Abstract
Idli is a cereal-pulse based fermented food. This study profiles the qualitative and quantitative analysis of biogenic amines formed in the fermented idli batter prepared using varying proportions of rice to black gram dal at 1:1, 2:1, 3:1 and 4:1 (w/w) ratios and stored at 30 and 4 °C for 7 days. Histamine, tyramine, putrescine, cadaverine, spermidine, and spermine were investigated for the first time in the idli batter using HPLC technique. Putrescine and cadaverine were the predominant biogenic amines found in both prepared and market samples. Histamine and spermine were not detected in all batter samples. Increasing the proportion of rice in the idli batter resulted in the decrease in the total biogenic amines formed in the fermented batter with batter having more black gram dal (1:1) showing the maximum formation of total biogenic amines. Idli is a safe, easily digestible food to consume as the highest total biogenic amines quantified (366.87 µg/g) in 1:1 variant batter was below the harmful limit (1000 µg/g).
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03609-9) contains supplementary material, which is available to authorized users.
Keywords: Biogenic amines, Idli batter, Histamine, Putrescine, Cadaverine, Tyramine, Spermidine
Introduction
Biogenic amines are organic nitrogenous compounds of low molecular weight which have potential biological significance in microbial, vegetable, and animal cells. These organic compounds are not toxic except when large quantities are taken or the natural mechanism of their catabolism are suppressed (Peñas et al. 2010). Biogenic amines are mainly formed by the reactions involving decarboxylation of amino acids as well as transamination of aldehydes and ketones (Santos 1996; Bai et al. 2013). Based on their chemical structure, it can be classified as heterocyclic (histamine and tryptamine), aliphatic (putrescine and cadaverine) or aromatic (tyramine and phenylethylamine) (Spano et al. 2010). Biogenic amines in food systems are formed by the inherent enzymes (Histidine decarboxylase, putrescine carbamoyl transferase, carbamate kinase, lysine decarboxylase, etc.) present in the raw material or are generated by the indigenous microflora (Spano et al. 2010; Collins et al. 2011). Therefore, they are likely to occur in fermented foods where they can be formed by the metabolic activity of the associated microbes.
Histamine and tyramine are the most toxic biogenic amines in food when present in excess. Food containing more than 1000 mg/kg of histamine is considered toxic and unsafe for human consumption (Witczak and Sikorski 2017). The consumption of foods rich in histamine can cause a headache, flushing, itching, hypotension, nasal secretion, bronchospasm, tachycardia, urticarial, pruritus, and asthma. Tyramine rich food can also cause hypertension and headache along with other symptoms such as a migraine, vomiting, perspiration, and pupil dilatation (Collins et al. 2011; Del Rio et al. 2017). Putrescine and cadaverine are less potent than histamine and tyramine. Spermidine and spermine are not toxic in themselves but they can increase the effect of histamine and tyramine (Naila et al. 2010; Peñas et al. 2010). Therefore, analysis of biogenic amines in fermented foods is important because of their potential toxicity and the possibility of using them as indicators of the degree of spoilage or freshness of food. High-performance liquid chromatography (HPLC) based methods are reliable, sensitive and can be used to quantify the concentrations of all biogenic amines present in various fermented food (Onal 2007).
“Idli” is a fermented food widely consumed in the Indian subcontinent and recently gaining recognition as part of the global cuisine (Durgadevi and Shetty 2012; Shrivastava and Ananthanarayan 2015). It is a steamed product, renowned for its appearance, characteristic sour taste, spongy texture, and typical flavor. As a part of traditional nutritional heritage and practices, idli is a favored food for both babies and elderly, especially with those of compromised digestion. It is prepared from a spontaneously fermented batter of rice (Oryza sativa L.) and split black gram dal (Phaseolus mungo), in a proportion of 2:1–4:1 (w/w). (Steinkraus 1995; Shrivastava and Ananthanarayan 2015). Lactic acid bacteria (LAB) utilize the available carbohydrates into organic acids such as lactic acid, reducing the pH of the batter, and favoring the growth of yeast (Sridevi et al. 2010; Shrivastava and Ananthanarayan 2015). The shelf life of the fermented idli batter is short, at 30 °C for 1 day, before becoming unpalatable, and it cannot be stored more than 5 days under refrigerated conditions (Nisha et al. 2005; Regubalan and Ananthanarayan 2018).
LAB and yeast are the dominant indigenous microbes playing a vital role in idli batter fermentation (Shrivastava and Ananthanarayan 2015). The indigenous microbes responsible for the fermentation of idli batter are Lactobacillus brevis, Lactobacillus fermentum, Lactobacillus delbrueckii, Lactobacillus lactics, Lactobacillus casei, Leuconostoc mesenteroides, Pediococcus pentosaceus, Pediococcus cerevisiae, and Enterococcus faecalis. Yeasts such as Saccharomyces cerevisiae, Debaryomyces hansenii, Candida glabarata, Candida tropicalis, Candida fragilola, Hansenula anomala, Torulopsis holmii, Geotrichum candidum, and Trichosporon beigelli have also been reported in idli fermentation (Steinkraus 1995; Sridevi et al. 2010; Saravanan et al. 2015). LAB have the ability to produce biogenic amines in fermented foods (Arena and Manca de Nadra 2001). However, yeast and molds cannot massively form biogenic amines (Collins et al. 2011).
