Abstract
In amyotrophic lateral sclerosis (ALS) cognitive impairment may occur. This could detrimentally influence communication between patient and health-care professionals and make clinical assessment difficult. Given the short life expectancy after diagnosis, it is crucial to accurately identify ALS patients early. Although suitable cognitive screening tools for patients with ALS are available, they have not been evaluated in a Norwegian population. Interpretation of scores for available tests and practical application of scoring is also not well established. The protocol described here involves two related studies that aim to improve the quality of ALS clinical testing instruments used in the Norwegian population. The first is a validation study that evaluates the psychometric properties of the ECAS-Norwegian. The second is a prospective cohort study that evaluates the ECAS-Norwegian as a tool to predict early changes in ability to work, drive a car and the need for advanced therapy. Study 1 is a multicenter study using international quality criteria. Patients with ALS, healthy control subjects, and control subjects with dementia will be included. Primary outcome is ECAS-Norwegian scores. In study 2, patients with ALS will be included. ECAS-Norwegian compared to Clinical Dementia Rating score and Montreal Cognitive Assessment scores will be used as a prognostic tool for working, driving, and initiating advanced life-prolonging therapy. Before clinical implementation, the ECAS-Norwegian needs to be evaluated and validated. Successful validation and implementation of the ECAS-Norwegian may provide early identification of cognitive impairment in ALS, leading to more proactive, individualized treatment.
Keywords: Amyotrophic lateral sclerosis, Cognitive, Screening, Validation, Predictor, ECAS
1. Introduction
Amyotrophic Lateral Sclerosis (ALS) is typically thought of as a neurodegenerative disease of motor neurons [1]. In Norway, as in the rest of Europe, the incidence rate of ALS is about 2–3 cases per 100.000 individuals [2,3]. However, this rate will likely increase in the future for two reasons: The disease usually presents clinically between the ages of 60 and 75 years; and, globally, more and more people are living to older ages [3]. Men are affected slightly more often than women [2,4]. Currently, there is no cure for ALS, and the mean life expectancy is 30 months after onset [5].
During the last decades, evidence has emerged that ALS is a multisystem disease rather than a pure motor system disease [6,7]. This new perspective has implications beyond the traditional view that focuses on motor impairment. Research shows that cognitive impairment is present in 30–50% of patients with ALS [6]. Deficits related to verbal fluency and language, followed by changes in social cognition, verbal memory and executive functions are most common [6]. Complicating diagnosis and decisions on treatment is the findings that 5–15% of patients with ALS meet the criteria for frontotemporal dementia, meaning behavioral changes are present [6,7]. Specifically, reduced concerns for hygiene, pronounced irritability, newly emerging unusual habits, and increased apathy are most frequently observed in patients with ALS [8]. Despite these compelling findings, the cognitive and behavioral status of most ALS patients in Norway has not been objectively evaluated. One reason may be due to lack of appropriate ALS screening tools.
Screening of cognitive and behavioral impairment is clearly recommended for ALS-specific health care [9,10]. Thus, a rapid screening tool valid for use in Norway is urgently needed. However, cognitive assessment of patients with ALS using traditional assessment tools can be difficult to perform due to the complexity of their cognitive impairment, and due to motor challenges they have with writing, drawing, and speaking. Therefore, only ALS-specific multi-domain screening instruments with integrated behavioral sections should be used [7,11].
Internationally, the Edinburgh Cognitive and Behavioral ALS Screen (ECAS) [12] is recommended as a valid, reliable test [[12], [13], [14], [15], [16]]. Introduction of the ECAS has probably contributed to achieving a more nuanced picture of cognitive impairment in ALS than was previously drawn [12]. The ECAS has been translated and culturally adapted into the Norwegian language and culture (known as ECAS-N). Despite this advance, health-care professionals in Norway currently have no clear recommendations for its interpretation and practical application of its scoring.
One of the major challenges in ALS management is deciding on the kind and course of advanced therapy. There is a lack of knowledge about how cognitive impairment in ALS might interfere with complex medical treatment that will affect quality of life [17]. This uncertainty has significant implications not only for patients with ALS and the community but also for their families, especially spouses. Thus, further investigation of the psychometric properties of the ECAS-N and its potential for clinical use is needed.
2. Materials and methods
2.1. Aims and objectives
The overall aims of this project are to provide validated tools for ALS-specific cognitive screening and to gain experience in their clinical use. The specific objectives are:
-
1.
To investigate whether the ECAS-N adequately characterizes cognitive impairment (internal consistency). Moreover, is it sufficiently robust to measure errors related to different testing times (test-retest reliability) and different scorers (interrater reliability).
-
2.
To investigate whether the ECAS-N can distinguish people with ALS-specific cognitive impairment, and people who have no known cognitive impairment, and those who have cognitive impairment related to other disorders (construct validity).
-
3.
To evaluate whether the ECAS-N predict early problems related to driving a car, continuing working, and initiating advanced life-prolonging therapy.
Two studies addressed these objectives, with Study 1 addressing points 1 and 2, and Study 2 addressing point 3.
