Abstract
Brain metastasis (BM) of non-small cell lung cancer (NSCLC) is relatively common and has a poor prognosis. Moreover, identifying which patients are more likely to develop BM is challenging. Akt, a serine/threonine-specific protein kinase, can be activated in various tumors, including lung cancer, and may be associated with poor prognosis. Here, we used immunohistochemistry to evaluate phosphorylated-Akt (p-Akt) expression in tumor tissues of 99 NSCLC patients. We also analyzed the genotype of the patients for two single nucleotide polymorphisms (SNPs) of the AKT1 gene, rs2498804 and rs2494732. We found that p-Akt expression differs between NSCLC patients and correlates with the risk of BM. Indeed, patients exhibiting medium to high p-Akt expression had a higher incidence of BM than those exhibiting low to no p-Akt expression (39% vs 16%). Our data also show that patients with the rs2498804 GT/GG and rs2494732 CT/TT variant genotypes were more likely to exhibit higher levels of p-Akt expression than those with the rs2498804 TT and rs2494732 CC variant genotypes (35% vs. 24% and 37% vs. 25%, respectively). Our results suggest that the level of expression of p-Akt, which may be affected by the AKT1 genotype, is correlated with the risk of BM. However, further studies are needed to establish p-Akt as a predictive marker for BM in NSCLC patients.
Keywords: Akt, Non-small cell lung cancer, Brain metastasis, Single nucleotide polymorphism, Predict biomarker
Abbreviations: BM, Brain metastases; NSCLC, Non-small cell lung cancer; p-Akt, Akt phosphorylation; PCI, Prophylactic cranial irradiation; CDH2, N-cadherin; SNPs, Single nucleotide polymorphisms; KPS, Karnofsky performance status
Highlights
•The level of expression of p-Akt differs significantly between NSCLC patients.
-
•
Patients with high p-Akt expression have a higher incidence of brain metastasis.
-
•
AKT1 SNP variant genotypes are associated with higher expression levels of p-Akt.
-
•
p-Akt is a prospective marker to predict brain metastasis in NSCLC patients.
1. Introduction
Non-small cell lung cancer (NSCLC) accounts for 85%–90% of all lung cancers, which are the leading cause of cancer-related deaths [1]. Brain metastasis (BM) is noted in 13%–54% of NSCLC patients [2]. With the rapid development of surgery, chemotherapy, and radiotherapy, the control of extracranial lesions has significantly improved for lung cancer patients, but the incidence of BM has not declined. Survival after BM diagnosis remains poor with an average ranging from 1.5 to 9.5 months [3,4]. Therefore, it is essential to improve the prediction and prevention of BM.
The benefit of prophylactic cranial irradiation (PCI) in the treatment of patients with acute lymphoblastic leukemia or small cell lung cancer has been well established, and PCI is now a part of the standard therapeutic course [5]. However, the results of previous studies evaluating PCI for NSCLC were less than satisfactory. Indeed, in most trials, PCI reduced the incidence of BM, but had no impact on overall survival [6,7]. This observation may reflect the fact that not all patients with NSCLC require PCI [2]. Moreover, PCI can be associated with acute side effects, including neurotoxicity. Therefore, it is essential to develop reliable tools to identify the subset of patients at the highest risk of BM, and most likely to benefit from PCI.
The pretreatment factors that have been used to predict a high incidence of BM include histology, disease extent, and young age. However, the data relating to these factors are not consistent and often contradictory [8,9]. To date, only a few inherited genetic markers have been shown to detect NSCLC patients at a high risk of BM. While a previous study showed that the expression levels of the CDH2 (N-cadherin), KIFC1, and FALZ genes correlate with the risk of BM in NSCLC patients [10], we have recently shown that single nucleotide polymorphisms (SNPs) in AKT1 can also predict the risk of BM in NSCLC patients [11].
