Abstract
Background
Animal studies suggest sphingolipids as an early marker of impaired glucose metabolism; however, research in humans is limited. We evaluated whether individual sphingolipid species were associated with fasting plasma glucose and incident impaired fasting glucose in a longitudinal cohort study.
Methods
We measured 15 sphingolipid species from blood samples collected in 2001–2003 from 2145 participants without prevalent diabetes in the Strong Heart Family Study. Fasting plasma glucose was measured in blood samples collected at baseline and follow-up (mean 5.5 years after baseline).
Findings
The average age of study participants was 38 years; 41% were men. Ceramide, sphingomyelin, and glucosylceramide species levels were higher in older participants; lactosyl-ceramide levels were higher in participants with lower BMIs. In adjusted analyses, greater concentrations of most ceramide species and lower lactosyl-ceramide with palmitic acid (LC-16) were associated with higher glucose levels at baseline. We did not observe associations of sphingomyelin species or glucosyl-ceramide species with glucose levels. Associations of sphingolipid levels with fasting glucose levels at follow-up were similar but had greater uncertainty than associations with baseline glucose. Although no statistically significant associations of sphingolipids with incident impaired fasting glucose were present, results were similar to glucose analyses.
Interpretation
We identified several ceramide species associated with higher fasting glucose levels and one sphingolipid, LC-16, that was associated with lower fasting glucose levels. These findings compliment previous research, which linked these sphingolipids with fasting insulin levels, and suggest that higher levels of these ceramides and lower LC-16 may be an early marker of impaired glucose metabolism.
Fund
US National Institutes Health.
Research in context.
Evidence before this study
Animal studies have suggested that sphingolipids play a role in insulin resistance and glucose metabolism, which are important factors in the development of diabetes. Human research in this area is limited; previous work in the Strong Heart Family study linked several ceramides with fasting plasma insulin levels; but associations of sphingolipids with markers of glucose metabolism in humans remain underexplored.
Added value of this study
We identified several ceramide species (Cer-18, Cer-22) that were associated with higher fasting glucose levels and one sphingolipid, LC-16, that was associated with lower fasting glucose levels.
Implications of all the available evidence
Combined with existing evidence, our findings suggest that higher levels of these ceramides and lower LC-16 may be an early marker of impaired glucose metabolism. These sphingolipid species may provide a useful target for therapeutic interventions among people at risk of diabetes.
Alt-text: Unlabelled Box
1. Introduction
Diabetes is an important global health problem, afflicting over 435 million people worldwide [1]. Among adults, approximately 95% of diabetes cases are Type 2, and the discovery of early metabolite markers of Type 2 diabetes may lead to specific targets for novel therapies to help reduce this high burden [2].
Sphingolipids, a family of cellular and circulating lipids that include ceramides and sphingomyelins, may be such a marker. Very long chain saturated fatty acids (with 20 or more carbons) are an important component of sphingolipids, and previous research has reported that higher levels of the circulating fatty acid 16:0 are associated with an increased risk of diabetes, while higher levels of very long chain saturated fatty acids are associated with lower risk of diabetes [3]. Although cell culture studies have demonstrated that ceramides block translocation of the GLUT4 glucose transporter and inhibit insulin-stimulated glucose uptake [4], human subjects research is limited, and the role that sphingolipids play in glucose homeostasis remains uncertain. Recent population-level research has linked levels of several ceramides with insulin levels; however, it is uncertain whether sphingolipids are associated with later markers of metabolic abnormalities, such as glucose intolerance, or with diabetes incidence itself [[5], [6], [7], [8]].
American Indians (AIs) experience a higher incidence of diabetes than many other ethnic groups, making diabetes prevention a public health priority in these communities [9]. The goal of this study was to examine associations of plasma ceramide and sphingomyelin species containing different saturated fatty acids with early metabolic markers of progression to diabetes, including fasting glucose and impaired fasting glucose, among participants in the Strong Heart Family Study (SHFS), a population-based cohort study comprised of AIs from 12 communities.
2. Methods
2.1. Study design and setting
The SHFS is a population-based longitudinal study of the genetics and risk factors for cardiovascular disease in several American Indian communities in Arizona, North Dakota, South Dakota and Oklahoma. Details of the study design have been described previously [10]. Briefly, 2768 men and women from 92 large families completed a baseline examination between 2001 and 2003. A follow-up examination was conducted in 2006–2009, during which 91% of those who completed the baseline examination also participated. Each examination included a personal interview, physical examination, medication review, and extensive laboratory work-up. The institutional review board from each Indian Health Service region and each of the included communities approved the study, and written informed consent was obtained from all participants at each exam.
