Abstract
Moringa seed protein isolate (MPI) was prepared by aqueous salt extraction followed by watering-out to precipitate proteins. Extraction and precipitation steps were optimized to achieve maximum MPI yield. Besides, MPI was characterized based on its composition and functional properties. Among the multiple salts examined, Na2SO4 (69.9%), KCl (66.2%), NaCl (65.4%), and NaBr (63.5%) displayed better protein extractability as well as higher MPI yield (~ 52%) with a protein content of > 90% d.b. However, NaCl was preferred considering its wider acceptance. Based on response surface methodology analysis, solvent-to-flour ratio, 22:1 (v/w), NaCl concentration, 0.4 M and temperature, 55 °C were found optimal for maximum protein extractability of 70.3%. Subsequent watering-out resulted in a maximum MPI yield of 56% (protein basis). MPI contained all the protein subunits (6.5, 14, 29 kDa) present in its source. It also scored over commercial soy protein isolate in many of the functional properties.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03690-0) contains supplementary material, which is available to authorized users.
Keywords: Moringa seed protein isolate, Response surface methodology, Watering-out, Functional properties
Introduction
Protein energy malnutrition is a serious problem in developing and underdeveloped countries due to the limited availability of protein sources and compounded by other socio-economic conditions (Guleria et al. 2017). Moreover, the availability of animal proteins is hindered due to their inadequate supply accompanied by higher cost (Che et al. 2017). Plant proteins can serve as a better alternative due to their good environmental sustainability, health-oriented composition, reliable origin, and attractive prices. However, even though large number of sources exists, the utilization is mainly centred around soybean, kidney bean and cowpea (Sridaran et al. 2012). Thus, research on unexploited plant protein sources have become important, and there is a necessity to look at all the potential sources of plant proteins.
Moringa oleifera is a highly valued vegetable plant owing to its nutritional and medicinal values (Makkar and Becker 1997). Moringa seed meal (MSM) is used as a natural coagulant and plays a significant role in water purification industry. MSM contains considerably good quantity of high-quality protein (~ 52%) with all the essential amino acids and may serve as a potential source of functional protein isolate. Moringa seed protein isolate can be used as an alternative to other proteins for human food use due to its balanced amino acid profile. The present authors have filed a patent application (3529/DEL/2014) entitled “A process for the preparation of protein isolate from defatted moringa seed meal flour”. In the current study, authors have attempted to determine optimal conditions for protein isolate preparation and to characterize the isolate in term of its constituent proteins and functional properties.
Hofmeister (1888) reported that protein solubility is influenced by the concentration and type of salts present, based on the precipitation studies of egg yolk protein and other colloids. The increase in protein solubility generally followed the Hofmeister series, accordingly:
Moringa seed meal protein (MSMP) extraction using salts (NaCl and CaCl2) was rated to be better compared to alkaline and water extraction (Govardhan et al. 2011). However, the attempts were limited to only two salts, while there could be a scope to improve the protein extractability by examining a wide range of salts. Besides protein surrounding environment (ion type and ion concentration) also has an influence on the conformational property of the protein, and ultimately affect micelle formation in the subsequent protein precipitation step.
Protein solubility in salt solution may be affected by various parameters including pH, temperature, ionic force, salt or solvent type, extraction time, solid–solvent ratio and presence of components causing inter-moleclar linking (Jain et al. 2015). When many factors and interactions affect desired response, response surface methodology (RSM) is an effective tool for optimizing the process (Triveni et al. 2001).
The objective of the present study is to understand the influence of Hofmeister ions on the moringa seed protein extraction and optimize protein extraction from seed meal with selected salt using RSM. Further, protein concentration and precipitation steps were assessed to achieve maximum protein isolate yield. Additionally, protein isolate obtained under optimized conditions was comparatively assessed with a commercial soy protein isolate in terms of functional properties.
Materials and methods
Materials
Dried moringa seeds were purchased from the local market. All the chemicals and solvents used were of analytical grade and purchased from reputed companies in the country.
Methods
Preparation of Moringa oleifera seed meal
Moringa seed flour was defatted with hexane until the fat content was reduced below 0.5%. The defatted flour was air-dried at ambient temperature (25–28 °C) for about 24 h and ground to a fine flour in a precision laboratory mill (Quadrumat; Brabender, Buisburg, Germany) fitted with a stainless steel control sieve (100 μm mesh). Moringa seed meal (MSM) thus obtained after milling was stored at ambient temperature in an airtight container until experimental use.
Single-factor experiments
Solvent-to-flour ratio
The impact of solvent-to-flour ratio on protein extractability from MSM was determined by dispersing 0.5 g of MSM in 0.75 M NaCl solution at various solvent-to-flour ratios (5:1, 10:1, 15:1, 20:1, and 25:1). Samples were stirred in a shaking water bath for 1 h at ambient temperature without any pH adjustment (natural pH 5.5). The solubilized liquor was separated from insoluble material by centrifugation at 6000g for 15 min at ambient temperature and protein estimated.
Incubation time
Protein extractability was examined over a range of extraction periods 5–60 min at a solvent-to-flour ratio of 20:1 under otherwise similar conditions as described in the earlier section of solvent-to-flour ratio.
Incubation temperature
Protein extractability was examined over a range of extraction temperature 30–100 °C for 10 min under otherwise similar conditions as described in the earlier section of incubation time.
