Abstract
Enzyme modified white cheese (EMWC) was produced to use as flavouring ingredient. White cheese curd coupled with low fat was hydrolysed using combination of proteinases/peptidase to produce a range of proteolysed products followed by lipolysis. The results revealed that lowering pH 5.6 known to impart flavour strength of cheese. The inclusion of enzyme preparations significantly elevated free amino acids and free fatty acids. Developed EMWC had relatively higher levels of volatiles and improved sensory characteristics including less negative attributes such as, astringent, bitter, pungent, rancid, smoky, and more positive attributes, such as the strength of buttery, sweaty, caramel and nutty notes. Spray-dried EMWC powders had low moisture content and water activity values whereas, scanning electron micrographs showed spherical with a uniform distribution and large microparticles size. Because consumers like low fat products with cheese flavour, EMWCs are important products. Thus, process demonstrates the potential to be a cost-effective to produce EMWC flavour as ingredient and may suited to the products in which added.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3526-y) contains supplementary material, which is available to authorized users.
Keywords: Enzyme-modified white cheese, Enzyme preparations, Proteolysis, Lipolysis, Spray-drying, Bioaroma powder
Introduction
Among food sources particular attention is paid to dairy ingredients due to their intense and unique flavour. White cheese is widely consumed and manufactured in many regions around the world, where it is known by different names, i.e. Telema, Feta, Danni, Jibnah, Iranian, Bulgarian and Turkish white cheese. It is a soft cheese with a salty and acidic taste and is brine ripened (4–5 °C) for 3 months (Akalin and Karaman 2011). The use of normal white cheese as a food ingredient has certain limitations, such as high cost due to the relatively long ripening time, deficiencies in texture, low flavour stability due to biochemical and microbiological changes during storage, low flavour consistency, and the large amount required to impart a given cheese flavour intensity to foodstuffs (Karaman and Akalin 2013). Meanwhile, owing to the risks associated with excessive dietary intake of fat and the increasing rates of obesity worldwide, there is increased consumer interest in low-fat dairy products. Enzyme-modified cheeses (EMCs) are concentrated cheese flavours and are natural in origin. Production of EMCs is a process manufactured by mixing raw cheese curds, water, low fat, proteins and salts into a homogeneous paste in conjunction with the thermal energies and proteinase/peptidase and/or lipase digestion (El-Bakry et al. 2011; Kilcawley et al. 2006; Miri and Najafi 2011). EMCs production technology provides minimized preparation time, a cost-effective alternative to natural cheeses, a high degree of cheese flavour intensity, extended product shelf life and a boarder choice for customers (Wilkinson et al. 2011). EMCs are used as cheese flavour ingredients in a wide range of foodstuffs, such as bakery products, creams, sauces, snack coatings, chips, dressings, and directly as flavouring on hot dishes like spaghetti and soups (Kilcawley et al. 1998; Miri and Najafi 2011). The usage of EMCs in these convenience foods confers the preferred flavour without an increase in fat content, as addition at the levels of 0.1% can strengthen dairy or cheesy notes for about 15–30 times higher than those of natural cheese, while contributing < 0.07% fat (Kilcawley et al. 1998). A novel, emerging application of EMCs are also explored as a source of bioactive peptides which arise from the extensive enzymatic hydrolysis of milk proteins. These include two angiotensin I-converting enzyme (ACE)-inhibitory peptides that have been demonstrated to have antihypertensive properties (Amighi et al. 2013; 2016). Thus, production of EMCs is an important industrial activity due to a greater demand for convenience foods together with the health-related benefits (Miri and Najafi 2011). However, despite economic importance there is a lack of detailed studies on the types of enzymes used and their specificities, enzyme dosage levels, curd substrate characteristics and process optimization (Miri and Najafi 2011; Wilkinson et al. 2011). EMC variants of many natural cheeses, such as Cheddar, Danish, Romano, Parmesan, Colby, Camembert, Mozzeralla, Brick and Emmental have been developed, which are commercially available with differing in flavour intensity (Kilcawley et al. 1998, 2000, 2006, 2012; Miri and Najafi 2011; Wilkinson et al. 2011). However, studies regarding to optimization of the production of enzyme modified white cheese (EMWC) flavours combined with low fat addition are relatively scarce. It was also author’s interest to produce a range of flavours desired in EMWC to spur further applications in white cheese-flavoured bakery products. Therefore, in this study we aimed (1) to generate proteolysed products and rank these products based on sensory preference, (2) to process the highest-ranking proteolysis product with different commercial lipases to generate a range of EMWC flavours, and (3) further investigated the microstructure, physical and thermal stability of EMWC bioaroma microparticles obtained by spray drying.
Materials and methods
Curd substrate manufacture
Fresh raw milk containing 3.5% milk fat was obtained from (Tianzi Dairy Company, Wuxi, Jiangsu, China), batch pasteurised at 70 °C in a water bath, cooled to 37 °C and supplemented with a freeze-dried 1.5% mixed culture containing Lactobacillus lactis subsp. lactis and Lactobacillus lactis subsp. cremoris (Danisco Co., Shanghai, China). 0.4 g CaCl2 per 2000 ml milk was added each batch to ensure sufficient calcium for the aggregation to obtain a proper flocks. For starter maturation milk was incubated at 37 °C for 1 h, after that rennet (Chymax-Plus, Chr Hansen, Cork, Ireland) was added to coagulate milk at the typical level of white cheese curd production. Following coagulation, curd was cut crossways, stirred, agitated and poured onto sieve, covered with cheese cloth for wheying-off. Curd was pressed for 2 h (35 kg final curd) and mixed to a homogenous mixture for the preparation of substrate. Batches of substrate (8.8 kg) were formulated as follows: 5 kg curd, 1.1 kg anhydrous milk fat, 2.5 l deionised water, 19 g NaCl (Merck Chemicals), 80 g Na2 HPO4, 40 g Na3PO4, 15.5 g potassium sorbate and 48.5 g trisodium citrate were blended to a homogeneous paste and heated at 80 °C for 10 min. The mixture was then cooled and stored at 4 °C using sterile sealed bags until required.
Enzyme modified white cheese flavour manufacture
A two-phase batch process was carried out (Fig. S1), for producing a range of new flavour ingredients using milk as raw material and commercial proteases, peptidase and lipases. In the first phase: 2 kg of each proteolysed products (1–5) was produced by the hydrolysis of white cheese curd substrate using proteinases in combination with peptidase (Fig. S1), and a control without enzyme using 2-l fermenter (Baoxing Co. Shanghai, China). The enzyme concentrations were incorporated as: Protease A “Amano” 2SD of 100,000 u/g and Peptidase R 420 u/g, Protease P “Amano” 3SD 300,000 u/g and Peptidase R 420 u/g, ProteAX 1400 u/g and Peptidase R 420 u/g, Neutrase® 0.5 l 1883 u/g and Peptidase R 420 u/g and Flavourzyme 1000 l® 1000 u/g and Peptidase R 420 u/g, respectively. The concentration of proteinase and peptidase to each preparation were identical in order to achieve proteolysis (pH 4.6-WSN) for incubation at 45 °C, for 24 h, and 500 rpm. Proteolysis was terminated at 80 °C and all products were stored at − 18 °C for the analysis. In the second phase: one of the selected proteolysed products based on ranked sensory preference analysis was further lipolysis (Fig. S1), to production of targeted EMWCs flavours (1–3). The lipase preparations to each product were incorporated as: Lipase AY “Amano”30SD 30,000 u/g, Lipase MER “Amano” 7500 u/g and Lipase A “Amano”12 120,000 u/g. Lipolysis was carried out at 45 °C, 24 h, 500 rpm and terminated at 80 °C for 20 min; finally the products were stored at − 18 °C for further analysis. The rationale behind the enzyme concentration levels, choice of incubation temperature, time and agitation speed for the proteolysis and lipolysis phases were selected based on the enzymes activity according to manufacturer’s guidelines and literature surveyed (Kilcawley et al. 1998, 2002, 2006; Amighi et al. 2016).
Compositional analysis
Samples were analysed in triplicate for moisture, protein, fat, ash, salt, calcium and phosphorous using IDF standard methods (Kilcawley et al. 2006). pH was measured using slurry prepared by macerating a 10 g of each sample with 10 ml H2O.