2.2. Sphingolipid measurement
Blood samples were collected at baseline and follow-up exams after a 12-hour fast and were stored at −70 °C until analyzed. The same collection protocols were used at baseline and follow-up exams. A detailed description of the sphingolipid measurement was previously reported [5]; briefly, lipids were extracted from baseline blood samples using organic protein precipitation in a mixture of methyl tert-butyl ether, methanol, and isopropanol. Ten microlitres of each sample was mixed with 190 μL of precipitation solvent in a polypropylene microtiter plate. The samples were filtered, then mixed with 450 μL of 65% methanol/25% isopropanol (v:v). A volume of 5 μL was injected using an autosampler and resolved using reversed-phase chromatography at 50 °C on an Acquity BEH 300 C4 1.7 μ 2.1 × 50 analytical column (Waters, Cat.No 186004495) equipped with a VanGuard BEH 300 C4 1.7 μm guard column (Waters, Cat. No. 186004623). The resolved analytes were then introduced to the mass spectrometer (Sciex 6500) and analyzed using optimized mass spectrometric parameters for each compound. Internal standards were included in the precipitation solvent at a concentration of 19.4 nM (Ceramide/Sphingolipid Internal Standard Mixture I, 25 μM, Avanti Polar Lipids, LM-6002), to control for variability in extraction efficiency, pipetting, and ion suppression.
We measured 22 sphingolipids that carry a saturated fatty acid acylated to the sphingoïd backbone, including palmitic acid (16:0 [16 carbons, 0 double bonds]), stearic acid (18:0), arachidic acid (20:0), behenic acid (22:0) and lignoceric acid (24:0). Measured levels for fifteen species had coefficients of variation of 20% or less and were included in the analysis. These included six ceramides: ceramide with 16:0 (Cer-16), ceramide with 18:0 (Cer-18), ceramide with 20:0 (Cer-20), ceramide with 22:0 (Cer-22), and a composite concentration of Cer-24 computed as the sum of the concentrations of two species of ceramides with 24:0 having the distinct “d181” and “d182” sphingoïd backbones. Also included were six sphingomyelins: SM-14, SM-16, SM-18, SM-20, SM-22, SM-24; three glucosyl-ceramides (GC): GC-16, GC-22, and GC-24; and one lactosyl-ceramide (LC): LC-16.
2.3. Plasma glucose measurement and impaired fasting glucose classification
The blood samples for glucose measurement were drawn into NaF-EDTA tubes, mixed gently, placed on ice, spun, and then aliquoted within 1 h for most samples, but within 2 h for every sample. The same enzymatic methods were used to measure glucose from baseline and follow-up plasma samples [9]. Impaired fasting glucose (IFG) was classified as a fasting plasma glucose level of between 100 and 125 mg/dL [11].
2.4. Assessment of other covariates
The baseline examination included a physical examination, laboratory testing, medication review, a 1-week pedometer log, and an in-person interview to collect information on physical characteristics, medical conditions, education, smoking, and alcohol consumption. Diabetes was defined as use of insulin or oral anti-diabetic medication or a fasting plasma glucose concentration ≥ 126 mg/dL [11]. Body mass index (BMI) was calculated as body weight divided by height squared (kg/m2). Waist circumference was measured at the umbilicus while supine. Smoking was classified as never, ever, or current. Steps per day were calculated from the 1-week pedometer log, and body fat was measured using a Quantum II Impedence Meter.
2.5. Statistical analysis
Analyses were limited to participants without prevalent diabetes at baseline. Associations of sphingolipid levels with fasting glucose levels were assessed using linear mixed models that included a family-specific random-effect to account for familial aggregation and a subject-specific random-effect with covariance among family members proportional to the kinship coefficient, to account for genetic similarity among family members. We evaluated three main outcomes: fasting glucose at baseline, fasting glucose at follow-up, and the change in fasting glucose level from between to follow-up. Due to potential high influence of very large values, fasting glucose levels at follow-up exceeding 200 mg/dL (n = 36; 98th percentile) were Winsorized at 200 mg/dL [12]. Models were adjusted for age, sex, study site, education, BMI, waist circumference, smoking, and physical activity; models of glucose change also adjusted for baseline glucose level. Results are presented per two-fold higher concentration of each sphingolipid, which is comparable to the difference between the 90th and 10th percentiles of each sphingolipid species [5].