Salt selection
Hofmeister cations
The impact of cations (Na+, K+, Mg2+, Ca2+) on protein extractability from MSM was determined by dispersing 0.5 g of MSM in 10 mL of their chlorine salt solution (NaCl, KCl, CaCl2, MgCl2) at different salt concentrations 0.15, 0.25, 0.50, 0.75 and 1.0 M without any pH adjustment. Samples were stirred for 1 h at ambient temperature. The homogenate was separated from the insoluble residue by centrifugation and protein extractability was estimated. Another homogenate prepared from 0.25 M salt solution maintaining the rest of the conditions same was used for protein precipitation using watering-out. Watering-out was performed by diluting (tenfold) the homogenate with chilled water having a temperature of 8–10 °C, and the solution was centrifuged at 12,000g for 10 min at 10 °C. Protein precipitate obtained was dried and weighed. Protein precipitate yield was calculated by using the following formula:
Hofmeister anions
The impact of anions (SO42−, HPO42−, CH3COO−, Cl−, Br−, I− and SCN−) on the extraction efficiency of MSMP was determined by dispersing 0.5 g of MSM in 10 mL of their sodium salt solution (Na2SO4, Na2HPO4, CH3COONa, NaCl, NaBr, NaI and NaSCN) at 0.25 M salt concentration without any pH adjustment. Samples were stirred for 1 h at ambient temperature. The homogenate was separated from the insoluble residue by centrifugation and protein extractability was estimated. Another homogenate prepared from 0.25 M salt solution under similar conditions was used for protein precipitation using watering-out and protein precipitate yield was calculated as explained in the earlier section of “Hofmeister cations”.
NaCl concentration
Protein extractability was examined over at a wide range of NaCl concentration 0.05–3.00 M for 10 min under otherwise similar conditions as described in the earlier section of “Hofmeister anions”.
Extraction pH
Protein extractability was examined over a range of pH (2–11) in 0.25 M NaCl solution under otherwise similar conditions as described in earlier section of NaCl concentration. The pH of the solution was adjusted to the required working pH (2–11) with HCl or NaOH of high-low normality to limit dilution as well as to maintain accuracy.
Experimental design for protein extraction optimization using RSM
Box–Behnken experimental design with three factors and three levels, including three replicates at the center point, was employed to fit a second-order response surface. Solvent-to-flour ratio (X1), NaCl concentration (X2) and temperature (X3) were chosen as independent variables in this design. Based on the single-factor experiments, X1 (5, 15, 25), X2 (0.05, 0.25, 0.45 M) and X3 (30, 55, 80 °C) were delineated as critical levels. A broader range was selected to adequately take care of the inter-dependent effects of experimental variables.
The variables were coded according to the following equation
| 1 |
where xi is the coded value of an independent variable, Xi is the real value of an independent variable, X0 is the real value of an independent variable at the center point and ΔXi is the step change. Experiments were randomized to minimize bias.
The average yield of the duplicate values obtained was taken as the dependent variable or response, Yi. The model proposed for the response is given below:
| 2 |
where Y is the predicted response (extractability of protein), β0 is offset term, β1, β2 and β3 are linear effect terms, β11, β22 and β33 are squared effects and β12, β13 and β23 are interaction effects.
The proportion of variance explained by the polynomial models obtained is given by the multiple coefficients of determination, R2. The significance of each coefficient was determined using the Students t test and p value. The behavior of the surface was investigated for the response function protein extractability, using the regression Eq. (2). The optimum conditions were validated by conducting experiments under these conditions. Responses were monitored, and results compared with model predictions.
Effect of dilution on precipitate yield
Seed meal protein was extracted from MSM under RSM optimized conditions. The extract obtained was diluted at different dilution fold (1–12) with chilled water (8–10 °C). The solutions were centrifuged at 12,000g for 10 min at 10 °C. Protein precipitates obtained were dried and precipitate yield was calculated as described in the earlier section of “Hofmeister cations”.
Membrane processing
Seed meal protein was extracted from MSM under RSM optimized conditions. Extract obtained (50 mL) was ultrafiltered to concentrate proteins at a volume concentration ratio of 4. The ultrafiltration was carried out in a stirred membrane cell unit fitted with a 25–30 kDa MWCO membrane at 0.4 MPa. The retentate containing concentrated solubilized protein was diluted (sixfold) with chilled water (≤ 10 °C) and the solution was centrifuged at 12,000g for 10 min at 10 °C. Protein precipitate obtained was dried and the precipitate yield was calculated as described in the earlier section of “Hofmeister cations”.
Functional properties of obtained protein isolate
Nitrogen solubility
Nitrogen solubility of moringa seed protein isolate and moringa seed meal at different pH (2–11) was determined according to the method of Adebiyi et al. (2007) with slight modifications. Samples were dispersed in distilled water (2% w/v), and the pH of the solution was adjusted to the required working pH (2–11) with HCl or NaOH as required. After a 30 min equilibrium period at ambient temperature and readjustment of pH if necessary, the samples were centrifuged at 5000 rpm. Solubility of nitrogen in the supernatant was determined by Kjeldahl method. The nitrogen solubility (NS) was calculated according to the formula:
Foaming capacity and foam stability
Foaming properties were determined by the method of Fernandez-Quintela et al. (1997), with minor modifications. Aliquots (100 mL) of sample solutions (1% in 0.4 M NaCl solution, w/v) were homogenized with a homogenizer (ART-MiccRA, Mullheim, Germany) at 19,000 rpm for 1 min. Foaming capacity was calculated as the percent increase in volume of the protein dispersion upon mixing whereas foam stability was estimated as the percentage of foam remaining after 30 min.
Water absorption capacity
Water absorption capacity (WAC) was determined using the method by Tomotake et al. (2002), with slight modifications. 1 g of protein isolate sample was dispersed in 10 mL of distilled water and mixed for 30 s every 10 min using a glass rod and the mixing was repeated four times. The mixture was centrifuged at 4000g for 20 min and the supernatant was carefully discarded. WAC was calculated as the increased percentage of the sample weight.
Oil absorption capacity
Oil absorption capacity (OAC) was determined by the method of Sze-Tao and Sathe (2000). Protein isolate sample (0.5 g) and groundnut oil (3.0 mL) were mixed and stirred for 1 min. After a holding period of 30 min, the mixture was centrifuged at 3000g for 20 min and excess oil was discarded. OAC was expressed as the percentage of oil absorbed by the protein isolate.