Proteolytic analysis
Samples with pH 4.6-water-soluble fractions (pH 4.6-WSFs) were prepared as described by (Hou et al. 2014). Proteolysis was monitored by measuring the percentage of total soluble N (pH 4.6-SN) or 5% phosphotungstic acid N (PTA-SN) of each pH 4.6-WSFs as described (Kilcawley et al. 2006). Nitrogen was determined by the macro-Kjeldahl method (IDF 1993). Molecular weight distribution of pH 4.6-WSFs was analysed on a Shodex Protein KW-804 column (Showa, Kyoto, Japan) using a LC system (Shimadzu, Kyoto, Japan) as described of (Wang et al. 2014). The gradient elution was carried with sodium phosphate buffer (0.05 M, pH 6.8) containing SDS (0.2%) and a flow rate of 0.7 ml/min. The thermostat was set at 30 °C and the detection wavelength was 214 nm. For peptide profile of each pH 4.6-WSFs, samples were diluted with mobile phase A (0.1% TFA in water) to a peptide concentration of 0.4 mg ml−1 and separated by a Nucleosil 300-5 C8 column (Machery-Nagel, Duren, Germany) using reverse phase-high performance liquid chromatography (RP-HPLC) as described by Kilcawley et al. (2006). Free amino acids (FAAs) were measured on 12% trichloroacetic acid filtrates prepared from the pH 4.6-WSFs and analysed using an amino acid analyzer (L-8800; Hitachi, Japan). All analyses were evaluated in triplicate for each sample.
Volatiles and free fatty acid analysis
For the analysis of volatile compounds in proteolysed product (5) and production EMWC (1–3) flavours, 3 g of above each sample was taken and placed in screw-capped headspace vial (20 ml) and sealed up with a Teflon cover. The vial was kept warm at 40 °C for 20 min with agitation, in order to reach compounds adsorption and desorption equilibrate. Extraction was achieved by using a 75 μm carboxen poly (dimethyl siloxane)-coated SPME fibre (Supelco, Bellefonte, PA, USA) into the vial and exposing it to the headspace for 30 min at 40 °C prior to adsorption. Headspace volatile compounds were analysed using an Thermo Scientific ISQ GC–MS (Trace GC Ultra gas chromatograph combined with ISQ, Thermo Scientific, USA) under the following conditions: injection temperature 280 °C; splitless mode; 5 min desorption time; DB5 MS column (30 m × 0.25 mm × 0.25 μm film thickness) (Restek, Bellefonte, PA, USA); oven temperature programmed: 35 °C start, 15 °C min−1 to 180 °C, then 20 °C/min to 260 °C and held for 30 s; He was carrier gas, flow rate 1 ml min−1 with the detector temperature 280 °C. Compounds were identified based on spectra matched of mass spectra with the NIST 2011 libraries. The abundance of sample compounds also compared with known standard amount to ensure both the SPME extraction and MS detection performed within specification. Peak areas and their relative abundance were calculated from the total ion current. Each sample was analysed in triplicate.
Lipid extraction and GC–MS analysis of free fatty acids (FFAs) were also carried out separately for each sample as described by De Jong and Badings (1990). GC-17A gas chromatograph (Shimadzu Scientific Instruments Inc., Columbia, MD, USA), equipped with an on-column injector and a flame ionization detector (FID) was used. The column was SGE, BP21-FFAP (15 m × 0.53 mm × 0.5 m i.d.). The quantification of the FFA levels of samples was performed using the internal standardization technique, i.e. C19:0 as an internal standard. The standard curve was constructed using six concentrations (0.002–0.2 mg ml−1) with [r2 = 0.99]. FFAs were further identified by its retention time and their concentrations were calculated by comparing the peak areas of the samples with those of the standards. Results were expressed as mg/kg. Samples were evaluated in triplicate.
Ranked preference and descriptive sensory analysis
The ranked preference and descriptive sensory sessions were conducted in panel booths, in a fluorescent-lighted room at the Sensory Analysis Laboratory of the School of Food Science and Technology, Jiangnan University (Jiangsu, Wuxi, China). For the ranked preference of proteolysed products (1–5), participants (n = 20, ages 24–33 years) familiar with the cheese taste and aroma were asked to rank the proteolysed products based on preference test using a method of Meilgaard et al. (1999). 10 g of each concentrate proteolysed products (1–5) diluted to 10% (w/w) curd substrate and coded with randomly selected 3-digit numbers were presented participants to rank the products. Data from preference analysis was computed for their statistical significance (P < 0.05) using Friedman’s test, Kendall’s coefficient and multiple comparison procedure (Meilgaard et al. 1999) to determine which product differed to each other (Meilgaard et al. 1999).
For the descriptive sensory analysis panellists (n = 22, ages 23–30 years) were asked to examine the production EMWCs (1–3). The individuals from panellist group were then trained to assess the cheeses using the descriptors outlined in Fig. 2b with defined descriptive terms (Table S2). Briefly, proteolysed product P-5 (control) and production WEMC flavours (1–3) kept at − 20 °C were thawed at 4 °C and presented to the panellists at an ambient temperature (~ 21 °C). Each sample (10 g) was immediately served to each individual assessor and coded with randomly selected 3-digit codes in a glass tumbler covered with a clock glass. Assessors were provided with water and plain crackers for palate cleansing to avoid error between the samples. The assessors then participated in ranking descriptive analysis using the consensus list of sensory descriptors (Hulin-Bertaud et al. 2000; Kilcawley et al.,2006), which was measured on a 10-cm line scale. The order of the presentation of all samples was randomized to prevent carryover effects, and all samples were presented in triplicate.
Fig. 2.
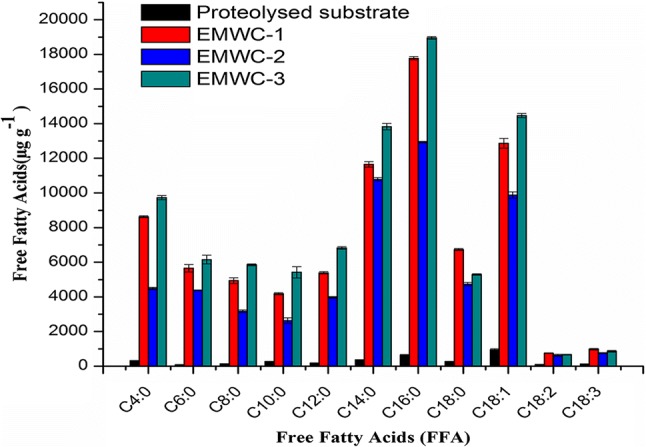
Free fatty acids profile of proteolysed substrate, developed white EMCs (1–3)
Drying process of enzyme modified white cheeses (EMWCs)
EMWCs were spray-dried using a GEA Niro spray dryer (Model Mobile Minor TM, Søborg, Denmark). Before each sample run, the dryer was route with distilled water for 10 min in order to achieve the desirable steady-state conditions. The drying air of chamber was circulating by the aspirating motor fan. The paste samples were dried at inlets drying air temperatures (150 °C), feeding pump powers (10%) and spraying air flows (600 l/h) as the most optimum cheese drying conditions (Amighi et al. 2016). Dried powder samples were collected, weighed and stored in airtight sterile bottles at 4 °C until analysis.
EMWCs physical properties analysis
The residual moisture content of each spray-dried EMWCs powder was determined gravimetrically at 105 °C.
Powder yield (%) was calculated as:
where P is the volume of powder collected in cyclone and vessel attached to the bottom of the dryer chamber, L is feed flow rate, Sp is total solid content in the resulting powder and Sf is total solid in the EMWCs paste feed (Chegini and Ghobadian 2007).
EMWCs powder solubility (%) was measured by weight difference as described by (Cono-Chauca et al. 2004).
S = solubility of powder, M1 = weight of empty dish, M2 = weight of dish and dried powder.
The water activity (aw) of EMWCs powder was measured at 25 °C using a digital water activity meter (Aqualab 4 TE, Decagon Devices Inc., USA). All analyses were performed for each sample in triplicate.
Thermogravimetric analysis (TGA)
Thermal stability of the EMWCs spray-dried powder was evaluated using thermogravimeter analyzer (TGA/SDTA851e; Mettler Toledo Instrument Co, Ltd, Switzerland). About 3.0 mg of each spray dried powder sample was used. The scans were run with heating from 50 to 600 °C at increasing rate of 10 °C/min under nitrogen atmosphere.