Akt, also known as protein kinase B (PKB), is a serine/threonine-specific protein kinase that plays a central role in fundamental biological processes, including cell proliferation, glucose metabolism, cell migration, apoptosis, cell cycle regulation, and angiogenesis [12]. Akt is an integral component of the PI3K/Akt/mTOR signaling pathway but also mediates other signal transduction pathways, such as the epidermal growth factor receptor pathway and Wnt signaling cascade. Indeed, the activated form of Akt, phosphorylated-Akt (p-Akt), can regulate numerous downstream factors, including NF-κB, BAD, GSK-3, and Wee 1 [13]. Importantly, previous studies have shown that aberrant Akt activation is associated with the development of many tumors. Consequently, Akt inhibition shows great therapeutic potential in the treatment of many malignancies. Furthermore, p-Akt expression level has been associated with the prognosis of several cancers [14,15], including NSCLC where p-Akt overexpression has been reported as an indicator of poor prognosis [[16], [17]].
While the role of p-Akt in the development of extracranial lesions in NSCLC patients is well-established [18], its role in the development of BM remains to be fully elucidated. Moreover, further studies are needed to understand the complex mechanisms underlying Akt activation. We previously reported that genetic variations in the AKT1 gene could be used to predict the risk of BM [11]. To validate further and expand our findings, in the present study, we examined p-Akt expression at the protein level in NSCLC tumor tissues and analyzed its association with the risk of BM and SNPs in AKT1.
2. Materials and methods
2.1. Study population and data collection
The study was based on a consecutive case series of 99 NSCLC patients, who were all found to have pathological tissue masses in our previous study and treated surgically at Tongji Hospital between 2007 and 2011. All the tissue samples analyzed came from paraffin-embedded tumor masses. The Karnofsky fitness score (KPS) of all patients was above 70, and all had a life expectancy exceeding six months. Epidemiological data were collected with questionnaires before enrollment and included information on demographics, smoking history, alcohol consumption, medical history, family history of cancer, and occupational exposure to potential carcinogens. Clinical and follow-up data on treatment regimens, disease stage, pretreatment performance status, and survival status at the time of analysis were obtained from patients' medical records. Please refer to our previous study for the definitions of disease staging, smoking status, diagnosis of brain metastasis, time to brain metastasis, and follow-up time [11]. The study was approved by the Ethics Committee of Tongji Medical College. Written informed consent was obtained from all patients before interview.
2.2. Polymorphism selection and genotyping
We selected two SNPs in AKT1 (rs2498804 and rs2494732) that were associated with BM in our previous study. The SNPs were genotyped as described previously [11]. Briefly, Genomic DNA was isolated from peripheral blood lymphocytes by using a QuickGene DNA whole blood kit S (Fuji Film) according to the manufacturer's protocol, and stored at −80 °C until use. AKT1: rs2494732 was genotyped using TaqMan assay. AKT1: rs2498804 was genotyped by using matrix-assisted laser desorption/ionization-time of flight mass spectrophotometry to detect allele-specific primer extension products with the MassARRAY platform (Sequenom, Inc.). Assay data were analyzed using Sequenom TYPER software (version 4.0). The individual call rate threshold was at least 95%.
2.3. Tissue immunohistochemical staining
First, paraffin-embedded sections were incubated for 2 h in a 65 °C oven and rinsed three times for 5 min each with phosphate-buffered saline (PBS). Then, the samples were dewaxed by immersing in xylene three times for 10 min each and hydrated by immersing in 100%, 95%, 75% ethanol for 5 min each and deionized water for 10 min. After blocking endogenous peroxidase with 3% hydrogen peroxide for 15 min, the samples were rinsed three times for 5 min each with PBS. For antigen retrieval, the samples were immersed in sodium citrate (pH 8.0) for 2 min and rinsed three times for 5 min each with PBS.
