Abstract
Acute deterioration of liver cirrhosis (e.g., infections, acute‐on‐chronic liver failure [ACLF]) requires an increase in cardiac contractility. The insufficiency to respond to these situations could be deleterious. Left ventricular global longitudinal strain (LV‐GLS) has been shown to reflect left cardiac contractility in cirrhosis better than other parameters and might bear prognostic value. Therefore, this retrospective study investigated the role of LV‐GLS in the outcome after transjugular intrahepatic portosystemic shunt (TIPS) and the development of ACLF. We included 114 patients (48 female patients) from the Noninvasive Evaluation Program for TIPS and Their Follow‐Up Network (NEPTUN) cohort. This number provided sufficient quality and structured follow‐up with the possibility of calculating major scores (Child, Model for End‐Stage Liver Disease [MELD], Chronic Liver Failure Consortium acute decompensation [CLIF‐C AD] scores) and recording of the events (development of decompensation episode and ACLF). We analyzed the association of LV‐GLS with overall mortality and development of ACLF in patients with TIPS. LV‐GLS was independently associated with overall mortality (hazard ratio [HR], 1.123; 95% confidence interval [CI],1.010‐1.250) together with aspartate aminotransferase (HR, 1.009; 95% CI, 1.004‐1.014) and CLIF‐C AD score (HR, 1.080; 95% CI, 1.018‐1.137). Area under the receiver operating characteristic curve (AUROC) analysis for LV‐GLS for overall survival showed higher area under the curve (AUC) than MELD and CLIF‐C AD scores (AUC, 0.688 versus 0.646 and 0.573, respectively). The best AUROC‐determined LV‐GLS cutoff was −16.6% to identify patients with a significantly worse outcome after TIPS at 3 months, 6 months, and overall. LV‐GLS was independently associated with development of ACLF (HR, 1.613; 95% CI, 1.025‐2.540) together with a MELD score above 15 (HR, 2.222; 95% CI, 1.400‐3.528). Conclusion: LV‐GLS is useful for identifying patients at risk of developing ACLF and a worse outcome after TIPS. Although validation is required, this tool might help to stratify risk in patients receiving TIPS.
Abbreviations
- A
peak late diastolic atrial filling velocity
- ACLF
acute‐on‐chronic liver failure
- AST
aspartate aminotransferase
- AUC
area under the curve
- CLIF‐C AD
Chronic Liver Failure Consortium acute decompensation
- E
peak early diastolic filling velocity
- E′
peak early diastolic mitral annular velocity
- GLS
global longitudinal strain
- HR
hazard ratio
- LV
left ventricular
- MELD
Model for End‐Stage Liver Disease
- NEPTUN
Noninvasive Evaluation Program for TIPS and Their Follow‐Up Network
- STE
speckle tracking echocardiography
- TIPS
transjugular intrahepatic portosystemic shunt
- TTE
transthoracic echocardiography
Portal hypertension in liver cirrhosis is frequent, causes serious complications, and predisposes for the development of acute‐on‐chronic liver failure (ACLF).1 Insertion of a transjugular intrahepatic portosystemic shunt (TIPS) induces a prompt decompression of the portal venous system and effectively treats complications of portal hypertension; however, careful selection of patients is required.2, 3 TIPS insertion increases central blood volume despite a severe aggravation of preload.4 These changes increase cardiac workload and encroach on cardiac reserves, which are especially decreased in patients with decompensated cirrhosis.5, 6, 7 In addition to TIPS, other physical stress situations (e.g., infections) could require a further increase in cardiac output, which might not be possible in an impaired hemodynamic situation.7 Therefore, assessment of the left ventricular function might help in the selection of patients for TIPS and enable stratification of management after TIPS.
In the development of ACLF, circulatory dysfunction has been defined only by arterial pressure and/or use of vasopressors8, 9; this describes a severely deranged hemodynamic situation but may not be sensitive enough in earlier but still relevant stages. Diastolic dysfunction (assessed by the early peak diastolic filling velocity to the peak late diastolic mitral annular velocity ratio [E:A] and the early peak diastolic filling velocity to the peak early diastolic mitral annular velocity [E:E′] ratio) could be associated with outcome.10, 11 However, its prognostic value is still debated.12 Moreover, the assessment of this parameter is angle dependent and limited by frame rate. Systolic function, usually assessed by ejection fraction, is easily misinterpreted in hyperdynamic circulation, e.g., in decompensated cirrhosis. Two‐dimensional speckle tracking echocardiography (STE) is an objective surrogate of cardiac contractility.13, 14 Left ventricular global longitudinal strain (LV‐GLS) is associated with outcome in patients with cirrhosis.4, 15 In the present study, we evaluated for the first time the prognostic value of LV‐GLS for the development of ACLF and mortality in patients with cirrhosis treated with TIPS.
