51–135 | ||
| Gravidity | 2.11 | 1.02 | 1–5 | ||
| Parity | 1.29 | 1.06 | 0–4 | ||
| Married | 56 | 100 | |||
| White Race | 56 | 100 | |||
| Current Smoker | 0 | 0 | |||
| Former Smoker | 6 | 10.7 | |||
| Education | |||||
| High school | 4 | 7.1 | |||
| Some College | 34 | 60.7 | |||
| College Graduate | 18 | 32.1 | |||
| Income ($USD) | |||||
| <49,000 | 8 | 14.3 | |||
| 50,000–100,000 | 22 | 39.3 | |||
| >100,000 | 26 | 46.4 | |||
| Gestational age at delivery (weeks) | 39.7 | 1.02 | 37.6–41.7 | ||
| Infant birth weight (g) | 3552 | 396.6 | 2805–4685 | ||
| Mode of delivery | |||||
| Vaginal | 37 | 66 | |||
| Cesarean | 19 | 34 | |||
| Infant sex | |||||
| Female | 29 | 52 | |||
| Male | 27 | 48 | |||
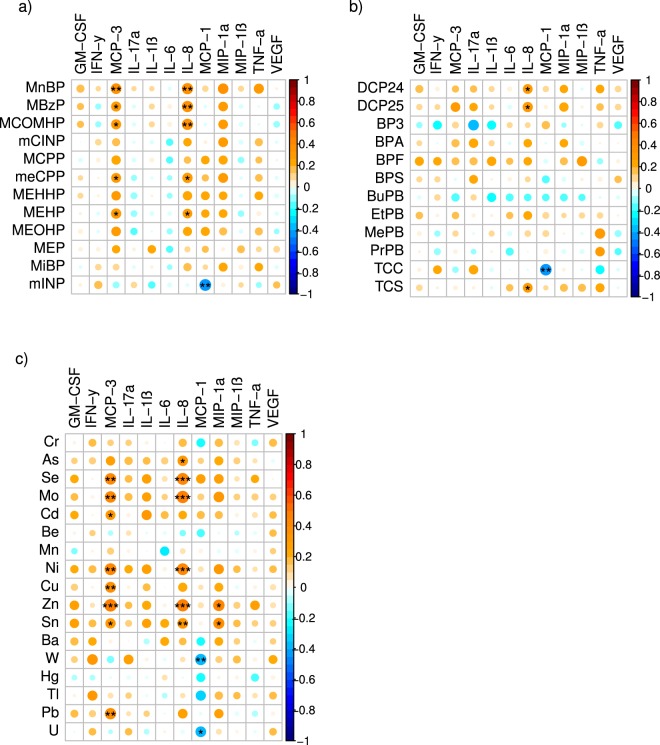
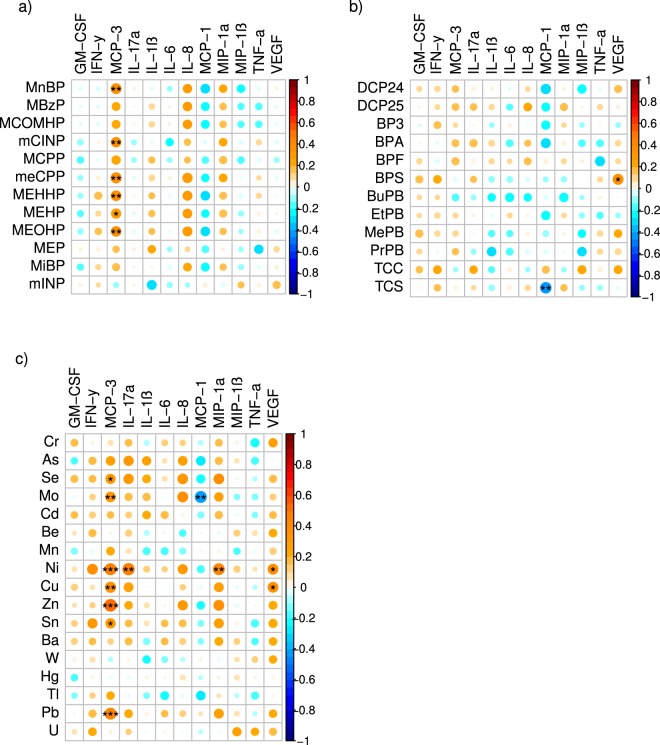
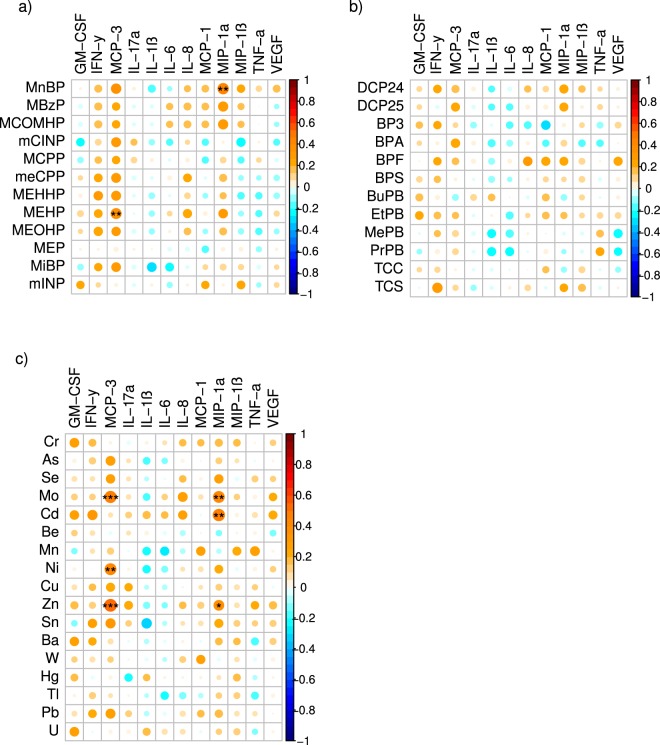
First trimester measures of several individual EDCs, particularly metals and phthalates, were independently associated with maternal first trimester and term inflammatory markers using Spearman correlation coefficients (Figs 1 and 2). Positive correlations were frequently observed between individual EDC exposures and maternal levels of MCP-3, IL-8, and MIP-1α. There were fewer observed correlations between individual EDC exposures and inflammatory markers in cord blood (Fig. 3).