Following 20 min incubation with 5% BSA Protein Blocking Buffer, the slides were incubated overnight at 4 °C with primary rabbit antibodies against p-Akt (1:200, Ser473, D9E, IHC specific, Cell Signaling, USA). Then, the slides were washed with PBS, incubated for 30 min at 37 °C with mouse anti-rabbit IgG secondary antibodies, and stained with diaminobenzidine (DAB) for 1–10 min. After washing once, the slides were counterstained for 2 min with hematoxylin, differentiated for 1 s in hydrochloric acid-ethanol solution, returned to blue for 6 s in ammonia water, and gradually dehydrated in 75%, 95%, and 100% ethanol. Finally, the slides were air-dried in a fume hood and sealed with a neutral resin.
2.4. Assessment of p-Akt expression level
Two pathologists performed a blind, semi-quantitative scoring of the stained sections. Cells with cytoplasmic and/or nuclear staining were considered positive. We used two indexes to estimate the level of expression of p-Akt, the percentage of positive cells (PP) and arbitrary staining intensity (SI). In brief, five high magnification fields were randomly selected, the PP for 200 cells in each field was determined, and the mean value was calculated. The SI was scored as follow: 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The level of expression of p-Akt was finally estimated using the H value. The H value was obtained by multiplying the PP by the SI score, resulting in a maximum H value of 300 (100% × 3). For the subsequent statistical analysis, the H value was categorized as negative (0–9), low (10–100), medium (101–200), and high (201–300) [19].
2.5. Statistical analysis
All statistical analyses were performed using the SPSS software package version 16.0. The relationship between p-Akt and AKT1 variant genotypes was analyzed using the chi-square test (Phi correlation). Kaplan–Meier curves were plotted to assess the cumulative probability of BM. The log-rank test was used to examine differences between groups. The Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence intervals (CIs) for the influence of p-Akt expression level on the risk of BM. The model was adjusted for disease stage, tumor histology, and smoking status. All P-values were two-sided, and P-values below 0.05 were considered statistically significant.
3. Results
3.1. Patients’ clinicopathological features
The clinicopathological features of the 99 patients (66 men and 33 women) are listed in Table 1. The median age of all patients was 58 years (range, 32–77 years), 76% had stage I to IIIA disease, 75% had adenocarcinoma, and 53% had smoked tobacco (77% of the men and <1% of the women). Twenty-three patients had developed BM at 24 months of mean follow-up (range, 0–80 months). We performed Chi-square analyses of the association between clinicopathological features of the tumors and the level of expression of p-Akt. In this small group study, we only found a correlation between the patients’ sex and the level of expression of p-Akt (Table 1).