Fermentation of idli batter leads to an increase in free sugar, amino acid, nicotinic acid, methionine and choline content (Ghosh and Chattopadhyay 2011; Shrivastava and Ananthanarayan 2015). The availability of free amino acid is essential for decarboxylase positive microorganism to form biogenic amines (Santos 1996). The black gram dal contains 21.57% of protein (Smartt and Nwokolo 2012) which provide major protein source to idli batter. Increase in the proportion of black gram dal could increase the free amino acid in the fermented idli batter which may act as precursors to increase the level of biogenic amines in idli batter. In general, market idli batter samples may contain various proportion of the raw materials used and it may be stored under varying storage conditions with potential for temperature abuse. According to Sharma et al. (2017), idli batter fermented at 35 °C for 19 h showed total biogenic amines content of 636 µg/g using a spectrophotometric technique with the accuracy level of 200–2000 ppm.
There is not much research reported about the formation of individual biogenic amines in the idli batter as a function of storage conditions and time. In order to ensure food safety of idli batter, there is a great need to determine the levels of biogenic amines formed both in freshly fermented and stored sample. The objective of this research involves estimation of different biogenic amines formed in the idli batter prepared from rice and black gram dal taken in different proportions of 1:1, 2:1, 3:1 and 4:1 (w/w) as a function of storage temperature.
Materials and methods
Chemicals and solvents
Spermine tetrahydrochloride (SPR), putrescine dihydrochloride (PUT), histamine dihydrochloride (HIS), cadaverine dihydrochloride (CAD), tyramine hydrochloride (TYR) and spermidine trihydrochloride (SPD) were procured from SRL Chemicals, Mumbai, India. Dansyl chloride was purchased from Sigma Chemical Co. (St. Louis, MO) Mumbai. Tri-chloroacetic acid, sodium bicarbonate, sodium hydroxide, glutamic acid, acetonitrile, and acetone were obtained from Merck, Mumbai. All solvents used were of HPLC grade.
Preparation of idli batter
The raw materials, parboiled rice (O. sativa), split black gram (P. mungo), TATA salt, and six marketed idli batters were all purchased from the local supermarket of Mumbai. Parboiled rice and black gram were taken individually in 1:1, 2:1, 3:1, 4:1 (w/w) ratio and soaked in distilled water at 30 °C for 4 h. The rice was ground to a coarse consistency and black gram dal was ground separately to a fine paste. These two batters were mixed with addition of 0.9% w/w table salt. The resulting batter was incubated at 30 °C for half a day for fermentation (Regubalan and Ananthanarayan 2018). The fermented batter was transferred to plastic containers and stored at 30 °C and 4 °C for 7 days to monitor the level of biogenic amines formation. Six idli batter samples were obtained from the local market, coded as IB1, IB2, IB3, IB4, IB5, and IB6 and immediately analyzed.
Measurement of pH
The pH of stored (30 °C and 4 °C) idli batter was measured using a digital EUTECH pH meter.
Measurement of acid content
Generally, 10 g of idli batter was taken in 25 ml of distilled water and titrated against freshly prepared 0.1 N NaOH using phenolphthalein as an indicator. The acid content of the idli batter was calculated in terms of anhydrous lactic acid produced during fermentation (Horwitz 1980).
Preparation of standard solutions of biogenic amines
In general, 5000 mg L−1 stock solutions of each tested biogenic amines were prepared in deionized water and kept in amber colored glass bottles at − 20 °C. Standard solutions were prepared by diluting this stock solution and used for plotting the standard curve.
Extraction and derivatization of biogenic amines from idli batter samples
The biogenic amines were extracted from 5 g of idli batter sample by mixing in 25 mL of 5% tri chloro acetic acid. The mixture was homogenized for 20 min, centrifuged at 6,296 g at 4 °C for 5 min and the supernatant was filtered through Whatman No. 1 filter paper. The dansyl chloride derivatives of the extracted biogenic amines were prepared by taking one mL of above extract in a test tube, to which 200 µL of a saturated solution of NaHCO3, 20 µL of 2 N NaOH and 2 mL of dansyl chloride (5 mg/mL) was added. The test tube was sealed and mixed properly and incubated at 70 °C for 15 min in dark. The unbound dansyl chloride was removed by addition of 1 mL of glutamic acid (2 mg/mL in deionized water) and this solution was left in the dark for 1 h. The final volume was adjusted to 5 mL with acetonitrile and filtered through 0.22 µm PVDF membrane filter (Saaid et al. 2009).
Analytical method
All the biogenic amines quantification was performed using Jasco HPLC system (UV-1575, PU-1580) by a standardized method reported by Saaid et al. (2009). Chromatographic separation was done by injecting 20 µL of pre-derivatized and filtered (0.22 µm) sample into Waters Spherisorb 5 µm ODS2 column (250 × 4.5 mm). The analysis was carried out in isocratic mode with the mobile phase composition of acetonitrile:water; 70:30 (v/v) at a flow rate of 1 mL/min. The column effluent was monitored at 254 nm. Reproducibility and repeatability were evaluated by injecting five times each of the six standard mixtures on the same day. Limit of detection and limit of quantification were determined based on the method described by Campos et al. (2017). Biogenic amines were spiked at 10, 40, and 80 ppm in the unfermented idli batter samples which were extracted, derivatized, and analyzed by HPLC to determine the percentage recovery. All the experiments were done in triplicates and results are expressed in mean and percentage relative standard deviation (%RSD).