Emulsification capacity
Emulsion capacity was determined according to the method of Pearce and Kinsella (1978). About 2 g flour sample was dispersed in 100 mL distilled water and homogenized in a blender-mixer until the proteins dispersed completely. As the dispersion was being homogenized, refined groundnut oil was added gradually at the rate of 0.5 mL/s from a burette. As the process continued, oil addition was interrupted after each addition of 5–10 mL to prevent a sudden rise in temperature until the emulsion became thick and attained maximum viscosity. Then, the rate of oil addition was reduced and continued dropwise thereafter until the emulsion reached breakpoint, at which oil and water separated into two phases. Emulsion capacity was measured as mL of oil emulsified and held per gram of flour.
Analyses
Protein estimation
Nitrogen content was determined according to AOAC micro-kjeldahl procedure (AOAC, Method 32.1.22, 2005). Protein content was estimated by multiplying nitrogen content by 6.25.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
Molecular mass determination under denaturing conditions was carried out by discontinuous SDS-PAGE using a vertical system (Laemmli 1970). A 12% polyacrylamide gel prepared in 1.5 M Tris–HCl buffer, pH 8.8, containing 1% SDS was used. Samples (moringa seed meal, NaCl extract, moringa protein isolate) were dissolved in 0.5 M Tris–HCl buffer, pH 6.8 (containing 2% SDS, 1% dithiothreitol) and boiled at 95 °C, for 5 min, before electrophoresis. Protein bands were stained with Coomassie Blue R-250.
Antinutritional factors determination
Glucosinolate
Glucosinolate content was determined based on the detection of the glucosinolate hydrolysis products (Slominski and Campbell 1989) since the samples contained both intact and hydrolyzed glucosinolates. The concentration of thiocyanate ion was determined colorimetrically using potassium thiocyanate (KSCN) as a standard.
Saponin
Saponin content was determined using the spectrophotometric method as described by Hiai et al. (1976). The results were expressed as diosgenin equivalent from a standard curve of diosgenin prepared in 80% aqueous methanol.
Statistical analysis
All the experiments and analyses were carried out in duplicate. The means of each pair of data were compared at 5% significance level by using t-test, in Microsoft Excel Data Analysis Tool Pack. Data from the BBD were used for determining the regression coefficients of the second-order multiple regression models. The results were analyzed with one-way Analysis of Variance (ANOVA) using Design Expert software version 9. Contour plots were created keeping one variable constant at center point while varying the other two variables.
Results and discussion
Effect of individual extraction parameter
Single factor experiments were conducted to analyze the individual parameters that have significant effect on protein extractability from MSM.
Effect of solvent-to-flour ratio
Solvent-to-flour ratio showed a significant influence on protein extractability from MSM (Fig. 1a). Protein extractability increased from 48.4 to 67.4% with an increase in solvent-to-flour ratio from 5:1 to 20:1. However, further increase in solvent-to-flour ratio (25:1) did not have any significant effect. Therefore, solvent-to-flour ratio of 20:1 was used in the subsequent studies.
Fig. 1.
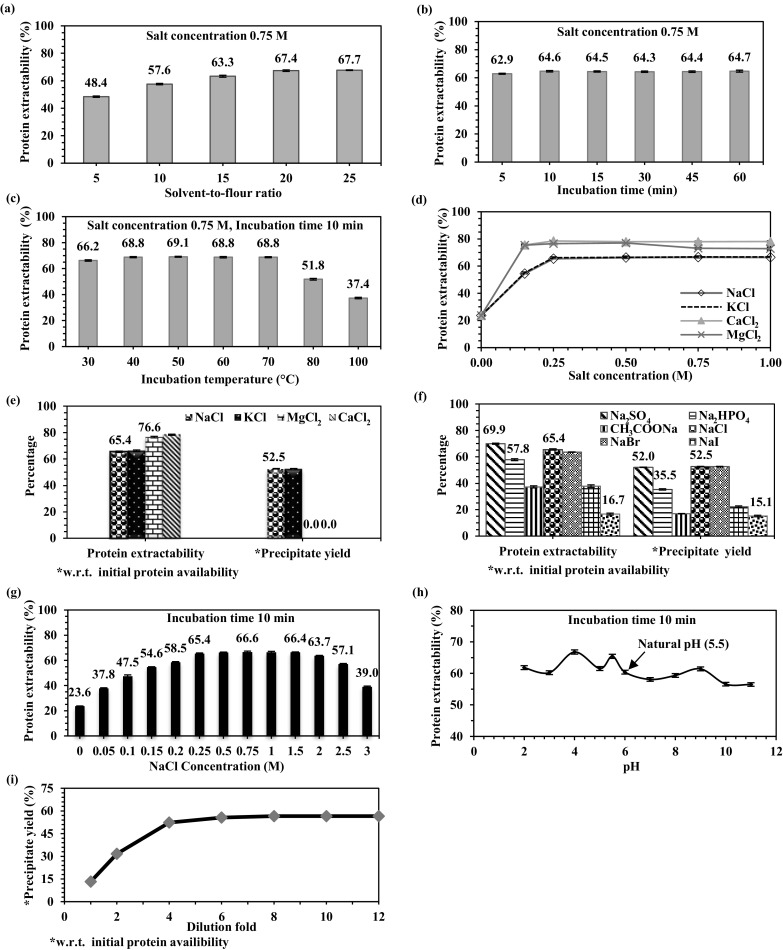
Single factor experiments. a Effect of solvent-to-flour ratio on protein extractability. b Effect of incubation time on protein extractability. c Effect of incubation temperature on protein extractability. d Effect of Hofmeister cationic salts on protein extractability. e Effect of Hofmeister cationic salts on protein extractability and protein precipitation. f Effect of Hofmeister anionic salts on protein extractability and protein precipitation. g Effect of NaCl concentration on protein extractability. h Effect of pH on protein extractability, i effect of dilution on protein precipitate yield upon extraction under RSM optimized conditions. Extraction conditions unless specified: Solvent-to-flour ratio 20:1, Incubation time 60 min, Ambient temperature, Salt concentration 0.25 M, Natural pH 5.5
Effect of incubation time
The influence of incubation time on protein extractability from MSM is shown in Fig. 1b. Virtually, all the proteins were extracted within 10 min, which indicated that the bulk of MSMP solubilized within a relatively short period.