Scanning electron microscope (SEM)
EMWCs spray-dried powder morphology was observed by scanning electron microscope (LEO 1455VP, Cambridge, UK) at an accelerating voltage of 20 kV. Powders were attached onto the aluminum specimen holder with double-sided adhesive tape, spread and coated with a thin layer of gold for 60 s under vacuum followed by taking micrographs with magnifications of 2000 × and 10,000 × .
Statistical analysis
Data was analysed statistically using SPSS statistical software program version 16.0 (SPSS Inc. Chicago, IL, USA). Analysis of variance (ANOVA) and Duncan’s multiple range tests was used to determine significant (P < 0.05) differences among the results. Principal component analysis (PCA) was performed using a covariance matrix and varimax rotation between the desired samples using SPSS.
Results and discussion
Composition properties
The compositional parameters of the curd substrate, proteolysed products (1–5) and developed EMWCs (1–3) are presented in Table 1. The pH values of the proteolysed products and EMWCs flavors ranged from ~ 7.0–5.6 to ~ 5.5–5.1, and the protein content ranged from ~ 12.1–14.3 to ~ 13.2–14.7%, respectively. pH was decreased, while there was a slight increase in moisture content observed during the experimental products, which generally favors the enzymes activity and likely related to an increase in FAAs and FFAs due to the high levels of proteolysis and lipolysis (Kilcawley et al. 2006; Noronha et al. 2008). A homogenous level of NaCl distribution in EMWCs (1–3) was also observed, which confers a salty flavour to the end products. In cheese development, lower the Ca contents may disrupt calcium-mediated protein interactions thus weaken the protein matrix. However, an adequate amount of Ca and P in the products was also observed, which acts as a strong promoter of protein-to-protein interactions (Noronha et al. 2008), and suggested a homogenous conversion of substrate proteins via enhanced enzyme–substrate interactions (Kilara 1985a; Kilcawley et al. 2000).
Table 1.
Compositional parameters of the curd substrate, proteolysed products (1–5) and developed EMWCs (1–3)
| Products | PH | Moisture (%)b | Protein (%)b | Fat (%)b | NaCl (%)b | Ash (%)b | WSN TN%)a | PTA-SN (TN%)a | Ca (mg g−1) | P (mg g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Curd substrate | 7.0 ± 0.02 | 62.3 ± 0.11 | 12.1 ± 0.09 | 51.2 ± 0.18 | 4.1 ± 0.01 | 8.1 ± 0.11 | 5.7 ± 0.34 | 4.83 ± 0.07 | 37.2 ± 0.61 | 26.2 ± 1.23 |
| Product -1 | 6.2 ± 0.04 | 64.5 ± 0.08 | 13.1 ± 0.06 | 54.2 ± 0.04 | 3.9 ± 0.08 | 7.6 ± 0.61 | 52.3 ± 0.91 | 12.8 ± 0.16 | 37.2 ± 0.37 | 24.2 ± 0.67 |
| Product- 2 | 6.4 ± 0.00 | 63.3 ± 0.20 | 14.3 ± 0.00 | 55.3 ± 0.02 | 3.9 ± 0.17 | 7.5 ± 0.18 | 53.6 ± 0.31 | 09.2 ± 0.24 | 37.2 ± 1.28 | 26.0 ± 0.19 |
| Product- 3 | 5.8 ± 0.01 | 64.1 ± 0.34 | 14.3 ± 0.10 | 54.2 ± 0.22 | 3.7 ± 0.06 | 7.5 ± 0.33 | 52.7 ± 0.28 | 10.3 ± 0.61 | 36.9 ± 0.16 | 25.9 ± 0.83 |
| Product -4 | 6.5 ± 0.03 | 64.0 ± 0.06 | 13.2 ± 0.08 | 55.3 ± 0.16 | 4.0 ± 0.00 | 7.3 ± 0.29 | 54.3 ± 0.64 | 11.2 ± 0.13 | 37.0 ± 0.13 | 25.8 ± 0.71 |
| Product -5 | 5.6 ± 0.05 | 65.4 ± 0.07 | 13.2 ± 0.14 | 55.4 ± 0.05 | 3.2 ± 0.05 | 7.5 ± 0.06 | 55.6 ± 0.33 | 13.9 ± 0.41 | 37.2 ± 0.51 | 26.2 ± 0.29 |
| WEMC -1 | 5.3 ± 0.01 | 63.2 ± 0.51 | 14.6 ± 0.21 | 55.0 ± 0.13 | 3.5 ± 0.14 | 7.1 ± 0.10 | 51.2 ± 0.18 | 12.6 ± 0.21 | 37.1 ± 0.27 | 26.1 ± 0.55 |
| WEMC -2 | 5.5 ± 0.00 | 66.4 ± 0.38 | 13.9 ± 0.07 | 54.9 ± 0.14 | 3.6 ± 0.21 | 7.2 ± 0.63 | 50.4 ± 0.27 | 13.4 ± 0.10 | 37.0 ± 0.38 | 25.0 ± 0.37 |
| WEMC -3 | 5.1 ± 0.03 | 64.0 ± 0.14 | 14.7 ± 0.11 | 55.1 ± 0.27 | 3.3 ± 0.09 | 7.3 ± 0.28 | 52.6 ± 0.82 | 13.1 ± 0.09 | 37.2 ± 0.19 | 24.1 ± 0.64 |
Values were expressed as mean of triplicate determinations ± SE (n = 3)
EMWCs Enzyme modified white cheeses
aTN Total nitrogen
bDry weight basis
Proteolysis
The extent of proteolysis was described by pH-4.6 WSN%, PTA-SN%, size-exclusion distribution, peptide profiles, and amino acid contents. The pH-4.6 WSN level in the proteolysed products (1–5) was significantly (p < 0.05) increased compared to the substrate and varied from ~ 52.3 to 55.6%, whereas PTA-SN% had at least three-fold higher levels and ranged from ~ 4.8 to 13.9% (Table 1). SE-HPLC has been widely used for protein size fractionation and protein solubility demonstration for the degree of hydrolysis. The SE-HPLC profiles of the proteolysed products can be divided into three fractions, included high-molecular weight (HMW) proteins over 97,400 Da (F1), low-molecular weight (LMW) proteins 20,000–97,400 Da (F2) and peptides and amino acids under 20,000 Da (F3) with six well separated major peaks (Fig. 1a). Both the peak area and peak height of F3 fraction significantly increased and was attributed to higher accumulation of amino acids and peptides through the hydrolysis. While, no obvious changes were detected for the proportion of F1 as EMC paste are mostly composed of short and medium chain peptides and amino acids rather than HMW proteins (Amighi et al. 2016). The differences in proteolytic assay also showed the extensive accumulation of low molecular weight peptides in the proteolysed products, and were evident from the RP-HPLC peptide profiles (Fig. 1b) and free amino acids (FAAs) contents (Fig. 1c). The RP-HPLC peptide profiles of the proteolysed products were apart from early eluting peptides (RT ~ 50), indicating that the products treated with different proteinases combined with peptidase had a higher proportion of peptides released, which likely resulted from the action of endo-peptidases (Kilcawley et al. 2006). It is worth noting that the proteolytic degradation of the cheese substrate into small and medium molecular weight peptides and FAAs significantly contributes to the intense background flavour and perception of cheeses (McSweeney and Sousa 2000). The effect of the different proteolytic treatments on mean concentrations of FAAs in products (1–5) were evaluated statistically (P < 0.05) (Fig. 1c). Compared to the base substrate, FAA levels of proteolysed products were significantly (p < 0.05) higher and this can be attributed to the characteristic cheese flavour and also served as precursors for other catabolic reactions that produced keto acids, ammonia, amines, aldehydes, acids and alcohols which are essential to the cheese taste and aroma (Iličić et al. 2012). The FAAs contents in proteolyzed products 1, 2 and 3 were higher than those observed in product 4 but differed from product 5 which was generated with Flavourzyme® 1000 l. This is consistent with the results of a study by Park and Lee (2015), in which soy yogurt treated with Flavourzyme® 1000 l also accumulated higher FAA contents. Thus, comparatively lower and higher FAA content in proteolysed products was probably due to the different enzymes used in their production. Similarly, Kilcawley et al. (2006) also reported that the enzymes used in the production of cheddar-type EMCs have a major role in FAA production. The proteolysed products also differed in individual FAA content. Different proteolytic preparations used to generate products 1–5 did not significantly affect the release of Asp, Ser, Arg and Pro. Products 2 and 4 differed (p < 0.05) with respect to His, Tyr and Met, whereas products 1 and 4 differed with respect to Asp, Gly, Tyr, Lys, Val, Leu and Ile. A particularly important finding in this study was the glutamic (Glu) acid a natural flavour potentiate which was the highest in all products and suggesting that there was a high level of glutaminase activity in the enzymes used. In product 5, Glu acid accounted for 12% of FAAs. This increased level of Glu acid was potentially important because it is added as a flavour enhancer to reduce bitterness and impart specific flavour notes to the end point products (Kilara 1985b; Kilcawley et al. 2006). Thus, it was apparent that extensive proteolysis was an integral part of the EMWCs process development.