Table 1.
p-AKT expression levels and clinicopathological features of 99 patients.
| Characteristic | No. of Patients | p-AKT expression |
X2 | P Value | |
|---|---|---|---|---|---|
| Negative + Low | Medium + High | ||||
| Sex | |||||
| Female | 33 | 16 (49) | 17 (51) | 9.393 | 0.002 |
| Male | 66 | 52(79) | 14 (21) | ||
| Age, years | |||||
| ≥60 years | 42 | 31(74) | 11 (26) | 0.890 | 0.345 |
| <60 years | 47 | 37 (65) | 20 (35) | ||
| Disease stage at diagnosis | |||||
| I, II, IIIA | 75 | 51 (68) | 24 (32) | 0.068 | 0.794 |
| IIIB, IV | 24 | 17 (71) | 7 (29) | ||
| Tumor histology | |||||
| Squamous cell | 25 | 21 (84) | 4 (16) | 3.646 | 0.056 |
| Adenocarcinoma | 74 | 47 (64) | 27 (36) | ||
| Smoking Status | |||||
| Current or Former | 44 | 40 (77) | 12 (23) | 3.455 | 0.063 |
| Never | 47 | 28 (60) | 19 (40) | ||
3.2. p-Akt expression level in NSCLC
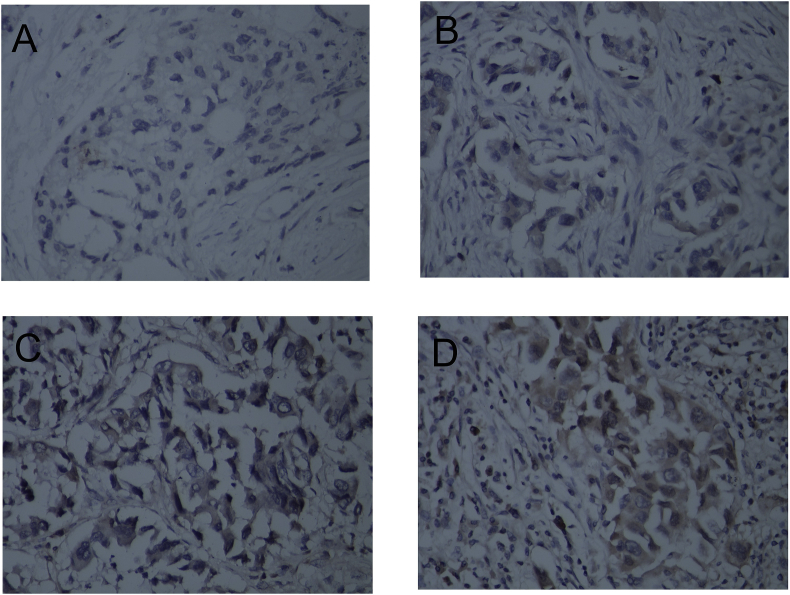
Overall, p-Akt was mainly expressed in the cytoplasm of the tumor cells and was only detected in the nucleus for one of 99 cases. Remarkably, our data show that p-Akt expression could be detected in 67% of the NSCLC cases tested (Table 2). Of the 99 patients, 33 (33%) were negative for p-Akt (H value between 0 and 9, Fig. 1A), 35 (36%) exhibited low p-Akt expression (H value between 10 and 100, Fig. 1B), 16 (16%) medium p-Akt expression (H value between 101 and 200, Fig. 1C), and 15 (15%) strong p-Akt expression (H value between 201 and 300, Fig. 1D).
Table 2.
p-AKT expression levels.
| H score | Negative (0–9) | Low (10–100) | Medium (101–200) | High (201–300) |
|---|---|---|---|---|
| p-AKT | 33 (33%) | 35 (36%) | 16 (16%) | 15 (15%) |
Fig. 1.
P-AKT expression levels in 99 NSCLCs. A. P-AKT was negative; B. P-AKT was low; C. P-AKT was moderate; D. P-AKT was high. All pictures are ×400 magnification.
3.3. p-Akt expression level and risk of BM
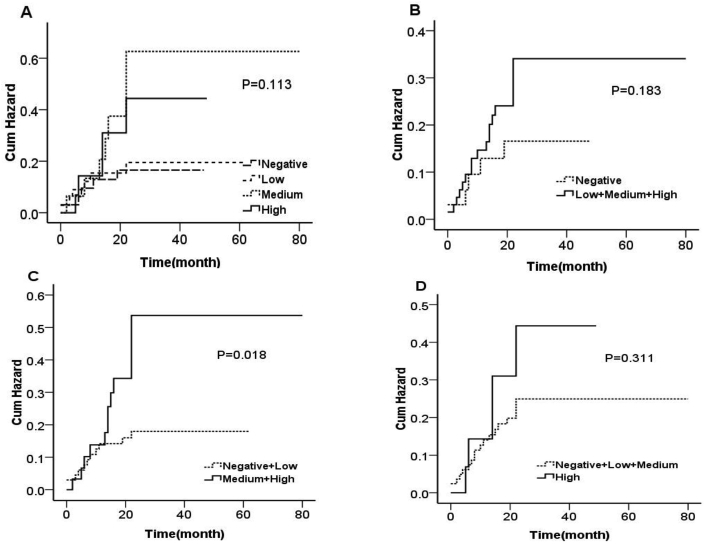
To assess the association between the level of expression of p-Akt and the risk of BM, we performed Kaplan-Meier and Cox proportional hazards analyses. Our data indicate that NSCLC patients with high levels of expression of p-Akt had higher cumulative probabilities of developing BM (Fig. 2). Indeed, compared to patients with low to no p-Akt expression, the patients with medium to high p-AKT expression exhibited a higher incidence of brain metastases (16% vs. 39%, Table 3). Moreover, multivariate Cox proportional hazards analyses showed that patients with medium to high p-Akt expression had a higher risk of developing BM than patients with low to no p-Akt expression (HR, 2.558, 95% CI, 1.095–5.831, P = 0.030, Table 3).