Statistical analysis
All experiments were done in triplicates and results are expressed as mean ± SD. Statistical differences between samples were compared by Duncan’s test using IBM©SPSS® version 20 at a p value of 0.05.
Results and discussion
Analytical characteristics of the method for estimation of biogenic amines
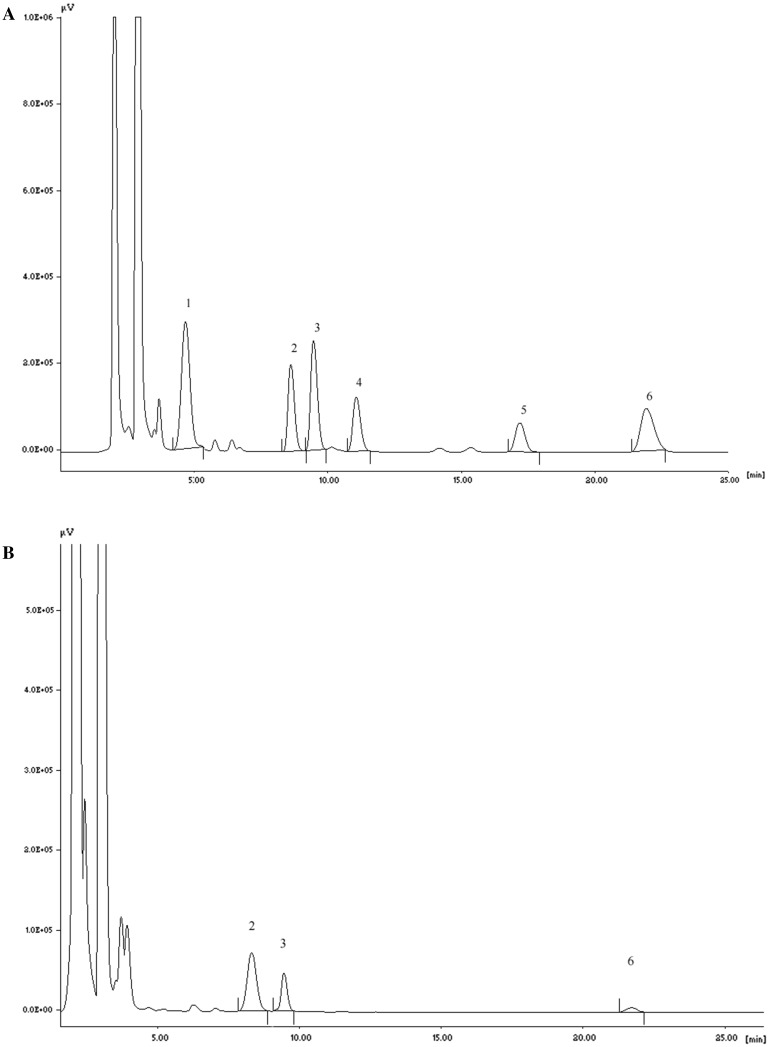
The HPLC method developed for simultaneous estimation of biogenic amines revealed well resolved chromatographic peaks. The representative chromatogram of the standard biogenic amines (A) and idli batter made from 3:1 (w/w) rice:black gram dal ratio stored at 30 °C for 7 day sample extract (B) are shown in Fig. 1. The analytical characteristics of the HPLC method used for quantification of biogenic amines in idli batter are shown in Table 1. The HPLC method was found to be linear over concentration range of 5–140 µg/mL with regression coefficients > 0.997.
Fig. 1.
Chromatogram of a standard biogenic amines and bidli batter made from 3:1 (w/w) rice:black gram dal ratio stored at 30 °C for 7 day. Peak identity: spermine (1), putrescine (2), cadaverine (3), histamine (4), tyramine (5), spermidine (6)
Table 1.
Analytical characteristics of the HPLC method used for quantification of biogenic amines
| Biogenic amines | tR (min) | R2 | LOD (µg/ml) | LOQ (µg/ml) | Linear range (µg/ml) | Intraday (% RSD) n = 3 | % Recovery | |||
|---|---|---|---|---|---|---|---|---|---|---|
| tR | Area | 10 (µg/ml) | 20 (µg/ml) | 40 (µg/ml) | ||||||
| Spermine | 4.93 | 0.997 | 0.77 | 2.31 | 5–80 | 0.06 | 0.68 | 91.52 (1.80) | 103.34 (1.26) | 97.62 (0.40) |
| Putrescine | 8.92 | 0.998 | 0.78 | 2.37 | 5–80 | 0.34 | 0.56 | 102.68 (0.56) | 103.24 (1.15) | 104.72 (1.53) |
| Cadaverine | 10.05 | 0.999 | 0.42 | 1.28 | 5–80 | 0.16 | 1.13 | 99.05 (5.20) | 99.39 (3.52) | 100.3 (1.64) |
| Histamine | 11.24 | 0.999 | 0.85 | 2.59 | 5–120 | 0.21 | 0.84 | 106.53 (0.22) | 102.52 (0.84) | 99.10 (2.42) |
| Tyramine | 19.23 | 0.999 | 0.59 | 1.79 | 5–140 | 0.64 | 2.39 | 93.48 (3.42) | 99.64 (2.39) | 96.66 (2.69) |
| Spermidine | 23.41 | 0.997 | 1.38 | 4.19 | 5–100 | 1.31 | 1.27 | 95.65 (4.40) | 100.80 (3.16) | 97.91 (2.28) |
Values in bracket is relative standard deviation (%RSD)
tR Retention time, R2 square of regression coefficient, LOD limit of detection, LOQ limit of quantification, RSD relative standard deviation
Limit of detection and limit of quantification were determined to know the sensitivity of the method. Good repeatability was achieved in both retention times (%RSD < 1.31) and the peak area (%RSD < 2.39) (Table 1). Acceptable recovery of all biogenic amines was obtained (91.52–106.53%).