Effect of incubation temperature
Figure 1c shows the effect of incubation temperature on protein extractability from MSM at different temperatures from 30 to 100 °C. Extractability increased significantly with an increase in temperature from 30 to 40 °C and became constant in the temperature range 40–70 °C. However, with further increment of incubation temperature (70–100 °C), extractability showed a drastic decrease probably due to protein denaturation.
Salt selection
Effect of Hofmeister cations
The effect of cations (Na+, K+, Mg2+, Ca2+) on protein extraction from MSM as well as protein precipitation using watering-out was studied with their chlorine salts (NaCl, KCl, CaCl2, MgCl2). Salting-in strength of Hofmeister cations is shown in Fig. 1d. There was a significant increase in protein extractability with monovalent cationic salts till 0.25 M salt concentration. Afterwards it remained constant in the range of concentration (0.25–1 M) examined. Whereas divalent salts showed improvement only up to 0.15 M. Therefore, 0.25 M salt concentration was used uniformly in the subsequent studies.
Results showed that protein extractability with cations followed a direct Hofmeister series (Na+ ~ K+< Mg2+ < Ca2+). Although divalent cationic salts (MgCl2, CaCl2) exhibited higher protein extractability compared to monovalent salts (NaCl and KCl), they did not result in protein precipitation upon watering-out (Fig. 1e). The reason could be that these salts provide higher ionic strength compared to monovalent salts at the same ionic concentration, therefore, requiring more dilution to reduce the ionic strength in the watering-out process. However, this would increase the distance between the exposed hydrophobic groups and thereby leading to limited inter-hydrophobic interaction, drifting away from a delicate balance of hydrophilic-hydrophobic non-covalent forces which is critical for the protein precipitation and their subsequent association. Consequently, monovalent cations were selected for subsequent studies since divalent cationic salts are not suitable for the critical precipitation step in the case of moringa seed protein.
Effect of Hofmeister anions
Hofmeister series is more pronounced for anions as compared to cations because anions have a stronger interaction with water than cations of the same size and absolute charge density (Kunz 2010). Therefore, few sodium salts were tested to study the effect of anions on protein extractability from MSM as well as precipitate yield and the results obtained are shown in Fig. 1f.
Results showed that protein extractability followed a reverse Hofmeister series except for Cl− and Br− ions which advanced their position. The descending order of protein extractability was of the following order:
There are studies that reported Hofmeister series reversal and some of them are contradictory. Zhang and Cremer (2009) reported a reversed anion series at low salt concentrations and a direct series at high salt concentrations. Whereas Salis et al. (2012) reported increase in salt concentration or change in pH could lead to Hofmeister series reverse. A partial or total reversal of the series could be ascribed to changes in the polarity or charge of protein surface (Schwierz et al. 2010). In a systematic study using small angle X-ray scattering (SAXS), Finet et al. (2004) demonstrated the occurence of Hofmeister reversal at pH < pI for lysozyme, α-crystallins, β -crystallins, ATCase and Brome Mosaic Virus. The major moringa seed protein (6.5 kDa) is cationic in nature due to its high pI greater than 10 (Gassenschmidt et al. 1995; Jerri et al. 2012). Therefore, moringa protein would have a net positive charge on its surface at the extraction pH (5.5) as it is lower than its pI value. This could be the possible reason for Hofmeister series reversal observed in the present system.
Na2SO4 (70%), NaCl (65%), and NaBr (64%) exhibited greater protein extractability but resulted in nearly the same precipitate yield (52%) with more than 90% purity. These results suggested that any one of these three salts could be used for preparation of M. oleifera seed protein isolate. However, NaCl was preferred considering its wider acceptance in various applications. So, NaCl was used in the subsequent studies for protein extraction optimization using response surface methodology (RSM).
Effect of NaCl Concentration
Figure 1g shows the effect of NaCl concentration on protein extractability from MSM. Protein extractability increased significantly with an increase in the salt concentration up to 0.25 M beyond which that there was no significant change in extractability till salt concentration reached 2.0 M. Further increase in the salt concentration affected the protein extractability significantly.
Effect of extraction pH
Figure 1h demonstrated that protein extractability was comparatively higher at its natural pH. Besides, any altered pH could affect the protein structure and functionality owing to the change effected in its surface charge. Hence, it is preferable to extract native protein at natural pH (5.5) without any pH adjustment.
Protein extraction optimization using response surface methodology (RSM)
Single factor experimental results concluded that the protein extractability from MSM dependent on various parameters including solvent-to-flour ratio, salt type, salt concentration and temperature; however, extraction time and pH did not have much sensitivity to the process. Since many factors and interactions affect the desired response, RSM was adopted for optimizing the process after appropriate selection of parameters and their limits.
Fitting the models
The experimental conditions and their corresponding experimental, predicted and relative % deviation values from the experimental design are presented in Table 1.
Table 1.