Fig. 2.
Kaplan-Meier curves show cumulative brain metastases in patients with non-small cell lung cancer. Patients were grouped according to P-AKT expression levels. A. Negative, low, medium and high expression of 4 groups; B. Negative, positive expression (including low, medium, high) 2 groups; C. Points (negative + low) and (middle + high) expression of 2 groups; D. scores (negative + low + medium) and high expression of 2 groups; the results showed that patients with high P-AKT expression had an increased brain metastasis rate.
Table 3.
p-AKT expression levels and brain metastases.
| p-AKT | No. of Patients | No. of Events (%) | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | |||
| Negative + Low | 68 | 11 (16) | 1.000 | 1.000 | ||||
| Medium + High | 31 | 12 (39) | 2.573 | 1.134–5.839 | 0.024 | 2.558 | 1.095–5.831 | 0.030 |
Note: Multivariate analysis adjusted sex, age, disease stage, histological type and smoking status.
3.4. p-Akt expression level and SNPs in the AKT1 gene
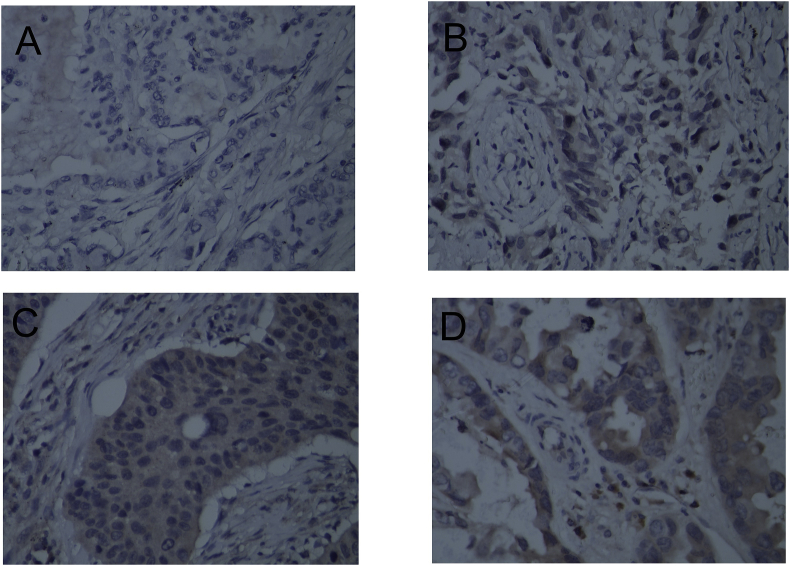
To further evaluate p-Akt as a potential predictive marker for BM, we used the Phi correlation coefficient to examine the relationship between the level of expression of p-Akt and several AKT1 variant genotypes. Our data clearly showed that two SNPs in AKT1 (rs2498804 and rs2494732) were associated with p-Akt expression. Compared to patients with the rs2498804 TT and rs2494732 CC variant genotypes, patients with the rs2498804 GT/GG and rs2494732 CT/TT variant genotypes exhibited higher levels of expression of p-Akt (24% vs. 35% and 25% vs. 37%, respectively; Fig. 3 and Table 4). However, in our small group study, this observed difference did not reach statistical significance (rs2498804: χ2 = 1.151, P = 0.283; rs2494732: χ2 = 1.554, P = 0.213; Table 4), and further studies are needed to confirm the association between these SNPs, the level of expression of p-Akt, and the risk of BM.
Fig. 3.
P-AKT expression levels in patients with different genotypes of AKT1:rs2498804. A. TT type P-AKT expression is negative; B. GT type P-AKT low level expression; C. GT type P-AKT expression level is moderate; D. GG type P-AKT high level expression. All pictures are ×400 magnification.
