Abstract
Methanolic, ethanolic and ethyl acetate extracts of five aonla varieties were analyzed for phytochemical characterization using RP-HPLC. Five compounds viz. ascorbic acid, gallic acid, ellagic acid, ethyl gallate and quercetin were identified in aonla extracts by RP-HPLC at 270 nm. Significant variations were observed in amount of identified phytochemical among the varieties. The highest level of phytochemical was observed in methanolic extract of variety Desi followed by varieties Kanchan, NA-7, Banarasi and Chakaiya. Among the solvent, methanol extracted the maximum phytochemicals while yield was least in ethyl acetate extract of aonla varieties. Gallic acid and ellagic acid were the most abundant phenolic compounds in extracts of aonla varieties. Ellagic acid (349.51 mg/100 g) and gallic acid (233.49 mg/100 g) were found maximum in methanolic extract of Desi variety. Results of the present study suggested that aonla is a good source of phenolic and flavonoid compounds.
Keywords: Quantification, HPLC, Phytochemical, Solvent, Characterization
Introduction
Recently, there has been visible spurt in number of studies aimed at characterizing the health promoting attributes of phenolic compounds exhibiting antioxidant and antimicrobial properties (Mradu et al. 2012). Phenolic phytochemicals are major and most extensively distributed category in plant kingdom out of three main groups: flavonoids, phenolic acid and polyphenols (Harborne 1973). Phenolic acid consist of two main acid named hydroxyl benzoic acid (ellagic and gallic acid) and hydroxycinnamic acid (Mass et al. 1991). Flavonoids are low molecular weight compound and generally bound to sugar group (King and Young 1999). Nowadays, main focus of researcher is shifting on screening and analyzing plants for the presence of compounds having medicinal properties. Various remedial potential along with antimutagenic and anticarcinogenic properties is exhibited by secondary metabolites synthesized in higher plants. Fruits and vegetables are good source of phenolic compounds (Singh et al. 2016).
Aonla, a member of family Eubhorbiaceae, also known as Indian gooseberry is widely distributed in tropical and subtropical areas of India, Indonesia, China and Malay Peninsula. Aonla is an important part of Indian medicine along with Chinese and Tibetan medicine system (Ayurvedic Formulation of India-Part-1 2003). Phenolic and flavonoid compounds reported to have radical scavenging activity, analgesic, anti-inflammatory and inmmuno-modulatory properties and these might be due to the presence of gallic acid, ellagic acid, tannic acid, quercetin etc. Aqueous extract of aonla showed anti-pyretic and tonic properties and also have antibacterial activity (Vinayagamoothy 1982). Aonla is proved to have nutraceutical properties which will increase popularity of fruit among literate population. Phenolic compound in aonla reduces loss of ascorbic acid due to oxidation during heating and at low temperature storage (Sachan et al. 2013).
In recent era, research regarding extraction of phenolic compounds from plant sources has attracted special interest (Pinelo et al. 2004). Extraction is an important step in isolation, identification and quantification of phenolics. There exists no single standard method of extraction (Ignat et al. 2011). Successful estimation of phytochemical from plant sources mainly depends on type of solvent used for extraction and polarity of solvent also play important role in extraction of bio-active constituents (Chiste and Benassi 2011). Phytochemicals are generally extracted from plant sources using methanol, ethanol, water and various combinations of alcohol and water. Extraction and separation of free and bound phenolic is complicated due to similarity in chemical nature of the compounds and also their standards are difficult to acquire (Filipiak-Szok et al. 2012).
From ancient time, different parts of medicinal plants consisted of phenolic, were used in treatment of various diseases. However, preclinical study is must for assessment of medicinal, phytochemical, toxic and biological attributes of any herbal drug before it’s clinical use. So, systematic qualitative and quantitative analysis of phenolic compound present in plant by scientific methodology and it’s comparison with standard of phenolic is required for establishing it’s effectiveness. The main aim of present investigation was to extract, identify and quantify the main phytochemical present in aonla using high performance liquid chromatography (HPLC) with gradient elusion system in mobile phase.
Materials and methods
Phytochemical extraction
Extraction was carried out using three different solvent
Methanolic extract
Extraction was done by the method of Liu et al. (2008) with some modification. Ten gram fresh aonla flesh was extracted with 100 ml methanol in conical flask using shaker at 25 °C and filtered through 0.45 µm filter paper. Residue was extracted twice with 100 ml methanol as mentioned above. Then combined extract was concentrated at 40 °C in rotary evaporator at low pressure. Finally the residue was lyophilised and stored at 4 °C till further use.
Ethanolic extract
Method of Ahmad et al. (1998) was adopted for preparation of extract with some modifications. Ten gram fresh aonla fruit was taken in conical flask and 100 ml of 95% ethanol was used for the extraction. The extract was filtered through 0.45 µm nylon filter paper and filtrate was concentrated in rotary evaporator at 40 °C after that sample was lyophilised and stored at 4 °C until use.
