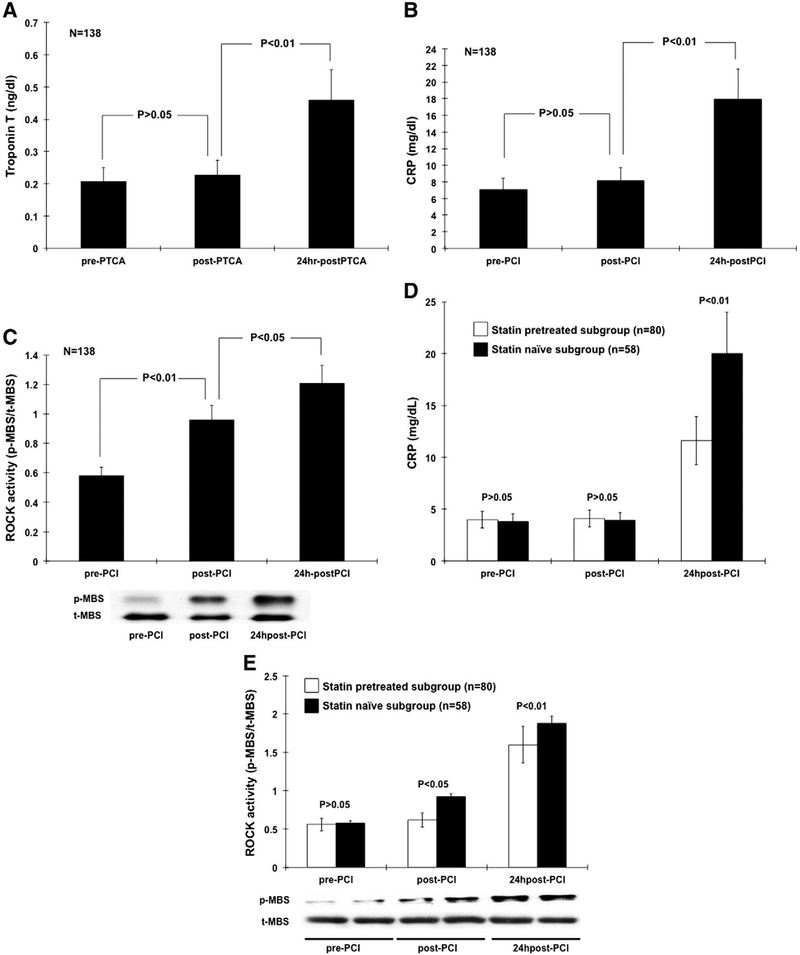
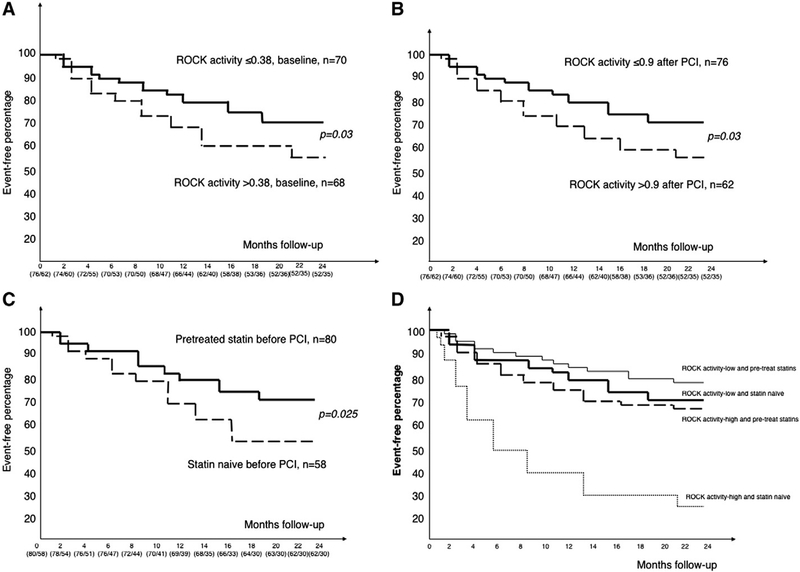
Statin therapy prior to PCI is associated with overall improvement of cardiovascular outcome, mortality and peri-procedural myocardial injury after PCI in coronary artery disease (CAD) patients [1–3]. In one meta-analysis report [4], statin pre-treated was better than nothing in the odds ratio of future coronary event outcomes. Interestingly, the level of low-density lipoprotein (LDL) cholesterol did not correlate with their outcome after PCI. This result probably implied that statin therapy has vascular protection beyond LDL lowering effect, but not for the whole group. However, it remained unknown what factors or conditions can be more beneficial from statin pretreatment during the PCI procedure. Mechanisms had been proposed as the inhibition of Rho-associated coiled-coil containing protein kinase (ROCK) [5–7] and inflammation reaction including c reactive protein (CRP) [8]. These so-called “pleiotropic” or cholesterol-independent effects of statins have been associated with the mechanisms via improvement in flow-mediated vasodilation [9,10], anti-inflammation [8,11] and also enhancement function of circulating vascular endothelial progenitor cells [12]. To test whether these important vascular biomarkers can predict those “high-risk” subgroup to be more beneficial to statin pretreatment strategy during PCI procedure, we conduct a clinical observational study based on the interplay of “pretreatment statin or not” and “the level of CRP or leukocyte ROCK activity” at baseline or after PCI, partially according our previous reports and methodologies [13–16]. We studied 138 patients who were documented as CAD with another 50 patients with normal coronary arteries who were compared as the control group. Patients’ characteristics were presented in Table 1. The prevalences of smoking behavior history, diabetes mellitus, hypertension, the levels of cholesterol as well as the baseline CRP and ROCK activity were all higher in the CAD group. There was no difference in the traditional cardiovascular risk factors, LDL-C levels and other inflammatory biomarkers when we divided the CAD group subjects into statin naïve or statin pretreated subgroups (Table S1). These data indicated that our study subgroups were well matched. The comparative procedural and angiographic characteristics of the subgroup defined by the pretreatment of statin or not were similar and presented in Table S1. The baseline ROCK activity did not correlate with baseline CRP levels (P = 0.33). Multiple logistic regression analysis with forward stepwise selection showed that in addition to hypertension (Odds ratio (OR) 3.2), smoking behavior (OR 2.3) and diabetes mellitus (OR 2.1), baseline higher levels of ROCK activity (>0.38) remained an independent predictor for CAD diagnosis (OR 2.8) (all P value b0.01). The sequential changes of leukocyte ROCK activity, CRP and troponin-T in control and CAD group were shown in Table S1 and Fig. 1A. Both groups had similar leukocyte ROCK activity after diagnostic-only angiography. However, it increased immediately after coronary PCI. In contrast, though the levels of CRP and troponin-T did not increase immediately after PCI, these 2 biomarkers had the highest level at the 24-hour delay after PCI. In control group, there were no significant changes for all biomarkers. These data support the effect of plaque rupture caused by balloon or stent procedure that could