2.2. Hypotheses
We will test predefined and specific hypotheses to assess construct validity (Study 1) and the use of ECAS-N as an early predictor (Study 2). For assessing construct validity we pose five hypotheses. i) A moderate positive association will be found between the scores on the ECAS-N and the Montreal Cognitive Assessment (MoCA). MoCA is a standardized screening test that is sensitive in detecting cognitive impairment in patients with ALS [18]. ii) A strong negative association will be found between the scores of patients with ALS and healthy controls on the ECAS-N. iii) A moderate positive association will be found between the scores of patients with ALS and non-ALS patients with cognitive impairment. iv) Higher ALS-specific scores will be found for patients with ALS than for patients with non-ALS related cognitive impairment. v) Non ALS-specific scores will be higher for patients with non-ALS related cognitive impairment than for patients with ALS.
In Study 2, we hypothesize that patients with low baseline scores on the ECAS-N will also have low scores on the Clinical Dementia Rating (CDR™). Additionally, we hypothesize that they will not be offered advanced life-prolonging treatment, and they will quit working and driving a car earlier than those who have high baseline scores on the ECAS-N. We hypothesize that their baseline ECAS-N scores and follow-up ECAS-N scores will likely not differ significantly. Similarly, we hypothesize that patients with low baseline MoCA scores will have low follow-up MoCA scores. However, we expect fewer patients with low MoCA scores than patients with low ECAS-N scores because these two tools have different sensitivity to ALS-specific cognitive problems.
2.3. Study designs
The Edinburgh cognitive and behavioral amyotrophic lateral sclerosis screen in Norway: A validation study project (Study 1) and the Edinburgh cognitive and behavioral amyotrophic lateral sclerosis screen in Norway: A prospective cohort study project (Study 2) are both registered at ClinicalTrials.gov (Study 1: registration number NCT03579017; https://clinicaltrials.gov/ct2/show/NCT03579017; Study 2: registration number NCT03578796; https://clinicaltrials.gov/ct2/show/NCT03578796). Participants will undergo a standardized program of cognitive tests at baseline and follow-up visits (see below).
We will use a validation design to investigate the psychometric properties of the ECAS-N in Study 1 [19]. We obtained approval from the ECAS’ copyright holder, Dr. Sharon Abrahams, to produce ECAS-N. The original ECAS was translated into Norwegian and culturally adapted according to the international guidelines described by Sousa et al. [20]. Briefly, these recommendations consider issues on translation and cultural adaption, sample size estimation, and validity assessment, which takes into account some distinctive characteristics of scale validation that demand special considerations [20]. Norwegian age- and education-adjusted norms for verbal fluency and cutoff scores for abnormal findings were established in compliance with recommendations in the ECAS manual [21]. Further validation of the ECAS-N will be in accordance with quality criteria applicable to health-measurement scales and proposed by Terwee et al. [22]. A cross-sectional approach will be used to investigate internal consistency, inter-rater reliability, and construct validity. A longitudinal approach will be used to investigate test-retest reliability.
Study 2 is a prospective cohort study. Here we will evaluate application of the ECAS-N as an early predictor of problems related to participants’ driving a car, working, and use of advanced life-prolonging therapy. Prospective cohort studies are considered to be the gold standard of observational research [23]. A group of patients with ALS will be evaluated for a period of 3 years, or until they begin to use permanent ventilation support, or until they die.
2.4. Setting of the study
Haukeland University Hospital (HUH) in Bergen, Norway is responsible for overseeing both studies. HUH will annually admit to the study 15–20 patients with newly diagnosed ALS. We sent recruitment invitations to specific sites, and we also mentioned the invitation at an oral presentation during the annual Norwegian network meeting in 2017. A letter of intent is signed by all stakeholders, meaning they understand their responsibilities and are willing to deliver data to the study.
HUH, St.Olavs Hospital, Trondheim University Hospital in Trondheim, Hospital of Southern Norway in Kristiansand, and Namsos Hospital in Namsos will be collecting data for Study 1. As with HUH, the other three hospitals offer high-quality multidisciplinary and ALS-specific health care, either in an ALS-specific outpatient clinic, in a hospital ward, and in some situations, in patients’ homes. Only HUH will be involved in Study 2.This is a pragmatic choice because of incompatible schedules in the clinics.
2.5. Participants’ eligibility and inclusion
Patients who, within 4 months after being diagnosed with ALS, seeks care at the ALS unit at HUH, St.Olavs Hospital, Hospital of Southern Norway, or Namsos Hospital will be eligible to participate in Study 1. One carer, chosen by the patient, will be eligible to participate also. Those who fulfill the inclusion criteria (see Table 1) will be included. To evaluate the specificity of ECAS-N in accurately identifying patients with ALS, we will use for comparison healthy individuals and patients with dementia, which will constitute the control groups. Patients and carers at HUH will be eligible to participate in Study 2.
Table 1.