Figure 1.
Spearman correlations between early pregnancy EDC exposures and first trimester inflammatory cytokines. Using the color spectrum, the orange color indicates a positive correlation, while the blue color indicates a negative correlation for cytokines with (a) phthalates, (b) phenols, and (c) metals. Circle size reflects the size of the correlation, with larger circles having correlations closer to 1 or −1. Shade of orange or blue also reflects the strength of the correlation coefficient. Significance is noted as follows: •••p < 0.01, ••p < 0.05, •p < 0.10.
Figure 2.
Spearman correlations between early pregnancy EDC exposures and term inflammatory cytokines. Using the color spectrum, the orange color indicates a positive correlation, while the blue color indicates a negative correlation for cytokines with (a) phthalates, (b) phenols, and (c) metals. Circle size reflects the size of the correlation, with larger circles having correlations closer to 1 or −1. Shade of orange or blue also reflects the strength of the correlation coefficient. Significance is noted as follows: •••p < 0.01, ••p < 0.05, •p < 0.10.
Figure 3.
Spearman correlations between early pregnancy EDC exposures and neonatal cord blood inflammatory cytokines. Using the color spectrum, the orange color indicates a positive correlation, while the blue color indicates a negative correlation for cytokines with (a) phthalates, (b) phenols, and (c) metals. Circle size reflects the size of the correlation, with larger circles having correlations closer to 1 or −1. Shade of orange or blue also reflects the strength of the correlation coefficient. Significance is noted as follows: •••p < 0.01, ••p < 0.05, •p < 0.10.
When adjusted for confounders using linear regression, individual EDCs were significantly associated with maternal IL-6 in the first trimester, and maternal MCP-1 and IL-17 at delivery. Table 2 demonstrates the directionality of these associations. The FDR Benjamini-Hochberg (BH) correction was used to control for false discoveries related to multiple comparisons, and it is standard to use a false discovery rate of 0.10. Thus, we used BH adjusted p-value < 0.10.
Table 2.
EDC associations with maternal cytokine levels in early and late pregnancy.
| Pregnancy Time Point | EDC | Category | Cytokine | Effect Size (SE) | BH-adjusted p-value |
|---|---|---|---|---|---|
| First trimester | BuPB | Phenol | IL-6 | −0.319 (0.106) | 0.097 |
| First trimester | Ba | Metal | IL-6 | 0.450 (0.150) | 0.097 |
| Term | Mo | Metal | MCP-1 | −0.859 (0.217) | 0.012 |
| Term | BPA | Phenol | MCP-1 | 0.816 (0.214) | 0.019 |
| Term | TCS | Phenol | MCP-1 | −0.509 (0.158) | 0.051 |
| Term | Ni | Metal | IL-17α | −0.265 (0.087) | 0.055 |
| Term | Se | Metal | IL-17α | 0.931 (0.310) | 0.066 |
| Term | As | Metal | IL-17α | 0.433 (0.145) | 0.066 |
| Term | Mo | Metal | IL-17α | 0.612 (0.219) | 0.075 |
| Term | Cu | Metal | IL-17α | 0.649 (0.237) | 0.075 |
Table 2 displays linear regression results for the association between EDC and maternal cytokine levels, after adjusting for confounding variables including urine dilution, maternal pre-pregnancy BMI, maternal age, history of smoking, mode of delivery, infant sex, and gestational age at delivery. Only statistically significant associations at a Benjamini-Hochberg adjusted p-value < 0.10 are displayed here. No significant associations with cord blood cytokines were observed.
Several positive correlations between inflammatory markers within each time point (first trimester, term, and post-delivery) were identified (Supplemental Fig. 2). For instance, in the first trimester, examples of significant, positive correlations include IFN-γ with IL-17, MCP-3 with IL-8, and MIP-1α with IL-8. In addition, several positive associations were observed between inflammatory marker levels in first trimester, at term, and in cord blood, with infant gestational age and birth weight at delivery (Table 3). For instance, first trimester and term levels of IL-8 were positively associated with birth weight, and cord blood VEGF was positively associated with gestational age.
Table 3.