Fig. 1.
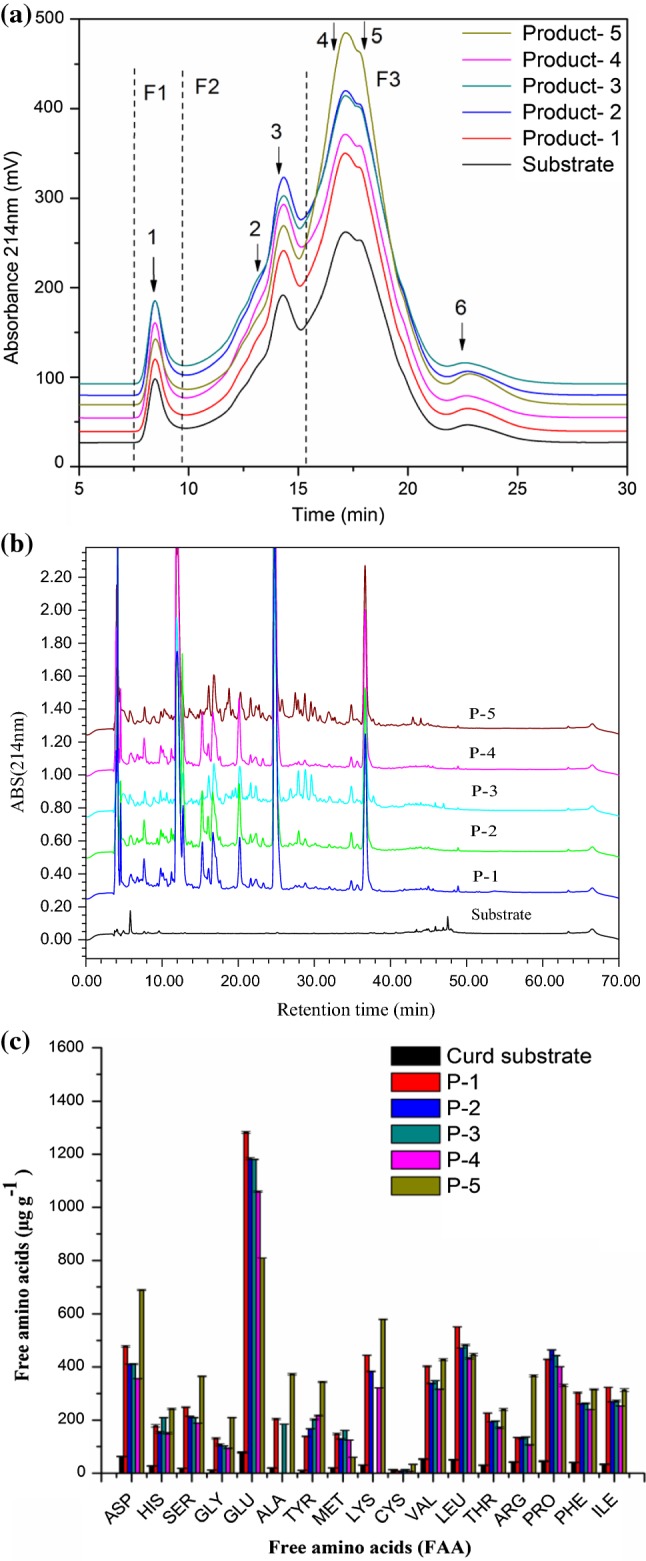
a Reverse-phase HPLC peptide profiles of the curd substrate and proteolysed products (1–5). b Molecular weight distribution profiles of the curd substrate and proteolysed products (1–5). Fractions: high-molecular-weight proteins over 97,400 (F1), low-molecular-weight proteins ranging from 20,000 to 97,400 (F2) and peptides and amino acids under 20,000 (F3). c Individual free amino acid contents of the base substrate, proteolysed products (1–5)
Ranked preference
Statistical analysis of the ranked preference using the Friedman test and Kendall’s coefficient revealed significant (p < 0.05) differences between proteolysed products (1–5) (Table S1). Overall, there was a good correlation between the aroma intensity in the proteolysed products. However, multiple comparison analysis for ranked data showed that only products 4 and 5 were different (p < 0.05) from their counterparts. Therefore, as product 5 gained the highest preference score, it was then further selected as the base substrate for lipolysis using different lipase preparations to produce a range of EMWC flavours.
Lipolysis
Lipolysis is an important component in the production of short and medium-chain free fatty acids (FFAs), particularly those that contribute to flavour and aroma intensity. Figure 2 shows the levels of FFA in the proteolysed substrate (P-5) and EMWCs (1–3). The general trend of FFA increased (p < 0.05) applied with different lipase preparations, of which ~ 36, ~ 32 and ~ 39% were aromatic short and medium-chain fatty acids (C4:0 to C12:0). This may be due to their preferential release from triglycerides by lipases with known differences in acyl and regio selectivity (Kilara 1985b; Kilcawley et al. 2006). The lipolytic enzymes cleave the ester linkage between a fatty acid and the glycerol backbone of triacylglycerides to generate key flavour compounds, such as FFAs. The FAAs that accumulated in the developed EMWCs (1–3) were very distinct. EMWC-1 and EMWC-3 had higher levels of short and medium-chain C4:0-C12:0, C14:0, C16:0, and C18:1 acids, whereas EMWC-2 had higher levels of C6:0, C16:0, C18:1 and C18:2. FFAs are typically used as a qualitative reference for cheese, milk, butter, and other dairy products (Mannion et al. 2015), and elevated FFA levels also contributed to the sensory aspects of the products.
Volatile compounds
GC–MS analysis of the proteolysed products and EMWCs (1–3) revealed 58 volatile compounds (Table 2). These volatiles consisted of several chemical classes: alcohols (12), aldehydes (8), ketones (10), esters (8), acids (11) and hydrocarbons (9). Most of these compounds have previously been identified to be present in white cheese as a result of the metabolism of carbohydrates, fats and amino acids (Akalin and Karaman 2011; Sahingil et al. 2014). EMWC-1 and 3 were characterized as containing high levels of aldehydes, hydrocarbons, acids, alcohol, ketones, and esters. The major volatile group was carboxylic acids that comprised of higher total values, since they are associated with sweet, fruity, cheesy, sharp, acrid, piquant odour notes and also act as precursors of methyl ketones, alcohols, aldehydes and esters (McSweeney and Sousa 2000; Zellner et al. 2008). However, all the cheeses (EMWC 1–3) contained high concentrations of volatile acitic, butanoic, pentanoic and hexanoic acids, the main dairy flavour and odour compounds. In cheese volatile acids are mainly released on the lipolysis of the fat and also produced through the catabolism of amino acids such as Val, Thr, Glu and Met (Sahingil et al. 2014; McSweeney and Sousa 2000). Alcohols was the quantitatively largest volatile group, whereas the formation of these compounds in cheese results from their proteolysis, lipolysis and are partly responsible for the sweet and fresh flavour (Kurtovic et al. 2016). The straight-chain aldehydes (hexanal, nonanal, decanal), 2-heptenal and 3-methyl-1-butanal were the most abundant aldehydes compounds and contributes to herbaceous aroma (Moio et al. 1993). Similar trends of 3-Methyl-1-butanal higher levels in white cheese also reported by (Sahingil et al. 2014). 2-nonanone, 2-heptanone and 2-undecanone were associated by a high level of ketones and give a creamy flavour. These compounds have been widely reported in white cheese, and the enzyme–substrate hydrolyze significantly affected their concentrations. Besides, decanoic acid ethyl ester, and acetic acid ethyl ester were the most abundant esters responsible for fruity note. Esters are formed by the deamination of the Ser, Ala, and Gly (Wallace and Fox 1997) and this reaction may be mediated by the enzymes used. Overall, data indicates that the EMWC products of lipolysis largely characterize the volatile profiles. This greater diversity of volatiles in EMWCs is in part and in good agreement with (Kilcawley et al. 2012; Omar et al. 2015), due to the enhanced levels of secondary proteolysis (Table 1), and lipolysis which increased the number of available peptides and FAA for products catabolism (Fig. 1a, c), and the amount of FAA (Fig. 2) released from substrate enzymatic lysis.