Inclusion and exclusion criteria for participants in Studies 1 and 2.
| Criteria | Participants |
|||
|---|---|---|---|---|
| Patients with ALS | Controls with dementia | Healthy controls | Carers | |
| Inclusion characteristics | ||||
| Voluntary informed consent | ✓ | ✓ | ✓ | ✓ |
| Native Norwegian speaker | ✓ | ✓ | ✓ | |
| Between 35 and 85 years old | ✓ | ✓ | ||
| Exclusion characteristics | ||||
| Great difficulties in writing or reading | ✓ | ✓ | ✓ | ✓ |
| Comorbid medical history | ✓ | ✓ | ✓ | ✓ |
| Neurological disorders | ✓ | ✓ | ✓ | ✓ |
| Comorbid psychiatric history | ✓ | ✓ | ✓ | ✓ |
Abbreviation: ALS, Amyotrophic Lateral Sclerosis.
Inclusion criteria of Studies 1 and 2 were selected to ensure voluntary participation and that all participants understand the instructions given. To closely match a typical ALS group in age, the control subjects should be between the ages of 35 and 85 years. Exclusion criteria include having severe cognitive impairment at time of testing, which would make it impossible to obtain accurate scores. For the same reason, people with great difficulties in writing or reading are also excluded. Specific inclusion and exclusion criteria are listed in Table 1.
2.6. Outcome measures
In Study 1, to investigate the psychometric properties of the ECAS-N, we will use scores on the ECAS-N and the Norwegian version of MoCA (version 7.1; available at www.mocatest.org) [24]. Internal consistency of the ECAS-N and its sub-scores will be evaluated by using scores obtained at baseline. Test-retest reliability of the ECAS-N and its sub-scores will be assessed on the basis of individual testers. We will compare their scoring on tests administered at baseline and follow-up assessment. For assessing inter-rater reliability of the ECAS-N and its sub-scores, we will compare scoring done by two independent testers. Both baseline and follow-up scores will be used. We will evaluate predefined hypotheses concerning ECAS-N sub-scores and total score by comparing ECAS-N sub-scores and total scores obtained at baseline to MoCA sub-scores and total scores obtained at baseline. This evaluation is to determine whether the ECAS-N can be used to distinguish people with ALS-specific cognitive impairment and those who have no known cognitive impairment and those who have cognitive impairment related to other disorders (construct validity of ECAS-N).
In Study 2, the primary outcome will be clinical diagnosis of dementia or lack of dementia, as assessed by using the CDR™ scale [25]. Secondary outcomes are scores assessing the participant's ability to work secularly and to drive a car. These are assessed using a modified questionnaire, originally designed for and used in the Norwegian ParkWest study, an investigation to determine the prognostic value of mild cognitive impairment in early Parkinson's disease (see below in section entitled modified ParkWest Questionnaire) [26]. Information about use of advanced life-prolonging therapy will be collected from a daily journal maintained by each participant. Baseline and follow-up scores on the ECAS-N and the MoCA will be used as predictors of incident cognitive impairment in ALS. We will evaluate the associations between the clinical diagnosis of dementia or lack of dementia and ECAS-N and MoCA scores after a follow-up period of 3 years, or when permanent ventilation support is initiated, or until the death of the participant, whichever comes first.
Background physical and medical information will be gathered using the ALS Functional Rating Scale [27]. Age, gender and highest level of education attained will come from a custom questionnaire specifically designed for these two studies.
2.6.1. ECAS-N
As with the original English version of the ECAS [12], the ECAS-N provides information on two different types of screens. One reflects the patient's cognitive disability (ECAS-cognitive screen), and another reflects the patient's behavioral disability (ECAS-behavioral screen). The ECAS-cognitive screen comprises 16 items organized into two sub-scales. An ALS-specific sub-scale taps into the cognitive domains of language, verbal fluency, and executive and social functions. A non-ALS-specific sub-scale specifically assesses memory and visuospatial function. The sub-scales of the ECAS-cognitive screen range, respectively, from 0 to 100 and from 0 to 36. Low scores indicate a greater deficit. An ECAS-total score (maximum score = 136) and dichotomized cutoff scores for normality are also provided. The ECAS-behavioral screen is an interview of the carer. It includes assessment of five domains of behavior, assessing changes that are characteristic of ALS. It also assesses three domains of psychotic changes. The sub-score for behavioral change ranges from 0 to 10. The sub-score for psychotic change ranges from 0 to 3. For both sub-scales, high scores indicate more problems.
2.6.2. Clinical Dementia Rating (CDR™)
The CDR™ is designed to assess global cognitive impairment, as well as possible diagnosis and severity of dementia [25]. The scores are based on patients' answers in a semi-structured interview and on their carers’ interview answers. The interview questions assess status in six different cognitive and behavioral domains: memory, orientation, judgment and problem solving, performance of self-care, performance of daily activities, as well as degree of active social engagement. The scoring table provides descriptive anchors that guide the clinician in making appropriate ratings based on interview data and his/her clinical judgment. The CDR™ is scored on a five-point scale (0–3): “0″ indicates no cognitive impairment is present, “0.5″ indicates questionable or very mild dementia is present, “1″ indicates mild dementia is present, “2″ indicate moderate dementia is present, and “3″ indicates severe dementia is present. A CDR™ score of 2 or above is an established cutoff for identifying patients at increased risk for unsafe driving [28].