Patients and Methods
Study Design And Patients
We included patients with sufficient quality of STE and structured follow‐up with the possibility of calculating major prognostic scores. A total of 110 patients were excluded from the study for the following reasons: poor quality of transthoracic echocardiography (TTE) before TIPS in 32 patients, prior history of ischemic heart disease in 31 patients, other chronic heart failure in 23 patients, severe valvular diseases in 18 patients, and atrial fibrillation in 6 patients. The main endpoints of the study were development of ACLF, as defined by the CANONIC study,8, 9 and overall mortality. This analysis included 114 patients (66 male adults) from the Noninvasive Evaluation Program for TIPS and Their Follow‐Up Network (NEPTUN) cohort. NEPTUN consists of patients with liver cirrhosis receiving TIPS at the Department of Internal Medicine I, University of Bonn, Germany, and receiving a structured routine evaluation and follow‐up. The patients included in this analysis received TIPS between June 2008 and June 2015. The diagnosis of cirrhosis was based on clinical, hemodynamic, and biochemical parameters as well as ultrasound and/or biopsy criteria. The local ethics committee of the University of Bonn (No. 121/14) approved the study in accordance with the Declaration of Helsinki, and the patients gave their signed written informed consent.
Conventional Echocardiography
TTE examination was performed using commercially available equipment (Vivid 7 [General Electric Medical Health, Waukesha, WI]; iE33 [Philips Medical Systems, Koninklijke N.V., Heerlen, the Netherlands]) with a 2.5‐MHz phased‐array transducer as described.15 Briefly, the Simpson method was used to calculate the LV ejection fraction from the four‐chamber view. To assess LV diastolic dysfunction, conventional pulsed‐wave Doppler techniques were used with the sample volume positioned at the tip of the mitral valve leaflets to measure mitral inflow velocities. E, A, and the E:A ratio were calculated. A sample volume was taken at the septal wall on a level with the mitral annulus to measure E′ and the E:E′ ratio to differentiate between normal and grade 2 impaired LV diastolic function as described.15
STE
STE was performed as described.15 Briefly, four‐chamber views were recorded during routine TTE in the context of evaluation for TIPS insertion. Image Arena 4.3 (TomTec Imaging Systems GmbH, 2001‐2010, Unterschleissheim, Germany) automatically measures frame by frame myocardial shortening of a distance between two points on the myocardium. Longitudinal and radial segmental two‐dimensional strains of the left ventricle were analyzed in the apical four‐chamber view. For this purpose, the endocardial boundary in the left ventricle was set manually. Each ventricle was automatically divided into six segments: basal lateral/septal, mid lateral/septal, and apical lateral/septal. LV‐GLS was calculated.
Statistical Analysis
We used the nonparametric Mann‐Whitney test for unpaired comparisons. Correlations were analyzed with Spearman's correlation coefficient. Univariate time‐to‐event analysis was performed to identify parameters that significantly predict survival. Multivariate Cox regression analysis (forward stepwise likelihood quotient) using the significant predictors in the univariate analysis was performed to identify independent predictors of survival. For the selection of cut‐off values, receiver operating characteristics (ROC) analysis with survival as endpoint was calculated. Kaplan‐Meier curves were used to compare the survival rates of patients using the log‐rank test. Statistical analysis was performed using SPSS 22 for Windows (SPSS, Inc., Chicago, IL).
Results
General Patient Characteristics
Clinical characteristics of the patients are listed in Table 1. There were 48 female and 66 male patients with a median age of 59 years (range, 18‐80 years). The etiology of cirrhosis was alcohol in 74 patients, chronic viral hepatitis in 14 patients, and other chronic liver disease in 26 patients. Twenty‐six patients presented with Child A, 76 with Child B, and 12 with Child C liver cirrhosis. The median Model for End‐Stage Liver Disease (MELD) score was 11. Forty patients showed no ascites at the time of TIPS insertion, 29 patients had a history of at least one episode of hepatorenal syndrome, and 22 patients had experienced at least one episode of hepatic encephalopathy (Table 1).