Table 2.
Mean values ± SD (area%) of volatile compounds identified in the substrate (Product-5) and developed EMWCs (1–3)
| Compounds | 1RT | Area2 (%) | |||
|---|---|---|---|---|---|
| Substrate (P-5) | EMWC-1 | EMWC-2 | EMWC-3 | ||
| Alcohols | |||||
| Ethanol* | 950 | 2.07 ± 0.13c | 2.67 ± 0.62a | 2.34 ± 0.65b | 2.11 ± 0.83c |
| 1-Dodecanol | 844 | 1.64 ± 0.08a | 0.61 ± 0.11d | 0.83 ± 0.08c | 1.01 ± 0.53b |
| 1-Hexanol* | 953 | 0.51 ± 0.07b | 0.54 ± 0.11b | 0.43 ± 0.08c | 0.76 ± 0.16a |
| 2,3-Butanediol | 943 | 1.11 ± 0.06c | 1.61 ± 0.04a | 1.43 ± 0.08b | 1.42 ± 0.02b |
| 1,2-Propanediol | 947 | 2.25 ± 0.18c | 3.21 ± 1.06b | 4.25 ± 0.67a | 4.26 ± 0.86a |
| 2-Pentanol | 875 | ND | 0.38 ± 0.08b | 0.23 ± 0.06c | 0.56 ± 0.13a |
| 1,3-Butanediol | 959 | 1.18 ± 0.27d | 2.14 ± 0.10b | 3.18 ± 1.61a | 1.86 ± 0.84c |
| 3-Methyl-1-butanol* | 906 | 0.41 ± 0.08b | 1.46 ± 0.61a | ND | ND |
| 1-Octanol* | 933 | 1.21 ± 0.03bc | 1.61 ± 0.17a | ND | 1.16 ± 0.08b |
| 1-Butanol* | 925 | 3.34 ± 1.02c | 3.18 ± 0.06d | 4.61 ± 0.21a | 3.81 ± 0.31b |
| 1-Heptanol* | 835 | 2.18 ± 0.84d | 4.92 ± 1.25b | 3.68 ± 0.94c | 5.08 ± 0.64a |
| Phenol* | 938 | 4.74 ± 2.11d | 5.51 ± 0.03b | 5.16 ± 0.13c | 5.85 ± 0.03a |
| Total | 20.64 | 27.84 | 26.14 | 27.88 | |
| Aldehydes | |||||
| Hexanal | 876 | 0.21 ± 0.04b | 0.69 ± 0.08a | 0.22 ± 0.10b | 0.23 ± 0.14b |
| Nonanal* | 944 | 0.52 ± 0.34b | ND | 0.62 ± 0.03a | 0.35 ± 0.03c |
| Decanal* | 894 | 0.13 ± 0.01c | 0.21 ± 0.03a | 0.18 ± 0.01ab | ND |
| (E)-2-Octenal | 920 | 0.13 ± 0.06d | 0.61 ± 0.11b | 0.51 ± 0.08c | 0.73 ± 0.24a |
| (Z)-2-nonenal | 909 | 0.19 ± 0.05 cd | 0.31 ± 0.08b | 0.47 ± 0.14a | 0.27 ± 0.07c |
| 2-Heptenal | 936 | 0.18 ± 0.04c | 0.22 ± 0.08b | ND | 0.43 ± 0.06a |
| 3-Methyl, butanal | 815 | 6.74 ± 0.19d | 12.41 ± 0.43a | 11.54 ± 0.61b | 10.84 ± 0.79c |
| (E)-2-Decenal | 809 | 0.37 ± 0.09c | 0.91 ± 0.16a | ND | 0.67 ± 0.21b |
| Total | 8.47 | 15.36 | 13.54 | 13.52 | |
| Ketones | |||||
| 1-Hydroxy-2-Propanone | 999 | 0.13 ± 0.05d | 1.56 ± 0.31a | 0.86 ± 0.03c | 1.12 ± 0.16b |
| 2-Nonanone* | 967 | 4.67 ± 0.61d | 5.42 ± 1.53c | 6.58 ± 1.43b | 8.26 ± 2.37a |
| 2-Undecanone | 911 | 1.21 ± 0.11d | 2.64 ± 0.18a | 1.63 ± 0.06c | 2.28 ± 0.31ab |
| 2-Dodecanone | 931 | 0.59 ± 0.11b | 0.74 ± 0.18a | 0.55 ± 0.04b | 0.39 ± 0.03c |
| 6-Methyl-3-Heptanone | 874 | ND | 0.97 ± 0.06a | 0.86 ± 0.21b | ND |
| 2-Octanone | 939 | 1.81 ± 0.06ab | 2.11 ± 0.37a | 1.84 ± 0.19ab | 1.42 ± 0.24c |
| 2-Pentanone* | 851 | 1.32 ± 0.10d | 2.82 ± 0.70a | 1.61 ± 0.20c | 1.84 ± 0.26b |
| 2-Heptanone* | 961 | 6.61 ± 0.61c | 7.42 ± 1.61b | 6.52 ± 0.94c | 9.21 ± 2.13a |
| 3-Hydroxy-2-Butanone* | 976 | 2.76 ± 0.21b | 2.51 ± 0.26b | 1.87 ± 0.31c | 3.11 ± 0.61a |
| 2-Pentadecanone | 929 | ND | 0.74 ± 0.16a | 0.46 ± 0.08bc | 0.53 ± 0.14b |
| Total | 19.23 | 26.93 | 22.71 | 28.16 | |
| Esters | |||||
| Octanoic acid, ethyl ester | 905 | 0.78 ± 0.14b | 0.53 ± 0.03c | 0.77 ± 0.11b | 0.93 ± 0.06a |
| Butanoic acid, 2-furanylmethyl ester* | 791 | 0.62 ± 0.07c | 0.43 ± 0.03d | 0.84 ± 0.13b | 0.91 ± 0.21a |
| Hexanoic acid, ethyl ester * | 941 | ND | 0.19 ± 0.02c | 0.48 ± 0.10a | 0.27 ± 0.06b |
| 2-Hydroxy-propanoic acid, ethyl ester | 989 | 0.25 ± 0.05c | 1.66 ± 0.11a | ND | 0.67 ± 0.08b |
| Decanoic acid, ethyl ester | 927 | 11.74 ± 1.37c | 10.33 ± 2.19d | 14.37 ± 2.09a | 12.82 ± 1.95b |
| Acetic acid, ethyl ester* | 912 | 6.93 ± 0.64d | 9.83 ± 1.37b | 8.64 ± 0.86c | 10.82 ± 2.19a |
| Ethyl Acetate* | 948 | 1.44 ± 0.08b | 1.64 ± 0.31a | 0.67 ± 0.18c | 1.21 ± 0.51b |
| Methyl Butyric Acid* | 782 | 0.21 ± 0.11b | 0.31 ± 0.04a | 0.13 ± 0.08c | ND |
| Total | 21.97 | 24.92 | 25.9 | 27.63 | |
| Volatile acids | |||||
| Acetic acid* | 981 | 4.73 ± 0.51d | 8.33 ± 1.20a | 6.64 ± 0.73c | 7.19 ± 1.27b |
| Decanoic acid* | 932 | 0.32 ± 0.03d | 1.45 ± 0.41ab | 1.69 ± 0.82a | 0.78 ± 0.07c |
| Butanoic acid* | 970 | 19.28 ± 0.67c | 17.47 ± 1.84d | 22.56 ± 3.11b | 25.38 ± 0.95a |
| Pentanoic acid | 954 | 2.59 ± 0.57a | 2.91 ± 0.74a | 2.23 ± 0.66b | 1.87 ± 0.28bc |
| Hexanoic acid* | 980 | 10.18 ± 1.05d | 13.43 ± 2.71b | 17.71 ± 3.11a | 11.94 ± 0.89c |
| Heptanoic acid* | 904 | 0.11 ± 0.03c | 0.57 ± 0.05a | 0.44 ± 0.07b | 0.59 ± 0.02a |
| Octanoic acid* | 972 | 13.71 ± 2.61ab | 12.26 ± 1.61c | 14.06 ± 3.18a | 13.03 ± 1.10b |
| Dodecanoic acid | 793 | 1.73 ± 0.66c | 2.69 ± 0.37b | 3.26 ± 1.23a | 1.37 ± 0.39c |
| Propanoic acid | 859 | ND | 0.73 ± 0.06b | 0.61 ± 0.14c | 0.81 ± 0.08a |
| Benzoic acid | 822 | 1.84 ± 0.31a | 0.62 ± 0.04d | 0.82 ± 0.08c | 1.25 ± 0.64b |
| 2-Methyl-Propanoic acid | 900 | 0.94 ± 0.31a | 0.37 ± 0.01 cd | 0.67 ± 0.16b | 0.48 ± 0.21c |
| Total | 55.97 | 60.83 | 70.69 | 64.69 | |
| Hydrocarbons | |||||
| Hexadecane | 907 | 0.53 ± 0.13b | 0.19 ± 0.06d | 0.67 ± 0.11a | 0.35 ± 0.07c |
| 3,5-Dimethyl-octane | 856 | 0.65 ± 0.24a | 0.41 ± 0.07c | 0.58 ± 0.09b | 0.37 ± 0.03 cd |
| 2-Methyl-decane | 845 | 0.21 ± 0.02a | 0.08 ± 0.03c | 0.15 ± 0.06b | ND |
| Undecane* | 887 | 0.28 ± 0.11a | 0.19 ± 0.07b | 0.17 ± 0.03b | 0.20 ± 0.04a |
| Dodecane* | 913 | 0.33 ± 0.18a | ND | 0.35 ± 0.21a | 0.26 ± 0.05b |
| Nonadecane | 922 | 2.85 ± 1.83b | 3.47 ± 2.13a | 3.05 ± 0.94a | 2.64 ± 0.38b |
| O-Xylene | 955 | ND | 0.24 ± 0.17a | 0.15 ± 0.03b | ND |
| 3-Ethyl-2-methyl-1-pentene | 866 | 1.27 ± 0.31a | 1.23 ± 0.61a | 0.42 ± 0.06c | 1.64 ± 0.22b |