2.6.3. Montreal Cognitive Assessment (MoCA)
The MoCA is used to assess cognitive function in the following domains: visuospatial/executive, naming, memory, attention, language, abstraction, delayed recall, and orientation [24]. The maximum score is 30. A score below 26 indicates cognitive impairment. Patients with 12 or less years of education are given an additional point in the scoring [18]. The MoCA may be predictive of driving fitness [29,30].
2.6.4. Modified ParkWest Questionnaire
We will use four items of the ParkWest Questionnaire related to the participants’ occupational situation, self-care, and their car driving. Answers are categorical, with the possibility of elaboration when needed for clarification. Elapsed time to change to reduced function in these items is recorded as data [26]. In addition, whether the patient is able to work or drive a car will be assessed by a physician. Whether inability is related to motor or cognitive deficits are also assessed.
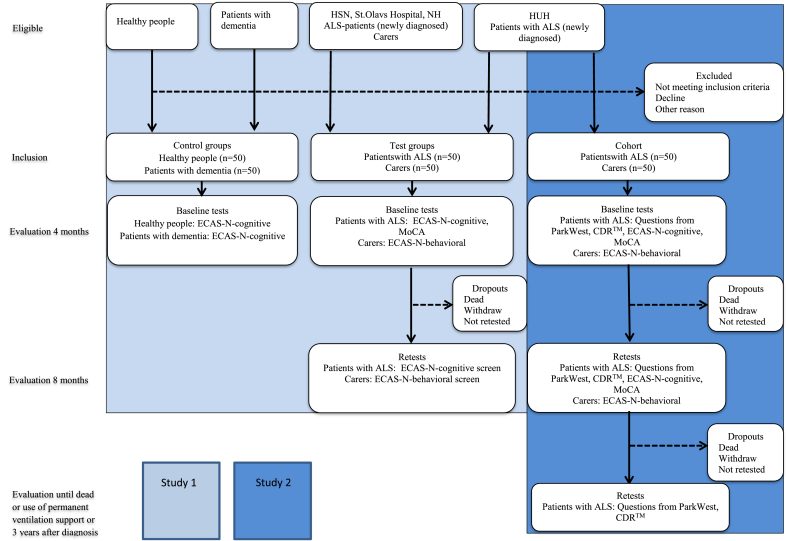
A flow diagram of all participants who will be included in Studies 1 and 2, outcome measures and scheduled data collection is shown in Fig. 1.
Fig. 1.
Flow diagram of all participant who will be included in Studies 1 and 2, measurements used, and time frame of implementation. Abbreviations: ALS, Amyotrophic Lateral Sclerosis; CDR™, Clinical Dementia Rating; ECAS-N, Edinburgh Cognitive and Behavioral ALS Screen-Norwegian version; HSN, Hospital of Southern Norway; HUH, Haukeland University Hospital; MoCA, Montreal Cognitive Assessment; NH, Namsos Hospital.
2.7. Sample size
In Study 1, a minimum of 50 participants are needed for the evaluation of psychometric characteristics of the ECAS-N. This sample size is in agreement with recommendations proposed by Terwee et al. [22]. This means that a minimum of 50 patients and their carers are wanted for estimation of inter-rater reliability, test-retest reliability, and internal consistency. For content validity, test scores are wanted from about 50 of the patients with ALS, 50 persons with cognitive impairment related to the diagnosis of dementia, and 50 healthy control subjects. In Study 2, we will enrol all patients from HUH during a period of 3 years. We expect 50–60 patients to be included. The handling of missing values will be determined when we have collected the data and know the structure of missing.
2.8. Recruitment
We will use a consecutive series for enrollment. On their first visit to the hospital (HUH, St.Olavs Hospital, Hospital of Southern Norway, or Namsos Hospital), each patient with ALS and their carer will be asked to participate. A member of the ALS-specific health-care team will make initial contact. Before this first visit, written information about the studies will be sent to the patient's home address. Testers will recruit healthy volunteers from among the ALS patients' family members or acquaintance. Patients with dementia will be recruited from an institution near HUH. Both control groups will be selected so that the demographics will closely match a typical ALS group in age, gender, and level of educational attainment. Specifically, we will aim to obtain an equal distribution of participants below and above the age of 60 years and low and higher level of education, across the three groups. About 60% of participants will be men.
2.9. Data collection
After being diagnosed with ALS, baseline tests will be administrated within 4 months, and the retest within 8 months. Timing is in line with routines at the hospitals involved, and tests are scheduled so that possible practice effects and withdrawal are limited as much as possible [31]. Further evaluation for Study 2 will be part of each follow-up visit in the ALS clinic at HUH until 3 years after being diagnosed, or until the patients with ALS begin permanent ventilation support, or until they die. Participants in the control groups will be tested once.