Ethyl acetate extract
Extraction was done using the method of Yokozawa et al. (2007) with modification. Ten gram fresh aonla flesh was extracted with 100 ml 80% ethyl acetate in conical flask using shaker at 25 °C and filtered through 0.45 µm filter paper. Residue was extracted twice with 100 ml solvent as mentioned above. Final extract was evaporated at 40 °C using rotary evaporator at low pressure. Concentrate was lyophilised and stored under refrigeration condition till further use.
Total polyphenol estimation of extracts
Total polyphenol content of the extract was determined by the method of Anesini et al. (2008) with some modification in the extraction protocol. One ml of the above prepared extract was used for the estimation of total polyphenol content.
Fourier transform infrared spectroscopy (FTIR)
Fourier Transform Infrared spectroscopy is a technique which is used to obtain infrared spectrum of absorption, Raman scattering, emission of a solid, liquid or gas. It simultaneously collects spectral data in broad spectrum range. Dried extracts of aonla varieties were studied by Fourier Transform Infrared spectroscopy. IR-spectral studies were carried out on Shimadzu IR affinity-I 8000 FT-IR spectrometer under dry air at room temperature using KBr pellets. One mg of samples were mixed with approx 300 mg KBr and formulated in the form of tablets. The samples were pressed directly on to attenuated reflectance KBr crystal into sampling unit. Spectra of the samples are recorded in the range 4000 – 400 cm−1 at 4 cm−1 and signal averaged over 32 scans.
Phytochemical characterization
Phytochemical characterization of different aonla extracts was done with the help of HPLC (high performance liquid chromatography) using method of Sawant et al. (2011) with some modification. HPLC (model: Waters e2695, e-alliance) having gradient elution system with C-18 reverse phase column: (Sunfire) having dimension (mm) as 250 × 4.6 and particle size as 5 µ and PDA detector (Waters 2998) and autosampler was used for qualitative and quantitative analysis of phytochemical present in the extracts of fresh aonla fruits. Extract were dissolved in methanol and filtered through 0.45 µm nylon syringe filter (Axiva). Twenty micro-litre of the sample extract was injected into HPLC with the help of auto sampler. Ten mg of each standard was dissolved in 100 ml methanol so as to prepare 100 ppm standard stock solution and 20 µl was injected into HPLC. The mobile phase was consisted of A: methanol:water:phosphoric acid (5:95:0.1, v/v/v), B: acetonitrile and eluted at a flow rate of 1.5 ml/min. A gradient program was performed by varying the portion of solvent A to solvent B (0–5 min: 100% A, 5–20 min: 85% A, 15% B, 20–30 min: 75% A, 25% B, 30–45 min: 70% A, 30% B, 45–50 min: 75% A, 25% B, 50–55 min: 80% A, 20% B, 55–60 min: 100% A) and detection was carried out at wavelength 270 nm. Retention time of the standards was used for the identification of different component in the extracts and area under respective peak was used for quantitative analysis.
Results and discussion
Extraction of phytochemicals
Quantification of naturally occurring bioactive compounds by modern analytical technique is required for establishment of authenticity, creditability, recommendation and end use. Present study was focused on identification and quantification of phytochemical present in different aonla varieties. Extraction of phytochemical in aonla fruit was carried out with different solvents (methanol, ethanol and ethyl acetate) and yield of extracts varied significantly (p < 0.05). As depicted in Table 1, yield of methanol extract was found maximum followed by ethanol and ethyl acetate extracts. Results of present study showed that methanol was the best solvent for extraction of phytochemicals. Similar trend was observed for extraction yield in Tamarindus indica L. using solvents: methanol, ethyl acetate and hexane by Razali et al. (2012). Results of present study clearly indicate that type of solvent influenced extraction protocol. Methanol was noted to be most effective solvent for extraction of total polyphenol content (TPC) followed by ethanol and ethyl acetate that might be due to difference in polarity of solvents. Compatibility between solvent and compound to be extracted affect the extraction rate (Teh et al. 2014; Filipiak-Szok et al. 2012). Total polyphenolic content in aonla extracts was estimated by Folin-Ciocalteu (FC) reagent and HPLC method. Methanol extract of aonla fruits showed significantly (p < 0.05) higher amount of TPC by HPLC as well as FC reagent method compared to ethanol and ethyl acetate extract. Total polyphenol content varied from 375.98 to 752 mg/100 g in methanol extract of aonla varieties by HPLC method (Table 2) and 109.8 to 159.4 mg/g by FC reagent method (Table 2). TPC in ethanol extract ranged from 324.64 to 533.29 mg/100 g by HPLC method (Table 2) and 104.1 to 139 mg/g (Table 2) by FC reagent method. Similarly, in ethyl acetate extract of aonla varieties TPC ranged from 104.46 to 299.85 mg/100 g (Table 2) by HPLC method and 70.6 to 89.3 mg/g (Table 2) by FC reagent method. Amount of TPC estimated by FC reagent method was significantly higher than that evaluated by HPLC method and that might be due to reactivity of FC reagent additionally with non-phenolic reducing compounds such as amino-acids, tertiary amine containing biological buffers, purines, hydroxylamine, some organic and inorganic reducing agents due to which TPC were over estimated by FC reagent method (Ikawa et al. 2003). Among the varieties, Desi variety showed the highest yield in all solvents followed by varieties Kanchan, NA-7, Banarasi and Chakaiya. Study by Singh et al. (2018) reported the presence of phenolic compounds in hydroalcoholic extract of aonla fruits.