As secondary analyses, we evaluated associations of sphingolipids with incident IFG at follow-up. These secondary analyses were limited to participants with normal fasting glucose levels (<100 mg/dL) at baseline who did not have diabetes at follow-up, and used generalized estimating equations with a Poisson distribution, log link, robust standard errors, and a family specific random effect. Included adjustment terms were identical to those included in the primary analyses of glucose levels.
Missing values of baseline physical activity measures (n = 177) were multiply imputed using information on age, sex, education, body fat, and triglycerides, in models that accounted for possible family effects. The Fully Conditional Expectation method implemented by the MICE package in R was used with the predictive mean matching method [13]. Variables included in the imputation were selected by minimizing the Bayesian Information Criterion (BIC) in models predicting physical activity in the complete-case data. Twenty imputed datasets were generated and model fitting results were pooled using standard methods [14]. To correct for multiple comparisons, we assessed statistical significance at a p < 0.0033 (0.05/15 sphingolipid species) threshold.
Because glucose levels at follow-up would be influenced by use of insulin or hypoglycemic agents that were initiated after baseline, in sensitivity analyses we evaluated additional models of follow-up glucose and glucose change that added a constant (60 mg/dL) to participants who reported use of insulin or hypoglycemic agents at follow-up, and models that excluded 206 participants with diabetes at follow-up. To assess whether associations with follow-up glucose differed with length of follow-up time, we evaluated models that included an interaction term with follow-up time. We also examined models that included interaction terms with BMI, age, and sex to assess whether these factors modified associations of sphingolipids with baseline glucose levels, and models that included further adjustment for LDL to verify that results represent associations of sphingolipids with glucose levels are independent of LDL levels. Finally, because previous studies of cardiovascular disease outcomes have reported divergent effects of Cer-16 and Cer-18 relative to Cer-24 [15,16], we assessed whether Cer-16 and Cer-18 associations with glucose levels may be independent of Cer-24 by evaluating models that included the ceramide ratios Cer-16/Cer-24 and Cer-18/Cer-24 as exposures, and models of log(Cer-16) and log(Cer-18) that included adjustment for log(Cer-24).
2.6. Data statement
Due to privacy agreements with the tribal communities involved in this study, access to study data are restricted. Further information can be found at https://strongheartstudy.org/.
2.7. Role of the funding source
The study sponsors did not have any role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper for publication. PNJ had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Of the 2713 SHFS participants with available sphingolipid measures, 510 had prevalent diabetes at baseline, 6 were missing fasting glucose measures, and 52 were missing adjustment covariate information and were excluded. Of these 2145 participants eligible for baseline analyses, 1860 participants completed the follow-up exam, which occurred an average of 5.5 years after baseline (range: 2.8–8.5 years).
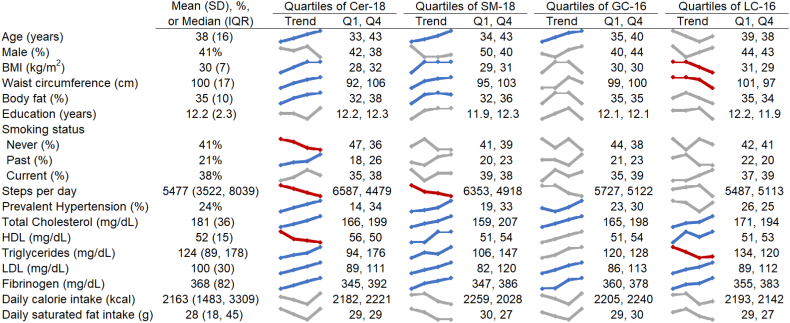
Baseline participant characteristics and unadjusted trends of these characteristics across quartiles of Cer-18, SM-18, GC-16, and LC-16 are presented in Fig. 1 (and across quartiles of each of the measured sphingolipids in Supplemental Figs. 1–3). Overall, the average age of participants was 38 years, 41% were male, and the average BMI was 30, with 23% having a BMI of 35 or greater. High-levels of physical activity were uncommon; only 14% of participants walked at least 10000 steps per day, and 25% walked fewer than 3500 steps per day. The average fasting plasma glucose was 94 mg/dL at baseline and 100 mg/dL at follow-up.