Cytokine associations with birth outcomes.
| Pregnancy Time Point | Cytokine | Birth Outcome | Effect Size (SE) | P-value |
|---|---|---|---|---|
| First trimester | MIP-1α | Birthweight | 0.198 (0.094) | 0.043 |
| First trimester | IL-8 | Birthweight | 0.184 (0.093) | 0.056 |
| First trimester | TNF-α | Birthweight | 0.185 (0.102) | 0.077 |
| First trimester | IL-6 | Gestational age | 1.907 (1.128) | 0.098 |
| Term | IL-8 | Birthweight | 0.248 (0.097) | 0.014 |
| Term | IL-1β | Gestational age | 1.859 (1.087) | 0.094 |
| Cord blood | MIP-1α | Birthweight | 0.205 (0.094) | 0.034 |
| Cord blood | IL-1 | Birthweight | 0.174 (0.101) | 0.094 |
| Cord blood | VEGF | Gestational age | 2.182 (1.142) | 0.063 |
Table 3 displays linear regression results for the association between inflammatory cytokines and birth outcomes. Adjustments were made for confounding variables including urine dilution, maternal pre-pregnancy BMI, maternal age, history of smoking, mode of delivery, and infant sex. Only associations considered significant with p-value < 0.10 are displayed here.
Tables 4–6 demonstrate each of the specific PC groupings which were significantly associated (p < 0.05) with maternal first trimester, maternal term, and delivery cord blood inflammation, respectively.
Ten of eleven PC groupings demonstrated statistically significant associations with inflammatory cytokines. Tables 4–6 demonstrate each of the specific PC groupings which were associated with maternal first trimester, maternal term, and delivery cord blood inflammation, respectively. For interpretation of these PCA results, the color heat map (Fig. 4) demonstrates the relative contribution of each EDC to a specific PC group. For instance, in PC1, metals and phthalates are positively weighted (red color), suggesting that higher metal and phthalate levels may be positively associated with first trimester IL-8 and IFN-γ (Table 4). Similarly, phenols are predominantly negatively weighted in PC6 (blue color), and in the first trimester, this PC grouping demonstrated negative associations with IL-1β, IL-17α, VEGF, and GM-CSF levels. Because of the similar trend in directionality, this suggests that phenols may be positively correlated with these individual cytokines (Table 4). Using the same 11 PC variables, there were no significant associations between EDC mixtures with infant birth weight or gestational age at delivery.
Table 5.
Association of EDC mixtures with maternal cytokines at term.
| PC variable | Maternal cytokines (term) | |
|---|---|---|
| Positive Association | Negative Association | |
| PC 1 | IL-17α, IFNγ | MCP-1 |
| PC 2 | IL-1β | |
| PC 4 | VEGF, IL-6, IL-17α, MCP-1, GM-CSF, MIP-1α | TNFα |
| PC 5 | IL-8, MIP-1α, MCP-3 | |
| PC 9 | IL-17α, IL-1β | |
| PC 10 | IL-17α | |
| PC 11 | IL-6 | MCP-1 |
Table 4.
Association of EDC mixtures with first trimester cytokines.
| PC variable | Maternal cytokines (first trimester) | |
|---|---|---|
| Positive Association | Negative Association | |
| PC 1 | IL-8, IFNγ | |
| PC 4 | IL-6, IL-8, GM-CSF | |
| PC 5 | IL-8 | |
| PC 6 | IL-1β, IL-17α, VEGF, GM-CSF | |
| PC 11 | IL-6 | IFNγ |
Table 6.
Association of EDC mixtures with cord blood cytokines.
| PC variable | Neonatal cytokines (cord blood) | |
|---|---|---|
| Positive Association | Negative Association | |
| PC 1 | MCP-3 | |
| PC 5 | MCP-3 | |
| PC 7 | VEGF, MIP-1α, GM-CSF | |
| PC 8 | IL-8, MCP-1 | |
| PC 10 | MIP-1α, IL-8 | |
| PC 11 | IL-6, IL-1β | |
Figure 4.
Principal component loading coefficients. This color heat map shows the loading coefficients of the exposures for each of the principal components (PC), demonstrating the relative contribution of each EDC to a specific PC group. Exposures that have a higher positive weight are darker red, whereas exposures that are negatively weighted are blue. Tables 4–6 demonstrate each of the specific PC groupings which were significantly associated with maternal first trimester, maternal term, and delivery cord blood inflammation, respectively. By analyzing the components of a significant PC, one can interpret the association results. For example, in Table 4, PC1 is shown to have positive association with IL-8 and IFN- γ. From Fig. 4, PC1 is positively weighted towards metals and phthalates (red color), suggesting that higher metal and phthalate levels may be positively associated with first trimester IL-8 and IFN-γ.
In the Linear Mixed Model (LMM) longitudinal analysis, there were statistically significant interactions between time of maternal inflammatory measurement and seven of the PC groupings. These interactions indicate an altered association from baseline to term, which are not captured by the cross-sectional analyses conducted by linear regression. Table 7 demonstrates these seven groupings and the related (p < 0.05) inflammatory cytokines. The positive values indicate an increased association at term from baseline. The main effects in the LMM model also demonstrated statistically significant associations between the PC groupings and the maternal baseline inflammatory levels. As expected, these associations were similar to the cross-sectional linear regression analysis on maternal first trimester cytokines (results not shown).