trigger an immediate activation of ROCK pathway and also a later effect on both inflammation as well as myonecrosis damage on the heart. To compare the effect of statin on the observed biomarkers and PCI outcome, we further divided the CAD patients into statin pretreated or statin naïve subgroups before PCI. In addition, the serial changes of LDL-C before and after PCI procedure were shown without any difference. The statin naïve subgroup showed a significant elevation of leukocyte ROCK activity after PCI. Interestingly, the elevation of ROCK activity was less obvious among the statin pretreated subgroup. (Table S1 and Fig. 1B). Because the statin naïve and pretreated subgroup subjects had similar baseline LDL-C and cholesterol levels, the serial changes of ROCK activity could thus be a “pleiotropic beneficial reaction” provided by statin pretreatment before PCI procedure. All patients received standard medical therapy following PCI and during follow-up. 236 patients after PCI were followed for a mean duration of 20.4 months (range 14 to 26 months). As for baseline leukocyte ROCK activity, the patients with higher levels of baseline leukocyte ROCK activity (>0.38, n = 68) had 28 coronary events during the follow-up period, whereas patients with lower levels (≤0.38, n = 70) had only 21 events (40.2% vs. 30%, P = 0.03) (Fig. 2A). This indicates that baseline ROCK activity itself is an outcome predictor after PCI. As for ROCK activity after PCI, those patients with higher levels of leukocyte ROCK activity after PCI (>0.9, n = 62) had 27 coronary events during the follow-up period, whereas patients with lower levels (≤0.9, n = 76) had only 22 events (Fig. 2B). In addition, statin pretreatment affected the prognosis after a 2-year follow-up. Those patients with statin pretreatment were observed to have less subsequent cardiovascular or coronary events during follow-up (Fig. 2C). These data support the hypothesis that statin pretreatment can not only lower the baseline ROCK activity, but also ameliorate the responsive increase of ROCK activity during PCI procedure. Multivariate forward stepwise Cox proportional hazard analysis showed that higher levels of baseline ROCK activity (>0.38) or after PCI (>0.9) and smoking were independent predictors of subsequent coronary events (Table 2). Finally, the protective effect of pretreated statin was not associated with the type, duration or dosage of statin nor the stent type used (data not shown, all P > 0.05). Interestingly, the outcome after PCI was not related to their baseline LDL level. However, the improvement in subsequent coronary events in patients with statin pretreatment was observed more significantly among patients with higher leukocyte ROCK activity (>0.9) after PCI. These results suggest that leukocyte ROCK activity after PCI predicts long-term outcome in patients especially among those without pre-treatment of statins. Our study was limited due to retrospective design, smaller size of population and lack of other associated inflammatory marker inputs. This research was supported in part by the Headquarters of University Advancement at the National Cheng Kung University, sponsored by the Ministry of Education and the National Science Council (95–2314-B-006–036-MY2, 98–2314-B-006–047-MY3 and 101–2314-B-006–075-MY2), and the National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH-10204018) also the grant funded by the Ministry of Health and Welfare in Taiwan (MOHW103-TDU-B-211–113002).