Fig. 1.
Participant characteristics, overall and according to Cer-18, SM-18, GC-16, and LC-16 (N = 2145).
The colored graphics show means or percentages of each characteristic across quartiles of Cer-18, SM-18, GC-16, and LC-16. Unadjusted linear and logistic regression models were used to assess statistically significant (p < .0026; 0.05/19 characteristics) associations of log-transformed Cer-18, SM-18, GC-16, and LC-16 with each characteristic; statistically significant positive trends are colored in blue, statistically significant negative trends are in red, grey indicates p > .0026.
In univariate analyses, presented as trend lines in Fig. 1, participants with higher Cer-18 and SM-18 concentrations were older, with higher BMIs, body fat percentages, and larger waist circumferences, walked fewer steps per day, were more likely to have prevalent hypertension, and had higher total cholesterol, LDL, and fibrinogen levels than those with lower Cer-18 and SM-18 concentrations. Those with higher Cer-18 also had lower HDL levels and were also more likely to be former smokers and less likely to have never smoked, while those with higher SM-18 had higher HDL levels. Participants with higher GC-16 were older and were more likely to have prevalent hypertension and higher total cholesterol, LDL, and fibrinogen levels than those with lower GC-16 concentrations, and participants with higher LC-16 had lower BMIs, smaller waist circumferences, higher total cholesterol, HDL, LDL, and fibrinogen levels and lower triglycerides than those with lower LC-16 concentrations.
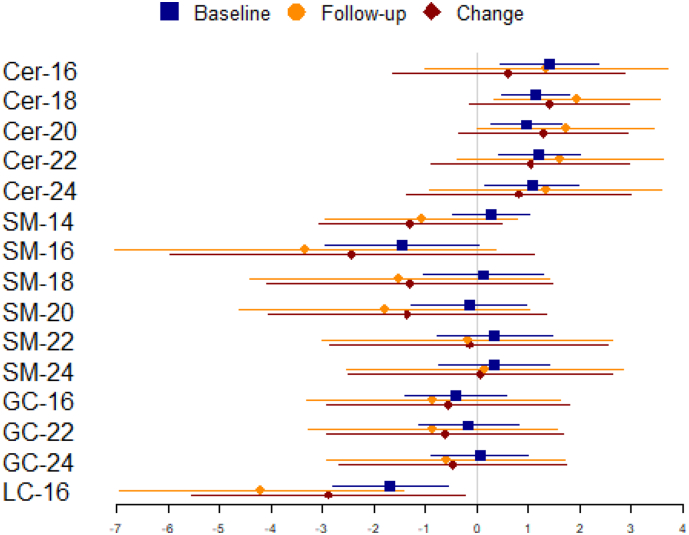
Results from adjusted models evaluating associations between sphingolipids and plasma fasting glucose levels at baseline, at the follow-up exam, and change in glucose between baseline and follow-up exam are presented in Fig. 2 in blue, orange, and red, respectively (and Supplemental Table 1). We observed that two-fold higher Cer-18 and Cer-22 levels were associated with 1.2–1.4 mg/dL higher fasting glucose levels at baseline, among people with similar covariate values. Among the remaining ceramides (Cer-16, Cer-20, and Cer-24), we observed similar associations with baseline fasting glucose; however, none of these met the Bonferroni corrected p-value threshold of p < .0033. Analyses of follow-up glucose levels resulted in similar point estimates (1.3–2.0 mg/dL) but with less precision. We did not observe associations of ceramides with change in glucose levels between baseline and follow-up.
Fig. 2.
Associations* of two-fold higher circulating plasma sphingolipids with fasting glucose (mg/dL) at baseline (phase 4), follow-up (phase 5), and change in fasting glucose from baseline to follow-up.
Markers represent point estimates; lines represent 95% confidence intervals.
*Adjusted for baseline age, sex, BMI, site, education, smoking, physical activity, and waist circumference; change model also includes adjustment for baseline glucose.