Table 7.
Change in association of EDC mixtures with maternal cytokines from first trimester to term.
| PC variable | Maternal cytokines (difference from baseline to term) | |
|---|---|---|
| Positive Association | Negative Association | |
| PC 1 | MCP-1, TNFα, MIP-1β | |
| PC 2 | IL-17α, MIP-1β | |
| PC 4 | VEGF | IL-1 |
| PC 6 | IL-1, MIP-1α, MIP-1β | |
| PC 9 | IL-1 | |
| PC 10 | MIP-1α, IL-8 | |
| PC 11 | IFNγ | |
Finally, we performed comparative analyses between several subgroups, using mean concentrations (95% CI) of EDC measures and inflammatory markers: maternal obese vs. non-obese, primiparous vs. multiparous, and neonatal sex (male vs. female). However, no significant differences were identified (data not shown).
Discussion
Women in this Michigan-based birth cohort demonstrated detectable exposure to an average of 30 chemicals and metals, out of 41 possible that were measured, in the first trimester. These results are comparable to population studies such as NHANES, which demonstrated a median detectable exposure of pregnant women to 50 chemical analytes, out of 71 possible exposures15. Pregnant women in the MMIP cohort demonstrated lower urinary levels of many exposures, such as Hg (geometric mean 0.07 ug/L [95% CI 0.06–0.09] in MMIP, vs geometric mean 0.45 ug/L in a San Francisco-based pregnancy cohort) and BPA (geometric mean 0.71 [0.52–0.98] in MMIP, vs 50th percentile 2.7 ug/L in NHANES)15,56. However, some exposure measures appeared to be greater in MMIP subjects, such as TCS (geometric mean 14.5 ug/L [8.28–25.40] vs 50th% percentile 8.2 ug/L in NHANES). These wide ranges of values, both within and between cohorts, may reflect differential exposure due to geography, socioeconomic status, occupation, and other environmental factors.
Furthermore, in this sample of pregnant women and their full-term newborns, inflammatory marker levels in early gestation and the peripartum period were differentially associated with (1) individual exposures and (2) exposure mixtures. These findings suggest that not only do these environmental exposures alter the maternal-fetal inflammasome throughout pregnancy, but that the combination of exposures is of particular importance. Furthermore, several positive associations between individual inflammatory measures and two birth outcomes, infant birth weight and gestational age, were also evident.
Under normal conditions, maternal inflammation is thought to increase throughout pregnancy. One proposed rationale is that systemic inflammation induces maternal insulin resistance, which increases the availability of glucose for fetal growth57,58. For the 12 inflammatory markers measured in our study, our subjects (excluding outliers) demonstrated similar cytokine levels in the first trimester and at term (Supplemental Fig. 1). These findings differ from a recently published study of 221 pregnant women, where serum levels of IL-8 and TNF-α were significantly higher in the third trimester compared to the first trimester59. Notably, that cohort had a larger sample size with a 13.9% preterm birth rate, which contrasts with our smaller cohort of full-term pregnancies. The different patterns of IL-8 and TNF-α levels may be explained by these population differences. Furthermore, inflammatory cytokine measures were only performed on plasma samples in our study. It is possible that cytokine profiles over time would have demonstrated more pronounced changes if these measures were also performed in amniotic fluid. Amniotic fluid cytokines are thought to have an important role in parturition, including rupture of fetal membranes, cervical dilation, and uterine contractility60. For instance, one study demonstrated that in healthy pregnant women, amniotic fluid samples had higher levels of IL-6, IL-8, and MCP-1 than matched blood samples, while levels of TNF-α, IFN-γ, and VEGF were higher in serum61. Although amniotic fluid cytokines may more accurately reflect fetal-placental inflammation, the invasive nature of amniocentesis limited the availability of these samples in our study.
Our findings also suggest that maternal exposure to EDCs may contribute to perturbations in antepartum and peripartum cytokine levels in not only the pregnant mother, but also the newborn. A unique aspect of our study is that maternal inflammatory markers were related to EDCs measured in early pregnancy (8–14 weeks’ gestation), a critical window of fetal and placental development. In relation to individual exposures, increased first trimester exposure to metals and phthalates was found to be positively associated with MCP-3 and IL-8 levels, while increased exposure to metals was negatively associated with MCP-1. However, when adjusted for confounders (urine dilution, maternal pre-pregnancy BMI, maternal age, history of smoking, mode of delivery, infant sex, and gestational age at delivery), first trimester IL-6 was the only cytokine with a significant association to environmental EDCs (positively correlated with Ba, and negatively correlated with BuPB). One published study involving a Puerto Rican pregnancy cohort45 found an association between TCS exposure and increased IL-6 levels at two time points in the second trimester (16–20 weeks, and 24–28 weeks) and a similar trend between the phthalate metabolite DEHP with IL-662. In contrast, in our cohort of Caucasian women, neither TCS nor DEHP metabolites were found to be individually associated with IL-6. These differences may relate to differences in the timing of EDC and inflammatory measures, in addition to ethnic differences. Another pregnancy cohort in Boston demonstrated a link between maternal BPA and IL-6 levels at various points in pregnancy, including the first trimester63. This finding was not observed in our MMIP population, possibly due to differences in patient demographics, regional exposures, and/or statistical power.
