Summary
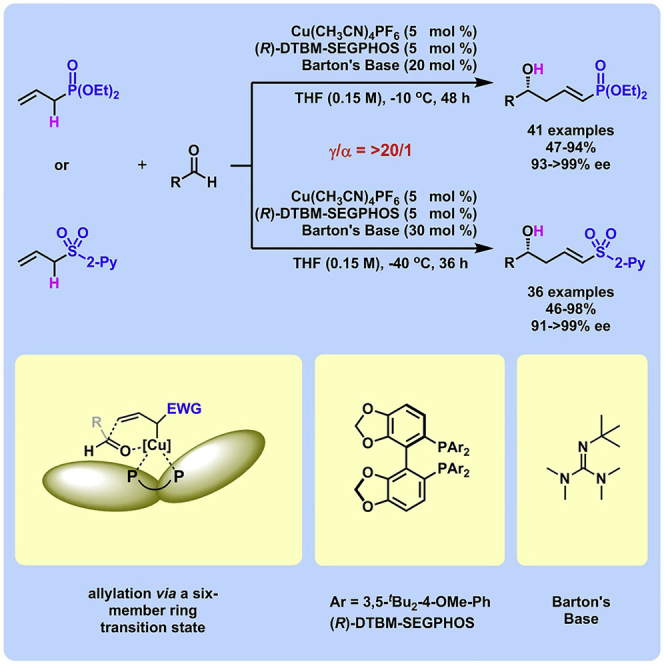
Two catalytic asymmetric vinylogous aldol-type reactions of aldehydes with allyl phosphonate and allyl sulfone have been uncovered in good to high yields for the first time. The bulky ligand—(R)-DTBM-SEGPHOS—was found to be the key to perfectly control both regio- and enantioselectivities. Transformations of the vinylogous products (including Horner-Wadsworth-Emmons and Julia olefinations) were successfully realized by virtue of the phosphonate and sulfone moieties. Moreover, the present methodology was successfully applied in the asymmetric synthesis of natural products.
Subject Areas: Chemistry, Organic Synthesis, Stereochemistry
Graphical Abstract
Highlights
-
•
Asymmetric vinylogous aldol
-
•
Excellent regioselectivity
-
•
HWE and Julia olefinations
Chemistry; Organic Synthesis; Stereochemistry
Introduction
Asymmetric vinylogous aldol reaction (VAR) has been one of the most important reactions in the synthesis of complex natural products, especially in the synthesis of polyketides. In the past decades, catalytic asymmetric VAR of aldehydes or ketones has evolved from classical Mukaiyama vinylogous aldol reaction (Denmark et al., 2005, Casiraghi et al., 2011; Pansare and Paul, 2011, Bisai, 2012, Kalesse et al., 2014, Hosokawa, 2018a, Hosokawa, 2018b) to direct vinylogous aldol reaction (DVAR) (Li and Yin, 2018, Otsuka et al., 2013, Zhu et al., 2013, Li et al., 2014, Han and Chang, 2016, Jing et al., 2016, Ray and Mukherjee, 2018), which enjoys the advantages of easy reaction protocol and high atom economy (Trost, 1991, Anastas and Crabtree, 2009, Newhouse et al., 2009). However, the nucleophiles in DVAR were mainly limited to unsaturated carbonyl compounds and their close derivatives (Bai et al., 2017). Unsaturated phosphonates or phosphine oxides, as well as unsaturated sulfones, have never been investigated as prenucleophiles in vinylogous aldol-type reactions.
In such reactions, synthetically versatile chiral vinylogous products containing α,β-unsaturated phosphonate or phosphine oxide moiety or α,β-unsaturated sulfone motif (Nishida et al., 2008, Xue et al., 2011, Konno et al., 2012, Lefevre et al., 2013, Hornillos et al., 2015, Lim and Hayashi, 2015, Lim and Hayashi, 2017, Wang and Hayashi, 2018, El-Awa et al., 2009, Alba et al., 2010, Nielsen et al., 2010, Zhu and Lu, 2010, Moteki et al., 2010, Quintard et al., 2011, Moure et al., 2011, Nishimura et al., 2012, Halskov et al., 2012, Zhou et al., 2012, Hernández-Toribio et al., 2012), would be produced. Furthermore, phosphonates and phosphine oxides had great applications in medicinal and agricultural chemistry (Mucha et al., 2011, Ordóñez et al., 2012, Corbridge, 2013, Horsman and Zechel, 2017). Sulfones, especially α,β-unsaturated sulfones, were widely distributed in biologically active compounds, even in the commercial pharmaceuticals (Meadows and Gervay-Hague, 2006, Dunny et al., 2013, Woo et al., 2014, Fang et al., 2016). Therefore it is highly desirable to achieve a vinylogous aldol-type reaction of unsaturated phosphonates or phosphine oxides, as well as unsaturated sulfones.
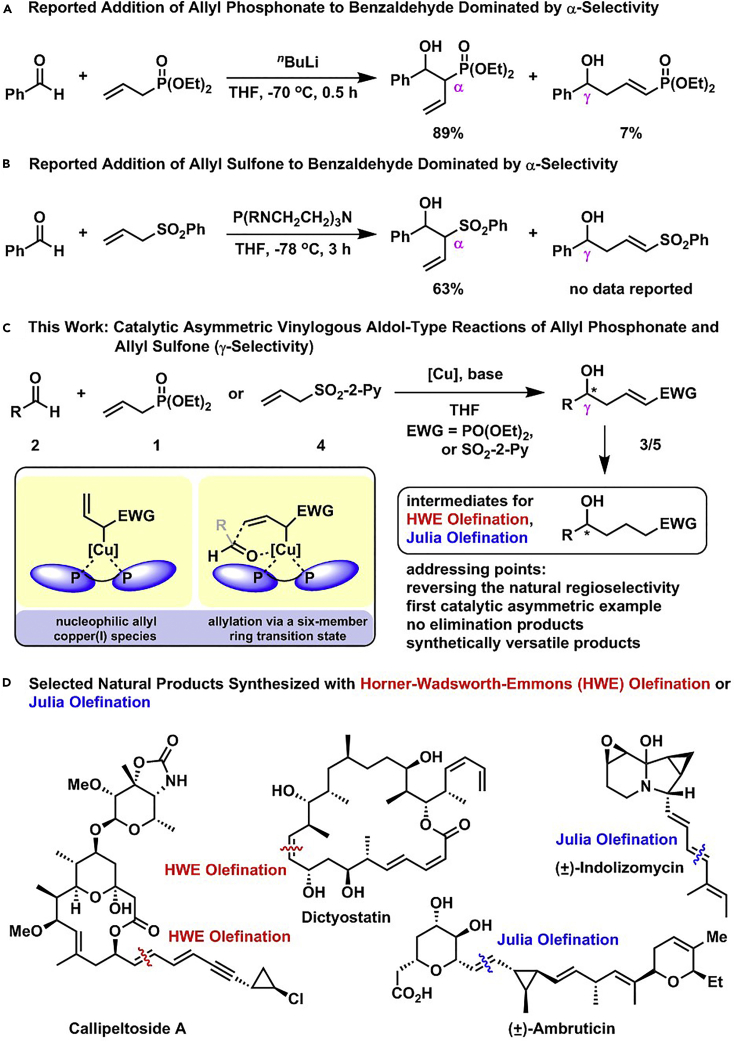
One inherent difficulty faced in the vinylogous aldol-type reaction of allyl phosphonate or allyl sulfone is the control of regioselectivity. The addition of allyl phosphonate to aldehyde was investigated by Yuan and co-workers in detail (Scheme 1A) (Yuan et al., 1990, Yuan et al., 1991). Treating allyl phosphonate with nBuLi in tetrahydrofuran (THF) at −70°C afforded the corresponding delocalized allylic carbanion, which reacted with benzaldehyde to give a mixture of α- and γ-adducts. α-Addition was the natural tendency and thus dominated the addition pathways, which led to the vinylogous product (γ-adduct) in significantly low yield. Increasing the steric hindrance of the alkyl group in phosphonate only led to a slight improvement of the γ-selectivity.