Table 4.
p-AKT expression levels and AKT gene single nucleotide polymorphisms.
| Polymorphisms and Genotypes | No. of Patients | p-AKT expression |
X2 | P Value | |
|---|---|---|---|---|---|
| Negative + Low | Medium + High | ||||
| AKT1: rs2498804 | |||||
| TT | 33 | 25 (76) | 8 (24) | ||
| GT + GG | 66 | 43 (65) | 23 (35) | 1.151 | 0.283 |
| AKT1: rs2494732 | |||||
| CC | 47 | 35 (75) | 12 (25) | ||
| CT + TT | 51 | 32 (63) | 19 (37) | 1.554 | 0.213 |
4. Discussion
BM is one of the most challenging aspects of the clinical treatment of NSCLC patients. Indeed, intracranial recurrence affects the survival of NSCLC patients, and the related symptoms also have a strong impact on their quality of life. In the present study, we examined the relationships between the level of expression of p-Akt in NSCLC and both the risk of BM and SNPs in AKT1. Our data support the notion that a high level of expression of p-Akt was associated with an increased risk of BM in NSCLC patients. Moreover, the level of expression of p-Akt was higher in patients with the AKT1 rs2498804 GG variant genotype. Further studies are needed to confirm this finding and complement our previous report on BM and genetic polymorphisms. Such studies would also advance our understanding of the mode of action of p-Akt in the biological behavior of BM in NSCLC patients.
Akt is overexpressed in many human tumors, and its oncogenic effects have been demonstrated previously [20]. Akt, which acts downstream of the phosphatidylinositol 3-kinase (PI3K), regulates cell survival mechanisms and can be activated by various growth factors, including insulin, the insulin-like growth factor, and the epithelial growth factor. The activation of Akt requires the phosphorylation of both thr308/309 on its kinase activation ring and ser473/474 on its carboxyl terminus [21]. Once activated, p-Akt is a powerful driver for cell survival and can counteract or inactivate intermediate components of the apoptosis cascades, such as the proapoptotic factors BAD and caspase-9, and members of the forkhead transcription factor family [22]. Moreover, Akt can also regulate angiogenesis and metastasis [23], two essential processes in tumor development. Previous studies have also confirmed the existence of structural activation of Akt in 90% of NSCLC cell lines, and, moreover, the relationship between p-Akt and metastasis has been reported for other tumors [24,25]. Altogether, these data support our finding that a high level of expression of p-Akt can increase the risk of BM in NSCLC patients.
We also provide evidence that, in NSCLC patients, high levels of expression of p-Akt can be associated with specific SNPs in AKT1, such as the AKT1 rs2498804 GG variant genotype. Multiple mechanisms may be associated with high levels of activation of the PI3K/AKT pathway, including mutations in the PIK3CA gene and loss of PTEN. Importantly, it was recently reported that SNPs in AKT1 and their haplotypes are associated with the expression levels of the Akt1 protein and the apoptotic ability of the cells [26].
PCI has been shown to reduce or delay the occurrence of BM in NSCLC patients, but none of the studies conducted so far have demonstrated any survival benefit [27,28]. This observed absence of survival benefit may be due to a lack of choice for a more suitable preventive treatment [2]. Moreover, because of the neurological damage associated with PCI, not all patients with NSCLC should receive PCI. Indeed, all patients underwent non-selective PCI because there is no test to determine which patients have a high risk of developing BM. Therefore, there is an urgent need to develop a model to predict the risk of BM in patients with NSCLC. Given that our findings will be validated in subsequent studies, these results, combined with clinicopathological data, could be the basis for a BM risk model and help predict which patients are at high risk of developing BM and should receive PCI.
When we examined the relationship between the level of expression of p-Akt and SNPs in AKT1, we found interesting trends that, unfortunately, were not validated by statistical analyses. This is most likely due to the small group size of our study and, therefore, special care should be taken when interpreting these results. Consequently, large randomized controlled trials are needed to verify and confirm our observations.