At term, just prior to delivery, we found that maternal MCP-3 maintained positive relationships with first trimester measures of metals and phthalates. Interestingly, the correlation between MCP-3 and first trimester metal exposures was also demonstrated in umbilical cord blood, although this correlation disappeared after adjusting for confounding variables. MCP-3 attracts monocytes and macrophages but its specific role in pregnancy has not been defined64. Its relevance to maternal-fetal outcomes warrants further investigation.
There is limited data on the relationship between EDC exposure with cord blood markers, despite the fact that cord blood samples are relatively easy to obtain. Measures of cord blood exposures may be an effective proxy for maternal exposures during pregnancy, as chemicals in maternal blood are often also detected in cord blood65. To our knowledge, ours is the first study to directly compare neonatal cord blood inflammation with early trimester maternal EDC measures. Assessing maternal exposure in early pregnancy, and not at the time of delivery, allows investigation of the maternal exposome during important periods in fetal development, which is a unique feature of our study.
Use of principal component analysis (PCA) identified several notable associations between EDC mixtures and maternal/neonatal cytokine levels. These findings contribute to a growing body of literature on the impact of chemical mixtures on human health66,67. Notably, comparison of individual EDCs to various groupings of EDC mixtures demonstrated different associations with cytokine levels. The principal component (PC) group 11 in our dataset provides an excellent example of this. As evidenced in Tables 4–6, PC 11 is weighted positively with TCS and BP3, and this particular chemical grouping is positively associated with IL-6 in maternal baseline, term, and fetal cord blood. However, when assessing TCS or BP3 as isolated exposures, no relationships with IL-6 were demonstrated. These differences reinforce the principle that EDCs may act in summation, negation, or synergy with one another, and that the chemical mixtures to which humans are exposed are critically important in assessing environmental exposure risk68. In addition, the observed longitudinal changes of inflammatory biomarkers from first trimester to term, in relation to EDC mixtures, suggest that EDC exposures may alter the physiologic cytokine shifts which occur throughout pregnancy. However, the exact contribution of these combined exposures to longitudinal cytokine levels, and the clinical implications of these findings, remain unknown at this time.
In practice, there are methodologic challenges to studying mixture interactions and their physiologic effects66,67. Some chemical combinations are more prominent than others depending upon socioeconomic, occupational, dietary, behavioral, and geographic factors of the study population69,70. More research is needed to identify the most prevalent exposure mixtures among specific populations, and their cumulative impact on physiologic processes66. Although the EDC exposure assessment undertaken in this study was comprehensive, with urinary measures of 41 chemicals, in reality humans are exposed to innumerable chemicals via various routes of administration including oral, inhaled, dermal, and ingested. The complex nature of these “real life” exposures, and the challenges of quantifying them, is demonstrated in assessing environmental exposure risks in the agriculture, oil and gas industries71,72. In addition, EDCs may modulate downstream molecular and cellular pathways in a dose-independent, non-monotonic, sex-specific manner, and may be influenced by other factors such as genetics, nutrition and stress2, which may be difficult to account for.
While several epidemiologic studies demonstrate an association between EDC burden and low birth weight in humans73–76, some studies demonstrate no association25,77,78. In our 56 subjects, we only observed associations between birth weight and two exposures, MCPP (positive) and BPS (negative)54. However, associations between inflammatory cytokine levels and birth outcomes were evident. Our findings revealed higher levels of first trimester MIP-1α, IL-8, and TNF-α to be associated with an increase in infant birth weight, while first trimester IL-6 was found to be associated with an increase in gestational age. In contrast, no significant associations were found between the inflammatory milieu at the time of delivery with birth outcomes. These observations have important translational value. Aberrant inflammation in pregnancy has been associated with a spectrum of adverse outcomes related to implantation, placentation, and fetal growth, including implantation failure, recurrent pregnancy loss, preeclampsia, intrauterine growth restriction, and preterm birth47,50,79–83. A link with inflammation is particularly well-established in the preterm population84–86. In our cohort, all newborns were delivered at term, which may explain why an association between gestational age and inflammation at delivery was not observed. Further, all newborns in our cohort were of normal birth weight. Although in the original DOHaD hypothesis87, low birth weight was the primary risk factor for adult-onset disease, there is increasing evidence that developmental programming may occur in a variety of pathways, including inflammatory, endocrine, epigenetic, nutritional, oxidative stress, and/or placental88–91. Our findings of an altered inflammatory milieu in utero may still confer long-term health programming effects on offspring, even in the setting of normal birth weight.
To our knowledge, this is the first study to support an association between EDC mixtures and alterations in the maternal and neonatal inflammatory environment. Similar to other studies16,92, we demonstrate that reproductive age women are widely exposed to multiple classes of environmental chemicals. Because gestational EDC exposures may have long-term health implications for adult offspring through developmental programming, potentially due to inflammatory mechanisms, this particular area of research has significant clinical and public health implications for women of childbearing age69,70,93.