Scheme 1.
Aldol-type Reactions of Aldehydes with Allyl Phosphonate and Sulfone and Selected Natural Products Synthesized with Horner-Wadsworth-Emmons and Julia Olefinations
Furthermore, the addition of allyl sulfone to benzaldehyde catalyzed by a phosphine-based strong base was studied by Verkade and co-workers, which delivered the α-adduct in 63% yield at −78°C (Scheme 1B) (Kisanga and Verkade, 2002). The same reaction promoted by stoichiometric nBuLi also afforded the α-adduct exclusively, which was employed to prepare acyclic 2-phenylsulfonyl 1,3-diene in 89% yield (Cuvigny et al., 1983, Cuvigny et al., 1986, Chinkov et al., 2003). Obviously, it is challenging to overcome the inherent α-selectivity in the aldol-type reactions of allyl phosphonate and allyl sulfone. The other difficulty in the DVAR is the remote asymmetric induction as the functional group (aldehyde, ketone, ester, and amide generally, and phosphonate or sulfone here) is far from the reactive γ-position in form, which was viewed as a challenge in asymmetric catalysis (Shirokawa et al., 2004). To the best of our knowledge, there is no reported enantioselective method to carry out a vinylogous aldol-type reaction of allyl phosphonate or allyl sulfone (Scheme 1C).
The α-carbanions of phosphonate and sulfone have been employed as nucleophiles in Horner-Wadsworth-Emmons (HWE) and Julia olefinations, which were identified as two prominent synthetic tools to assemble the carbon-carbon double bond in organic synthesis, especially in the total synthesis of complex natural products (Kobayashi et al., 2018, Ma et al., 2010) (such as callipeltoside A, dictyostatin, indolizomycin and ambruticin) (Scheme 1D) (Trost et al., 2002, Ho et al., 2013, Kim et al., 1993, Liu and Jacobson, 2001). The products from the saturation of vinylogous aldol-type products (3/5) would be suitable substrates for HWE and Julia olefinations for further structure elaboration. Herein, we report two asymmetric vinylogous aldol-type reactions of aldehydes with allyl phosphonate and allyl sulfone catalyzed by a bulky chiral copper(I) complex and an organic base. The deprotonation of allyl phosphonate or allyl sulfone would generate a nucleophilic allylcopper(I) species, which afforded the vinylogous product through an asymmetric allylation via a six-member ring transition state (Scheme 1C).
Results and Discussion
The reaction of allyl sulfone 4'/4 and benzaldehyde (2a) was investigated as a model reaction in the presence of Cu(CH3CN)4PF6, phosphine ligand, and Barton's base (Table 1). In all cases with 4′, the vinylogous product 5a′ was obtained with unsatisfactory regio- and enantioselectivities (entries 1–9). (R)-DTBM-SEGPHOS, an effective bulky ligand, led to good control of the enantioselectivity in our previously reported catalytic asymmetric aldol reaction of unsaturated esters and aldehydes (Zhang and Yin, 2018) and gave excellent control of the regioselectivity. The enantioselectivity was improved from 43% to 76% by switching phenyl to 2-pyridinyl (entry 10). Lowering the temperature to −40°C resulted in 97% enantiomeric excess (ee) for 5a (entry 11). The moderate yield was enhanced to 98% by changing the ratio of 2a/4, increasing the amount of Barton's base to 30 mol %, and prolonging the reaction time to 36 h (entries 12–14). Finally, the catalyst loading was successfully decreased to 3 mol % without changing both regio- and enantioselectivities (entry 15).
Table 1.
Optimization of the Reaction Conditionsa
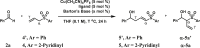 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Ar | Ligand | T (°C) | x | Total yieldb | γ/αb | ee of 5a′/5ac |
| 1 | Ph | (R)-BINAP | rt | 5 | 52% | 1/1.5 | 45% |
| 2 | Ph | (R)-TOL-BINAP | rt | 5 | 43% | 1/1 | 33% |
| 3 | Ph | (R)-SEGPHOS | rt | 5 | 42% | 1/1.5 | 8% |
| 4 | Ph | (R)-QUINAP | rt | 5 | 11% | 1/1 | 40% |
| 5 | Ph | (R,R)-QUINOXP* | rt | 5 | 48% | 1.5/1 | 8% |
| 6 | Ph | (R)-(S)-JOSIPHOS | rt | 5 | 51% | 1/1 | 58% |
| 7 | Ph | (R,Rp)-TANIAPHOS | rt | 5 | 47% | 1/2 | 51% |
| 8 | Ph | (R,R)-Ph-BPE | rt | 5 | 37% | 1/1 | 14% |
| 9 | Ph | (R)-DTBM-SEGPHOS | rt | 5 | 80% | >20/1 | 43% |
| 10 | 2-Py | (R)-DTBM-SEGPHOS | rt | 5 | 70% | >20/1 | 76% |
| 11 | 2-Py | (R)-DTBM-SEGPHOS | −40 | 5 | 76% | >20/1 | 97% |
| 12d | 2-Py | (R)-DTBM-SEGPHOS | −40 | 5 | 79% | >20/1 | 97% |
| 13d | 2-Py | (R)-DTBM-SEGPHOS | −40 | 30 | 92% | >20/1 | 97% |
| 14d,e | 2-Py | (R)-DTBM-SEGPHOS | −40 | 30 | 98% | >20/1 | 97% |
| 15d,e,f | 2-Py | (R)-DTBM-SEGPHOS | −40 | 30 | 82% | >20/1 | 97% |
HPLC, high-performance liquid chromatography; NMR, nuclear magnetic resonance
4/4′, 0.1 mmol; 2a, 0.2 mmol.