There was no evidence of associations of any of the sphingomyelin species with fasting glucose levels at baseline or follow-up, or with change in glucose levels between baseline and follow-up. Likewise, glucosyl-ceramides were not associated with any of the glucose measures. However, LC-16, the only lactosyl ceramide species measured, was associated with lower glucose levels at both baseline and follow-up exams, and with lower rate of change in glucose between the two exams. After adjustment for covariates, a two-fold higher LC-16 level at baseline was associated with a 1.7 mg/dL lower fasting glucose level at baseline and a 4.2 mg/dL lower fasting glucose at follow-up. The difference between baseline and follow-up fasting glucose per two-fold higher LC-16 at baseline was −4.0 mg/dL, but this did not reach the pre-specified threshold of significance.
Models that added a constant to glucose levels among participants using insulin or hypoglycemic agents at follow-up resulted in larger point estimates but greater uncertainty. Associations of Cer-18, Cer-22, and LC-16 were not significant after excluding participants with prevalent diabetes at follow-up, nor were any new associations detected (Supplemental Table 2). There was no evidence to suggest that BMI, age, sex, or length of time from baseline to follow-up modified associations of sphingolipids with fasting glucose levels (Supplemental Tables 3 and 4). Neither of the ceramide ratios Cer-16/Cer-24 or Cer-18/Cer-24 were associated with fasting glucose levels, and results from models of Cer-16 and Cer-18 that included adjustment for Cer-24 were similar to the primary analysis (Supplemental Tables 5 and 6).
Among the 1381 participants with normal fasting glucose levels at baseline who also had glucose measures at follow-up, 18% (n = 250) had incident IFG at follow-up. We did not see evidence in multivariable adjusted models that any of the measured sphingomyelins, glucosyl-ceramides, or lactosyl-ceramides were associated with incident IFG at follow-up (Table 1). Although it did not meet the pre-specified significance threshold, there was a suggestion that a two-fold higher Cer-24 level is associated with a 45% higher relative risk of incident IFG (RR: 1.45; 95% CI: 1.13, 1.88).
Table 1.
Relative risk of incident IFG at follow-up per two-fold higher sphingolipid level among 1381 participants with normal fasting glucose levels at baselinea.
| Incident IFG |
|||
|---|---|---|---|
| RR | 95 CI | p-Value | |
| Cer-16 | 1.29 | (1.01, 1.65) | 0.043 |
| Cer-18 | 1.25 | (1.05, 1.48) | 0.011 |
| Cer-20 | 1.24 | (1.03, 1.49) | 0.021 |
| Cer-22 | 1.34 | (1.08, 1.66) | 0.008 |
| Cer-24 | 1.45 | (1.13, 1.88) | 0.004 |
| SM-14 | 1.22 | (0.97, 1.53) | 0.088 |
| SM-16 | 1.55 | (0.99, 2.41) | 0.054 |
| SM-18 | 1.18 | (0.86, 1.61) | 0.306 |
| SM-20 | 1.00 | (0.72, 1.39) | 0.999 |
| SM-22 | 1.15 | (0.80, 1.65) | 0.455 |
| SM-24 | 1.29 | (0.89, 1.87) | 0.185 |
| GC-16 | 1.21 | (0.98, 1.50) | 0.080 |
| GC-22 | 1.07 | (0.81, 1.41) | 0.634 |
| GC-24 | 1.22 | (0.92, 1.62) | 0.161 |
| LC-16 | 1.09 | (0.80, 1.49) | 0.602 |
Adjusted for baseline age, sex, BMI, site, education, smoking, physical activity, and waist circumference.
4. Discussion
Among 2145 American Indians without prevalent diabetes, greater concentrations of circulating ceramides and lower lactosyl-ceramide with 16:0 were associated with higher fasting plasma glucose levels at baseline. We did not find evidence that sphingomyelins or glucosyl-ceramides were associated with fasting glucose levels. Associations of sphingolipids with fasting glucose levels at follow-up were similar but had greater uncertainty than associations with baseline glucose, and no associations were seen with change in glucose. No associations with incident IFG at follow-up reached statistical significance.
Our findings complement recent work that suggests ceramides as potential early metabolic markers of progression to diabetes. Insulin resistance, beta cell dysfunction, and hyperglycemia are all interrelated in the pathogenesis of type 2 diabetes, and while the relationship between these three states is complex, insulin resistance and beta cell dysfunction can induce impaired glucose tolerance [17]. Animal studies have provided a strong link between ceramides and insulin resistance; lean mice that were infused with LDL containing ceramides showed reduced insulin-stimulated glucose uptake [18,19]. In humans, small, cross-sectional studies have reported that circulating plasma ceramides are elevated in subjects with type II diabetes and indicate severity of insulin resistance [20]. A recent analysis in the SHFS identified several ceramide species that were associated with fasting insulin levels, HOMA-IR, and HOMA-B at baseline and at the follow up exam [5]. Among these, we observed that Cer-18 and Cer-22 were also associated with fasting glucose levels at baseline, indicating that these ceramides are associated with sequential markers in the progression to type-2 diabetes.