Box–Behnken experiment design matrix with experimental, predicted and relative % deviation values of the protein extractability from MSM
| Run | Solvent-to-flour ratio | NaCl Conc. (M) | Temp. (°C) | Protein extractability (%) | Relative deviation (%) | |
|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||
| X1 | X2 | X3 | ||||
| 1 | 5 | 0.05 | 55 | 35.2 | 30.1 | 14.3 |
| 2 | 5 | 0.45 | 55 | 39.9 | 43.3 | 8.6 |
| 3 | 25 | 0.05 | 55 | 48.8 | 45.3 | 7.1 |
| 4 | 25 | 0.45 | 55 | 70.1 | 75.1 | 7.2 |
| 5 | 15 | 0.05 | 30 | 41.1 | 43.8 | 6.4 |
| 6 | 15 | 0.05 | 80 | 59.4 | 53.5 | 9.8 |
| 7 | 15 | 0.45 | 30 | 20.4 | 26.3 | 28.6 |
| 8 | 15 | 0.45 | 80 | 62.1 | 59.5 | 4.2 |
| 9 | 5 | 0.25 | 30 | 33.8 | 36.2 | 7.1 |
| 10 | 25 | 0.25 | 30 | 61.5 | 62.3 | 1.3 |
| 11 | 5 | 0.25 | 80 | 33.8 | 33.0 | 2.4 |
| 12 | 25 | 0.25 | 80 | 56.3 | 53.9 | 4.3 |
| 13 | 15 | 0.25 | 55 | 67.3 | 67.4 | 0.1 |
| 14 | 15 | 0.25 | 55 | 67.5 | 67.4 | 0.2 |
| 15 | 15 | 0.25 | 55 | 67.3 | 67.4 | 0.1 |
| Average deviation | 6.8 | |||||
The regression equation obtained for protein extractability from MSM is as follows:
| 3 |
F-test checked the statistical significance of Eq. (3). Because of the model F value (10.95) and a small probability value (0.008), it could be deduced as highly significant. Further, a high coefficient of determination (R2) of 0.9517 suggested that the model proposed is relatively accurate. This implied that independent variables included in the study attributed to 95.17% of the variation for the protein extractability from MSM. The data proved that the developed model could adequately represent the real relationship among the parameters chosen. Besides, the average relative deviation, an indicator of fitness between experiment and model, was only 6.8%.
Effects of independent variables on responses and RSM model validation
The estimates of independent variables and their corresponding P values suggested that the independent variables X2 (salt concentration) and X3 (temperature) had a significant effect on Y (Protein extractability from MSM). Positive coefficient for X1, X2 and X3 revealed a linear effect to increase Y. All of the quadratic terms (X21, X22, X23) had a significant impact on Y (P < 0.05), whereas the cross-product terms (X1X2, X2X3 and X1X3) were not significant (P > 0.05). In this work, X2, X3, X21, X22 and X23 were significant model terms (see Supplementary Table S-1). The presence of optimum levels of solvent-to-flour-ratio, NaCl concentration and temperature for maximum protein yield can be seen in contour plots (see Supplementary Fig. S1).
RSM model was validated by extracting proteins from MSM under the optimal conditions obtained from the model. The experimental values were compared with the predicted values to ascertain the validity of the model. The stationary point giving the maximum protein extractability from MSM had the following critical values: solvent-to-flour ratio 22:1, NaCl concentration 0.4 M, and temperature 55 °C. The predicted protein extractability under these conditions was 76% while the experimental value was 70.3%. The corresponding percentage deviation of 8% is closer to the average relative percentage deviation (6.8%) indicating the agreement of the predicted values. Additionally, ten more experiments were conducted to further validate above RSM model (see Supplementary Table S-2). The results indicated that the experimental values agreed with the predicted ones with an average relative percentage deviation of 5.0%.
Effect of dilution on protein precipitate yield
Protein was extracted from MSM under the optimal conditions established (solvent-to-flour ratio 22:1, NaCl concentration 0.4 M, temperature 55 °C and incubation time 10 min) and the influence of dilution on precipitate yield was studied. There was a significant rise in precipitate yield up to sixfold dilution and reached 56%, beyond which it remained nearly constant (Fig. 1i). These results indicated that a sixfold dilution is optimal for precipitating proteins using watering-out process.
Membrane concentration of protein extract
Concentration of aqueous protein extract was attempted using membrane processing to explore whether the amount of water required in the subsequent precipitation process could be reduced. Aqueous seed extract having a protein content of 17.5 mg/mL was concentrated to 63 mg/mL with a protein recovery of 90% in the ultrafiltration process (25–30 kDa, 4 VCR). The retentate containing concentrated proteins upon dilution (sixfold) with water resulted in a protein precipitate yield of 56%. Although there was a 10% protein loss in the concentration process, it did not affect the overall precipitate yield. Further the reduction in extract volume reduced the amount of water required in the subsequent precipitation step by 75%.
Anti-nutritional factors in MSM and MPI
Anti-nutrients are natural or synthetic compounds that interfere with the absorption of nutrients. Glucosinolate and saponin, are the two major anti-nutritionals factors reported in moringa seed meal (Makkar and Becker 1997). Glucosinolate and saponin content reduced drastically (95% and 97%) in MPI compared to MSM owing to the precipitation process involving large amount of water taking away these water-soluble compounds (Table 2). The reduction was lower in the membrane isolate (84% and 93%) as the concentration step proportionally reduced the amount of water employed for protein precipitation to the extent of 75%. If desired, anti-nutritional factors content in these isolates could be further reduced by washing (3–4 times) the isolate with water as most of the anti-nutritional factors are water soluble in nature.
Table 2.