Determined by 1H NMR analysis of reaction crude mixture using mesitylene as an internal standard.
Determined by chiral-stationary-phase HPLC analysis.
4. 0.2 mmol; 2a. 0.1 mmol.
36 h.
Cu(CH3CN)4PF6 and ligand, 3 mol %, Barton's base = 2-tBu-1,1,3,3-tetramethylguanidine.
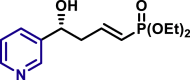
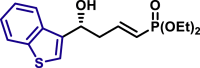
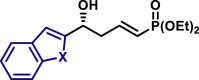
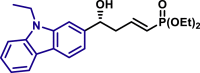
By modifying the optimized reaction conditions for 4 (3 equiv. 1, 20 mol % Barton's base, and −10°C), the substrate scope of aldehydes 2 in the reaction with allyl phosphonate 1 was studied (Table 2). Aromatic aldehydes with electron-withdrawing groups or electron-donating groups were competent substrates to generate the corresponding vinylogous products uniformly in good yields with both excellent regioselectivity (>20/1) and excellent enantioselectivity (≥95% ee) (3a-3o). Moreover, the reaction was not sensitive to the position of a substituent on the phenyl ring of the aromatic aldehydes. Even the sterically congested ortho-CF3-benzaldehyde afforded the product 3l in 91% yield with 95% ee. 1-Naphthaldehyde was also an excellent substrate (3p). Moreover, the present reaction conditions were applicable to various heteroaromatic aldehydes (3q-3x). Although the yields were moderate in some cases, both regio- and enantioselectivities were excellent. Particularly noteworthy are the aldehydes containing a pyridine motif (3t) and a carbazole motif (3x) as these functional groups potentially can coordinate to the metal center and thus deactivate the catalyst.
Table 2.
Substrate Scope of Aldehydes in the Reaction with 1a
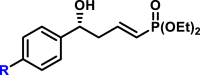 |
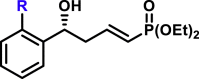 |
 |
|
| R = H, R = F, R = Cl, R = Br, R = I, R = Me, R = tBu, R = CH3S, R = CH3O, R = CF3O, |
3a, 85%, 99% ee;b 3b, 81%, 99% ee; 3c, 74%, 99% ee; 3d, 77%, 98% ee; 3e, 92%, 99% ee; 3f, 80%, 98% ee; 3g, 90%, >99% ee; 3h, 85%, >99% ee; 3i, 81%, 97% ee; 3j, 83%, 99% ee; |
R = F, 3k, 90%, 98% ee; R = CF3, 3l, 91%, 95% ee; R = OCH3, 3m, 91%, 98% ee; |
R = Cl, 3n, 81%, 98% ee; R = Br, 3o, 88%, 97% ee; |
 |
 |
||
| 3p, 94%, 99% ee; | X = O, 3q, 85%, >99% ee; X = S, 3r, 91%, >99% ee; |
||
 |
 |
 |
|
| 3S, 76%, 98% ee; | 3t, 55%, >99% ee; | 3u, 85%, >99% ee; | |
 |
 |
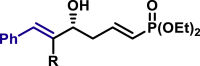 |
|
| X = O, 3v, 81%, 97% ee; X = S, 3w, 90%, >99% ee; |
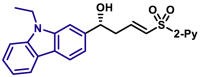
3x, 58%, >99% ee; | R = H, 3y, 76%, 97% ee; R = Me, 3z, 78%, 98% ee; |
|
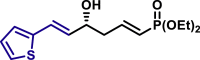 |
|||
| 3aa, 85%, 98% ee; | 3ab, 68%, 93% ee; | 3ac, 58%, 97% ee; | |
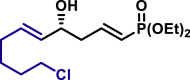 |
|||
| 3ad, 68%, 95% ee; | 3ae, 71%, 93% ee; | 3af, 71%, 97% ee; | |
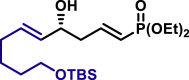 |
 |
||
| 3ag, 47%, 98% ee; | 3ah, 85%, 98% ee; | 3ai, 68%, 99% ee; | |
 |
 |
 |
|
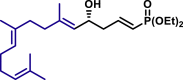
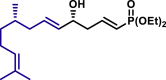
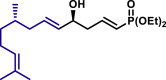
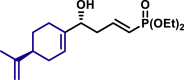
| 3aj, 52%, 15/1 dr; | 3aj′, 60%, >20/1 dr;c | 3ak, 58%, >20/1 dr; | |
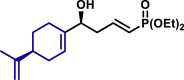 |
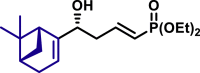 |
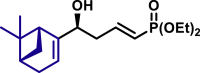 |
|
| 3ak′, 79%, >20/1 dr;c | 3al, 88%, >20/1 dr; | 3al′, 61%, >20/1 drc | |
HPLC, high-performance liquid chromatography; NMR, nuclear magnetic resonance
2, 0.3 mmol; 1, 0.9 mmol. Isolated yield was reported. Regio- and diastereoselectivity were determined by 1H NMR analysis of reaction crude mixture. Enantioselectivity was determined by chiral-stationary-phase HPLC analysis.
Gram-scale synthesis.
(S)-DTBM-SEGPHOS was used.
α,β-Unsaturated aldehydes also served as suitable substrates (3y-3ai). Aryls, heteroaryls, alkyls, and vinyls with substituent were well tolerated at the β-position of the α,β-unsaturated aldehydes. Moreover, functional groups, such as alkyl chloride (3af), tert-butyldimethylsilyl (TBS)-ether (3ag), alkynyl (3ah), and prenyl (3ai), remained intact in the present reaction conditions. These functional groups offer the opportunity for further structure elaboration. It is noteworthy that acrolein (2ab), susceptible to conjugate addition, served as a suitable substrate to give the vinylogous product 3ab in moderate yield with excellent enantioselectivity. The chiral aldehydes, including α,β-unsaturated aldehyde 2aj derived from (−)-citronellal, (−)-perillaldehyde (2ak), and (−)-myrtenal (2al), were also investigated with both (R)-DTBM-SEGPHOS and (S)-DTBM-SEGPHOS. In both cases, the products (3aj, 3aj′, 3ak, 3ak′, 3al, and 3al′) were obtained in good yields with excellent diastereoselectivity, which indicated that the asymmetric introduction was dominated by the copper(I)-catalyst. The absolute configuration of 3a was determined to be R by transforming it to a reported compound (for details, see Supplemental Information). The absolute configurations of other products were tentatively assigned by analogy.