Strengths of our study include our comprehensive assessment of the association between 41 first trimester exposures, with 12 inflammatory cytokines in early pregnancy and the peripartum period. Recruitment of pregnant women at their first prenatal appointment allowed assessment of the maternal inflammasome in the first trimester of pregnancy, when fetal development is particularly sensitive to insults. Our study is limited by a small sample size and a non-diverse patient population. However, our subject homogeneity does limit the potential for additional confounding variables including race, education, and socioeconomic status. As previously mentioned, EDCs may exert their effects in a sex-specific manner94, but due to small sample size, male and female newborns were not analyzed separately. Limitations of our study design include the fact that we did not account for hemodilution or hemoconcentration of plasma samples. Furthermore, EDC exposures were only measured at a single time point in pregnancy. Exposure measures may vary throughout pregnancy, and repeated measures of EDC levels over time may better represent average prenatal exposure95,96. By only measuring EDCs in the first trimester, we were unable to assess the impact of second or third trimester exposures on inflammatory processes. Other possible confounders, which were not addressed by our statistical design, include pre-pregnancy maternal comorbidities, maternal weight gain in pregnancy, parity, medication use, vitamin or supplement intake during pregnancy, and whether labor was initiated spontaneously or via induction. Finally, although our pilot study is unable to assess mechanisms or establish causality, our findings highlight the potential association of circulating inflammatory markers in pregnancy with EDC mixtures, which warrants additional research.
Conclusions
In conclusion, inflammatory cytokine levels throughout pregnancy and at delivery may be explained by unique mixtures of first trimester environmental toxicant exposures. More research is needed to validate these findings on a larger scale and to assess their impact on a wider range of pregnancy and birth outcomes.
Supplementary information
Acknowledgements
Financial support for this study provided by: U2CES026553, RO1ES017500, PO1ES022844, P3OES017885, RD 83543601. University of Michigan (UM) NIEHS/EPA Children’s Environmental Health and Disease Prevention Center P01 ES022844/RD 83543601, NIH Children’s Health Exposure Analysis Resource (CHEAR, 1U2C ES026553), Michigan Lifestage Environmental Exposures and Disease (MLEEaD) NIEHS Core Center (P30 ES017885), NIH/NIEHS UG3 OD023285. U2CES026553, RO1ES017500, PO1ES022844, P3OES017885, RD 83543601, UG3 OD023285.
Author Contributions
A.K.: data analysis and manuscript writing. M.B.: data analysis and manuscript writing. J.G.: study design, environmental exposure measures. D.D.: cohort development, environmental exposure measures. C.B.: cohort development, study design. S.D.: patient recruitment, cohort establishment, clinical oversight. Y.S.: clinical oversight. P.S.: data analysis, statistical oversight. V.P.: conception of study, study design and oversight, cohort establishment, funding procurement, manuscript writing.
Data Availability
The data analyzed during the current study are not publicly available, but can be made available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angela S. Kelley and Margaret Banker contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41134-z.
References
- 1.Trasande L, et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology. 2016;4:565–572. doi: 10.1111/andr.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore AC, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva MJ, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenberg LN, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ. Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calafat AM, et al. Co-exposure to non-persistent organic chemicals among American pre-school aged children: A pilot study. Int. J. Hyg. Environ. Health. 2017;220:55–63. doi: 10.1016/j.ijheh.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendryx, M. & Luo, J. Children’s environmental chemical exposures in the USA, NHANES 2003–2012. Environ. Sci. Pollut. Res. Int., 10.1007/s11356-017-0874-5 (2017). [DOI] [PubMed]
- 9.Marcoccia D, Pellegrini M, Fiocchetti M, Lorenzetti S, Marino M. Food components and contaminants as (anti)androgenic molecules. Genes Nutr. 2017;12:6. doi: 10.1186/s12263-017-0555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. EXS. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJP. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 12.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ. Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grandjean P, et al. Life-Long Implications of Developmental Exposure to Environmental Stressors: New Perspectives. Endocrinology. 2015;156:3408–3415. doi: 10.1210/EN.2015-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbuckle TE, et al. Exposure to free and conjugated forms of bisphenol A and triclosan among pregnant women in the MIREC cohort. Environ. Health Perspect. 2015;123:277–284. doi: 10.1289/ehp.1408187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ. Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosofsky A, et al. Exposure to multiple chemicals in a cohort of reproductive-aged Danish women. Environ. Res. 2017;154:73–85. doi: 10.1016/j.envres.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee W-C, Fisher M, Davis K, Arbuckle TE, Sinha SK. Identification of chemical mixtures to which Canadian pregnant women are exposed: The MIREC Study. Environ. Int. 2017;99:321–330. doi: 10.1016/j.envint.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Philips EM, et al. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004-5. Environ. Res. 2018;161:562–572. doi: 10.1016/j.envres.2017.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caserta D, et al. Environment and women’s reproductive health. Hum. Reprod. Update. 2011;17:418–433. doi: 10.1093/humupd/dmq061. [DOI] [PubMed] [Google Scholar]
- 20.Rouillon, S. et al. Endocrine Disruptors and Pregnancy: Knowledge, Attitudes and Prevention Behaviors of French Women. Int. J. Environ. Res. Public Health14 (2017). [DOI] [PMC free article] [PubMed]