Table 1.
Extraction of aonla phytochemicals using different solvents
| Variety/extract | Extraction yield (%) | ||
|---|---|---|---|
| Methanol | Ethanol | Ethyl acetate | |
| Desi | 1.69 ± 0.02c | 1.12 ± 0.03c | 0.88 ± 0.01c |
| Kanchan | 1.55 ± 0.01bc | 1.08 ± 0.02b | 0.82 ± 0.04ab |
| Chakaiya | 1.43 ± 0.03a | 1.02 ± 0.04a | 0.79 ± 0.03a |
| NA-7 | 1.53 ± 0.04b | 1.09 ± 0.01b | 0.84 ± 0.01b |
| Banarasi | 1.49 ± 0.02ab | 1.06 ± 0.05ab | 0.85 ± 0.02bc |
aThe values are mean ± SD of determination made in triplicates. Mean values followed by different letters within a same column differ significantly (p > 0.05)
Table 2.
Quantification of phytochemicals in aonla varieties using RP-HPLC at 270 nm
| Variety | Extract | Ascorbic acid (mg/100 g) | Gallic acid (mg/100 g) | Ethyl gallate (mg/100 g) | Ellagic acid (mg/100 g) | Quercetin (mg/100 g) | Total polyphenol content (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Desi | Methanol | 167.19 ± 1.39g | 233.49 ± 1.59j | 0.98 ± 0.01b | 349.51 ± 1.63k | 1.48 ± 0.03j | 752.65 |
| Kanchan | 126.42 ± 2.09e | 199.05 ± 0.90i | 0.27 ± 0.03a | 167.41 ± 1.29j | 0.55 ± 0.01e | 493.7 | |
| Chakaiya | 136.48 ± 1.13f | 83.74 ± 0.79g | 0.33 ± 0.02a | 155.16 ± 0.79i | 0.27 ± 0.01ab | 375.98 | |
| NA-7 | 206.75 ± 1.30i | 154.77 ± 1.29h | 0.39 ± 0.03a | 113.35 ± 0.89g | 1.94 ± 0.06k | 477.2 | |
| Banarasi | 178.96 ± 1.02h | 87.68 ± 0.83g | 0.33 ± 0.01a | 124.11 ± 1.07h | 0.34 ± 0.02c | 391.42 | |
| Desi | Ethanol | 375.97 ± 1.99m | 52.55 ± 0.34de | 41.96 ± 0.49f | 61.62 ± 0.58d | 1.19 ± 0.02i | 533.29 |
| Kanchan | 293.51 ± 1.08k | 46.72 ± 0.28c | 2.99 ± 0.14d | 33.98 ± 0.34a | 0.87 ± 0.03h | 378.07 | |
| Chakaiya | 228.25 ± 1.04j | 43.83 ± 0.75c | 1.76 ± 0.02c | 50.22 ± 0.34c | 0.56 ± 0.02e | 324.62 | |
| NA-7 | 342.96 ± 1.60l | 48.46 ± 0.50cd | 5.97 ± 0.02e | 38.70 ± 0.63ab | 0.24 ± 0.03a | 436.33 | |
| Banarasi | 224.82 ± 0.53j | 64.90 ± 1.02f | 2.92 ± 0.08d | 43.61 ± 0.05b | 0.47 ± 0.03d | 336.72 | |
| Desi | Ethyl acetate | 122.47 ± 1.03e | 83.16 ± 0.50g | 1.04 ± 0.01b | 92.44 ± 0.35j | 0.74 ± 0.02g | 299.85 |
| Kanchan | 53.55 ± 1.06c | 53.40 ± 0.45e | 0.24 ± 0.01a | 41.84 ± 0.50b | 0.32 ± 0.01bc | 149.35 | |
| Chakaiya | 65.82 ± 0.60d | 21.00 ± 0.08a | 1.77 ± 0.03c | 69.54 ± 0.30e | 0.34 ± 0.03c | 158.47 | |