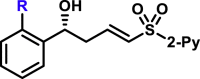
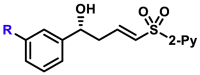
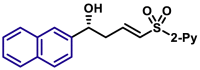
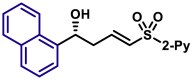
Moreover, the substrate scope of aldehydes in the reaction with allyl sulfone 4 was evaluated (Table 3). Various aromatic aldehydes with a substituent at ortho-, meta-, or para-position were suitable substrates. Both 1-naphthyl and 2-naphthyl aldehydes were well applicable. The vinylogous products (5a-5h, 5k, 5l, 5n, 5o, 5p, and 5am-5aq) were isolated in moderate to excellent yields with excellent regio- and enantioselectivities. Heteroaromatic aldehydes also served as competent substrates without compromising enantioselectivity (5q, 5r, 5u, 5x, 5ar and 5as). As for α,β-unsaturated aldehydes, aryls, heteroaryls, vinyls with substituent, and alkyls were accepted at β-position (5y, 5z, 5aa, 5ad, 5ae, 5at, 5au, and 5ah). Moreover, the reaction conditions were successfully applied to chiral natural products bearing α,β-unsaturated aldehyde moiety, such as (−)-perillaldehyde (2ak) and (−)-myrtenal (2al). The corresponding vinylogous products (5ak, 5ak′, 5al, and 5al′) were obtained in moderate yields with high diastereoselectivity. It is evident that in the case of (−)-myrtenal with (S)-DTBM-SEGPHOS, mismatch phenomenon was observed. The absolute configuration of 5a was assigned to be R by its transformation to a known compound (for details, see Supplemental Information). Analogically, the stereochemistry of other products was dictated tentatively. It should be pointed out that the gram-scale syntheses of both 3a and 5a were successfully carried out with constant results. Moreover, it should be mentioned that aliphatic aldehydes afforded α-adducts mainly in low yields at the present reaction conditions, which is a limitation of the present reactions. However, the vinylogous products of aliphatic aldehydes could be potentially accessed by the transformations of vinylogous products of various α,β-unsaturated aldehydes by means of the carbon-carbon double bond.
Table 3.
Substrate Scope of Aldehydes in the Reaction with 4a
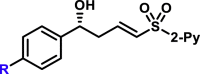 |
 |
 |
|||
| R = H, R = F, R = Cl, R = Br, R = I, R = Me, R = tBu, R = CH3S, R = CO2Me, |
5a, 95%, 97% ee;b 5b, 88%, 97% ee; 5c, 93%, 97% ee; 5d, 85%, 92% ee; 5e, 82%, 91% ee; 5f, 96%, 97% ee; 5g, 91%, 95% ee; 5h, 90%, 97% ee; 5am, 76%, 98% ee; |
R = F, R = Br, R = Me, R = CF3, |
5K, 95%, 98% ee; 5an, 85%, 92% ee; 5ao, 98%, 99% ee; 5l, 81%, 97% ee; |
R = F, R = Cl, R = Br, |
5ap, 80%, 97% ee; 5n, 81%, 98% ee; 5o, 76%, 98% ee; |
 |
 |
||||
| 5aq, 88%, 97% ee; | 5p, 97%, 95% ee; | ||||
 |
 |
 |
|||
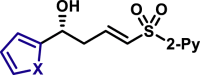
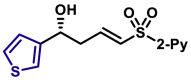
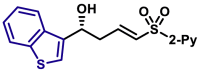
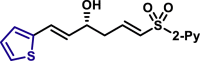
| X = O, 5q, 81%, 98% ee; X = S, 5r, 84%, 98% ee; |
5ar, 76%, >99% ee; | 5u, 85%, 95% ee; | |||
 |
 |
 |
|||
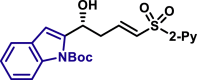
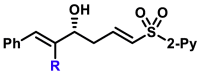
| 5as, 78%, 97% ee; | 5x, 46%, 93% ee; | R = H, 5y, 84%, 94% ee; R = Me, 5z, 51%, 95% ee; |
|||
 |
 |
||||
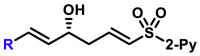
| 5aa, 80%, 92% ee; | R = Me, 5ad, 50%, 93% ee; R = nPr, 5ae, 70%, 97% ee; |
5at, 88%, 96% ee; | |||
 |
 |
||||
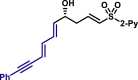
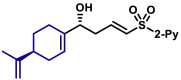
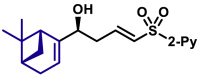
| 5au, 80%, 94% ee; | 5ah, 70%, 95% ee; | 5ak, 56%, >20/1 dr; | |||
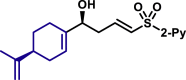 |
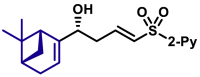 |
 |
|||
| 5ak′, 60%, >20/1 dr;c | 5al, 68%, >20/1 dr; | 5al′, 53%, 10/1 drc | |||
HPLC, high-performance liquid chromatography; NMR, nuclear magnetic resonance
2, 0.3 mmol; 4, 0.6 mmol. Isolated yield was reported. Regio- and diastereoselectivity were determined by 1H NMR analysis of reaction crude mixture. Enantioselectivity was determined by chiral-stationary-phase HPLC analysis.
Gram-scale synthesis.
(S)-DTBM-SEGPHOS was used.