- 21.Veiga-Lopez A, et al. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J. Clin. Endocrinol. Metab. 2015;100:E1394–403. doi: 10.1210/jc.2015-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauritzen HB, et al. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case-cohort study. Pediatr. Res. 2017;81:33–42. doi: 10.1038/pr.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bach CC, et al. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit. Rev. Toxicol. 2015;45:53–67. doi: 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- 24.Govarts E, et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ. Health Perspect. 2012;120:162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff MS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birks L, et al. Occupational Exposure to Endocrine-Disrupting Chemicals and Birth Weight and Length of Gestation: A European Meta-Analysis. Environ. Health Perspect. 2016;124:1785–1793. doi: 10.1289/EHP208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenters V, et al. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environ. Health Perspect. 2016;124:365–372. doi: 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berends LM, Ozanne SE. Early determinants of type-2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:569–580. doi: 10.1016/j.beem.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Martin, A., Connelly, A., Bland, R. M. & Reilly, J. J. Health impact of catch-up growth in low-birth weight infants: systematic review, evidence appraisal, and meta-analysis. Matern. Child Nutr. 13 (2017). [DOI] [PMC free article] [PubMed]
- 30.Mierzynski R, et al. Intra-uterine Growth Retardation as a Risk Factor of Postnatal Metabolic Disorders. Curr. Pharm. Biotechnol. 2016;17:587–596. doi: 10.2174/1389201017666160301104323. [DOI] [PubMed] [Google Scholar]
- 31.Casas M, et al. Exposure to Bisphenol A and Phthalates during Pregnancy and Ultrasound Measures of Fetal Growth in the INMA-Sabadell Cohort. Environ. Health Perspect. 2016;124:521–528. doi: 10.1289/ehp.1409190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marie C, Vendittelli F, Sauvant-Rochat M-P. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ. Int. 2015;83:116–136. doi: 10.1016/j.envint.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 34.Heindel JJ, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberg LN, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKay, H. & Abizaid, A. A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA). Horm. Behav., 10.1016/j.yhbeh.2017.11.001 (2017). [DOI] [PubMed]
- 37.Ribeiro, E., Ladeira, C. & Viegas, S. EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics5 (2017). [DOI] [PMC free article] [PubMed]
- 38.Feron VJ, Cassee FR, Groten JP, van Vliet PW, van Zorge JA. International issues on human health effects of exposure to chemical mixtures. Environ. Health Perspect. 2002;110(Suppl 6):893–899. doi: 10.1289/ehp.02110s6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler PA, et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol. Cell. Endocrinol. 2012;355:231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Johns LE, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod. Biol. Endocrinol. 2015;13:4. doi: 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2014;147:401–409. doi: 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietert RR. Misregulated inflammation as an outcome of early-life exposure to endocrine-disrupting chemicals. Rev. Environ. Health. 2012;27:117–131. doi: 10.1515/reveh-2012-0020. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999-2006. Environ. Res. 2011;111:718–726. doi: 10.1016/j.envres.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song H, et al. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environ. Res. 2017;158:490–498. doi: 10.1016/j.envres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Watkins DJ, et al. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health. 2015;218:212–219. doi: 10.1016/j.ijheh.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zota AR, et al. Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ. Int. 2018;115:9–20. doi: 10.1016/j.envint.2018.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cotechini T, Graham CH. Aberrant maternal inflammation as a cause of pregnancy complications: A potential therapeutic target? Placenta. 2015;36:960–966. doi: 10.1016/j.placenta.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Amaral LM, Wallace K, Owens M, LaMarca B. Pathophysiology and Current Clinical Management of Preeclampsia. Curr. Hypertens. Rep. 2017;19:61. doi: 10.1007/s11906-017-0757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. doi: 10.1136/bmj.j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyle AK, Rinaldi SF, Norman JE, Stock SJ. Preterm birth: Inflammation, fetal injury and treatment strategies. J. Reprod. Immunol. 2017;119:62–66. doi: 10.1016/j.jri.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Walker CL. Minireview: Epigenomic Plasticity and Vulnerability to EDC Exposures. Mol. Endocrinol. 2016;30:848–855. doi: 10.1210/me.2016-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: A preliminary analysis. Reprod. Toxicol. 2016;65:59–66. doi: 10.1016/j.reprotox.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montrose L, et al. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics. 2018;13:301–309. doi: 10.1080/15592294.2018.1448680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodrich J. M. et al. First trimester maternal exposures to endocrine disrupting chemicals and metals and fetal size in the Michigan Mother Infant Pairs Study. J DOHaD. In press (2018). [DOI] [PMC free article] [PubMed]
- 55.Lever J, Krzywinski M, Altman N. Principal component analysis. Nat. Methods. 2017;14:641. [Google Scholar]
- 56.Morello-Frosch R, et al. Environmental Chemicals in an Urban Population of Pregnant Women and Their Newborns from San Francisco. Environ. Sci. Technol. 2016;50:12464–12472. doi: 10.1021/acs.est.6b03492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Redman CWG, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response–a review. Placenta. 2003;24(Suppl A):S21–7. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 59.Ashford KB, et al. Patterns of Systemic and Cervicovaginal Fluid Inflammatory Cytokines throughout Pregnancy. Am. J. Perinatol. 2018;35:455–462. doi: 10.1055/s-0037-1608677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 61.Chow SSW, et al. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine. 2008;44:78–84. doi: 10.1016/j.cyto.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Ferguson KK, et al. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ. Sci. Technol. 2014;48:7018–7025. doi: 10.1021/es502076j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, Meeker JD. Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reprod. Toxicol. 2016;66:93–98. doi: 10.1016/j.reprotox.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu LL, et al. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur. J. Immunol. 1995;25:2612–2617. doi: 10.1002/eji.1830250931. [DOI] [PubMed] [Google Scholar]