To conclude, our study demonstrated that a high level of expression of p-Akt is associated with an increased risk of BM in NSCLC patients. We also found that the level of expression of p-Akt is related to the AKT1 genotype and that NSCLC patients with the rs2498804 GG variant genotype had a higher level of expression of p-Akt. However, large randomized controlled trials are needed to validate these findings and establish a marker for predicting BM in NSCLC patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was funded by two grants from the National Natural Science Foundation of China (grants 81502521 and 81472921).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100625.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Bovi J.A., White J. Radiation therapy in the prevention of brain metastases. Curr. Oncol. Rep. 2012;14:55–62. doi: 10.1007/s11912-011-0208-6. [DOI] [PubMed] [Google Scholar]
- 3.Langer C.J., Mehta M.P. Current management of brain metastases, with a focus on systemic options. J. Clin. Oncol. 2005;23:6207–6219. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J., Gong Z., Jia Q., Wu Y., Yang Z.Z., Zhu B. Programmed death ligand 1 expression and CD8(+) tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018;498:751–757. doi: 10.1016/j.bbrc.2018.03.053. [DOI] [PubMed] [Google Scholar]
- 5.Manapov F., Kasmann L., Roengvoraphoj O., Dantes M., Schmidt-Hegemann N.S., Belka C., Eze C. Prophylactic cranial irradiation in small-cell lung cancer: update on patient selection, efficacy and outcomes. Lung Canc. 2018;9:49–55. doi: 10.2147/LCTT.S137577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuschke M., Eberhardt W., Pottgen C., Stamatis G., Wilke H., Stuben G., Stoblen F., Wilhelm H.H., Menker H., Teschler H., Muller R.D., Budach V., Seeber S., Sack H. Prophylactic cranial irradiation in locally advanced non-small-cell lung cancer after multimodality treatment: long-term follow-up and investigations of late neuropsychologic effects. J. Clin. Oncol. 1999;17:2700–2709. doi: 10.1200/JCO.1999.17.9.2700. [DOI] [PubMed] [Google Scholar]
- 7.Precival C., Landy M., Poole C., Mullaney L. The role of prophylactic cranial irradiation for non-small cell lung cancer. Anticancer Res. 2018;38:7–14. doi: 10.21873/anticanres.12185. [DOI] [PubMed] [Google Scholar]
- 8.Sun D.S., Hu L.K., Cai Y., Li X.M., Ye L., Hou H.Y., Wang C.H., Jiang Y.H. A systematic review of risk factors for brain metastases and value of prophylactic cranial irradiation in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:1233–1239. doi: 10.7314/apjcp.2014.15.3.1233. [DOI] [PubMed] [Google Scholar]
- 9.Ji Z., Bi N., Wang J., Hui Z., Xiao Z., Feng Q., Zhou Z., Chen D., Lv J., Liang J., Fan C., Liu L., Wang L. Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:330–337. doi: 10.1016/j.ijrobp.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Grinberg-Rashi H., Ofek E., Perelman M., Skarda J., Yaron P., Hajduch M., Jacob-Hirsch J., Amariglio N., Krupsky M., Simansky D.A., Ram Z., Pfeffer R., Galernter I., Steinberg D.M., Ben-Dov I., Rechavi G., Izraeli S. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin. Cancer Res. 2009;15:1755–1761. doi: 10.1158/1078-0432.CCR-08-2124. [DOI] [PubMed] [Google Scholar]
- 11.Li Q., Yang J., Yu Q., Wu H., Liu B., Xiong H., Hu G., Zhao J., Yuan X., Liao Z. Associations between single-nucleotide polymorphisms in the PI3K-PTEN-AKT-mTOR pathway and increased risk of brain metastasis in patients with non-small cell lung cancer. Clin. Cancer Res. 2013;19:6252–6260. doi: 10.1158/1078-0432.CCR-13-1093. [DOI] [PubMed] [Google Scholar]