Anti-nutritional factors in MSM and MPI
| Sample | Protein content (%) | Glucosinolates (µM/g) | Saponins (%) | ||
|---|---|---|---|---|---|
| Total | Free | Bound | |||
| MSM | 52 ± 0.8 | 34.50 ± 0.4 | 7.27 ± 0.2 | 27.23 ± 0.2 | 1.73 ± 0.02 |
| MPI (without UF) | 93 ± 0.7 | 1.78 ± 0.1 | 0.50 ± 0.1 | 1.28 ± 0.1 | 0.06 ± 0.00 |
| UF-MPI | 93 ± 0.9 | 5.54 ± 0.04 | 1.51 ± 0.03 | 4.03 ± 0.04 | 0.13 ± 0.01 |
Molecular weight estimation
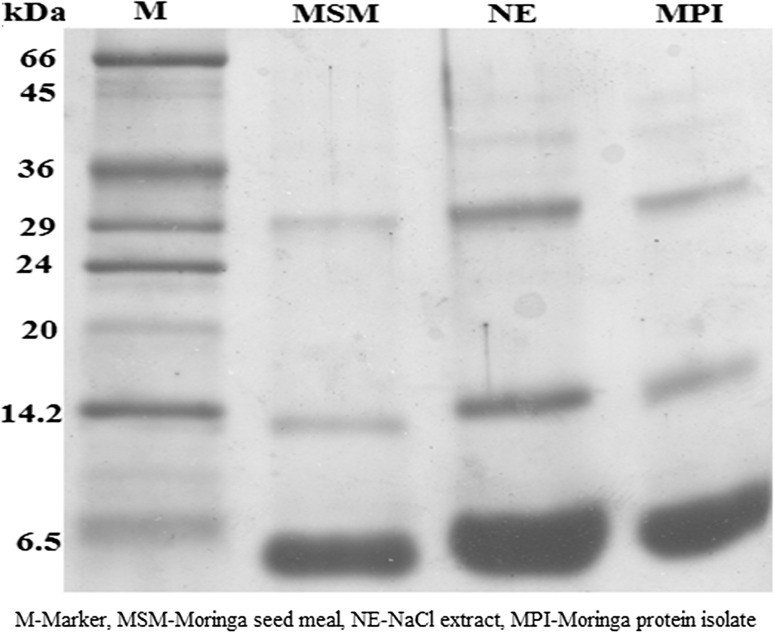
Moringa seed meal showed the presence of three major protein bands in reducing condition corresponding to molecular weights, 29, 14.2 and 6.5 kDa (Fig. 2). Previous researchers have shown the presence of many coagulating proteins in the moringa seed meal with different molecular weights (6.5–26.5 kDa) (Ndabigengesere et al. 1995; Santos et al. 2009; García-Fayos et al. 2010). The major protein bands corresponding to moringa seed meal were in agreement with earlier studies. All these three proteins were also present in the salt extract as well as in the MPI. Thus, the composition of MPI prepared from the process proposed construed to be identical to the protein components present in the source material.
Fig. 2.
Constituent proteins of moringa protein isolate and moringa seed meal
Functional properties of moringa seed protein isolate
Functional properties of food proteins denote those physicochemical properties that determine the behavior of foods during processing, preparation, storage and consumption. Functional properties affect the sensory character and physical behavior of foods or food ingredients. The type of functional properties required in a protein varies with the food system. The important functionality that determines the quality of the protein product includes nitrogen solubility, foaming capacity, foam stability, water absorption capacity, oil absorption capacity and emulsification property.
Nitrogen solubility
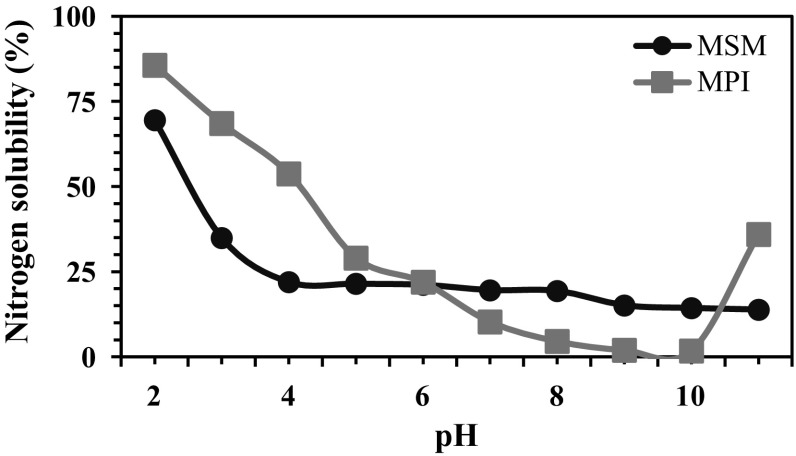
Solubility characteristics of proteins are among the most important functional properties since many functional performances of proteins depend upon their capacity to go into solution initially. Figure 3 shows the nitrogen solubility profile of MPI and MSM over a pH range of 2–11. Nitrogen solubility of MPI was significantly higher compared to MSM in the acidic pH range (2–5) widening its scope of practical application. The lower nitrogen solubility of the MPI and MSM in the pH range pH 7–10 may be since this pH range is closer to its isoelectric point (10). The lack of electric charge corresponding to the isoelectric point has been known to promote hydrophobic aggregation which translates to the minimum solubility (Damodaran 1996). Similar observations on nitrogen solubility and isoelectric point of protein had been reported for soy (Zhang et al. 2007) and groundnut (Jain et al. 2015).
Fig. 3.
Nitrogen solubility of MPI and MSM at different pH
Foaming capacity (FC) and foam stability (FS)
Foaming capacity and foaming stability are the important functional properties of protein isolates that determine their utilization in different food systems where aeration and overrun is required, for example whipped toppings, baked foods and ice-cream mixes (Shevkani et al. 2015).
The capacity of proteins to form stable foams with gas by forming impervious protein films is called as foaming capacity. The foaming capacity of MPI was 36% higher than that of SPI (Table 3). High protein solubility is a prerequisite for achieving better FC (Deng et al. 2011). Besides, the diffusion rates of protein at the air–water interface to unfold its structure as well as its capability to encapsulate air particles determine FC.
Table 3.