| 4-Methyl-Undecane | 807 | 0.41 ± 0.04b | 0.35 ± 0.11c | ND | 0.64 ± 0.31a |
| Total | 6.7 | 6.42 | 5.68 | 6.1 | |
The results are mean ± SD (n = 3) and different superscript letters in the same column are significantly different at P < 0.05
P-5; (Substrate), EMWC—1–3; (Enzyme modified white cheeses)
1RT = Retention Index
2Results are peak area percentages
ND Not detected
Compounds marked with (*) were reported to be present in White Cheese (Akalin and Karaman 2011; Sahingil et al., 2014)
Sensory profiles
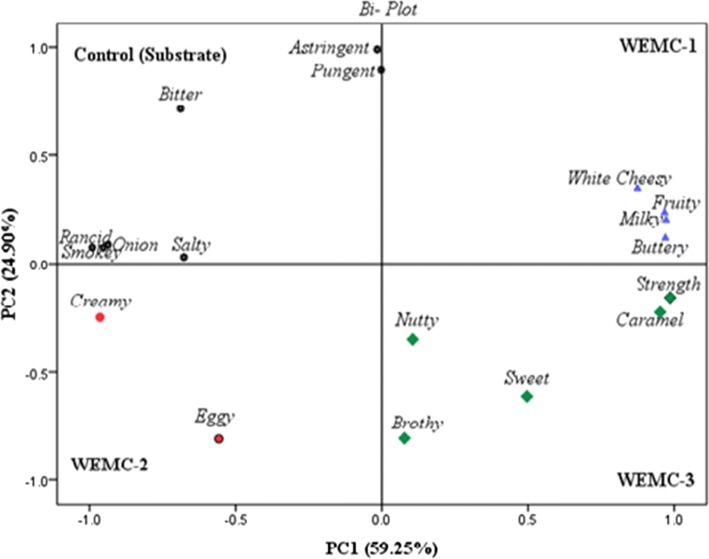
The study revealed a positive effect on the sensory properties of developed EMWCs. The PCA discriminates panelist data score for the sensory profile of developed EMWCs (1–3) in a bi-plot of PC1 and PC2 and accounts for a cumulative variation of 84% (Fig. 3). EMWC-2 which was produced using Lipase A “Amano”12” received lower scores, whereas the substrate (control) had a more bitter flavour than the developed EMWCs (1–3). Lowered levels of bitterness also have been reported in various EMCs studies where added milk fat and the introduction of peptidase and fungal lipases have a significant role in de-bittering (Kilcawley et al. 2006; Azarnia et al. 2011). The “creamy” attribute is associated with lipid content and the reduction levels in EMWCs (1, 3) was may be due to the formation of more carboxylic acids. The ‘nutty’, ‘brothy’ and ‘sweet’ sensory attributes were used to describe all EMWCs at significantly higher levels compared to the control (1–3). These terms were significantly correlated with EMWC-3, and also have role in bitterness reduction. A suitable explanation for the creamier and sweeter flavour of EMWC 2 and 3 may reside in their significantly higher concentrations of glutamate, leucine, proline, aspartate, butyric acid and hexanoic acid. Similarly, Kilcawley et al. (2006) carried out a comparable descriptive sensory study on Cheddar-type EMCs and concluded that the introduction of proteinases, peptidase and/or lipases are capable of modifying sensory qualities of EMCs with added milk fat. It is worth noting that none of the EMC products are deemed to be identical to natural cheese but with exaggeration of flavour profile and consumed as flavour ingredient in a food products (Kilcawley et al. 2006; Noronha et al. 2008). Overall, process enabled to have a significant impact on EMWCs products sensory characteristics.
Fig. 3.
Sensory profile of proteolysed substrate developed white EMCs (1–3)
Physical properties
The spray dried products were collected and analysed for yield, moisture, water activity (aw) and the degree of solubility. Powders obtained had yield of proteolysed substrate (P-5) 92.6% and developed EMWCs (1–3) 91.7, 94.3 and 93.1% whereas, the moisture content of substrate (P-5) was 8.7% and EMWCs (1–3) were 9.3, 8.6 and 9.1%. These results were in accordance with reported by (Gianfrancesco et al. 2008; Amighi et al. 2016) of drying infant food powder and Iranian white cheese. aw is a measure of the availability of free water in food system that can take part in any biochemical reaction. For food powders with a high level of aw (> 0.3%), the shelf life is limited (Kumar and Mishra 2004; Fazaeli et al. 2012). aw content of substrate (P-5) was 2.4% and for EMWCs (1–3) were 2.1, 2.7 and 2.6%, which can be considered microbiologically safe and stable during storage. Solubility is another important parameter as food powders are intended for rehydration and dissolve within a short period of time without lumps (Amighi et al. 2016). The solubility of substrate (P-5) was 72.3% and EMWCs (1–3) were 68.8, 74.6 and 73.3%. The differences in physical properties of spray dried products were not as significant as envisaged.
Thermogravimetry analysis
The thermogravimetry and differential thermal analysis (TG–DTA) is a technique for determining both the mass loss and the thermal response in various food products. Thermal stability of spray dried powder defined as the thermal stressing resistant capability of powder for a exposure time without appreciable deterioration. TG–DTA as a function of thermal response of proteolysed substrate (P-5) and developed EMWC 1–3 bioaroma powders were obtained at 10 °C/min under inert atmosphere (Fig. 4 a, b). A two-stage weight loss was observed in dried powder curves. The first stage was observed from 155 to 260 °C which related to a weight loss of substrate P-5 (31%) and EMWCs 1–3 (27, 24 and 21%), corresponding with the loss of water to the critical moisture level and volatile compounds on the surfaces of the microparticles (Gomes da Costa et al. 2015). The second stage was attributed to the major loss of substrate P-5 (68%) and EMWCs 1–3 (65, 64 and 61%) between 275 and 376 °C, degradation to carbonaceous residues. Gradually increasing in temperature may prompt changes in the molecular structure, allowing the intermolecular interactions of long chain fatty acids which in turn reduced the thermal decomposition temperature and providing a possible increase in the thermal stability of the microparticles (Gomes da Costa et al. 2015). Similarly, the lower weight loss (%), the more stable the material is towards thermal decomposition (Canevarolo 2003). Therefore, this study suggests that EMWCs demonstrated a higher thermal stability during the decomposition stage.