Metabolic activity in relation to pH and titratable acidity
pH and titratable acidity are important parameters which decide the sour taste of idli and also represent the metabolic activity of the microbes. The observed trends for decrease in pH and increase in titratable acidity during the storage of idli batter samples are depicted in Fig. 2. pH drastically decreased in all four variants of idli batter when stored at 30 °C for 7 days, whereas, refrigerated idli batter samples showed a slow reduction of pH and slow increase in acidity throughout the storage period of 7 days because of the inhibition of the metabolic activity of microbes at low temperature.
Fig. 2.

Change in the pH and titratable acidity in different fermented idli batter samples (0.5 day) stored at 30 and 4 °C for 7 days
Biogenic amines in idli batter sample stored at 30 °C
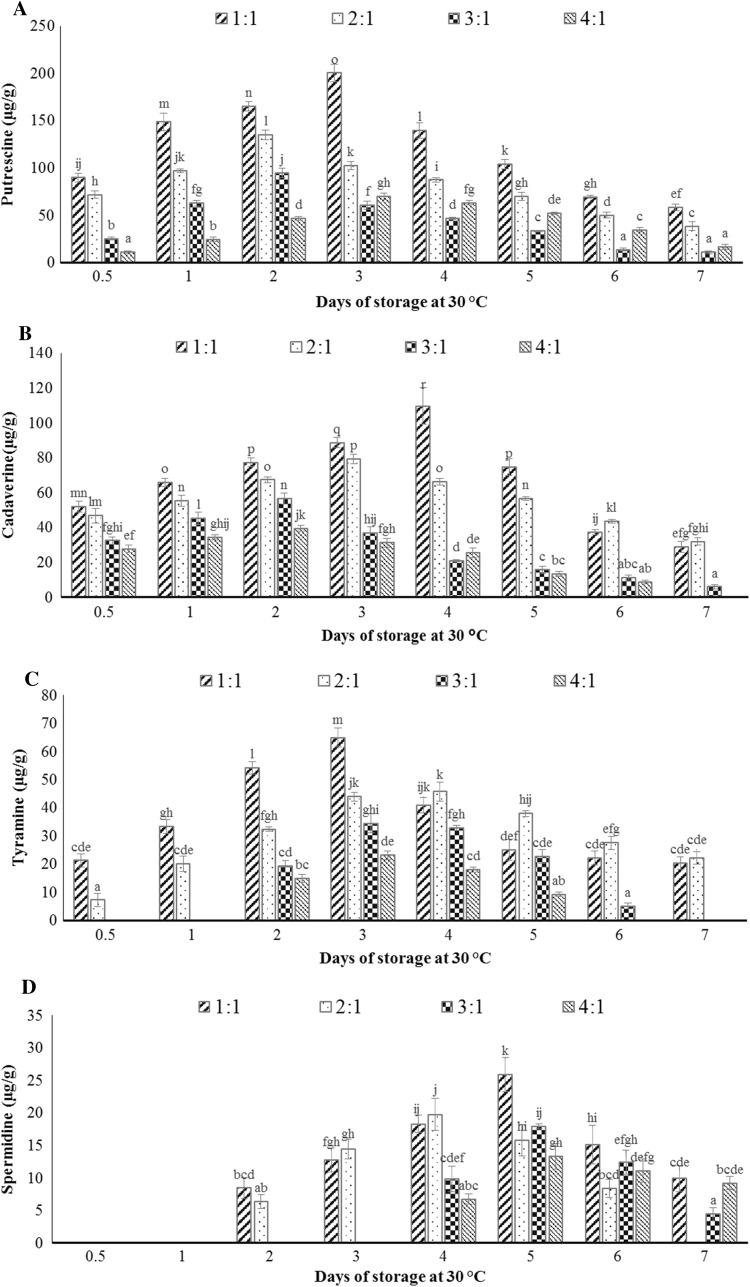
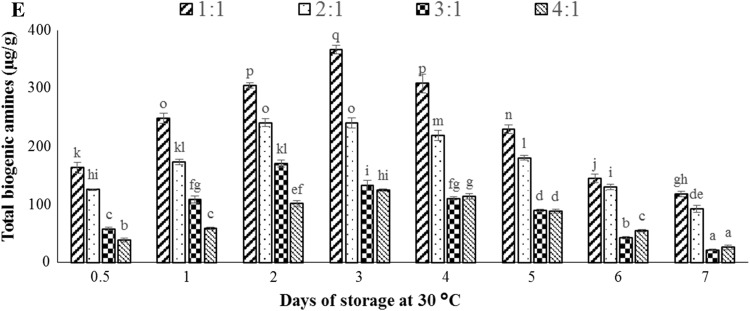
The biogenic amine profile of prepared idli batter samples stored at 30 °C for 7 days is shown in Fig. 3. Putrescine, cadaverine, tyramine, and spermidine were the major biogenic amines observed in idli batter. Histamine and spermine were not detected in the idli batter samples stored under these conditions. The biogenic amines were not formed in any variant of unfermented idli batter.