Functional properties of moringa seed protein isolate (MPI) and soy protein isolate (SPI)
| Property | MPI | SPI |
|---|---|---|
| FC (%) | 185 ± 10 | 120 ± 12 |
| FS (%) | ||
| 10 min | 170 ± 5 | 116 ± 5 |
| 20 min | 165 ± 4 | 114 ± 5 |
| 30 min | 165 ± 6 | 114 ± 6 |
| WAC (g g−1) | Not detected | 4.0 ± 0.2 |
| OAC (g g−1) | 1.9 ± 0.1 | 1.4 ± 0.1 |
| Emulsifying capacity (mL g−1) | 90 ± 6 | 120 ± 7 |
FS indicates the percentage of foam remaining after a given period, which is dependent upon the formation of a thick cohesive layer around the air bubble. Protein molecules should form continuous intermolecular polymers enveloping the air bubble to render good FS, since intermolecular cohesiveness and elasticity are important to produce stable foams (Kamara et al. 2009). Foams from MPI and SPI were quite stable till 30 min and MPI exhibited 29% higher foam stability than SPI (Table 3). The high FS could be attributed to the unfolding of the protein structure which facilitates surface hydrophobic association as well as reduced air leakage leading to prevention of rupture and coalescence (Wang et al. 2012; Shevkani et al. 2015). Although the WAC of MPI was rather very poor, it showed excellent foaming capacity in a salt system.
Water and oil absorption capacity
The ability of protein to absorb water and retain it against a gravitational force within a protein matrix is known as water absorption capacity (WAC). MPI did not display any water absorption capacity, while SPI displayed WAC of 4 g g−1 (Table 3). But poor WAC of MPI will not restrict its application because the salt content in the functional foods formulation would be sufficient to provide the desired solubility. Arrese et al. (1991) studied the functional properties of several commercial isolates and reported that isolates with more denaturation showed a greater water holding capacity. This may be attributed to the unfolding of the polypeptide chain resulting in a matrix that can trap the absorbed water. The process employed for the isolation of proteins also did not disrupt the protein structures to impart WAC to MPI.
An important functionality that influences the taste of the fried products is the ability of the protein to absorb oil. Oil absorption capacity (OAC) of MPI (1.94 g g−1) was 30% higher compared to SPI (1.36 g g−1). The differences in oil absorption capacity might be due to the presence of more non-polar amino acids in MPI than SPI. The presence of several non-polar side chains may bind the hydrocarbon chains of fats, thereby resulting in higher absorption of oil (Sathe et al. 1982). High OAC of MPI make them good ingredients in cold meat industry, particularly for sausages, where the protein usually bridges the fat and water to obtain good products.
Emulsifying capacity
The emulsifying capacity is a measure of the effectiveness of proteinaceous emulsifiers (Pearce and Kinsella 1978). Proteins are composed of charged amino acids, non-charged amino acids and nonpolar amino acids which make protein a possible emulsifier, to interact with both water and oil in food system. The emulsifying capacities of the protein are generally dependent on its ability to adsorb on the interface whereas emulsion stability is related to the properties of this adsorbed layer. Protein solubility and hydrophobicity are two major important factors that determine their initial adsorption and thus their emulsifying properties (Shevkani et al. 2015). Emulsifying capacity of MPI was 25% lower than SPI understandably due to its poor solubility in water. Nevertheless, emulsifying capacity of MPI was closer to the whey protein concentrate, 104–110 mL g−1 (El-Desoki 2009) which is second commonly used proteinaceous emulsifier after soy.
Conclusion
This paper provides information on the preparation and characterization of Moringa seed protein isolate with a purity of > 90% and maximum yield. Various extraction factors namely solvent-to-flour ratio, salt type, salt concentration, and temperature were found to have a significant effect on protein extractability from MSM; however, incubation time and pH did not have much influence. Ultrafiltration reduced the amount of water required in the subsequent precipitation step drastically without affecting the final precipitate yield. MPI preparation process retained the protein subunit profile (6.5, 14, 29 kDa) intact while reducing the major anti-nutritional factors (glucosinolates and saponins). MPI also showed superior oil absorption capacity, foaming capacity and foam stability compared to commercial SPI which could be exploited in food formulations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the Director, CSIR-CFTRI, Mysore for extending facilities for this study. Department of Biotechnology, Government of India, New Delhi is duly acknowledged for the financial support in the form of grant-in-aid project to Dr. C. Radha, Sanction No. BT/Bio-CARe/05/400/2010-2011 dated 16-02-2012.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adebiyi AP, Adebiyi AO, Ogawa T, Muramoto K. Preparation and characterization of high-quality rice bran proteins. J Sci Food Agric. 2007;87:1219–1227. doi: 10.1002/jsfa.2819. [DOI] [Google Scholar]
- AOAC (2005) International methods 925.10. In: Official methods of analysis of association of official analytical chemists, 18th edn. Washington
- Arrese EL, Sorgentini DA, Wagner JR, Anon MC. Electrophoretic, solubility and functional properties of commercial soy protein isolates. J Agric Food Chem. 1991;39:1029–1032. doi: 10.1021/jf00006a004. [DOI] [Google Scholar]
- Che J, Su B, Tang B, et al. Apparent digestibility coefficients of animal and plant feed ingredients for juvenile Pseudobagrus ussuriensis. Aquac Nutr. 2017 [Google Scholar]
- Damodaran S (1996) Amino acids, peptides and proteins. In: Food chemistry, 3rd edn. CRC Press, pp 321–329