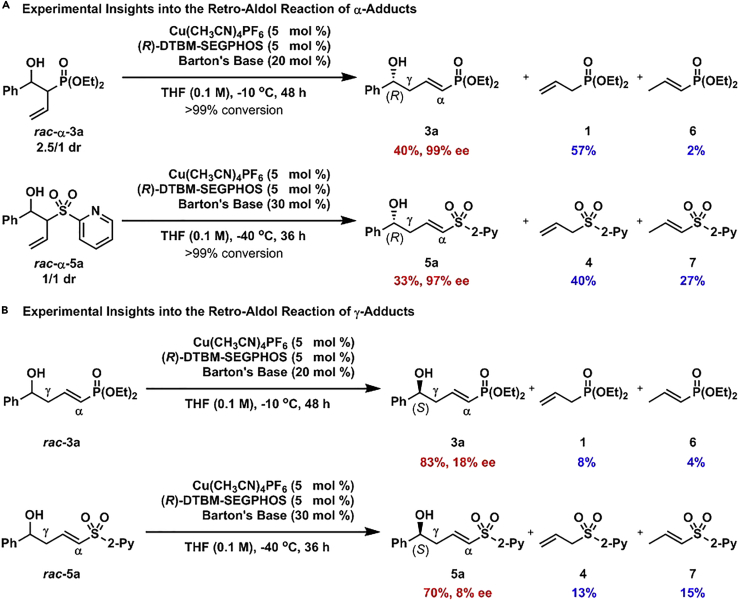
To get insights into the mechanism, rac-α-3a and rac-α-5a (racemic α-adducts) prepared according to a reported and a modified reaction procedure (for details, see Supplemental Information) were subjected to the standard reaction conditions, respectively (Scheme 2A). It was found that rac-α-3a was completely consumed and 3a was observed in 40% yield with 99% ee, together with benzaldehyde (2a), allyl phosphonate 1, and α,β-unsaturated phosphonate 6. These results clearly indicated that retro-aldol reaction of rac-α-3a proceeded to afford benzaldehyde (2a) and allyl phosphonate 1. One portion of allyl phosphonate 1 reacted with benzaldehyde (2a) to give the vinylogous product 3a in 40% yield with 99% ee in the presence of 5 mol % copper(I) catalyst and 20 mol % Barton's base. One portion of allyl phosphonate 1 isomerized to α,β-unsaturated phosphonate 6, whereas the other portion of allyl phosphonate 1 remained. The same tendency was also observed in the retro-aldol reaction of rac-α-5a. This phenomenon indicated that significantly reversible α-addition led to the transformation of α-adducts to γ-adducts, which finally led to excellent control of the regioselectivity.
Scheme 2.
Trials for retro-Aldol Reactions of α-Adducts and γ-Adducts
Rac-3a and rac-5a prepared by Cu(CH3CN)4PF6-rac-DTBM-SEGPHOS-catalyzed reactions were also submitted to the standard reaction conditions, respectively (Scheme 2B). Thin-layer chromatography, 1H nuclear magnetic resonance, and chiral high-performance liquid chromatographic analyses of the reaction crude mixtures indicated that slow and inefficient retro-vinylogous additions occurred, as 3a was obtained in 83% yield with −18% ee, whereas 5a was generated in 70% yield with −8% ee. It was obvious that the retro-vinylogous aldol reactions of both (R)-3a and (R)-5a proceeded selectively, which resulted in the slight enrichment of (S)-3a and (S)-5a in the reaction mixtures. However, these retro-vinylogous aldol reactions were very slow and inefficient, which would not have detrimental effect on the enantioselectivity in the catalytic asymmetric vinylogous aldol-type reactions of allyl phosphonate 1 and allyl sulfone 4. Based on these important experimental observations and literatures (Bazán-Tejeda et al., 2006, Yamaguchi et al., 2007, Bouaouli et al., 2018), a possible reaction pathway was proposed in Supplemental Information.
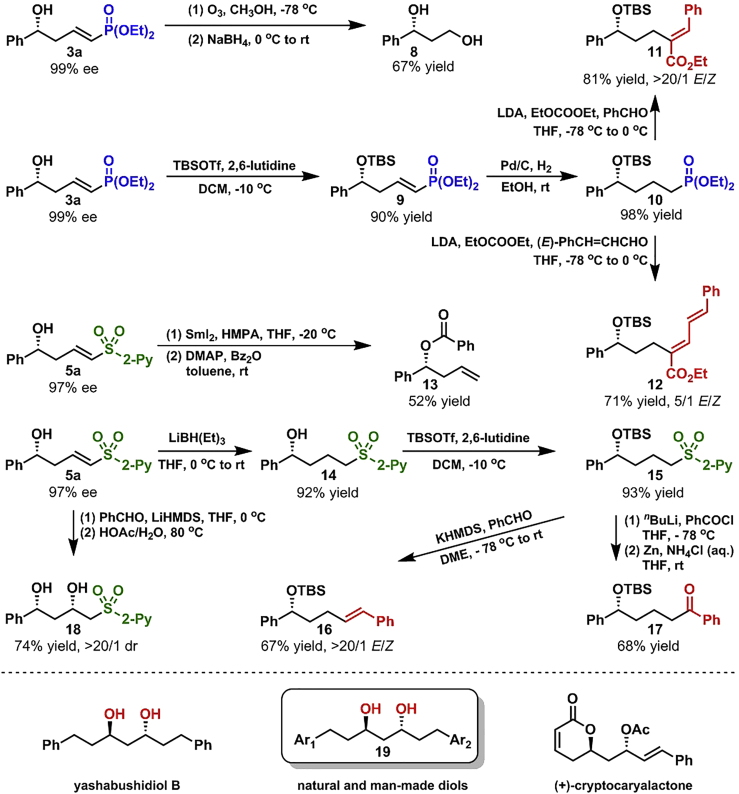
The transformations of the vinylogous aldol products (3a and 5a) were carried out as shown in Scheme 3. The cleavage of unsaturated double bond in 3a was easily achieved through ozonolysis to deliver diol 8 in 67% yield after the reduction of generated aldehyde moiety with NaBH4. After being protected as TBS-ether, 3a was reduced to phosphonate 10 with H2 in the presence of Pd/C. 10 Was easily transformed to α,β-unsaturated compounds 11 and 12 via α-functionalization and subsequent HWE olefination. The sulfone moiety in 5a was successfully removed without touching the double bond to afford ester 13 in 52% yield after the protection of the alcohol motif. Sulfone 15 was easily accessed through the reduction of the unsaturated double bond and the protection of the hydroxyl group in 5a, which was transformed to olefin 16 in 67% yield with >20/1 E/Z ratio through modified Julia olefination. Ketone 17 was prepared from 15 in 68% yield in two steps by α-functionalization and the following removal of the sulfone group. Moreover, chiral diol 18 was synthesized from 5a in 74% yield with >20/1 diastereoisomeric ratio (dr) in two steps through intramolecular oxo-Michael addition and the cleavage of the generated acetal motif. Furthermore, the synthetic utilities of the present methodology were showcased by its applications in the asymmetric synthesis of yashabushidiol B and the formal asymmetric synthesis of (+)-cryptocaryalactone (for the details, see Supplemental Information). Moreover, our synthetic route provided a straightforward method for the asymmetric synthesis of various chiral diols 19. Some of the diols 19 (both natural and man-made) exhibited significant anti-proliferative activity on some human cancer cell lines (Narasimhulu et al., 2009, Yokosuka et al., 2002).