Fig. 3.
The amount of the individual and total biogenic amines detected in fermented idli batter (0.5 day) stored at 30 °C for 7 days. Putrescine (a), cadaverine (b), tyramine (c), spermidine (d), and total biogenic amines (e). Different letters indicate statistically significant differences (p < 0.05)
Parboiled rice contains 6% of protein and rice proteins are deficient in lysine (Shakuntala and Shadaksharaswamy 1987) whereas, the proximate analysis of black gram shows 21.57% of protein (Smartt and Nwokolo 2012) which contributes major proteins to the idli batter. According to Padhye and Salunkhe (1979), black gram contains lysine (6.0 mol%), arginine (5.2 mol%), and tyrosine (2.5 mol%) which are precursors for the biogenic amines formed during idli batter fermentation. Arginine is the precursor for the formation of putrescine, spermidine, and spermine. Similarly, lysine is the precursor for the formation of cadaverine (Santos 1996). Increase in the black gram dal proportion let to an increase in putrescine and cadaverine formation in the fermented idli batter (0.5 day). Tyramine was formed from tyrosine by tyrosine decarboxylase enzyme (Collins et al. 2011). Tyramine was detected in 1:1 and 2:1 (w/w) ratio of fermented idli batter (0.5 day), however, it was found on the second day of storage for 3:1 and 4:1 (w/w) ratio. The decrease in the proportion of black gram dal delays the formation of tyramine in idli batter. The highest concentration of tyramine was found to be 64.91 µg/g in 1:1 variant stored at 30 °C for 3 days. Spermidine was the only polyamine detected in 1:1 and 2:1 (w/w) ratio of idli batter from the second day of storage at 30 °C; similarly it was found in 3:1 and 4:1 (w/w) ratio from the fourth day of storage. The highest concentration of spermidine was found to be 25.91 µg/g in 1:1 variant stored at 30 °C for 5 days. Putrescine is the precursor for the formation of spermidine, and spermine. The conversion of putrescine into spermidine may be one of the reasons for the observed reduction of putrescine concentration in all the variants of idli batter stored at 30 °C beyond 3 days.
Putrescine and cadaverine were the predominant biogenic amines found in all four variants of idli batter after fermentation (0.5 day). These biogenic amines may be potentially used as a bio-marker to quantify the proportion of black gram dal in idli batter. Biogenic amines detected from all four variants of idli batter were significantly reduced on the seventh day of storage at 30 °C. The maximum concentration of cadaverine, putrescine, tyramine, and spermidine were produced in 1:1 (w/w) ratio of idli batter stored at 30 °C. Idli batter is known to have wide varieties of LAB strains. The LAB culture that could produce amine oxidizing enzymes namely mono amine oxidase (MAO, EC 1.4.3.4) and diamine oxidase (DAO, EC 1.4.3.6) may be able to metabolize more than one biogenic amine leading to the degradation of biogenic amines in idli batter stored at 30 °C for 7 days. Tosukhowong et al. (2011) reported that L. plantarum BCC 9546, has an ability to decrease tyramine formation in a Thai fermented sausage called Nham.
The fermented idli batter (0.5 day) made up of 1:1, 2:1, 3:1 and 4:1 (w/w) ratios of rice:black gram dal showed total biogenic amine concentration of 163.19, 125.93, 57.87 and 38.71 µg/g respectively. The highest concentration of total biogenic amines was 366.87 µg/g in 1:1 variant of idli batter stored at 30 °C for 3 days. Total biogenic amine levels of 1000 mg/kg in food were considered harmful to human health (Kim et al. 2012). Thus under adverse conditions of storage (30 °C), the total biogenic amine content in idli batter was found to be less than harmful level.