- 65.Aylward LL, et al. Relationships of chemical concentrations in maternal and cord blood: a review of available data. J. Toxicol. Environ. Health B Crit. Rev. 2014;17:175–203. doi: 10.1080/10937404.2014.884956. [DOI] [PubMed] [Google Scholar]
- 66.Braun JM, Gennings C, Hauser R, Webster TF. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ. Health Perspect. 2016;124:A6–9. doi: 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rider CV, Carlin DJ, Devito MJ, Thompson CL, Walker NJ. Mixtures research at NIEHS: an evolving program. Toxicology. 2013;313:94–102. doi: 10.1016/j.tox.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carpenter DO, Arcaro K, Spink DC. Understanding the human health effects of chemical mixtures. Environ. Health Perspect. 2002;110(Suppl 1):25–42. doi: 10.1289/ehp.02110s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang A, Padula A, Sirota M, Woodruff TJ. Environmental influences on reproductive health: the importance of chemical exposures. Fertil. Steril. 2016;106:905–929. doi: 10.1016/j.fertnstert.2016.07.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Renzo GC, et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int. J. Gynaecol. Obstet. 2015;131:219–225. doi: 10.1016/j.ijgo.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kassotis CD, Tillitt DE, Lin C-H, McElroy JA, Nagel SC. Endocrine-Disrupting Chemicals and Oil and Natural Gas Operations: Potential Environmental Contamination and Recommendations to Assess Complex Environmental Mixtures. Environ. Health Perspect. 2016;124:256–264. doi: 10.1289/ehp.1409535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans NP, et al. Reproduction Symposium: does grazing on biosolids-treated pasture pose a pathophysiological risk associated with increased exposure to endocrine disrupting compounds? J. Anim. Sci. 2014;92:3185–3198. doi: 10.2527/jas.2014-7763. [DOI] [PubMed] [Google Scholar]
- 73.Baibergenova A, Kudyakov R, Zdeb M, Carpenter DO. Low birth weight and residential proximity to PCB-contaminated waste sites. Environ. Health Perspect. 2003;111:1352–1357. doi: 10.1289/ehp.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Washino N, et al. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Health Perspect. 2009;117:660–667. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dadvand P, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ. Health Perspect. 2013;121:267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padmanabhan V, et al. Maternal bisphenol-A levels at delivery: a looming problem? J. Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woods MM, Lanphear BP, Braun JM, McCandless LC. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environ. Health. 2017;16:115. doi: 10.1186/s12940-017-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clin. Rev. Allergy Immunol. 2017;53:40–53. doi: 10.1007/s12016-016-8596-x. [DOI] [PubMed] [Google Scholar]
- 80.Kim CJ, Romero R, Chaemsaithong P, Kim J-S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015;213:S53–69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LaMarca B, et al. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;311:R1–9. doi: 10.1152/ajpregu.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Makhseed M, et al. Circulating cytokines and CD30 in normal human pregnancy and recurrent spontaneous abortions. Hum. Reprod. 2000;15:2011–2017. doi: 10.1093/humrep/15.9.2011. [DOI] [PubMed] [Google Scholar]
- 83.Peraçoli JC, Rudge MVC, Peraçoli MTS. Tumor necrosis factor-alpha in gestation and puerperium of women with gestational hypertension and pre-eclampsia. Am. J. Reprod. Immunol. 2007;57:177–185. doi: 10.1111/j.1600-0897.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 84.Keelan JA, et al. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24(Suppl A):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 85.Wei S-Q, Fraser W, Luo Z-C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet. Gynecol. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 86.Di Renzo, G. C., Tosto, V. & Giardina, I. The biological basis and prevention of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol, 10.1016/j.bpobgyn.2018.01.022 (2018). [DOI] [PubMed]
- 87.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental Programming, a Pathway to Disease. Endocrinology. 2016;157:1328–1340. doi: 10.1210/en.2016-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burton GJ, Fowden AL, Thornburg KL. Placental Origins of Chronic Disease. Physiol. Rev. 2016;96:1509–1565. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Segovia, S. A., Vickers, M. H. & Reynolds, C. M. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J. Dev. Orig. Health Dis. 1–12 (2017). [DOI] [PubMed]
- 91.Fleming TP, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391:1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agay-Shay K, et al. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ. Health Perspect. 2015;123:1030–1037. doi: 10.1289/ehp.1409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robinson O, Vrijheid M. The Pregnancy Exposome. Curr Environ Health Rep. 2015;2:204–213. doi: 10.1007/s40572-015-0043-2. [DOI] [PubMed] [Google Scholar]
- 94.Rosenfeld CS. Sex-Specific Placental Responses in Fetal Development. Endocrinology. 2015;156:3422–3434. doi: 10.1210/en.2015-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y-H, Ferguson KK, Meeker JD, McElrath TF, Mukherjee B. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ. Health. 2015;14:9. doi: 10.1186/1476-069X-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferguson KK, McElrath TF, Ko Y-A, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int. 2014;70:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study are not publicly available, but can be made available from the corresponding author on reasonable request.