Table 1.
General Patient Characteristics of the Cohort
| Parameters | Values* | |
|---|---|---|
| General Characteristics | Sex (male/female) | 66/48 |
| Indication (bleeding/ascites/both) | 40/71/3 | |
| Age median (range) | 59 (18‐80) | |
| Etiology (alcohol/viral/other) | 74/14/26 | |
| Child category (A/B/C) | 26/76/12 | |
| MELD score median (range) | 11 (6‐40) | |
| Ascites (absent/present) | 40/74 | |
| Previous HRS (no/yes) | 85/29 | |
| Previous HE (0/I‐II/III‐IV) | 92/21/1 | |
| Central venous pressure (mm Hg) | 8 (0‐23) | |
| Portal pressure (mm Hg) | 28 (10‐49) | |
| Pressure gradient (mm Hg) | 19 (7‐42) | |
| CLIF‐C AD score | 21 (7‐27) | |
| Biochemical Characteristics | Sodium (mmol/L) | 138 (114‐146) |
| Creatinine (mg/dL) | 1.3 (0.5‐8.5) | |
| Bilirubin (mg/dL) | 1.0 (0.2‐30.3) | |
| CRP (mg/L) | 10.3 (0.3‐107.0) | |
| Albumin (g/L) | 31.1 (14.6‐43.9) | |
| INR | 1.1 (0.9‐2.3) | |
| TWC (G/L) | 6.0 (1.7‐20.7) | |
| Hb (g/dL) | 10.3 (6.5‐15.7) | |
| Platelets (G/L) | 120 (28‐679) | |
| GGT (U/L) | 126 (23‐788) | |
| ALT (U/L) | 24 (8‐120) | |
| AST (U/L) | 39 (11‐361) | |
| Cardiac Parameters | EDV (mL) | 97 (39‐195) |
| ESV (mL) | 34 (12‐85) | |
| EF (%) | 67 (51‐85) | |
| Diastolic dysfunction present (no/yes) | 61/53 | |
| LV‐GLS (%) | −16.6 (−7.0 to −26.7) |
Unless otherwise specified, values represent median (range).
Abbreviations: ALT, alanine aminotransferase; CRP, C‐reactive protein; EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; GGT, gamma‐glutamyltransferase; Hb, hemoglobin; HE, hepatic encephalopathy; HRS, hepatorenal syndrome; INR, international normalized ratio; TWC, total white blood cell count.
The more frequent indication for TIPS was refractory ascites (62%, 71 patients). The median portosystemic pressure gradient was 19 mm Hg. The median Chronic Liver Failure Consortium acute decompensation (CLIF‐C AD) score was low at 21, indicating that these decompensated patients were stabilized at the time of TIPS insertion (Table 1). The CLIF‐C AD score includes surrogate parameters of systemic inflammation, which is missing in the MELD score.
In TTE and STE, diastolic dysfunction was present in 53 patients (46%), and none of the patients had a compromised ejection fraction (Table 1). LV‐GLS ranged from −7% to −26.7%, with a median of −16.6%. More detailed information on the TTE data of the cohort is shown in Supporting Table S1. Importantly, no correlation between the baseline parameters and LV‐GLS could be assessed (data not shown).
The Association Of Lv‐Gls With Development Of Aclf And Mortality
During the 24 months of structured per protocol follow‐up, 54 patients developed ACLF according to European Association for the Study of the Liver‐CLIF criteria. ACLF grade I was present in 17 patients, grade II in 19 patients, and grade III in 18 patients. The median time span from insertion of TIPS to occurrence of ACLF was 65 days. More detailed information regarding the different ACLF grades is provided in Supporting Table S3.
The most often occurring type of organ failure was kidney injury. In 28 out of 54 patients developing ACLF, ACLF was the cause of death. LV‐GLS of patients developing ACLF after TIPS was similar compared to LV‐GLS of patients who did not develop the syndrome. Interestingly, LV‐GLS, together with a MELD score above 15, was independently associated with development of ACLF (Table 2). This showed the time association between LV‐GLS and ACLF.
Table 2.