All testers have been specially trained for administering the ECAS-N, and the project leader is a certificated ECAS tester. For obtaining data on ECAS-N's inter-rater reliability, two testers will independently score the participants at the same time. The time frame and outcome measures used in Studies 1 and 2 are presented in Table 2.
Table 2.
Time frame and outcome measures used in Studies 1 and 2.
| Outcome measures | Baseline (4 month) | Follow-up (8 month) | Further evaluation |
|---|---|---|---|
| ECAS-N | ✓ | ✓ | – |
| MoCA | ✓ | ✓ | – |
| ParkWest Questionnaire | ✓ | ✓ | ✓ |
| CDR™ | ✓ | ✓ | ✓ |
| ALS-FRS-R | ✓ | ✓ | ✓ |
Abbreviations: ALS-FRS, Amyotrophic Lateral Sclerosis Functional Rating Scale; CDR™, Clinical Dementia Rating; ECAS, Edinburgh Cognitive and Behavioral Amyotrophic Lateral Sclerosis Screen; MoCA, Montreal Cognitive Assessment.
2.10. Data management
All information will be processed and used without any information that is directly identifiable. The original forms from all sites will be stored in a locked cabinet in the office of the project leader. All data collected will be entered contemporaneously with collection and analyzed by the project leader using the statistical computer programs SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp) and Matlab® (MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, Massachusetts, United States).
Data for Study 1 will be secured in the quality register of HUH (project code: 2016/03166). Data for Study 2 will be in the research database of HUH (project code: 2016–02187). All data will be kept on the HUH secure research server. Only primary investigators will have access to these files. The codes for identifying participants will be kept separate from the data. All data published will be anonymous.
2.11. Statistical methods
Descriptive statistics will be used for demographic variables and other baseline characteristics for included participants and those who are drop-outs. Test-retest reliability and inter-rater reliability will be evaluated by using intraclass correlation (ICC) for continuous variables and a kappa coefficient for categorical variables. Additionally, Bland-Altman plots will be used for continuous variables. Cronbach's alpha will be used as an estimate of internal consistency. In Study 2, linear mixed-effects models will be used to assess the hypothesis related to association between ECAS-N and CDR™ and secondary outcome measures. All models will be estimated, unadjusted and adjusted for age, gender, and level of educational attainment. A model containing appropriate adjustments will be used for interpretation.
3. Ethical considerations
We will make testing accommodations for patients with ALS and patients with dementia, since taking part in research studies may be exhausting for them. Therefore all patients and carers will be informed in writing about the study prior to their first visit at the hospitals. Informed consent will be obtained for all participants in this study. They will be able to withdraw from the study at any time, with no explanation and with no consequences of prejudicial treatment in the future. All tests will be conducted by healthcare workers highly trained and experienced in handling the testing situation of vulnerable patients. All participants are encouraged to ask questions during the test session and by phone.
Major modification of the protocol that may impact the performance of the study or the participants will require a formal amendment to the protocol. The project group will have to approve the amendments. Additionally, for Study 1 the Data Privacy Unit at each hospital involved will have to give approval. For Study 2, the Regional Committees for Medical and Health Research Ethics will have to approve any major modifications. Any administrative changes having minor effects on the study are to be agreed upon by the project group and documented in a memorandum. The Ethics Committee/Data Privacy Unit may be notified of these changes.
4. Dissemination of study results
We plan to publish at least two articles in international peer-reviewed journals using the data collected in this study. One article will be related to objectives 1 and 2 of this project. Another article will be related to objective 3. The results will also be disseminated in national and international multidisciplinary networks of health-care professionals and international conferences/congresses within the field of ALS and motor neuron disease. To communicate publicly with patients and their relatives, we will publish the results in lay media channels that are easily accessible to the participants, such as websites and lay community meetings arranged by the foundation ALS Norwegian support group.
5. Discussion of methodological limitations and strengths
The non-randomized study design is subject to the risk of selection bias, observer bias and confounding factors. This situation implies there is a possible threat to the internal validity of the two studies [23].
Studying health outcomes in patients with rare diseases is also challenging because it is difficult to obtain a sufficiently large study sample. To deal with this, Study 1 is designed as a multicenter study, and the trial duration will be extended for Study 2. This is to allow more participants to be enrolled. In accordance to Terwee, 50 patients in each sub-group analysis is recommended. Especially, in the dementia group this may be challenging. To avoid missing the cooperating institution will be followed close and supervised. However, if the admission is far less than expected and we realize this early, we will consider involving any of the cooperators from study 1. In clinical studies, missing is always a threat. It will be described in the original paper when analysis is done. Trial duration is based on the actual number of patients with newly diagnosed ALS at the participating hospitals in recent years. In order to avoid testing on the same day as being diagnosed and imposing more burden on patients traveling to hospitals over long distances, baseline tests may conducted up to 4 months after patients are diagnosed. This implies there is a risk that data may not necessarily come from patients with the shortest course of disease. This also means that those severely affected by ALS may not be tested. Although some patients, or their carer, view the results of the ECAS-N as a welcomed explanation for their medical difficulties, obtaining the results for some is viewed as a burden. This situation may contribute to the numbers of dropouts being disproportionately greater among those participants who are cognitive impaired. In order to discourage dropping out, all participants will receive careful explanation about the nature of the study prior to actual testing and will be referred to follow-up treatment in the community when needed. They will also be invited to call the study coordinators to clarify any issues and ask questions. In addition, participants will be personally invited for follow-ups in writing and, in some cases, by phone. Participants who have long distances to travel or have poor health may stay in a hospital ward, or in some cases, testing may be conducted in the patient's home.