The role of sphingomyelins in the progression to diabetes, through insulin resistance and glucose tolerance, has not been well established [[21], [22], [23]]. Previous work in the SHFS reported associations of sphingomyelins with fasting insulin, HOMA-IR, and HOMA-B levels were modified by BMI, with higher SM-18, -20, -22, and -24 species being associated with lower insulin, HOMA-IR, and HOMA-B levels at baseline and follow-up for normal BMIs but associated with higher levels at higher BMIs [5]. In our analysis, we did not find evidence of associations of sphingomyelins with glucose levels, or evidence of similar interactions.
Glucosyl and lactosyl ceramide are both precursors to larger glycosphingolipids such as gangliosides, but their individual biological properties are not well understood. Mouse studies have shown that when glucosylceramide synthase is inhibited, gangliosides appear to interfere with insulin action in adipocytes, but not in myotubes, where they promote insulin sensitivity [24,25]. In contrast with Cer-18 and SM-18, lower levels of LC-16 were found in those with higher BMIs, larger waist circumferences, and higher triglyceride levels. Although no associations were observed with glucosyl ceramides, higher levels of the lactosyl ceramide LacCer-16 were associated with lower fasting glucose.
Although we did not observe statistically significant associations of any of the measured sphingolipids with incident IFG, results were consistent with the primary analysis, and there was evidence to suggest that ceramides may provide an early marker of impaired glucose tolerance. However, there are several reasons why we were less able to detect associations of sphingolipids with incident IFG than with glucose levels. First, our IFG analyses had a smaller sample size than glucose level analyses, because models of IFG were limited to participants with normal fasting glucose levels at baseline who did not develop diabetes. Secondly, IFG is a binary outcome, which would result in diminished statistical power when compared to the outcome of glucose levels, which is a continuous measure. Therefore, further research is needed to replicate and further investigate these findings.
In univariate analyses, which may reflect underlying associations with age or other characteristics, none of the sphingolipids were associated with sex or caloric or saturated fat intake, but ceramides were lower in those with greater level of physical activity and who did not smoke. In addition, all of the sphingolipids were correlated with total cholesterol and LDL levels, and results were not changed with the inclusion of LDL as an adjustment variable in our models. Ceramides and sphingomyelins in the blood circulate in lipoproteins, likely explaining the associations with LDL [26]. However, ceramides and LC-16 show divergent associations with triglycerides, measures of adiposity and HDL.
There are a number of reasons why follow-up results differed slightly from baseline results. Participants who at baseline had fasting glucose levels ≥126 mg/dL or were using insulin were excluded from all analyses, but participants with high glucose levels or diabetes medication use at the follow-up exam were included in the longitudinal analyses. This resulted in greater variability of follow-up glucose levels, which corresponded with more uncertain estimates in the longitudinal models. Sensitivity analyses restricted to participants without prevalent diabetes at follow-up produced more precise point estimates, but those point estimates are conservative due to their exclusion of those with highest glucose values at follow-up; further studies are needed to investigate associations of sphingolipids with diabetes risk. While sphingolipids and baseline glucose were measured from the same blood samples, and thus reflect dietary and behavioral patterns from the same period of time, the interval between baseline and follow-up blood samples varied between individual participants. Although we did not detect effect modification by length of follow-up time, some participants had more time than others to advance in diabetes progression, which may have diluted observed associations of sphingolipids with follow-up glucose levels.
Strengths of this study include the large sample size, a study population with a high underlying risk of diabetes, objective laboratory measurements, and the assessment and control of multiple potential confounders. This study also has a number of limitations. The ethnically homogenous study population may impair generalizability to other populations, and the observational nature of the study prohibits assessments of causality. Although underivatized ceramides and sphingomyelins with saturated fatty acids were measured with enough precision, some ceramide species containing saturated fatty acids, such as lactosyl and glucosyl ceramides, were not included in this analysis due to high laboratory assay measurement error. Finally, the average length of time between baseline and follow-up was 5.5 years, which may not have been a long enough period of time for detectable changes in glucose tolerance to occur in this relatively young population.