| NA-7 | 29.89 ± 0.02a | 29.30 ± 0.01b | 0.26 ± 0.02a | 44.52 ± 0.15bc | 0.49 ± 0.01d | 104.46 | |
| Banarasi | 36.99 ± 0.03b | 67.57 ± 0.12j | 0.53 ± 0.02a | 58.57 ± 0.06d | 0.64 ± 0.03f | 164.3 |
aThe values are mean ± SD of determination made in triplicates. Mean values followed by different letters within a same column differ significantly (p > 0.05)
Phytochemical identification
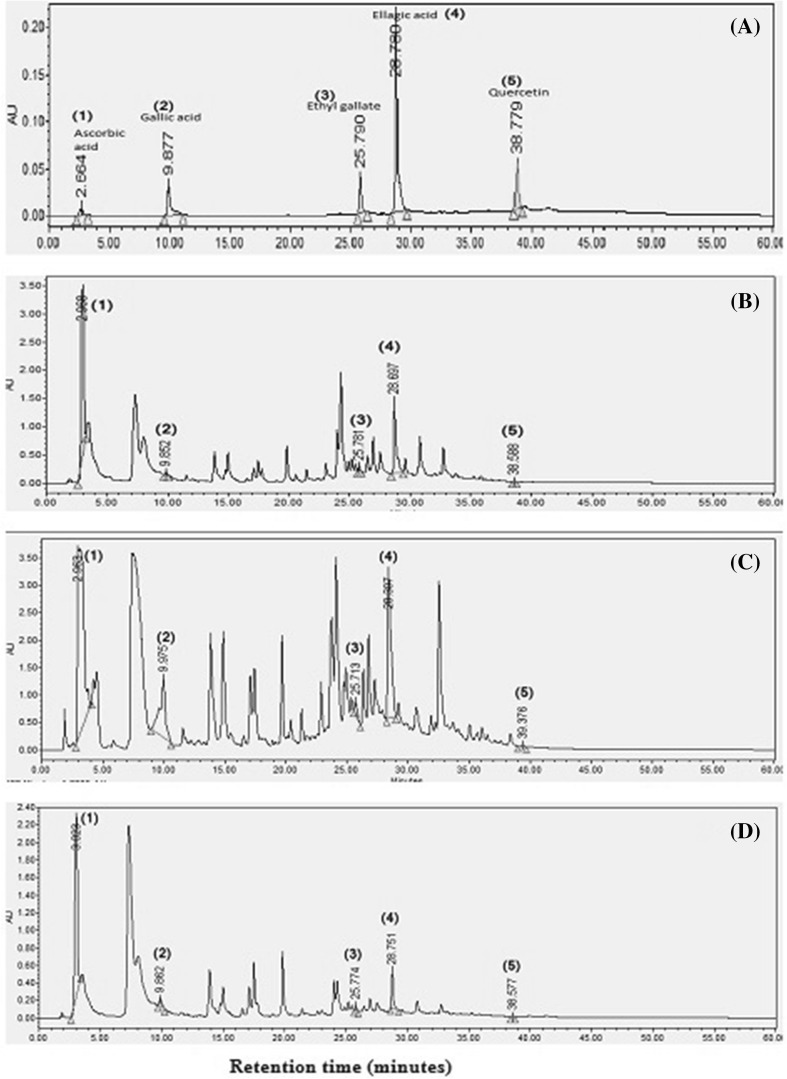
Phytochemicals are the secondary metabolites naturally occurring in plants and reported to have various therapeutic properties (Krishnaiah et al. 2009). Plants show variation in content and structure of phenolic compounds. Therefore, identification, quantification and characterization of phytochemical is required for the application of their extracts as food additives (Maisuthisakul et al. 2007). RP-HPLC chromatogram for methanol, ethanol and ethyl acetate extract from aonla using PDA detector are presented in Fig. 1b, c and d respectively while chromatogram of different standards is shown in Fig. 1a Different peaks of interest on chromatograms were identified using retention time of reference standard. Due the complex nature of natural/crude extracts and unavailability of standards, identification and estimation of each peak on chromatogram was not possible. A total of five compounds were identified in aonla varieties. The compounds identified in aonla extracts by retention time (minutes) of respective standard using RP-HPLC at 270 nm were: ascorbic acid (2.66), gallic acid (9.87), ellagic acid (25.79), ethyl gallate (28.78) and quercetin (38.77). Five identified compound were detected in different extracts of aonla varieties. Peak 1 on the chromatogram was identified as ascorbic acid having retention time 2.8 comparable to the retention of standard ascorbic acid. Similarly, peaks 2, 3, 4, 5 on the chromatogram were identified as gallic acid, ethyl gallate, ellagic acid and as quercetin with retention time 10.4, 26.2, 28.8 and 39.2 respectively by comparing retention time with that of respective standard. Results of present study were in accordance to data reported by Yokozawa et al. (2007) in HPLC profiling of ethyl acetate extract of aonla. Identification of five compounds was also confirmed using FTIR.
Fig. 1.