Univariate Time‐To‐Event Analysis of Collected Data to Predict Aclf Occurrence and Multivariable Cox Regression Analysis (Forward Stepwise Likelihood Quotient) using the Variable from Univariate Analysis to Predict Aclf Occurrence
| Parameters | Univariate Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI for HR | P | HR | 95% CI for HR | |||
| Lower | Upper | Lower | Upper | |||||
| Sex | n.s. | 1.1 | 0.632 | 1.9 | ||||
| Age | n.s. | 1 | 0.986 | 1.03 | ||||
| Bilirubin | n.s. | 1 | 0.997 | 1.0 | ||||
| Albumin | n.s. | 1 | 0.937 | 1.1 | ||||
| AST | n.s. | 1 | 0.97 | 1.1 | ||||
| MELD score above 15 | <0.001 | 2.348 | 1.482 | 3.719 | 0.001 | 2.222 | 1.400 | 3.528 |
| LV‐GLS | 0.016 | 1.740 | 1.109 | 2.731 | 0.039 | 1.613 | 1.025 | 2.540 |
Abbreviation: n.s., nonsignificant.
In univariate time‐to‐event analysis, a number of parameters were identified to be associated with overall survival. In addition to a MELD score above 15, serum bilirubin, albumin, aspartate aminotransferase (AST), CLIF‐C AD score, and LV‐GLS were also significantly associated with overall survival (Table 3). In multivariable Cox regression analysis, only AST, CLIF‐C AD score, and LV‐GLS were independently associated with overall mortality (Table 3). These effects might be due to the strong effect in the subgroup of patients receiving TIPS for ascites (Supporting Table S2). Still, the overall result remains when controlling for the indication of TIPS.
Table 3.
Univariate Time‐to‐Event Analysis of Collected Data to Predict Survival and Multivariable Cox Regression Analysis (Forward Stepwise Likelihood Quotient) using the Variable from Univariate Analysis to Predict Survival
| Parameters | Univariate Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI for HR | P | HR | 95% CI for HR | |||
| Lower | Upper | Lower | Upper | |||||
| Sex | n.s. | 1.071 | 0.549 | 2.092 | ||||
| Age | 0.035 | 1.032 | 1.002 | 1.064 | ||||
| MELD score | 0.025 | 1.474 | 1.247 | 1.909 | ||||
| Bilirubin | 0.002 | 1.131 | 1.045 | 1.224 | ||||
| Albumin | 0.051 | 0.952 | 0.906 | 1.000 | ||||
| AST | 0.002 | 1.008 | 1.003 | 1.014 | <0.001 | 1.009 | 1.004 | 1.014 |
| LV‐GLS | 0.017 | 1.441 | 1.224 | 1.866 | 0.03 | 1.123 | 1.010 | 1.250 |
| CLIF‐C AD score | 0.014 | 1.136 | 1.030 | 1.260 | 0.01 | 1.080 | 1.018 | 1.137 |
Abbreviation: n.s., nonsignificant.
ROC analysis with mortality as the endpoint was performed to define a cutoff for LV‐GLS. The best cutoff for LV‐GLS was −16.6%. Kaplan‐Meier analysis stratified for median LV‐GLS showed that patients with low contractility (higher LV‐GLS) developed ACLF significantly earlier than patients with higher cardiac contractility (lower LV‐GLS) (Fig. 1). Kaplan‐Meier analysis stratified for LV‐GLS showed higher mortality in patients with high LV‐GLS (Fig. 2). Interestingly, LV‐GLS (area under the curve [AUC], 0.688; P = 0.03) showed a higher AUC compared to the MELD score (AUC, 0.646; P = 0.08) and CLIF‐C AD score (AUC, 0.573; P = 0.39).
Figure 1.
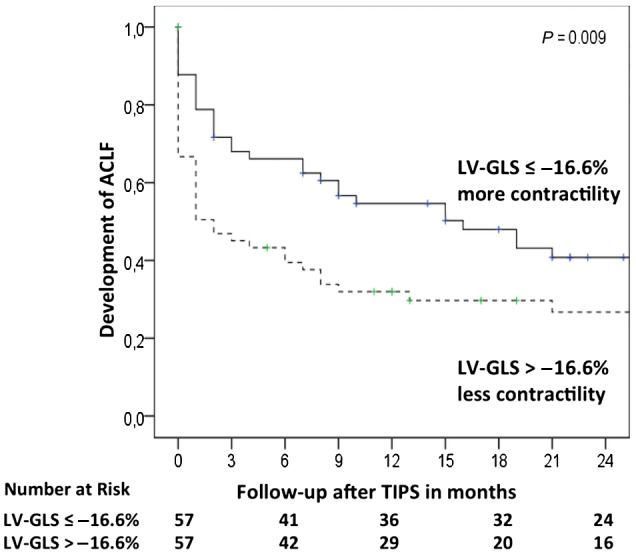
Time association of LV‐GLS and ACLF development. Kaplan‐Meier analysis shows development of ACLF in patients stratified by LV‐GLS (cutoff, −16.6%). Lower LV‐GLS levels mean better cardiac contractility, whereas higher LV‐GLS levels reflect worse cardiac contractility. Rates of ACLF development are shown using Kaplan‐Meier plots and are analyzed by the log‐rank test.