Scheme 3.
Transformations of the Vinylogous Products
Limitations of Study
Aliphatic aldehydes were not applicable in the present reactions as α-adducts were obtained in low yields and no vinylogous products were generated. Fortunately, α,β-unsaturated aldehydes served as competent substrates and their vinylogous products could be potentially converted to the vinylogous products of aliphatic aldehydes through the transformations of carbon-carbon double bond.
Conclusion
In summary, two copper(I)-(R)-DBTM-SEGPHOS complex-catalyzed asymmetric vinylogous aldol-type reactions of aldehydes with allyl phosphonate and allyl sulfone were disclosed. These two reactions enjoyed advantages of 100% atomic economy, mild reaction conditions, easy reaction protocol, broad substrate scope, excellent regioselectivity, and excellent enantioselectivity. The mechanistic studies revealed a significantly reversible α-addition process and a slightly reversible γ-addition process, which accounted for the perfect control of the regioselectivity in these two vinylogous aldol-type reactions. Finally, various transformations of the vinylogous products (including HWE and Julia olefinations) were successfully carried out by means of phosphonate and sulfone. Application of the present methodology in the asymmetric synthesis of complex natural products is currently on the way in our laboratory.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We gratefully acknowledge the financial support from the “Thousand Youth Talents Plan,” the National Natural Science Foundation of China (No. 21672235 and No. 21871287), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000), CAS Key Laboratory of Synthetic Chemistry of Natural Substances, and the Shanghai Institute of Organic Chemistry.
Author Contributions
L.Y. conceived the project and designed the experiments. W.-J.Y. and C.-Y.Z. performed and analyzed the experiments. L.Y. wrote the manuscript. W.-J.Y. wrote the Supplemental Information and contributed other related materials. All the authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.03.010.
Supplemental Information
References
- Alba A.-N.R., Companyó X., Rios R. Sulfones: new reagents in organocatalysis. Chem. Soc. Rev. 2010;39:2018–2033. doi: 10.1039/b911852g. [DOI] [PubMed] [Google Scholar]
- Anastas P.T., Crabtree R.H. Wiley-VCH: Weinheim; 2009. Handbook of Green Chemistry-Green Catalysis. [Google Scholar]
- Bai X., Zeng G., Shao T., Jiang Z. Catalytic enantioselective γ-selective additions of 2-allylazaarenes to activated ketones. Angew. Chem. Int. Ed. 2017;56:3684–3688. doi: 10.1002/anie.201700190. [DOI] [PubMed] [Google Scholar]
- Bazán-Tejeda B., Bluet G., Broustal G., Campagne J.-M. α,β-Unsaturated δ-lactones from copper-catalyzed asymmetric vinylogous Mukaiyama reactions of aldehydes: scope and mechanistic insights. Chem. Eur. J. 2006;12:8358–8366. doi: 10.1002/chem.200600335. [DOI] [PubMed] [Google Scholar]
- Bisai V. Organocatalytic asymmetric vinylogous aldol reactions. Synthesis. 2012;44:1453–1463. [Google Scholar]
- Bouaouli S., Spielmann K., Vrancken E., Campagne J.-M., Gérard H. Mechanism of enolate transfer between Si and Cu. Chem. Eur. J. 2018;24:6617–6624. doi: 10.1002/chem.201800099. [DOI] [PubMed] [Google Scholar]
- Casiraghi G., Battistini L., Curti C., Rassu G., Zanardi F. The vinylogous aldol and related reactions: ten years of progress. Chem. Rev. 2011;111:3076–3154. doi: 10.1021/cr100304n. [DOI] [PubMed] [Google Scholar]
- Chinkov N., Majumdar S., Marek I. Stereoselective preparation of dienyl zirconocene complexes via a tandem allylic C–H bond activation-elimination sequence. J. Am. Chem. Soc. 2003;125:13258–13264. doi: 10.1021/ja036751t. [DOI] [PubMed] [Google Scholar]
- Corbridge D.E.C. Sixth Edition. CRC Press; 2013. Phosphorus: Chemistry, Biochemistry and Technology. [Google Scholar]
- Cuvigny T., Hervé du Penhoat C., Julia M. Synthesis with sulfones (XXX): stereoselective synthesis of arenesulfonyl-1,3-dienes. Tetrahedron Lett. 1983;24:4315–4318. [Google Scholar]
- Cuvigny T., Hervé du Penhoat C., Julia M. Syntheses with sulfones XLVI: stereoselective preparation of 2-benzenesulfonyl-1,3-dienes and 2-benzenesulfonyl-1,4-dienes. Tetrahedron. 1986;42:5329–5336. [Google Scholar]
- Denmark S.E., Heemstra J.R., Jr., Beutner G.L. Catalytic, enantioselective, vinylogous aldol reactions. Angew. Chem. Int. Ed. 2005;44:4682–4698. doi: 10.1002/anie.200462338. [DOI] [PubMed] [Google Scholar]
- Dunny E., Doherty W., Evans P., Malthouse J.P.G., Nolan D., Knox A.J.S. Vinyl sulfone-based peptidomimetics as anti-trypanosomal agents: design, synthesis, biological and computational evaluation. J. Med. Chem. 2013;56:6638–6650. doi: 10.1021/jm400294w. [DOI] [PubMed] [Google Scholar]
- El-Awa A., Noshi M.N., du Jourdin X.M., Fuchs P.L. Evolving organic synthesis fostered by the pluripotent phenylsulfone moiety. Chem. Rev. 2009;109:2315–2349. doi: 10.1021/cr800309r. [DOI] [PubMed] [Google Scholar]
- Fang Y., Luo Z., Xu X. Recent advances in the synthesis of vinyl sulfones. RSC Adv. 2016;6:59661–59676. [Google Scholar]
- Halskov K.S., Johansen T.K., Davis R.L., Steurer M., Jensen F., Jørgensen K.A. Cross-trienamines in asymmetric organocatalysis. J. Am. Chem. Soc. 2012;134:12943–12946. doi: 10.1021/ja3068269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-L., Chang C.-H. An asymmetric assembly of spirooxindole dihydropyranones through a direct enantioselective organocatalytic vinylogous aldol-cyclization cascade reaction of 3-alkylidene oxindoles with isatins. Chem. Commun. (Camb.) 2016;52:2322–2325. doi: 10.1039/c5cc08883f. [DOI] [PubMed] [Google Scholar]