Table 1.
Baseline characteristics of control and CAD patients, subdivided into statin pretreatment or naïve before PCI procedures.
| Control (n = 50) | CAD (n = 138) | Statin naive (n = 58) | Statin pretreated (n = 80) | |
|---|---|---|---|---|
| Age, years | 62 ± 10 | 61 ± 13 | 61 ± 15 | 61 ± 15 |
| Male, sex (%) | 35 (70) | 94 (68.1) | 40 (68.9) | 54 (67.5) |
| Smoking (%) | 20 (40) | 67 (48.6)* | 27 (46.5) | 40 (50.0) |
| Diabetes mellitus (%) | 5 (10) | 27 (19.5)** | 10 (17.2) | 14 (17.5) |
| Hypertension (%) | 12 (24) | 76 (55.0)** | 30 (52) | 43 (53.7) |
| Total cholesterol, mg/dl | 196 ± 40 | 206 ± 34* | 208 ± 25 | 205 ± 30 |
| HDL-cholesterol, mg/dl | 52 ± 14 | 46 ± 20* | 47 ± 26 | 46 ± 14 |
| LDL-cholesterol, mg/dl | 123 ± 38 | 131 ± 40** | 132 ± 33 | 131 ± 47 |
| Triglycerides, mg/dl | 133 ± 42 | 135 ± 43 | 136 ± 55 | 135 ± 53 |
| BM1, kg/m2 | 22.1 ± 4.2 | 23.0 ± 5.1 | 23.1 ± 4.3 | 23.0 ± 3.1 |
| Number of risk factors | 1.3 ± 1.5 | 2.3 ± 1.7** | 2.3 ± 1.9 | 2.5 ± 2.3 |
| CRP, mg/dl | 0.46 (0.18–2.56) | 3.25** (1.28–5.88) | 3.23 (1.28–4.98) | 3.26 (1.33–5.88) |
| ROCK activity (pMBS/tMBS ratio) | 0.21 (0.12–0.62) | 0.40** (0.27–1.02) | 0.42 (0.27–0.89) | 0.40 (0.28–1.02) |
| Previous MI (%) | - | 21 (15.2) | 8 (13.7) | 13 (16.2) |
Values are expressed as mean ± SD or number, or median with inter-quartile range. BMI = body mass index; CRP = C – reactive protein; MI = myocardial infarction; PCI = percutaneous coronary intervention; LDL = low-density lipoprotein; HDL = high-density lipoprotein; NA = not analyzed; ROCK = Rho kinase.
P < 0.05,
P < 0.01 compared between normal control and CAD groups; there was no difference between statin naïve and statin pretreated subgroups.
Fig. 1.
Serial changes of biochemical markers before and after PCI. (A) and (B): Troponin-T and C-reactive protein (CRP) elevated significantly only at 24 h after PCI. (C): Rho kinase (ROCK) activity on isolated peripheral leukocytes increased immediately after PCI and persistent till 24 h after PCI. (D): Pretreated statin subgroup showed less elevation of C-reactive protein (CRP) at 24 h after PCI than the statin naïve subgroup. (E): Pretreated statin showed less elevation of Rho kinase (ROCK) activity elevation on isolated peripheral leukocytes both immediately after PCI and also at 24 h after PCI than the statin naïve subgroup. PCI = percutaneous coronary intervention; p-MBS = phosphor-myosin binding subunit; t-MBS = total myosin binding subunit.