RP-HPLC chromatogram for standards and different aonla extracts detected at 270 nm. a = standard, b = ethyl acetate extract, c = methanol extract and d = ethanol extract
Fourier transform infra red spectroscopy analysis of aonla extracts
FTIR spectroscopy was performed to obtain finger prints of five aonla varieties. Figure 2 presents the FTIR spectra of extracts of aonla varieties. Presence of peak at 3295, 1726, 1617.96, 1538, 1239, 1107, 1059 and 864.33 cm−1 indicated presence of gallic acid in aonla (Nirmaladevi et al. 2010). Peak present around 1092 and 1663 cm−1 represents aromatic bending and stretching (Heo et al. 2013) that was observed in slightly different location at i.e., 1622.37 cm−1 and 1058.55 cm−1 confirms the presence of quercetin in aonla. In FTIR spectra of aonla –OH (hydroxyl) stretch at 3240 cm−1 is shifted to 3295 cm−1, C–O (carbonyl group) alcohol stretch at 1038 cm−1 is shifted to 1060 cm−1, the acidic –C–O stretch at 1234.35 cm−1 is shifted to 1302 cm−1, C=0 stretch at 1728 cm−1 is shifted to 1725.97 cm−1 and =CH stretch at 694.32 cm−1 is shifted to 696.50 cm−1. Presence of higher peak intensity of functional group confirmed the higher concentration of indentified different phenolic and flavonoid group in aonla. (Fig. 3)
Fig. 2.
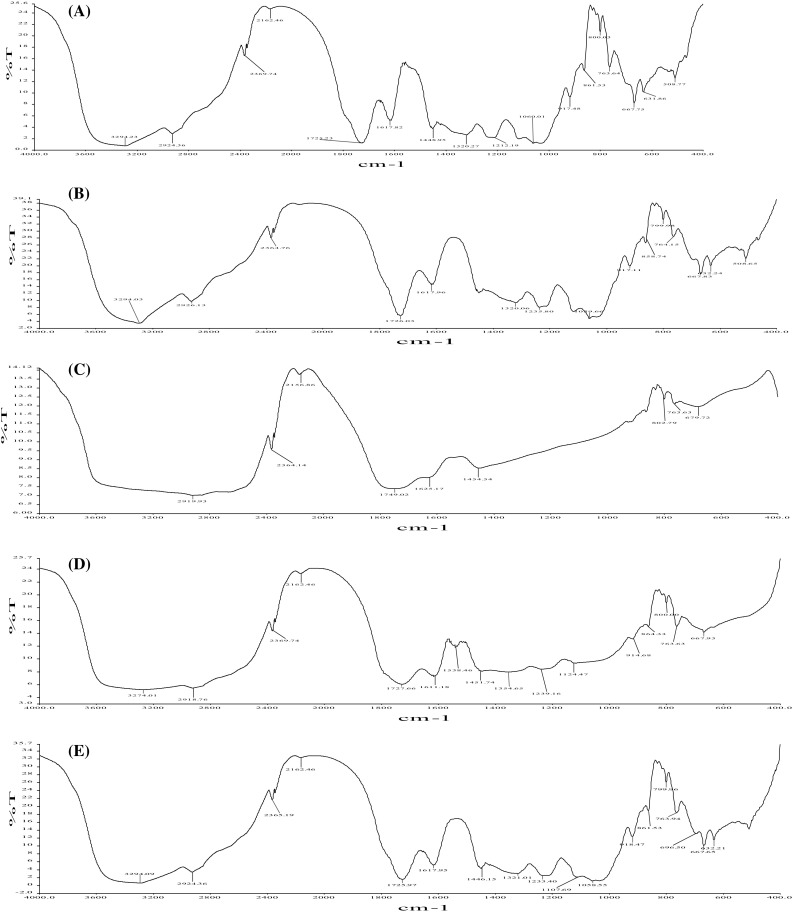
FTIR spectra of extracted phytochemicals of aonla varieties (a = Banarasi, b = Chakaiya, c = Desi, d = Kanchan and e = NA-7)
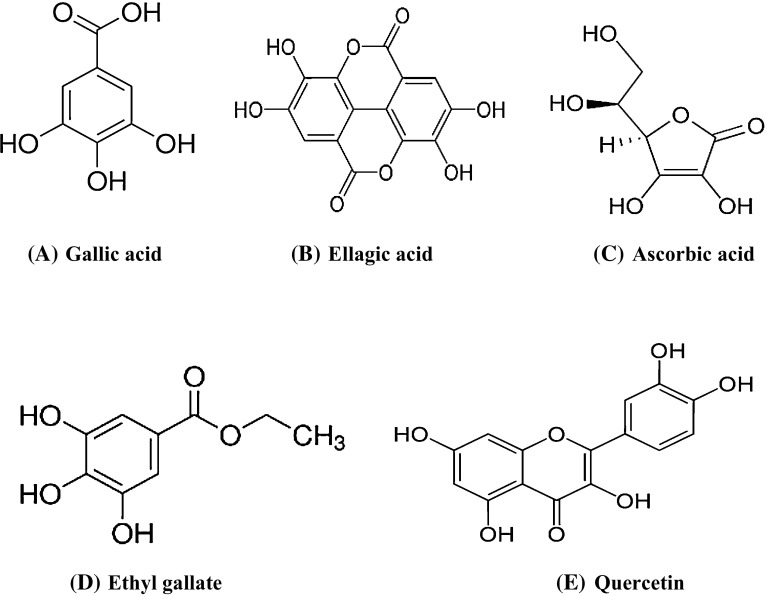
Fig. 3.