Biogenic amines in idli batter sample stored at 4 °C
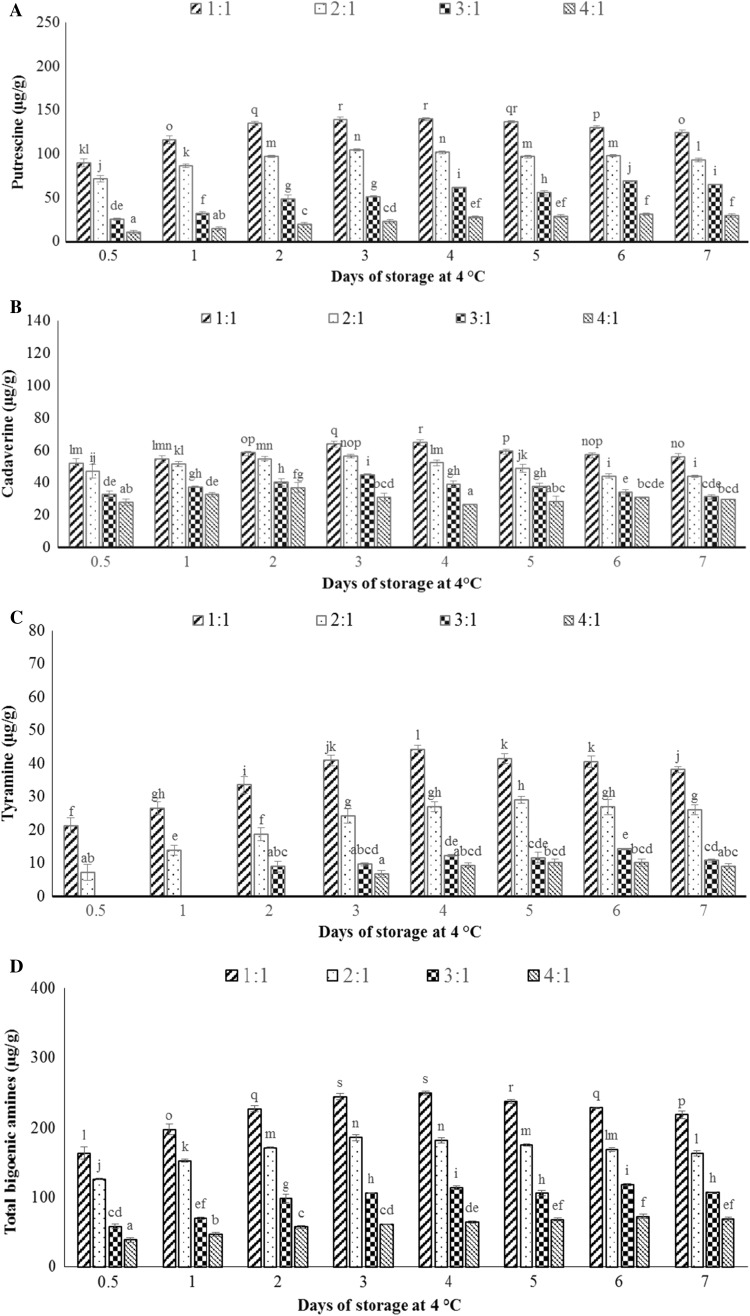
The biogenic amines content measured from fermented idli batter (0.5 day) stored at 4 °C for 7 days is depicted in Fig. 4. Histamine, spermidine, and spermine were not detected throughout the storage at 4 °C in all variants of idli batter. Putrescine, cadaverine, and tyramine were detected during the storage of fermented idli batter (0.5 day) at 4 °C for 7 days. In the present work, the highest concentration of putrescine obtained was 140 µg/g which was same (p < 0.05) for 1:1 ratio of idli batter stored at 4 °C for 3, 4 and 5 days. Cadaverine concentration was gradually increased in all variant of idli batter up to 3 days when stored at 4 °C. The highest concentration of cadaverine was found to be 58 µg/g in 1:1 variant of idli batter. Tyramine was formed in fermented idli batter (0.5 day) in 1:1 and 2:1 variants. It was formed on the second day of storage in 3:1 ratio and third day of storage in 4:1 ratio of idli batter. It can be concluded that the reduction in the proportion of black gram dal delayed the formation of tyramine under refrigerated storage conditions. According to Patat et al. (1995), 1100 mg of tyramine was found to be an effective dose (ED50) at which 50% of the individuals responded. Highest tyramine content was found to be 44.31 µg/g in 1:1 variant of batter stored at 4 °C for 4 days, which is far lesser than the effective dose.
Fig. 4.
The amount of the individual and total biogenic amines detected in fermented idli batter (0.5 day) stored at 4 °C for 7 days. Putrescine (a), cadaverine (b), tyramine (c), and total biogenic amines (d). Different letters indicate statistically significant differences (p < 0.05)
The highest total biogenic amine concentration was found to be 249.13 µg/g in idli batter prepared from 1:1 (w/w) ratio after 4 days of storage at 4 °C. Overall, idli batter stored under refrigerated conditions showed a gradual increase in the individual and total biogenic amines concentrations in the initial period of storage (up to 4 days) and subsequently a slight decline was observed. Refrigerated storage condition (4 °C) may lead to the reduction in enzymatic and metabolic activity of the microbes which could explain the slow increase and decline of biogenic amine formation at 4 °C as compared to storage of idli batter at 30 °C. The same phenomenon was observed by Peñas et al. (2010), who reported that the individual biogenic amine levels underwent a gradual increase in the sauerkraut when stored at 4 °C for 3 months.
Correlation of pH, titratable acidity and LAB count vs total biogenic amines
Despite pH, titratable acidity, LAB count and concentration of biogenic amines, all being output effects of fermentation, R2 value was found to be 0.039 between pH and total biogenic amines formed in idli batter stored at 30 °C for 7 days (Supplementary Fig. 1). The regression value was 0.026 for idli batter stored at 4 °C for 7 days. Similarly, the regression value was found to be less than 0.1 between titratable acidity and total biogenic amines under both storage conditions (Supplementary Fig. 2). Similarly, R2 value was found less than 0.35 between LAB count and total biogenic amines in idli batter stored at 30 °C and 4 °C. It appears that formation of total biogenic amines is not correlated with pH and percent titratable acidity which are indicators of metabolic activity of the fermenting microflora. The Japanese fermented food “nattao” also showed the R2 value less than or around 0.1 between percent titratable acidity and total biogenic amines found in fermented nattao (Kim et al. 2012).