5. Conclusions
We identified several ceramide species associated with higher fasting glucose levels and one sphingolipid, LC-16, that was associated with lower fasting glucose levels. These findings compliment previous research, which linked these sphingolipids with fasting insulin levels, and suggest that higher levels of these ceramides and lower LC-16 may be an early marker of impaired glucose metabolism.
Declaration of interests
The authors have no conflicts of interest to disclose. P.N.J. drafted the manuscript and analyzed the data. A.M.F., C.Y., C.M.S., and B.M. contributed to the data analysis and critically reviewed the manuscript. A.H. performed biospecimen measurements and critically reviewed the manuscript. J.U., B.V.H., and R.N.L. contributed to the conception and design of the study, the acquisition of data, and critically reviewed the manuscript. D.S.S., I.B.K., and N.S. contributed to the interpretation of results and critically reviewed the manuscript.
Funding sources
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under Award Number R01-DK103657 and P30 DK035816 and by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL109284. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online athttps://doi.org/10.1016/j.ebiom.2018.12.046.
Appendix A. Supplementary data
Supplemental Figures and Tables
References
- 1.Global Burden of Disease Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Atlanta, GA: 2017. National Diabetes Statistics Report, 2017. [Google Scholar]
- 3.Lemaitre R.N., Fretts A.M., Sitlani C.M. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101(5):1047–1054. doi: 10.3945/ajcn.114.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summers S.A., Garza L.A., Zhou H., Birnbaum M.J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18(9):5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaitre R.N., Yu C., Hoofnagle A. Circulating sphingolipids, insulin, HOMA-IR and HOMA-B: the Strong Heart Family Study. Diabetes. 2018;67(8):1663–1672. doi: 10.2337/db17-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pories W.J., Dohm G.L. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care. 2012;35(12):2438–2442. doi: 10.2337/dc12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corkey B.E. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes. Diabetes Care. 2012;35(12):2432–2437. doi: 10.2337/dc12-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weyer C., Hanson R.L., Tataranni P.A., Bogardus C., Pratley R.E. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094–2101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 9.Lee E.T., Welty T.K., Fabsitz R. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 10.North K.E., Howard B.V., Welty T.K. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157(4):303–314. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastings C., Mosteller F., Tukey J.W., Winsor C.P. Low moments for small samples – a comparative study of order statistics. Ann. Math. Stat. 1947;18(3):413–426. [Google Scholar]
- 13.Van Buuren S., Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J. Stat. Softw. 2011;45(3) [Google Scholar]
- 14.Schafer J.L. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 15.Anroedh S., Hilvo M., Akkerhuis K.M. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J Lipid Res. 2018;59(9):1729–1737. doi: 10.1194/jlr.P081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laaksonen R., Ekroos K., Sysi-Aho M. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967–1976. doi: 10.1093/eurheartj/ehw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerf M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. (Lausanne) 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavez J.A., Summers S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Boon J., Hoy A.J., Stark R. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62(2):401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haus J.M., Kashyap S.R., Kasumov T. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauschert S., Uhl O., Koletzko B. Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. J Clin Endocrinol Metab. 2016;101(3):871–879. doi: 10.1210/jc.2015-3525. [DOI] [PubMed] [Google Scholar]
- 22.Xu F., Tavintharan S., Sum C.F., Woon K., Lim S.C., Ong C.N. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab. 2013;98(6):E1060–E1065. doi: 10.1210/jc.2012-4132. [DOI] [PubMed] [Google Scholar]
- 23.Hanamatsu H., Ohnishi S., Sakai S. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes. 2014;4:e141. doi: 10.1038/nutd.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennemann R., Grone H.J. Cell-specific in vivo functions of glycosphingolipids: lessons from genetic deletions of enzymes involved in glycosphingolipid synthesis. Prog Lipid Res. 2013;52(2):231–248. doi: 10.1016/j.plipres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Chavez J.A., Siddique M.M., Wang S.T., Ching J., Shayman J.A., Summers S.A. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J Biol Chem. 2014;289(2):723–734. doi: 10.1074/jbc.M113.522847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammad S.M. Blood sphingolipids in homeostasis and pathobiology. Adv Exp Med Biol. 2011;721:57–66. doi: 10.1007/978-1-4614-0650-1_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures and Tables