Structure of phytochemicals present in aonla: a Ascorbic acid, b Gallic acid, c Ethyl gallte, d Ellagic acid and e Quercetin
Phytochemical quantification
Phenolic compounds, ubiquitous in plant sources, are one of the most widely distributed class of phytochemicals. In recent years, owing to bioactivity phenolic compounds are attaining popularity among the researchers (Abu-Reidah et al. 2012). Fruits and vegetables are rich source of flavonoids, which exhibit various health promoting properties (Lila and Raskin 2005). Quantification of identified compound was performed based on external standard of known concentration using RP-HPLC. Area under peak of compound was measured and compared to peak area of respective standards. Data pertaining to quantity of phytochemical found in different aonla varieties and their extract is presented in Table 2. Significant (p < 0.05) variation was observed in phenolic content in different aonla extracts. Total phytochemicals were extracted maximum in methanol extract followed by ethanol and ethyl acetate extract. Ascorbic acid content varied from 29.89 to 375.97 mg/100 g among extracts of aonla varieties. A significant (p < 0.05) varietal difference was observed among aonla varieties. Ethanol solvent was the most efficient solvent for extracting ascorbic acid followed by methanol and ethyl acetate. Maximum ascorbic acid content was noted in ethanol extract of Desi variety (375.97 mg/100 g). Similarly, Nambiar et al. (2015) reported 220.183 mg/100 g ascorbic acid content in methanol extract of aonla pulp. Data observed in present study was supported by data reported by Raghu et al. (2007) in fresh aonla fruit (236.2 mg/100 g) studied using HPLC. Aqueous aonla extract was found to have 32.5 mg/g of ascorbic acid estimated using HPLC (Khopde et al. 2001). Among the phenolic compounds, ellagic acid was present in the highest amount followed by gallic acid and ethyl gallate (Table 2). Quantity of quercetin (flavonoid) was least compared to all phenolic compounds. In aonla extract, content of gallic acid varied from 21 to 233.49 mg/100 g. As depicted in Table 2 gallic acid content was observed the highest in methanol extract followed by ethanol and ethyl acetate irrespective of aonla variety.
Nambiar et al. (2015) observed 10.0078 µg/mg of gallic acid and 9.609 µg/mg of quercetin content in methanol extract of aonla pulp. Ethyl acetate extract of aonla was reported to have 5.847% gallic acid and 1.603% ellagic acid (Yokozawa et al. 2007). Study by Nampoothiri et al. (2011) confirmed presence of gallic acid, ellagic acid and ascorbic acid in methanol, ethyl acetate and hexane extract of aonla using LC–MS. Study by Kumar et al. (2006) reported gallic and tannic acid to be prominent antioxidant in phenolic fraction of aonla extract. Ellagic acid present in different fruit and vegetables significantly increased effectiveness of quercetin in reducing proliferation and inducing apoptosis (Mertens-Talcott et al. 2003). Ellagic acid was major phenolic, present in aonla extracts with varied concentration from 33.98 to 349.51 mg/100 g (Table 2). Study by Singh et al. (2017) showed that maximum extraction of GA in acetone as solvent followed by methanol, ethanol and water.
Kanchan, Chakaiya, Banarasi and NA-7. Ethyl gallate content determined in different aonla extracts ranged from 0.24 to 41.96 mg/100 g among the aonla varieties. Maximum ethyl gallate content was noted in ethanol extract of Desi variety. Sawant et al. (2011) studied phytoconstituent of methanolic extract of fresh aonla fruit and observed ascorbic acid (2.15%), gallic acid (0.44%), Ethyl gallate (0.14) and ellagic acid (0.15%). Flavonoid quercetin content varied from 0.24 to 1.94 mg/100 g among aonla extracts of different varieties (Table 2). As given in Table 2, methanol extract of variety NA-7 showed maximum quercetin content followed by Desi, Kanchan, Banarasi and Chakaiya. Similarly, study by Filipiak-Szok et al. (2012) reported 0.87–0.93 mg/100 g quercetin in ethanolic extract of aonla using HPLC–DAD.