In order to minimize the problem of observer bias, all tests will be conducted by health-care professionals who have been trained for these studies. In addition, regular trouble-shooting video-meetings will be conducted with the ECAS testers during the data collection period. The results of the ECAS-N will be blinded to those testers who administer the CDR™. Incompatible scheduling across the different clinics involved in the study means that, within the limits defined in the protocol, the elapsed time between baseline testing and follow-up testing may vary some. However, we do not anticipate this will be a problem, since cognitive impairment is already present in early stages of the disease of ALS and appears to be stable over time [31]. Thus, this pragmatic choice is important for participant recruitment and testing and is justified.
The impact of possible practice effects of repeated ECAS testing in longitudinal studies is unresolved [31]. Even if the time frame between baseline and follow-up is adjusted to minimize possible practice effects, use of alternate forms of the ECAS in follow-up testing may mitigate the effects [32]. However, these newly developed alternate forms of the ECAS are currently not available in Norwegian. Regardless, practice effects on this test may have little impact [31].
A major strength of the studies is the choice of ALS-specific outcome measures. In addition, the ECAS-N will be compared with another available test, the MoCA, to evaluate its specificity. Cognitive assessment of patients with ALS can be difficult due to motor challenges they have with writing, drawing, and speaking. However, the ECAS is designed to accommodate motor disabilities that are common in ALS [12]. This makes it possible to differentiate between ALS patients with cognitive and/or behavioral changes from those with pure motor-system involvement. While many tests fall short by testing only single domains of cognitive function, the ECAS taps into several, allowing a range of cognitive and behavioral changes to be assessed that are common in patients with ALS [33,34].
6. Conclusions
Effective screening and management of cognitive decline in ALS, which is relevant for clinical practice, is much needed. Screening and appropriately managing the decline will become more imperative in coming years, since the incidence of ALS will likely increase as more people are living to older ages [3]. Introducing the ECAS-N in clinical practice is a first step in more accurately diagnosing ALS and managing the cognitive impairment associated with it. In the longer term, this can help to more precisely determine the incidence, kind, and severity of cognitive impairment in ALS in Norway [12], which has significant implications for society, patients, carers, and health-care professionals. Health-care professionals and carers may find information on a patient's cognitive status helpful in discussions related to life-prolonging treatment, as well as to decisions about the patient continuing to work and drive [7,35,36]. Early identification of cognitive status in ALS may contribute to a more proactive treatment, one better tailored to patients' individual needs, as well as proactive discussions about end-of-life wishes [17].
Acknowledgements
We thank Dr. Sharon Abrahams, the copyright holder of the ECAS, for essential cooperation during the process of translation and adaption of the ECAS into Norwegian language and culture. We thank the patients, their carer, and the health-care professionals involved in the process. We also thank our collaborators at St.Olavs Hospital in Trondheim, Hospital of Southern Norway in Kristiansand and Namsos Hospital in Namsos. Western Norway University of Applied Sciences, Bergen, Norway contributed research time necessary for developing the grant application.
Contributor Information
Tina Taule, Email: tina.taule@helse-bergen.no.
Annbjørg Spilde Morland, Email: annbjorg.spilde.morland@helse-bergen.no.
Marit Arnevik Renså, Email: marit.elise.arnevik.rensa@helse-bergen.no.
Jörg Aßmus, Email: Jorg.Assmus@helse-bergen.no.
Ole-Bjørn Tysnes, Email: ole-bjorn.tysnes@helse-bergen.no.
Tiina Rekand, Email: tiina.rekand@helse-bergen.no.
List of abbreviations
- ALS
Amyotrophic Lateral Sclerosis
- CDM™
Clinical Dementia Rating
- ECAS
Edinburgh Cognitive and Behavioral ALS screen
- HUH
Haukeland University Hospital
- MoCA
Montreal Cognitive Assessment
Declarations
Funding
This work is funded by grants from the Western Regional Norwegian Health Authority [grant number: 912158, 2017]; the foundation ALS Norwegian support group [2015]; and the Norwegian Association of Occupational Therapy [grant number: 200, 2015].
Authors' contributions
TT conceived the study. She is the project leader and is responsible for carrying out the studies. All authors contributed to the study design, refinement of the study protocol, and approved the final manuscript. ASM and MAR are responsible for testing and data collection, JA provides statistical expertise and conduct primary statistical analyses. TR and OBT are supervisors.