Biogenic amines in marketed samples of idli batter
The levels of pH, titratable acidity and biogenic amines of six marketed idli batter samples are shown in Table 2. LAB plays a significant role in the fermentation of idli batter producing lactic acid as a result of their metabolic activity. The formation of lactic acid during fermentation leads to a drop in pH and increase in titratable acidity. An optimally fermented idli batter generally demonstrates a pH of 4.0 to 4.5. Thus pH and titratable acidity are good measures of degree of fermentation of idli batter. The market samples of idli batter procured for this study showed pH in the range of 4 to 4.5 implying that they were adequately fermented. This could support our findings of biogenic amines in these batters as these amines are formed due to the metabolic activity of LAB. Putrescine and cadaverine were the two predominant amines detected in all market samples except IB4 which may be due to the low proportion of black gram dal used in the preparation of batter which is also implied by the lower price of this batter sample. Tyramine was found only in IB6 sample. Tyramine formation is highly influenced by the proportion of black gram dal used in batter preparation (Figs. 3c, 4c). Tyramine formation was very less during the refrigerated condition of storage as shown in Fig. 4c.
Table 2.
Levels of pH, % titratable acidity, and biogenic amines in market samples of idli batter
| Market sample | Price (INR/kg) | pH | Acidity (%) | Biogenic amines (µg/g) | Total biogenic amines (µg/g) | ||
|---|---|---|---|---|---|---|---|
| PUT | CAD | TYR | |||||
| IB1 | 60 | 3.96 ± 0.03b | 1.05 ± 0.02d | 35.27 ± 1.98d | 19.87 ± 1.56b | N.D. | 55.14 ± 3.54b |
| IB2 | 60 | 4.42 ± 0.01d | 0.75 ± 0.03a | 10.99 ± 1.65a | 15.43 ± 2.11a | N.D. | 26.42 ± 3.76a |
| IB3 | 70 | 4.19 ± 0.02c | 0.91 ± 0.01c | 29.22 ± 2.70c | 34.89 ± 1.99d | N.D | 64.11 ± 0.71c |
| IB4 | 30 | 3.87 ± 0.04a | 1.16 ± 0.03e | N.D. | N.D. | N.D. | N.D. |
| IB5 | 70 | 4.21 ± 0.01c | 0.82 ± 0.02b | 24.11 ± 3.07b | 30.77 ± 2.45 cd | N.D. | 54.88 ± 5.52b |
| IB6 | 60 | 4.00 ± 0.03b | 1.03 ± 0.04d | 21.67 ± 2.37b | 28.65 ± 3.19c | 5.08 ± 2.76 | 55.40 ± 1.94b |
PUT putrescine, CAD cadaverine, TYR tyramine. Histamine, spermine, and spermidine were not detected; ND not detected
Different letters indicate a significant difference (p < 0.05) within the same column
Histamine, spermidine, and spermine were not detected in all four variants of idli batter stored at 4 °C for 7 days likewise these three biogenic amines were not detected in any of the six market samples. The IB3 market sample showed the highest concentration of total biogenic amines (64.11 µg/g), while IB4 showed no formation of biogenic amines. Overall, the total biogenic amines concentration estimated in market batter samples was far below the toxic dosage level.
Conclusion
The present work was conducted to investigate the biogenic amines formed in different variants of fermented idli batter stored at 30 °C and 4 °C for 7 days. Cadaverine and putrescine were the predominant amines found in the fermented idli batter (0.5 day) which also showed formation of tyramine. Histamine, spermidine, and spermine were not found in all variants of batter stored under refrigerated conditions. Traces of tyramine were found in only one of the market batter samples. The total biogenic amines content was increased for 3 days and decreased from the fourth day of storage at 30 °C for 7 days, whereas a gradual increase of total biogenic amines content was observed at 4 °C for 7 days. It was found that total biogenic amines quantified in the idli batter was substantially less than the toxic dosage which showed that idli is a safe food to consume. Thus biogenic amines found in idli batter were majorly influenced by the proportion of raw materials used and the storage conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to University Grants Commission for the funding.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Baburaj Regubalan, Email: baburaj1326@gmail.com.
Laxmi Ananthanarayan, Phone: +91 22 33612506, Email: l.ananthanarayan@ictmumbai.edu.in.