Inhibitory activity of DNA, RNA and protein synthesis was shown by quercetin in NY68-infected chick embryo fibroblast (Jullien et al, 1984). Bansal et al. (2015) studied the phenolic and flavonoid compound present in aonla juice using RP-HPLC. Result of study reported ascorbic acid (1039–1066 mg/100 ml), gallic acid (26.94–37.95 mg/100 ml), ellagic acid (69.81–71.20 mg/100 ml) and quercetin (2.01–2.40 mg/100 ml) in aonla juice. As depicted in Table 2, extractability of various phenolic and flavonoid compounds in extracts depend on solvent polarity i.e. higher the polarity of solvent, higher the extraction of phenolic compounds. On the other hand, lower the polarity of solvent used, higher will be the extraction of organic compound (less polar in nature) (Chiste and Benassi 2011).
Conclusion
Results of present study revealed that aonla fruit is a potential source of phenolic and flavonoid compounds. Overall, methanol was the most effective solvent for extraction of phenolic and flavonoid. Among varieties, Desi variety had the highest phytochemical content irrespective of type of solvent used for extraction. Gallic acid, ellagic acid, ethyl gallate, ascorbic acid and quercetin were identified as major phytochemical in aonla extract using RP-HPLC. FTIR spectra of aonla confirmed the presence of the identified phytochemicals. Further studies are required for isolation and identification of more bioactive phytochemicals from crude extract of aonla fruits that will help in better understanding of aonla fruit as potential source of phytochemicals. Additionally, purification of phenolic and flavonoid compounds is needed to substantiate therapeutic and nutraceutical value of aonla.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Reidah IM, Arraez-Roman D, Quirantes-Pine R, Fernandez-Arroyo S, Segura-Carretero A, Fernandez-Gutierrez A. HPLC-ESI-Q-TQF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res Int. 2012;46:108–117. doi: 10.1016/j.foodres.2011.11.026. [DOI] [Google Scholar]
- Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol. 1998;62(2):183–193. doi: 10.1016/S0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem. 2008;56(19):9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- Ayurvedic Formulation of India-Part-1 (2003) 2nd edn Government of India, Ministry of Health and Family Planning. Department of Health, New Delhi
- Bansal V, Sharma A, Ghanshyam C, Singla ML. Rapid HPLC method for determination of vitamin c, phenolic acids, hydroxycinnamic acid, and flavonoids in seasonal samples of emblica officinalis juice. J Liq Chromatogr Relat Technol. 2015;38(5):619–624. doi: 10.1080/10826076.2014.936608. [DOI] [Google Scholar]
- Chiste RC, Benassi MT, Mercadante AZ. Effect of solvent type on the extractability of bioactive compounds, antioxidant capacity and colour properties of natural annatto extracts. Int J Food Sci Technol. 2011;46:1863–1870. doi: 10.1111/j.1365-2621.2011.02693.x. [DOI] [Google Scholar]
- Filipiak-Szok A, Kurzawa M, Sztyk E. Determination of anti-oxidant capacity and content of phenols, phenolic acids and flavonols in Indian and European gooseberry. Chem Pap. 2012;66(4):259–268. doi: 10.2478/s11696-012-0151-5. [DOI] [Google Scholar]
- Harborne JB. Phytochemical methods, a guide to modern techniques of plant analysis. London: Chapman and Hall; 1973. pp. 33–41. [Google Scholar]
- Heo BG, Jang HG, Cho JY, Namiesnik J, Jastrzebski Z, Vearasilp K, Aguliar GG, Ayala ALM, Suhaj M, Gorenstein S. Partial characterization of Indigo (Polygonum tinctorium Ait.) plant seeds and leaves. Ind Crops Prod. 2013;42:429–439. doi: 10.1016/j.indcrop.2012.06.029. [DOI] [Google Scholar]
- Ignat I, Volf I, Popa VI. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Schaper TD, Dollar CA, Sasner JJ. Utilization of Folin–Ciocalteau phenol reagent for the detection of certain nitrogen compounds. J Agric Food Chem. 2003;51(7):1811–1815. doi: 10.1021/jf021099r. [DOI] [PubMed] [Google Scholar]
- Jullien M, Villandy J, Golde A, Harel L. Inhibition by quercetin of the release of density dependent inhibition of cell growth in RSV-transformed chicken cell. Cell Biol Interact Rep. 1984;8:939–947. doi: 10.1016/0309-1651(84)90192-9. [DOI] [PubMed] [Google Scholar]
- Khopde SM, Priyadarsini KI, Mohan H, Gawandi VB, Satav JG, Yakhmi JV, Banavaliker MM, Biyani MK, Mittal JP. Characterizing the antioxidant activity of amla (Phyllanthus emblica) extract. Curr Sci. 2001;81(2):185–190. [Google Scholar]