Competing interest
The project group was involved in the translation and adaption of the ECAS into Norwegian (ECAS-N).The authors report no further conflicts of interest. The authors alone are responsible for the content and writing of this protocol.
Ethical approval
Study 1 was considered by the Regional Committees for Medical and Health Research Ethics (reference number 2015/1221/REK vest), but found to be beyond their mandate. Thereafter, Study 1 was approved by the Data Privacy Unit at HUH, Bergen, Norway (reference numbers 2015/11598 and 2016/3166). Study 2 was approved by the Regional Committees for Medical and Health Research Ethics (reference number 2016/2187/REK vest). This project adheres to the Declaration of Helsinki [37].
References
- 1.Talbott E.O., Malek AM D.L. The epidemiology of amyotrophic lateral sclerosis. Handb. Clin. Neurol. 2016;138:225–238. doi: 10.1016/B978-0-12-802973-2.00013-6. https://doi.org/doi:10.1016/B978-0-12-802973-2.00013-6 [DOI] [PubMed] [Google Scholar]
- 2.Gundersen M., Yaseen R., Midgard R. Incidence and clinical features of amyotrophic lateral sclerosis in Møre and Romsdal County, Norway. Neuroepidemiology. 2011;37(1):58–63. doi: 10.1159/000329523. [DOI] [PubMed] [Google Scholar]
- 3.Chiò A., Logroscino G., Traynor B.J., Collins J., Simeone J.C., Goldstein L.A. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118–130. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakken O., Lindstrøm J.C., Tysnes O.-B., Holmøy T. Assessing amyotrophic lateral sclerosis prevalence in Norway from 2009 to 2015 from compulsory nationwide health registers. Amyotroph Lateral Scler and Frontotemp. Degener. 2017;22:1–8. doi: 10.1080/21678421.2017.1418004. [DOI] [PubMed] [Google Scholar]
- 5.Hardiman O., Al-Chalabi A., Brayne C., Beghi E., Van den Berg L., Chio A. The changing picture of amyotrophic lateral sclerosis: lessons from European registers. J. Neurol. Neurosurg. Psychiatry. 2017;88:557–563. doi: 10.1136/jnnp-2016-314495. [DOI] [PubMed] [Google Scholar]
- 6.Beeldman E., Raaphorst J., Twwennaar M., deVisser M., Schmand B., de Haan R. The cognitive profile of ALS: a systematic review and meta-analysis update. J. Neurol. Neurosurg. Psychiatry. 2016;87(6):611–619. doi: 10.1136/jnnp-2015-310734. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein L.H., Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12(4):368–380. doi: 10.1016/s1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- 8.Burke T., Pinto-Grau M., Lonergan K., Bede P., O'Sullivan M., Heverin M. A Cross-sectional population-based investigation into behavioral change in amyotrophic lateral sclerosis: subphenotypes, staging, cognitive predictors, and survival. Ann. Clin. Transl. Neurol. 2017;4(5):305–317. doi: 10.1002/acn3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motor Neurone Disease: Assessment and Management. National Institute for Health and Care Excellence (NICE); 2016. https://www.nice.org.uk/guidance/ng42/resources/motor-neurone-disease-assessment-and-management-1837449470149 [Internet] Available from. [PubMed] [Google Scholar]
- 10.Lee C. Reviewing evidences on the management of patients with motor neuron disease. Hong Kong Med. J. 2012;18(1):48–55. https://PMID/22302912 [PubMed] [Google Scholar]
- 11.Strong M.J., Grace G.M., Freedman M., Lomen-Hoerth C., Woolley S.C., Goldstein L.H. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler.: Off. Publ. World Fed. Neurol. Res. Group Motor Neuron Dis. 2009;10:131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- 12.Abrahams S., Newton J., Niven E., Foley J., Bak T. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler. Frontotemp. Degener. 2014;15(1–2):9–14. doi: 10.3109/21678421.2013.805784. [DOI] [PubMed] [Google Scholar]
- 13.Poletti B., Solca F., Carelli L., Madotto F., Lafronza A., Faini A. The validation of the Italian Edinburgh cognitive and behavioural ALS screen (ECAS) Amyotroph Lateral Scler. Frontotemp. Degener. 2016;17(7–8):489–498. doi: 10.1080/21678421.2016.1183679. [DOI] [PubMed] [Google Scholar]
- 14.Lulé D., Burkhardt C., Abdulla S., Böhm S., Kollewe K., Uttner I. The Edinburgh cognitive and behavioural amyotrophic lateral sclerosis screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler. Frontotemp. Degener. 2015;16(1–2):16–23. doi: 10.3109/21678421.2014.959451. [DOI] [PubMed] [Google Scholar]
- 15.Ye S., Li C., Liu X., Fan D. The Edinburgh cognitive and behavioural ALS screen in a Chinese amyotrophic lateral sclerosis population. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niven E., Newton J., Foley J., Colville S., Swingler R., Chandran S. Validation of the Edinburgh cognitive and behavioural amyotrophic lateral sclerosis screen (ECAS): a cognitive tool for motor disorders. Amyotroph Lateral Scler. Frontotemp. Degener. 2015;16(3–4):172–179. doi: 10.3109/21678421.2015.1030430. [DOI] [PubMed] [Google Scholar]