References
- Arena ME, Manca de Nadra MC. Biogenic amine production by Lactobacillus. J Appl Microbiol. 2001;90:158–162. doi: 10.1046/j.1365-2672.2001.01223.x. [DOI] [PubMed] [Google Scholar]
- Bai X, Byun BY, Mah J. Formation and destruction of biogenic amines in Chunjang (a black soybean paste) and Jajang (a black soybean sauce) Food Chem. 2013;141:1026–1031. doi: 10.1016/j.foodchem.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Campos WEO, Rosas LB, Neto AP, et al. Extended validation of a sensitive and robust method for simultaneous quantification of aflatoxins B1, B2, G1 and G2 in Brazil nuts by HPLC-FLD. J Food Compos Anal. 2017;60:90–96. doi: 10.1016/j.jfca.2017.03.014. [DOI] [Google Scholar]
- Collins JD, Noerrung B, Budka H, et al. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011;9:2393. doi: 10.2903/j.efsa.2011.2393. [DOI] [Google Scholar]
- Del Rio B, Redruello B, Linares DM, et al. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017;218:249–255. doi: 10.1016/j.foodchem.2016.09.046. [DOI] [PubMed] [Google Scholar]
- Durgadevi M, Shetty PH. Effect of ingredients on texture profile of fermented food, idli. APCBEE Procedia. 2012;2:190–198. doi: 10.1016/j.apcbee.2012.06.034. [DOI] [Google Scholar]
- Ghosh D, Chattopadhyay P. Preparation of idli batter, its properties and nutritional improvement during fermentation. J Food Sci Technol. 2011;48:610–615. doi: 10.1007/s13197-010-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists, Horwitz W (1980) Changes in official methods of analysis made at the ninety-third annual meeting, October 15–18, 1979. 1st supplement to 13th edition official methods of analysis-AOAC. Association of Offical Analytical Chemists
- Kim B, Byun BY, Mah J. Biogenic amine formation and bacterial contribution in Natto products. Food Chem. 2012;135:2005–2011. doi: 10.1016/j.foodchem.2012.06.091. [DOI] [PubMed] [Google Scholar]
- Naila A, Flint S, Fletcher G, et al. Control of biogenic amines in food—existing and emerging approaches. J Food Sci. 2010;75:R139–R150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisha P, Ananthanarayan L, Singhal RS. Effect of stabilizers on stabilization of idli (traditional south Indian food) batter during storage. Food Hydrocoll. 2005;19:179–186. doi: 10.1016/j.foodhyd.2004.03.007. [DOI] [Google Scholar]
- Onal A. A review: current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007;103:1475–1486. doi: 10.1016/j.foodchem.2006.08.028. [DOI] [Google Scholar]
- Padhye VW, Salunkhe DK. Biochemical studies on black gram (Phaseolus mungo L.) seeds: amino acid composition and subunit constitution of fractions of the proteins. J Food Sci. 1979;44:606–614. doi: 10.1111/j.1365-2621.1979.tb03846.x. [DOI] [Google Scholar]
- Patat A, Berlin I, Durrieu G, et al. Pressor effect of oral tyramine during treatment with befloxatone, a new reversible monoamine oxidase—a inhibitor, in healthy subjects. J Clin Pharmacol. 1995;35:633–643. doi: 10.1002/j.1552-4604.1995.tb05022.x. [DOI] [PubMed] [Google Scholar]
- Peñas E, Frias J, Sidro B, Vidal-valverde C. Impact of fermentation conditions and refrigerated storage on microbial quality and biogenic amine content of sauerkraut. Food Chem. 2010;123:143–150. doi: 10.1016/j.foodchem.2010.04.021. [DOI] [Google Scholar]
- Regubalan B, Ananthanarayan L. Shelf life improvement of idli batter by addition of mustard essential oil as bio-preservative. J Food Sci Technol. 2018;55:3417–3426. doi: 10.1007/s13197-018-3247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaid M, Saad B, Hashim NH, et al. Determination of biogenic amines in selected Malaysian food. Food Chem. 2009;113:1356–1362. doi: 10.1016/j.foodchem.2008.08.070. [DOI] [Google Scholar]
- Santos MHS. Biogenic amines: their importance in foods. Int J Food Microbiol. 1996;29:213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Gopu V, Shetty PH. Diversity and functional characterization of microflora isolated from traditional fermented food idli. J Food Sci Technol. 2015;52:7425–7432. doi: 10.1007/s13197-015-1791-6. [DOI] [Google Scholar]
- Shakuntala MN, Shadaksharaswamy M. Food: facts and principles. New Delhi: Mohinder Singh Sejwal Publ New Delhi/India Wiley East Ltd; 1987. [Google Scholar]
- Sharma A, Kumari S, Nout MJ, Sarkar PK. Minimization of antinutrients in idli by using response surface process optimization. J Food Process Preserv. 2017;41(5):e13099. doi: 10.1111/jfpp.13099. [DOI] [Google Scholar]
- Shrivastava N, Ananthanarayan L. Use of the backslopping method for accelerated and nutritionally enriched idli fermentation. J Sci Food Agric. 2015;95:2081–2087. doi: 10.1002/jsfa.6923. [DOI] [PubMed] [Google Scholar]
- Smartt J, Nwokolo E. Food and feed from legumes and oilseeds. New York: Springer; 2012. [Google Scholar]
- Spano G, Russo P, Lonvaud-Funel A, et al. Biogenic amines in fermented foods. Eur J Clin Nutr. 2010;64(Suppl 3):S95–100. doi: 10.1038/ejcn.2010.218. [DOI] [PubMed] [Google Scholar]
- Sridevi J, Halami PM, Vijayendra SVN. Selection of starter cultures for idli batter fermentation and their effect on quality of idlis. J Food Sci Technol. 2010;47:557–563. doi: 10.1007/s13197-010-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus K. Handbook of indigenous fermented foods, revised and expanded. Boca Raton: CRC Press; 1995. [Google Scholar]
- Tosukhowong A, Visessanguan W, Pumpuang L, et al. Biogenic amine formation in Nham, a Thai fermented sausage, and the reduction by commercial starter culture, Lactobacillus plantarum BCC 9546. Food Chem. 2011;129:846–853. doi: 10.1016/j.foodchem.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Witczak A, Sikorski Z. Toxins and other harmful compounds in foods. Boca Raton: CRC Press; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.