- Deng Q, Wang L, Wei F, et al. Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 2011;124:1458–1465. doi: 10.1016/j.foodchem.2010.07.108. [DOI] [Google Scholar]
- El-Desoki W. Influence of acidity and sodium chloride on the function properties of whey protein powder. World J Dairy Food Sci. 2009;4:150–153. [Google Scholar]
- Fernandez-Quintela A, Macarulla MT, Del Barrio AS, Martinez JA. Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum Nutr. 1997;51:331–342. doi: 10.1023/A:1007936930354. [DOI] [PubMed] [Google Scholar]
- Finet S, Skouri-Panet F, Casselyn M, et al. The Hofmeister effect as seen by SAXS in protein solutions. Curr Opin Colloid Interface Sci. 2004;9:112–116. doi: 10.1016/j.cocis.2004.05.014. [DOI] [Google Scholar]
- García-Fayos B, Arnal J, Verdú G, Rodrigo I (2010) Purification of a natural coagulant extracted from Moringa oleifera seeds: isolation and characterization of the active compound. In: International conference on food innovation
- Gassenschmidt U, Jany KD, Tauscher B, Niebergall H. Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochem Biophys Acta. 1995;1243:477–481. doi: 10.1016/0304-4165(94)00176-X. [DOI] [PubMed] [Google Scholar]
- Govardhan SR, Ogunsina BS, Radha C. Protein extractability from defatted Moringa oleifera Lam. seeds flour. Ife J Sci. 2011;13:121–127. [Google Scholar]
- Guleria P, Kumar V, Guleria S. Genetic engineering: a possible strategy for protein-energy malnutrition regulation. Mol Biotechnol. 2017 doi: 10.1007/s12033-017-0033-8. [DOI] [PubMed] [Google Scholar]
- Hiai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976;29:116–122. doi: 10.1055/s-0028-1097639. [DOI] [PubMed] [Google Scholar]
- Hofmeister F. Zur Lehre von der Wirkung der Salze. Archiv für Experimentelle Pathologie und Pharmakologie. 1888;24:247–260. [Google Scholar]
- Jain A, Prakash M, Radha C. Extraction and evaluation of functional properties of groundnut protein concentrate. J Food Sci Technol. 2015;52:6655–6662. doi: 10.1007/s13197-015-1758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerri HA, Adolfsen KJ, McCullough LR, et al. Antimicrobial sand via adsorption of cationic Moringa oleifera protein. Langmuir. 2012;28:2262–2268. doi: 10.1021/la2038262. [DOI] [PubMed] [Google Scholar]
- Kamara MT, Huiming Z, Kexue Z, et al. Comparative study of chemical composition and physicochemical properties of two varieties of defatted foxtail millet flour grown in China. Am J Food Technol. 2009;4:255–267. doi: 10.3923/ajft.2009.255.267. [DOI] [Google Scholar]
- Kunz W. Specific ion effects in colloidal and biological systems. Curr Opin Colloid Interface Sci. 2010;15:34–39. doi: 10.1016/j.cocis.2009.11.008. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makkar HPS, Becker K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J Agric Sci. 1997;128:311–322. doi: 10.1017/S0021859697004292. [DOI] [Google Scholar]
- Ndabigengesere A, Narasiah K, Talbot B. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995;29:703–710. doi: 10.1016/0043-1354(94)00161-Y. [DOI] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Salis A, Cugia F, Parsons DF, et al. Hofmeister series reversal for lysozyme by change in pH and salt concentration: insights from electrophoretic mobility measurements. Phys Chem Chem Phys. 2012;14:4343–4346. doi: 10.1039/c2cp40150a. [DOI] [PubMed] [Google Scholar]
- Santos AFS, Luz LA, Argolo ACC, et al. Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem. 2009;44:504–508. doi: 10.1016/j.procbio.2009.01.002. [DOI] [Google Scholar]
- Sathe SK, Deshpande S, Salunkhe DK. Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. J Food Sci. 1982;47:491–497. doi: 10.1111/j.1365-2621.1982.tb10110.x. [DOI] [Google Scholar]
- Schwierz N, Horinek D, Netz RR. Reversed anionic hofmeister series: the interplay of surface charge and surface polarity. Langmuir. 2010;26:7370–7379. doi: 10.1021/la904397v. [DOI] [PubMed] [Google Scholar]
- Shevkani K, Singh N, Kaur A, Rana JC. Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll. 2015;43:679–689. doi: 10.1016/j.foodhyd.2014.07.024. [DOI] [Google Scholar]
- Slominski BA, Campbell LD. Formation of indole glucosinolate breakdown products in autolyzed, steamed, and cooked brassica vegetables. J Agric Food Chem. 1989;37:1297–1302. doi: 10.1021/jf00089a020. [DOI] [Google Scholar]
- Sridaran A, Karim AA, Bhat R. Pithecellobium jiringa legume flour for potential food applications: studies on their physico-chemical and functional properties. Food Chem. 2012;130:528–535. doi: 10.1016/j.foodchem.2011.07.062. [DOI] [Google Scholar]
- Sze-Tao KWC, Sathe SK. Functional properties and in vitro digestibility of almond (Prunus dulcis L.) protein isolate. Food Chem. 2000;69:153–160. doi: 10.1016/S0308-8146(99)00244-7. [DOI] [Google Scholar]
- Tomotake H, Shimaoka I, Kayashita J, et al. Physicochemical and functional properties of buckwheat protein product. J Agric Food Chem. 2002;50:2125–2129. doi: 10.1021/jf011248q. [DOI] [PubMed] [Google Scholar]
- Triveni R, Shamala TR, Rastogi NK. Optimised production and utilisation of exopolysaccharide from Agrobacterium radiobacter. Process Biochem. 2001;36:787–795. doi: 10.1016/S0032-9592(00)00279-X. [DOI] [Google Scholar]
- Wang JM, Xia N, Yang XQ, et al. Adsorption and dilatational rheology of heat-treated soy protein at the oil–water interface: relationship to structural properties. J Agric Food Chem. 2012;60:3302–3310. doi: 10.1021/jf205128v. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cremer PS. The inverse and direct Hofmeister series for lysozyme. Proc Natl Acad Sci USA. 2009;106:15249–15253. doi: 10.1073/pnas.0907616106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li Y, Ren Y. Research on the phosphorylation of soy protein isolate with sodium tripoly phosphate. J Food Eng. 2007;79:1233–1237. doi: 10.1016/j.jfoodeng.2006.04.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.