Fig. 4.
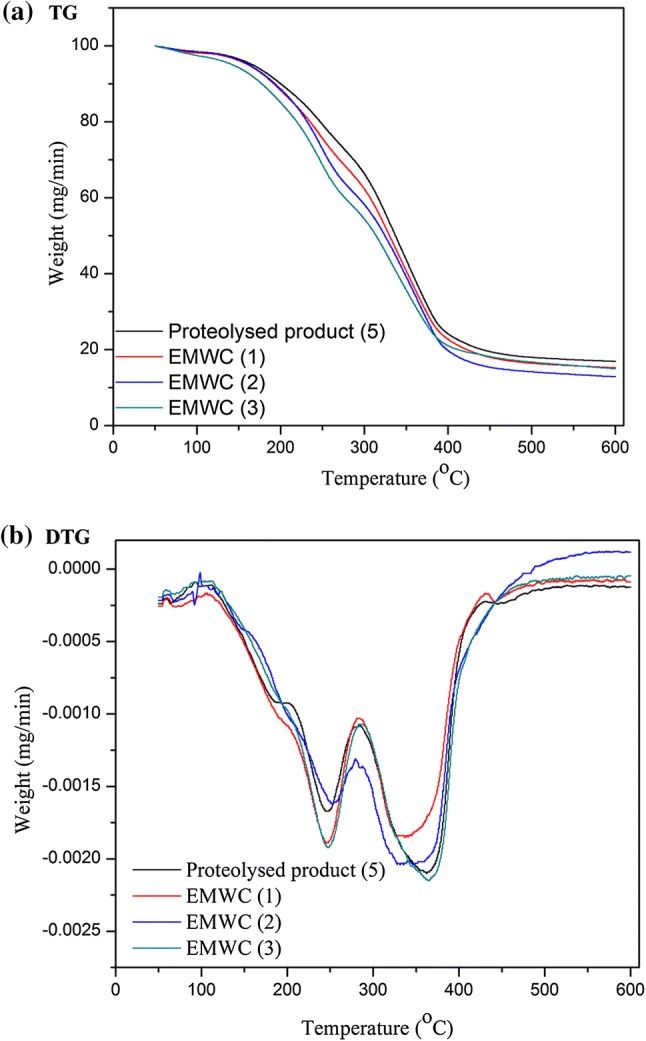
a TGA (thermal gravity analysis) curves showing the weight loss of proteolysed substrate and developed White EMCs (1–3) bioaroma powders. b The DTG curve (derivative thermal gravity) showing the derivative weight loss as a function of temperature of proteolysed substrate and developed White EMCs (1–3) bioaroma powders
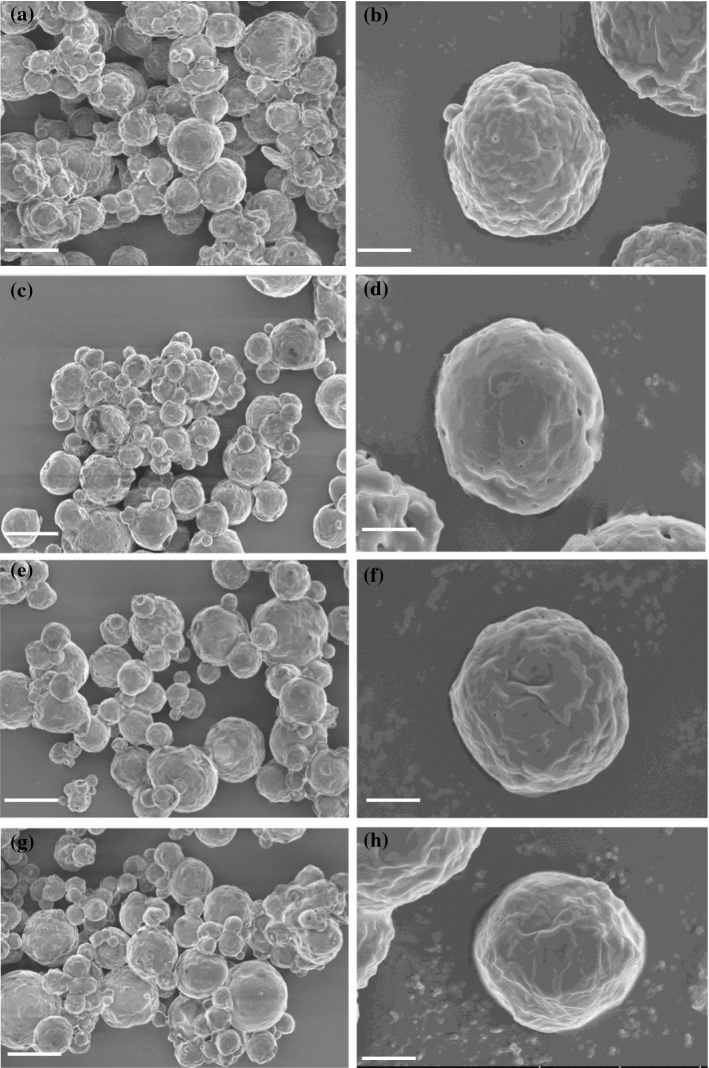
Microstructure
SEM micrograph of proteolysed substrate (product-5) and developed WEMCs (1–3) bioaroma powders produced with significant changes or disruption are presented in Fig. 5. Different powders produced were similar in appearance indicating that the anhydrous milk fat (AMF) addition did not affect the morphology of the particles. The shape of the particle was spherical and amorphous structure with uniform dispersed size distributions and had a flat and smooth surface which can be attributed to the rapid evaporation of the water. However, EMWCs powder obtained of relatively well-separated particles without aggregates and clumps, similar results were observed for Iranian white cheese (Amighi et al. 2013).
Fig. 5.