- 17.Khin E., Minor D., Holloway A., Pelleg A. Decisional capacity in amyotrophic lateral sclerosis. J. Am. Acad. Psychiatr. Law. 2015;43:210–217. https://PMID/26071511 [PubMed] [Google Scholar]
- 18.Osborne R., Sekhon R., Johnston W., Kalra S. Screening for frontal lobe and general cognitive impairment in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 2014;336(1–2):191–196. doi: 10.1016/j.jns.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 19.Arafat Y. Validation study can be a separate study design. Int. J. Med. Sci. Public Health. 2015;5(11):1–2. doi: 10.5455/ijmsph.2016.19042016471. [DOI] [Google Scholar]
- 20.Sousa V., Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J. Eval. Clin. Pract. 2011;17(2):268–274. doi: 10.1111/j.1365-2753.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 21.Abrahams S., Bak T. The University of Edinburgh; 2013. Edinburgh Cognitive and Behavioural ALS Screen - ECAS English Version 2013 Edinburgh Research Archive.https://www.era.lib.ed.ac.uk/handle/1842/6592 [updated 2013.03.19; cited 2015 2015.04.28]. Available from: [Google Scholar]
- 22.Terwee C., Bot S., de Boer M., van der Windt D., Knol D., Dekker J. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Polit D.E., Beck C.T., Nursing research . The Point; Philadelphia: 2008. Generating and Assessing Evidence for Nursing Practice. [Google Scholar]
- 24.Nasreddine Z.S., Phillips N.A., Bèdirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 26.Vossius C., Nilsen O., Larsen J. Parkinson's disease and hospital admissions: frequencies, diagnoses and costs. Acta Neurol. Scand. 2010;121(1):38–43. doi: 10.1111/j.1600-0404.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 27.Cedarbaum J., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999;169(1–2):13–21. doi: 10.1016/S0022-510X(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 28.Iverson D.J., Gronseth G.S., Reger MAC S., Dubinsky R.M., Rizzo M. Evaluation and management of driving risk in dementia. Report of the quality standards subcommittee of the american academy of neurology. Neurology. 2010;74:1316–1324. doi: 10.1212/WNL.0b013e3181da3b0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis A.M., Duncanson H., Kapust L.R., Xi P.M., O'Connor M.G. Validity of the mini-mental state examination and the Montreal cognitive assessment in the prediction of driving test outcome. J. Am. Geriatr. Soc. 2015;63(5):988–992. doi: 10.1111/jgs.13384. [DOI] [PubMed] [Google Scholar]
- 30.Kwok JCW I.G., Benoit D., Chilingaryan G. Predictive validity of the Montreal Cognitive Assessment (MoCA) as a screening tool for on-road driving performance. Br. J. Occup. Ther. 2015;78(2):100–108. doi: 10.1177/0308022614562399. [DOI] [Google Scholar]
- 31.Burkhardt C., Neuwirth C., Weber M. Longitudinal assessment of the Edinburgh cognitive and behavioural amyotrophic lateral sclerosis screen (ECAS): lack of practice effect in ALS patients? Amyotroph Lateral Scler. Frontotemp. Degener. 2017;18(3–4):202–209. doi: 10.1080/21678421.2017.1283418. [DOI] [PubMed] [Google Scholar]
- 32.Crockford C.J., Kleynhans M., Wilton E., Radakovic R., Newton J., Nieven E.H. ECAS A-B-C: alternate forms of the Edinburgh cognitive and behavioural ALS screen. Amyotroph Lateral Scler. Frontotemp. Degener. 2018;19(1–2):57–64. doi: 10.1080/21678421.2017.1407793. [DOI] [PubMed] [Google Scholar]
- 33.Gordon P.H., Wang Y., Doorish C., Lewis M., Battista V., Mitsumoto H. A screening assessment of cognitive impairment in patients with ALS. Amyotroph Lateral Scler. 2007;8:362–365. doi: 10.1080/17482960701500817. [DOI] [PubMed] [Google Scholar]
- 34.Flaherty-Craig C., Eslinger P., Stephens B., Simmons Z. A rapid screening battery to identify frontal dysfunction in patients with ALS. Neurology. 2006;67(2017–2) doi: 10.1212/01.wnl.0000247667.89251.43. [DOI] [PubMed] [Google Scholar]
- 35.Helsedirektoratet . Avdeling omsorgstjenester; 2012. Nasjonal veileder for langtids mekanisk ventilason. Helsedirektoratet. [Google Scholar]
- 36.Helsedirektoratet . Avdeling rehabilitering og sjeldne tilstander; 2013. Beslutningsprosesser ved begrensning av livsforlengde behandling. Helsedirektoratet. [Google Scholar]
- 37.World medical association World medical association declaration of Helsinki - ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. https://doi.org/doi:10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]