- 12.Brazil D.P., Park J., Hemmings B.A. PKB binding proteins. Getting in on the Akt, Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 13.Manning B.D., Toker A. Akt/Pkb signaling: Navigating the Network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray J.W. PI3 kinase pathway mutations in human cancers. JAMA Oncol. 2016;2:1543–1544. doi: 10.1001/jamaoncol.2016.0875. [DOI] [PubMed] [Google Scholar]
- 15.Steelman L.S., Stadelman K.M., Chappell W.H., Horn S., Basecke J., Cervello M., Nicoletti F., Libra M., Stivala F., Martelli A.M., McCubrey J.A. Akt as a therapeutic target in cancer. Expert Opin. Ther. Targets. 2008;12:1139–1165. doi: 10.1517/14728222.12.9.1139. [DOI] [PubMed] [Google Scholar]
- 16.David O., Jett J., LeBeau H., Dy G., Hughes J., Friedman M., Brody A.R. Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin. Cancer Res. 2004;10:6865–6871. doi: 10.1158/1078-0432.CCR-04-0174. [DOI] [PubMed] [Google Scholar]
- 17.Lim W.T., Zhang W.H., Miller C.R., Watters J.W., Gao F., Viswanathan A., Govindan R., McLeod H.L. PTEN and phosphorylated AKT expression and prognosis in early- and late-stage non-small cell lung cancer. Oncol. Rep. 2007;17:853–857. [PubMed] [Google Scholar]
- 18.Yang Y., Luo J., Zhai X., Fu Z., Tang Z., Liu L., Chen M., Zhu Y. Prognostic value of phospho-Akt in patients with non-small cell lung carcinoma: a meta-analysis. Int. J. Cancer. 2014;135:1417–1424. doi: 10.1002/ijc.28788. [DOI] [PubMed] [Google Scholar]
- 19.Adamo B., Deal A.M., Burrows E., Geradts J., Hamilton E., Blackwell K.L., Livasy C., Fritchie K., Prat A., Harrell J.C., Ewend M.G., Carey L.A., Miller C.R., Anders C.K. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011;13:R125. doi: 10.1186/bcr3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffer P.J., Jin J., Woodgett J.R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 1998;335(Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pahar B., Pan D., Lala W., Kenway-Lynch C.S., Das A. Transforming growth factor-beta1 regulated phosphorylated AKT and interferon gamma expressions are associated with epithelial cell survival in rhesus macaque colon explants. Clin. Immunol. 2015;158:8–18. doi: 10.1016/j.clim.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 23.Sun J., Zhang D., Bae D.H., Sahni S., Jansson P., Zheng Y., Zhao Q., Yue F., Zheng M., Kovacevic Z., Richardson D.R. Metastasis suppressor, NDRG1, mediates its activity through signaling pathways and molecular motors. Carcinogenesis. 2013;34:1943–1954. doi: 10.1093/carcin/bgt163. [DOI] [PubMed] [Google Scholar]
- 24.Fumarola C., Bonelli M.A., Petronini P.G., Alfieri R.R. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem. Pharmacol. 2014;90:197–207. doi: 10.1016/j.bcp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Grille S.J., Bellacosa A., Upson J., Klein-Szanto A.J., van Roy F., Lee-Kwon W., Donowitz M., Tsichlis P.N., Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- 26.Zhang W., Jiang W., Luan L., Wang L., Zheng X., Wang G. Prophylactic cranial irradiation for patients with small-cell lung cancer: a systematic review of the literature with meta-analysis. BMC Canc. 2014;14:793. doi: 10.1186/1471-2407-14-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox J.D., Stanley K., Petrovich Z., Paig C., Yesner R. Cranial irradiation in cancer of the lung of all cell types. J. Am. Med. Assoc. 1981;245:469–472. [PubMed] [Google Scholar]
- 28.Gore E.M., Bae K., Wong S.J., Sun A., Bonner J.A., Schild S.E., Gaspar L.E., Bogart J.A., Werner-Wasik M., Choy H. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J. Clin. Oncol. 2011;29:272–278. doi: 10.1200/JCO.2010.29.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.