- Hernández-Toribio J., Padilla S., Adrio J., Carretero J.C. Catalytic asymmetric synthesis of α-quaternary proline derivatives by 1,3-dipolar cycloaddition of α-silylimines. Angew. Chem. Int. Ed. 2012;51:8854–8858. doi: 10.1002/anie.201203828. [DOI] [PubMed] [Google Scholar]
- Ho S., Bucher C., Leighton J.L. A highly step-economical synthesis of dictyostatin. Angew. Chem. Int. Ed. 2013;52:6757–6761. doi: 10.1002/anie.201302565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornillos V., Vila C., Otten E., Feringa B.L. Catalytic asymmetric synthesis of phosphine boronates. Angew. Chem. Int. Ed. 2015;54:7867–7871. doi: 10.1002/anie.201502987. [DOI] [PubMed] [Google Scholar]
- Horsman G.P., Zechel D.L. Phosphonate biochemistry. Chem. Rev. 2017;117:5704–5783. doi: 10.1021/acs.chemrev.6b00536. [DOI] [PubMed] [Google Scholar]
- Hosokawa S. Remote asymmetric induction reactions using a E,E-vinylketene silyl N,O-acetal and the wide range stereocontrol strategy for the synthesis of polypropionates. Acc. Chem. Res. 2018;51:1301–1314. doi: 10.1021/acs.accounts.8b00125. [DOI] [PubMed] [Google Scholar]
- Hosokawa S. Recent development of vinylogous Mukaiyama aldol reactions. Tetrahedron Lett. 2018;59:77–88. [Google Scholar]
- Jing Z., Bai X., Chen W., Zhang G., Zhu B., Jiang Z. Organocatalytic enantioselective vinylogous aldol reaction of allyl aryl ketones to activated acyclic ketones. Org. Lett. 2016;18:260–263. doi: 10.1021/acs.orglett.5b03412. [DOI] [PubMed] [Google Scholar]
- Kalesse M., Cordes M., Symkenberg G., Lu H.-H. The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis. Nat. Prod. Rep. 2014;31:563–594. doi: 10.1039/c3np70102f. [DOI] [PubMed] [Google Scholar]
- Kim G., Chu-Moyer M.Y., Danishefsky S.J., Schulte G.K. The total synthesis of indolizomycin. J. Am. Chem. Soc. 1993;115:30–39. [Google Scholar]
- Kisanga P.B., Verkade J.G. P(RNCH2CH2)3N-Catalyzed 1,2-addition reactions of activated allylic synthons. J. Org. Chem. 2002;67:426–430. doi: 10.1021/jo0106492. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tanaka K., III, Kogen H. Recent topics of the natural product synthesis by Horner-Wadsworth-Emmons reaction. Tetrahedron Lett. 2018;59:568–582. [Google Scholar]
- Konno T., Shimizu K., Ogata K., Fukuzawa S. Rhodium-catalyzed enantioselective hydrogenation of unsaturated phosphonates by Click-Ferrophos ligands. J. Org. Chem. 2012;77:3318–3324. doi: 10.1021/jo300129m. [DOI] [PubMed] [Google Scholar]
- Lefevre N., Brayer J.-L., Folléas B., Darses S. Chiral α-amino phosphonates via rhodium-catalyzed asymmetric 1,4-addition reactions. Org. Lett. 2013;15:4274–4276. doi: 10.1021/ol402059w. [DOI] [PubMed] [Google Scholar]
- Li H., Yin L. Recent progress on direct catalytic asymmetric vinylogous reactions. Tetrahedron Lett. 2018;59:4121–4135. [Google Scholar]
- Li T.-Z., Jiang Y., Guan Y.-Q., Sha F., Wu X.-Y. Direct enantioselective vinylogous aldol-cyclization cascade reaction of allyl pyrazoleamides with isatins: asymmetric construction of spirocyclic oxindole-dihydropyranones. Chem. Commun. (Camb.) 2014;50:10790–10792. doi: 10.1039/c4cc04235b. [DOI] [PubMed] [Google Scholar]
- Lim K.M.-H., Hayashi T. Rhodium-catalyzed asymmetric arylation of allyl sulfones under the conditions of isomerization into alkenyl sulfones. J. Am. Chem. Soc. 2015;137:3201–3204. doi: 10.1021/jacs.5b00216. [DOI] [PubMed] [Google Scholar]
- Lim K.M.-H., Hayashi T. Dynamic kinetic resolution in rhodium-catalyzed asymmetric arylation of phospholene oxides. J. Am. Chem. Soc. 2017;139:8122–8125. doi: 10.1021/jacs.7b04570. [DOI] [PubMed] [Google Scholar]
- Liu P., Jacobson E.N. Total synthesis of (+)-Ambruticin. J. Am. Chem. Soc. 2001;123:10772–10773. doi: 10.1021/ja016893s. [DOI] [PubMed] [Google Scholar]
- Ma J.-H., Wang F., Wang J.-X., You Q.-D. Progress of Julia olefination in total synthesis of natural products. Chin. J. Org. Chem. 2010;30:1615–1623. [Google Scholar]
- Meadows D.C., Gervay-Hague J. Vinyl sulfones: synthetic preparations and medicinal chemistry applications. Med. Res. Rev. 2006;26:793–814. doi: 10.1002/med.20074. [DOI] [PubMed] [Google Scholar]
- Moteki S.A., Xu S., Arimitsu S., Maruoka K. Design of structurally rigid trans-diamine-based Tf-amide organocatalysis with a dihydroanthracene framework for asymmetric conjugate additions of heterosubstituted aldehydes to vinyl sulfones. J. Am. Chem. Soc. 2010;132:17074–17076. doi: 10.1021/ja107897t. [DOI] [PubMed] [Google Scholar]
- Moure A.L., Arrayás R.G., Carretero J.C. Catalytic asymmetric conjugate boration of α,β-unsaturated sulfones. Chem. Commun. (Camb.) 2011;47:6701–6703. doi: 10.1039/c1cc11949d. [DOI] [PubMed] [Google Scholar]
- Mucha A., Kafarski P., Berlicki Ł. Remarkable potential of the α-aminophosphonate/phosphinate structure motif in medicinal chemistry. J. Med. Chem. 2011;54:5955–5980. doi: 10.1021/jm200587f. [DOI] [PubMed] [Google Scholar]
- Narasimhulu M., Reddy T.S., Mahesh K.C., Krishna A.S., Rao J.V., Venkateswarlu Y. Synthesis of yashahushidiol and its analogues and their cytotoxic activity against cancer cell lines. Bioorg. Med. Chem. Lett. 2009;19:3125–3127. doi: 10.1016/j.bmcl.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Newhouse T., Baran P.S., Hoffmann R.W. The economies of synthesis. Chem. Soc. Rev. 2009;38:3010–3021. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M., Jacobsen C.B., Holub N., Paixão M.W., Jørgensen K.A. Asymmetric organocatalysis with sulfones. Angew. Chem. Int. Ed. 2010;49:2668–2679. doi: 10.1002/anie.200906340. [DOI] [PubMed] [Google Scholar]