Fig. 2.
Kaplan–Meier survival curve of the proportion of patients remaining free of subsequent coronary events after PCI (A): Baseline leukocyte Rho kinase (ROCK) activity on isolated peripheral leukocytes (cutoff value: 0.38 (pMBS/tMBS ratio from Western blot)); (B) level of ROCK activity after PCI (cutoff value: 0.9); (C): pretreated statin or not; (D): subanalysis according to the level of ROCK activity after PCI (cutoff value: 0.9 (pMBS/tMBS ratio from Western blot)) and the pretreatment of statin or not. *P < 0.05. PCI = percutaneous coronary intervention.
Table 2.
Univariate and multiple Cox proportional hazard model analysis for future coronary events after PCI.
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Univariate analysis | |||
| Higher ROCK activity after PCI (>0.9) | 2.3 | 1.3–4.6 | 0.01 |
| Higher baseline ROCK activity (>0.38) | 2.0 | 1.2–5.8 | 0.03 |
| Higher CRP after PCI (>7.5 mg/dl) | 1.8 | 1.4–4.8 | 0.02 |
| Severe CAD (≥2 vessel disease) | 2.2 | 1.9–6.3 | 0.04 |
| Smaller stent size (≤2.5 mm) | 1.8 | 1.5–4.7 | 0.02 |
| Diabetes mellitus | 1.9 | 1.2–8 | 0.01 |
| Smoking | 2.4 | 2.0–7.8 | 0.01 |
| Multivariate analysis | |||
| Higher ROCK activity after PCI (>0.9) | 2.2 | 1.3–9.0 | 0.01 |
| Higher baseline ROCK activity (>0.38) | 2.2 | 1.1–7.2 | 0.02 |
| Smoking | 3.1 | 1.2–6.3 | 0.01 |
Abbreviations please see Table 1.
Supplementary Material
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ijcard.2014.06.059.
References
- [1].Herrmann J, Lerman A, Baumgart D, et al. Preprocedural statin medication reduces the extent of periprocedural non-Q-wave myocardial infarction. Circulation 2002;106:2180–3. [DOI] [PubMed] [Google Scholar]
- [2].Briguori C, Colombo A, Airoldi F, et al. Statin administration before percutaneous coronary intervention: impact on periprocedural myocardial infarction. Eur Heart J 2004;25:1822–8. [DOI] [PubMed] [Google Scholar]
- [3].Chang SM, Yazbek N, Lakkis NM. Use of statins prior to percutaneous coronary intervention reduces myonecrosis and improves clinical outcome. Catheter Cardiovasc Interv 2004;62:193–7. [DOI] [PubMed] [Google Scholar]
- [4].Merla R, Reddy NK, Wang FW, Uretsky BF, Barbagelata A, Birnbaum Y. Meta-analysis of published reports on the effect of statin treatment before percutaneous coronary intervention on periprocedural myonecrosis. Am J Cardiol 2007;100:770–6. [DOI] [PubMed] [Google Scholar]
- [5].Noma K, Rikitake Y, Oyama N, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest 2008;118:1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang HW, Liu PY, Oyama N, et al. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. FASEB J 2008;22:3561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dong M, Liao JK, Yan B, Li R, Zhang M, Yu CM. A combination of increased Rho kinase activity and N-terminal pro-B-type natriuretic peptide predicts worse cardiovascular outcome in patients with acute coronary syndrome. Int J Cardiol 2013;167:2813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–8. [DOI] [PubMed] [Google Scholar]
- [9].Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006;27:1182–90. [DOI] [PubMed] [Google Scholar]
- [10].Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation 2009;119:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med 2008;14:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 2001;108:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chang CL, Lee PT, Chang WT, et al. The interplay between inflammation, physical activity and metabolic syndrome in a remote male geriatric community in Southern Taiwan: the Tianliao Old People (TOP) study 03. Diabetol Metab Syndr 2013;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu PY, Li YH, Tsai WC, et al. Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur Heart J 2003;24:1824–32. [DOI] [PubMed] [Google Scholar]
- [15].Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol 2007;49:1619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu PY, Liao JK. A method for measuring Rho kinase activity in tissues and cells. Methods Enzymol 2008;439:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.