- King A, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc. 1999;99(2):213–218. doi: 10.1016/S0002-8223(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Krishnaiah D, Devi T, Bono A, Sarbatky R. Studies on phytochemical constituents of six Malaysian medicinal plants. J Med Plants. 2009;3(2):067–072. [Google Scholar]
- Kumar GS, Nayaka H, Dharmesh SM, Salimath PV. Free and bound phenolic antioxidants in amla (Emblica officinalis) and turmeric (Curcuma longa) J Food Compost Anal. 2006;19:446–452. doi: 10.1016/j.jfca.2005.12.015. [DOI] [Google Scholar]
- Lila MA, Raskin I. Health related interactions of phytochemicals. J Food Sci. 2005;70(1):20–27. doi: 10.1111/j.1365-2621.2005.tb09054.x. [DOI] [Google Scholar]
- Liu X, Cui C, Zaho M, Wang J, Luo W, Yang B, Jiang Y. Identification of phenolics in the fruit of Emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem. 2008;109:909–915. doi: 10.1016/j.foodchem.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Maisuthisakul P, Pongsawatmanit R, Gordon MH. Characterization of the phytochemicals and antioxidant properties of extracts from teaw (Cratoxylum fromosum Dyer) Food Chem. 2007;100:1620–1629. doi: 10.1016/j.foodchem.2005.12.044. [DOI] [Google Scholar]
- Mass JL, Galletta GJ, Stoner GD. Ellagic acid, an anticarcinogenic in fruits, especially in strawberries: a review. Hort Sci. 1991;26(1):10–13. [Google Scholar]
- Mertens-Talcott S, Talcott S, Percival S. Low concentration of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLt-4 Human leukemia cells. J Nutr. 2003;133:2669–2674. doi: 10.1093/jn/133.8.2669. [DOI] [PubMed] [Google Scholar]
- Mradu G, Saumyakanti S, Sohini M, Mukherjee A. HPLC profiling of standard phenolic compounds present in medicinal plants. Int J Pharmacog Phytochem Res. 2012;43(3):162–167. [Google Scholar]
- Nambiar SS, Paramesha M, Shetty NP. Comparative analysis of phytochemical profile, antioxidant activities and foam prevention abilities of whole fruit, pulp and seeds of Emblica officinalis. J Food Sci Technol. 2015;52(11):7254–7262. doi: 10.1007/s13197-015-1844-x. [DOI] [Google Scholar]
- Nampoothiri SV, Prathapan A, Cherian OL, Raghu KG, Venugopalan VV, Sundaresan A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked type 2 diabetes. Food Chem Toxicol. 2011;49:125–131. doi: 10.1016/j.fct.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Nirmaladevi R, Padma PR, Kavita D. Analyzes the methanolic extract of the leaves of Rhinacanthus nasutus. J Med Plants Res. 2010;4:1554–1560. [Google Scholar]
- Pinelo M, Rubilar M, Sineiro J, Nunez MJ. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster) Food Chem. 2004;85:267–273. doi: 10.1016/j.foodchem.2003.06.020. [DOI] [Google Scholar]
- Raghu V, Platel K, Srinivasan K. Comparison of ascorbic acid content of Emblica officinalis fruits determined by different analytical methods. J Food Compost and Anal. 2007;20:529–533. doi: 10.1016/j.jfca.2007.02.006. [DOI] [Google Scholar]
- Razali N, Mat-Junit S, Abdul-Muthalib FA, Subramaniam S, Abdul-Aziz A. Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chem. 2012;131:441–448. doi: 10.1016/j.foodchem.2011.09.001. [DOI] [Google Scholar]
- Sachan NK, Gangwar SS, Sharma R, Kumar Y. An investigation into phytochemical profile and neutraceutical value of amla (Emblica officinalis) fruits. Int J Modern Pharm Res. 2013;2(1):1–12. [Google Scholar]
- Sawant L, Prabhakar B, Mahajan A, Pai N, Pandita N. Development and validation of HPLC method for quantification of phytoconstituents in Phyllanthus emblica. J Chem Pharm Res. 2011;3(4):937–944. [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh N, Thakur S, Kaur A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J Food Sci Technol. 2017;54(4):921–932. doi: 10.1007/s13197-016-2356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Verma V, Yadav R, Singh B. Pharmacognostical study of Amalaki (Emblica officinalis Gaertn.) J Pharmacogn Phytochem. 2018;7(3):3476–3480. [Google Scholar]
- Teh S, Bekhit AE, Birch J. Antioxidative polyphenols from defatted oilseed cakes: effect of solvents. Antioxidants. 2014;3:67–80. doi: 10.3390/antiox3010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayagamoothy T. Antibacterial activity of some medicinal plants of Srilanka Ceylon. J Sci Biol. 1982;11:50–55. [Google Scholar]
- Yokozawa T, Kim HY, Kim HJ, Tanaka T, Sugino H, Okubo T, Chu DC, Juneja LR. Amla (Emblica officinalis Gaertn.) attenuates age-related renal dysfunction by oxidative stress. J Agr Food Chem. 2007;55(19):7744–7752. doi: 10.1021/jf072105s. [DOI] [PubMed] [Google Scholar]