- Nishida G., Noguchi M., Hirano M., Tanaka K. Enantioselective synthesis of P-stereogenic alkynylphosphine oxides by Rh-catalyzed [2+2+2] cycloaddition. Angew. Chem. Int. Ed. 2008;47:3410–3413. doi: 10.1002/anie.200800144. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Takiguchi Y., Hayashi T. Effect of chiral diene ligands in rhodium-catalyzed asymmetric addition of arylboronic acids to α,β-unsaturated sulfonyl compounds. J. Am. Chem. Soc. 2012;134:9086–9089. doi: 10.1021/ja303109q. [DOI] [PubMed] [Google Scholar]
- Ordóñez M., Sayago F.J., Cativiela C. Synthesis of quaternary α-aminophosphonic acids. Tetrahedron. 2012;68:6369–6412. [Google Scholar]
- Otsuka Y., Takada H., Yasuda S., Kumagai N., Shibasaki M. Direct catalytic asymmetric addition of allylic cyanides to aldehydes for expeditious access to enantioenriched unsaturated δ-valerolactones. Chem. Asian J. 2013;8:354–358. doi: 10.1002/asia.201201021. [DOI] [PubMed] [Google Scholar]
- Pansare S.V., Paul E.K. The organocatalytic vinylogous aldol reaction: recent advances. Chem. Eur. J. 2011;17:8770–8779. doi: 10.1002/chem.201101269. [DOI] [PubMed] [Google Scholar]
- Quintard A., Alexakis A., Mazet C. Access to high levels of molecular complexity by one-pot iridium/enamine asymmetric catalysis. Angew. Chem. Int. Ed. 2011;50:2354–2358. doi: 10.1002/anie.201007001. [DOI] [PubMed] [Google Scholar]
- Ray B., Mukherjee S. Direct catalytic enantioselective vinylogous aldol reaction of allyl ketones to pyrazole-4,5-diones. J. Org. Chem. 2018;83:10871–10880. doi: 10.1021/acs.joc.8b01566. [DOI] [PubMed] [Google Scholar]
- Shirokawa S., Kamiyama M., Nakamura T., Okada M., Nakazaki A., Hosokawa S., Kobayashi S. Remote asymmetric induction with vinylketene silyl N,O-acetal. J. Am. Chem. Soc. 2004;126:13604–13605. doi: 10.1021/ja0465855. [DOI] [PubMed] [Google Scholar]
- Trost B.M. The atom economy—a search for synthetic efficiency. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- Trost B.M., Dirat O., Gunzner J.L. Callipeltoside A: assignment of absolute and relative configuration by total synthesis. Angew. Chem. Int. Ed. 2002;41:841–843. doi: 10.1002/1521-3773(20020301)41:5<841::aid-anie841>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wang Z., Hayashi T. Rhodium-catalyzed enantioposition-selective hydroarylation of divinylphosphine oxides with aryl boroxines. Angew. Chem. Int. Ed. 2018;57:1702–1706. doi: 10.1002/anie.201712572. [DOI] [PubMed] [Google Scholar]
- Woo S.Y., Kim J.H., Moon M.K., Han S.-H., Yeon S.K., Choi J.W., Jang B.K., Song H.J., Kang Y.G., Kim J.W. Discovery of vinyl sulfones as a novel class of neuroprotective agents toward Parkinson’s disease therapy. J. Med. Chem. 2014;57:1473–1487. doi: 10.1021/jm401788m. [DOI] [PubMed] [Google Scholar]
- Xue Z.-Y., Li Q.-H., Tao H.-Y., Wang C.-J. A facile Cu(I)/TF-BiphamPhos-catalyzed asymmetric approach to unnatural α-amino acid derivatives containing gem-bisphosphonates. J. Am. Chem. Soc. 2011;133:11757–11765. doi: 10.1021/ja2043563. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Aoyama N., Matsunaga S., Shibasaki M. Ba-catalyzed direct Mannich-type reactions of a β,γ-unsaturated ester providing β-methyl aza-Morita–Baylis–Hillman-type products. Org. Lett. 2007;9:3387–3390. doi: 10.1021/ol071380x. [DOI] [PubMed] [Google Scholar]
- Yokosuka A., Mimaki Y., Sakagami H., Sashida Y. New diarylheptanoids and diarylheptanoid glucosides from the rhizomes of tacca chantrieri and their cytotoxic activity. J. Nat. Prod. 2002;65:283–289. doi: 10.1021/np010470m. [DOI] [PubMed] [Google Scholar]
- Yuan C., Yao J., Li S. Studies on organophosphorus compounds XLIV. Structural effect of electrophiles on the regioselectivity of carbanion derived from dialkyl allylphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 1990;53:21–27. [Google Scholar]
- Yuan C., Yao J., Li S. Studies on organophosphorus compounds XLV. Structural effects of ester alkyl group on dialkyl allylphosphonate carbanion on the regioselectivity of electrophilic addition. Phosphorus Sulfur Silicon Relat. Elem. 1991;55:125–131. [Google Scholar]
- Zhang H.-J., Yin L. Asymmetric synthesis of α,β-unsaturated δ-lactones through copper(I)-catalyzed direct vinylogous aldol reaction. J. Am. Chem. Soc. 2018;140:12270–12279. doi: 10.1021/jacs.8b07929. [DOI] [PubMed] [Google Scholar]
- Zhou T., Peters B., Maldonado M.F., Govender T. Enantioselective synthesis of chiral sulfones by Ir-catalyzed asymmetric hydrogenation: a facile approach to the preparation of chiral allylic and homoallylic compounds. J. Am. Chem. Soc. 2012;134:13592–13595. doi: 10.1021/ja306731u. [DOI] [PubMed] [Google Scholar]
- Zhu Q., Lu Y. Stereocontrolled creation of all-carbon quaternary stereocenters by organocatalytic conjugate addition of oxindoles to vinyl sulfone. Angew. Chem. Int. Ed. 2010;49:7753–7756. doi: 10.1002/anie.201003837. [DOI] [PubMed] [Google Scholar]
- Zhu B., Zhang W., Lee R., Han Z., Wang W., Tan D., Huang K.-W., Jiang Z. Direct asymmetric vinylogous aldol reaction of allyl ketones with isatins: divergent synthesis of 3-hydroxy-2-oxindole derivatives. Angew. Chem. Int. Ed. 2013;52:6666–6670. doi: 10.1002/anie.201302274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.