Micrographs of proteolysed substrate (a, b) and developed White EMCs (1–3) bioaroma powders (c–h); Bars represent 2000 × in a, c, e, g; bars represent 10,000 × in b, d, f, h
Conclusion
The production of White EMCs was accomplished and their flavour was significantly improved. An increase in the mean level of FAAs particularly a natural flavour enhancer glutamic acid appeared to enhance flavour intensity and have a significant impact on sensory properties using Flavourzyme 1000 l® in combination with Lipase MER®. The release of free fatty acids, the production of more volatiles also significantly contributed to the flavour development. Spray-dried EMCs powder particles were large, spherical and uniform. Thus, the present study demonstrates significant advantages over traditional methods for making flavoured White cheese, which includes the ability to produce using a substrate by commercial enzyme. Furthermore, studies on the properties of White EMCs powder during storage should be accomplished.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by China Scholarship Council, the National Natural Science Support of China (No. 31872905) and the National key research and development program of the 13th five years plan of China (2018YFD0400604) are acknowledged for financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akalin AS, Karaman AD. Influence of packaging systems on the biochemical characteristics and volatile compounds of industrially produced Turkish White cheese. J Food Biochem. 2011;35:663–680. doi: 10.1111/j.1745-4514.2010.00409.x. [DOI] [Google Scholar]
- Amighi F, Emam-Djomeh Z, Madadlou A. Spray drying of ACE-inhibitory enzyme-modified white cheese. Int J Food Sci Tech. 2013;48:2276–2282. [Google Scholar]
- Amighi F, Emam-Djomeh Z, Madadlou A. Optimised production and spray drying of ACE-inhibitory enzyme modified Cheese. J Dairy Res. 2016;83:125–134. doi: 10.1017/S0022029915000424. [DOI] [PubMed] [Google Scholar]
- Azarnia S, Lee B, St-Gelais D, Kilcawley K, Noroozi E. Effect of free and encapsulatedrecombinant aminopeptidase on proteolysis indices and sensory characteristics of Cheddar cheese. LWT Food Sci Technol. 2011;44:570–575. doi: 10.1016/j.lwt.2010.08.022. [DOI] [Google Scholar]
- Canevarolo SV., Jr . Técnicas de Caracterização de Polímeros. São Paulo: Artliber; 2003. [Google Scholar]
- Chegini GR, Ghobadian B. Spray dryer parameters for fruit juice drying. World J Agric Sci. 2007;3:230–236. [Google Scholar]
- Cono-Chauca M, Stringheta PC, Sardagna LD Cal-Vidal J (2004) Mango juice dehydration spray drying using different carriers and functional characterisation. In: Proceedings of the 14th international drying symposium, 2005–2012
- De Jong C, Badings HT. Determination of free fatty acids in milk and cheese procedures for extraction, clean up and capillary gas chromatographic analysis. J Sep Sci. 1990;13:94–98. [Google Scholar]
- El-Bakry M, Beninati F, Duggan E, O’Riordan ED, O’Sullivan M. Reducing salt in imitation cheese: effects on manufacture and functional properties. Food Res Int. 2011;44:589–596. doi: 10.1016/j.foodres.2010.12.013. [DOI] [Google Scholar]
- Fazaeli M, Emam-Djomeh Z, Ashtari AK, Omid M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod Process. 2012;90:667–675. doi: 10.1016/j.fbp.2012.04.006. [DOI] [Google Scholar]
- Gianfrancesco A, Turchiuli C, Dumoulin E. Powder agglomeration during the spray-drying process: measurements of air properties. Dairy Sci Technol. 2008;88:53–64. doi: 10.1051/dst:2007008. [DOI] [Google Scholar]
- Gomes da Costa JM, Silva EK, Toledo Hijo AAC, Azevedo VV, Borges SV. Physical and thermal stability of spray-dried Swiss cheese bioaroma powder. Dry Technol Int J. 2015;33:346–354. doi: 10.1080/07373937.2014.952376. [DOI] [Google Scholar]
- Hou J, Hannon JA, Mcsweeney PL, Beresford TP, Guinee TP. Effect of curd washing on cheese proteolysis, texture, volatile compounds, and sensory grading in full fat Cheddar cheese. Int Dairy J. 2014;34:190–198. doi: 10.1016/j.idairyj.2013.08.008. [DOI] [Google Scholar]
- Hulin-Bertaud S, Kilcawley KN, Wilkinson MG, Delahunty CM. Sensory and compositional relationships between commercial cheddar-flavoured enzyme-modified cheeses and natural cheddar. J Food Sci. 2000;65:1076–1082. doi: 10.1111/j.1365-2621.2000.tb09421.x. [DOI] [Google Scholar]
- IDF (1993) Milk: determination of nitrogen content, IDF Standard No. 20B:1993, Parts 1 and 2. IDF, Brussels, Belgium
- Iličić MD, Milanovic´ SD, Caric´ MD, Kanuric´ KG, Vukic´ VR, Hrnjez DV, Ranogajec MI. Volatile compounds of functional dairy products. Acta Period Technol. 2012;43:11–19. [Google Scholar]
- Karaman AD, Akalin AS. Improving quality characteristics of reduced and low fat turkish white cheeses using homogenized cream. LWT Food Sci Techol. 2013;50:503–510. doi: 10.1016/j.lwt.2012.08.017. [DOI] [Google Scholar]
- Kilara A. Enzyme-modified protein food ingredients. Process Biochem. 1985;20:149–157. [Google Scholar]
- Kilara A. Enzyme-modified lipid food ingredients. Process Biochem. 1985;20:35–45. [Google Scholar]
- Kilcawley KN, Wilkinson MG, Fox PF. Review: enzyme-modified cheese. Int Dairy J. 1998;8:1–10. doi: 10.1016/S0958-6946(98)00010-7. [DOI] [PubMed] [Google Scholar]
- Kilcawley K, Wilkinson MG, Fox PF. A survey of the composition and proteolytic indices of commercial enzyme modified Cheddar cheese. Int Dairy J. 2000;10:181–190. doi: 10.1016/S0958-6946(00)00029-7. [DOI] [Google Scholar]
- Kilcawley KN, Wilkinson MG, Fox PF. Determination of key enzyme activities in commercial peptidase and lipase preparations from microbial or animal sources. Enzym Microb Technol. 2002;31:310–320. doi: 10.1016/S0141-0229(02)00136-9. [DOI] [Google Scholar]
- Kilcawley KN, Wilkinson MG, Fox PF. A novel two stage process for the production of enzyme-modified cheese. Food Res Int. 2006;39:619–627. doi: 10.1016/j.foodres.2005.12.006. [DOI] [Google Scholar]
- Kilcawley K, Nongonierma A, Hannon J, Doolan I, Wilkinson M. Evaluation of commercial enzyme systems to accelerate Cheddar cheese ripening. Int Dairy J. 2012;26:50–57. doi: 10.1016/j.idairyj.2012.03.015. [DOI] [Google Scholar]
- Kumar P, Mishra HN. Yoghurt powder—a review of process technology, storage and utilization. Food Bioprod Process. 2004;82:133–142. doi: 10.1205/0960308041614918. [DOI] [Google Scholar]
- Kurtovic I, Marshall SN, Cleaver HL, Miller MR. The use of immobilised digestive lipase from Chinook salmon (Oncorhynchus tshawytscha) to generate flavour compounds in milk. Food Chem. 2016;199:323–329. doi: 10.1016/j.foodchem.2015.12.027. [DOI] [PubMed] [Google Scholar]
- Mannion DT, Furey A, Kilcawley KN. Free fatty acids quantification in dairy products. Int J Dairy Technol. 2015;69:1–12. doi: 10.1111/1471-0307.12301. [DOI] [Google Scholar]
- McSweeney PLH, Sousa MJ. Biochemical pathways for the production of flavour compounds in cheeses during ripening: a review. Lait. 2000;80:293–324. doi: 10.1051/lait:2000127. [DOI] [Google Scholar]
- Meilgaard M, Civille GV, Carr BT. Sensory evaluation techniques. 3. Boca Raton: CRC Press Inc.; 1999. [Google Scholar]
- Miri AM, Najafi HBM. The effect of adding enzyme-modified cheese on sensory and texture properties of low- and high-fat cream cheeses. Int J Dairy Technol. 2011;64:92–98. doi: 10.1111/j.1471-0307.2010.00624.x. [DOI] [Google Scholar]
- Moio L, Dekimpe J, Etievant P, Addeo F. The neutral volatile compounds of water-buffalo milk. Ital J Food Sci. 1993;5:43–56. [Google Scholar]
- Noronha N, Cronin DA, O’Riordan ED, O’Sullivan M. Flavouring of production cheese with enzyme modified cheeses (EMCs): sensory impact and measurement of aroma active short chain fatty acids (SCFAs) Food Chem. 2008;106:905–913. doi: 10.1016/j.foodchem.2007.06.059. [DOI] [Google Scholar]
- Omar KA, Gounga ME, Liu R, Mlyuka E, Wang X. Effects of microbial lipases on hydrolyzed milk fat at different time intervals in flavour development and oxidative stability. J Food Sci Technol. 2015;53:1035–1046. doi: 10.1007/s13197-015-2158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MJ, Lee SY. Quality characteristics of soy yogurt produced using proteases and mixed microbial consortia. J Korean Soc Appl Biol Chem. 2015;58:761–769. doi: 10.1007/s13765-015-0105-z. [DOI] [Google Scholar]
- Sahingil D, Hayaloglu AA, Simsek O, Ozer B. Changes in volatile composition, proteolysis and textural and sensory properties of white-brined cheese: effects of ripening temperature and adjunct culture. Dairy Sci Technol. 2014;94:603–623. doi: 10.1007/s13594-014-0185-2. [DOI] [Google Scholar]
- Wallace JM, Fox PF. Effect of adding free amino acids to Cheddar cheese curd on proteolysis, flavour and texture development. Int Dairy J. 1997;7:157–167. doi: 10.1016/S0958-6946(96)00049-0. [DOI] [Google Scholar]
- Wang P, Chen H, Mohanad B, Xu L, Ning Y, Xu J, Xu X. Effect of frozen storage on physico-chemistry of wheat gluten proteins: studies on gluten-, glutenin-and gliadin-rich fractions. Food Hydrocoll. 2014;39:187–194. doi: 10.1016/j.foodhyd.2014.01.009. [DOI] [Google Scholar]
- Wilkinson GM, Doolan IA, Kilcawley KN. Reference module in food science: enzyme-modified cheese. 2. New York: Academic Press; 2011. pp. 799–804. [Google Scholar]
- Zellner BA, Dugo P, Dugo G, Mondello L. Gas chromatography–olfactometry in food flavour analysis. J Chromatogr A. 2008;1186:123–143. doi: 10.1016/j.chroma.2007.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.