Figure 2.
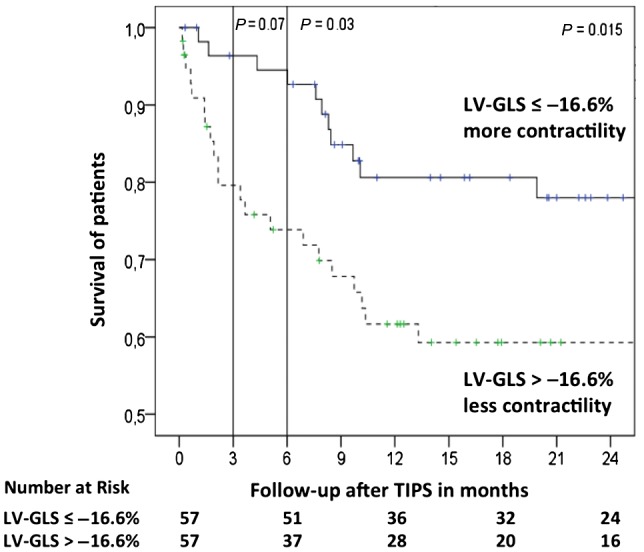
Survival after TIPS stratified by their cardiac contractility, assessed using LV‐GLS. Kaplan‐Meier analysis shows survival of patients stratified by LV‐GLS (cutoff, −16.6%). Lower LV‐GLS levels mean better cardiac contractility, whereas higher LV‐GLS levels reflect worse cardiac contractility. Survival rates are shown using Kaplan‐Meier plots and are analyzed by the log‐rank test.
Discussion
We show for the first time that LV‐GLS is independently associated with the development of ACLF and overall survival of patients with cirrhosis after TIPS. Therefore, LV‐GLS allows a better characterization of the circulatory and hemodynamic dysfunction in decompensated patients and thus enables stratification of patients at risk.
The CANONIC study has shown that circulatory failure, defined as use of vasopressors, is detrimental in patients with ACLF.8 However, there are no good parameters for early identification of patients at risk to develop circulatory failure. Moreover, the reduced effective arterial volume, which occurs particularly in patients who are acutely decompensated, possibly predisposes or even induces organ failure, especially renal failure, which is highly dependent on organ perfusion.3 STE parameters of the LV function have been shown in other large studies to be clearly related to the development of renal dysfunction.13 This possibly also explains the prediction of the need for liver transplantation by STE,15 which is MELD based and renal function dependent. Therefore, it is not surprising that STE parameters, and specifically LV‐GLS, independently predict development of ACLF in these patients who are decompensated and receiving TIPS mainly for refractory ascites.
ACLF is defined by the development of organ failures after precipitating events, which in many cases are infections.8, 9 Impaired organ perfusion, in particular, might be the reason for the development of organ failure, especially of kidney and brain.13, 16 Impaired organ perfusion may be at least partly a consequence of decreased cardiac contractility and less responsive myocardium as assessed by STE.13 Infections, the most frequent cause of ACLF, are known to require a higher cardiac reserve to maintain adequate circulation. Therefore, latent cardiac dysfunction could lead to the development of organ failure, ACLF, and death.17 The present study supports this hypothesis and further suggests that impaired cardiac contractility, reflected by higher LV‐GLS, predisposes to the development of ACLF and death in cirrhosis.
As TIPS insertion itself requires sufficient cardiac reserve, it is contraindicated in patients with severe cardiac pathologies, such as severe tricuspid regurgitation and severe pulmonary hypertension. However, the role of other cardiac parameters in patient selection has been a matter of debate.3, 18 Previous studies, which evaluated conventional TTE, reported TIPS insertion resulting in a significant increase of left atrial diameter, LV end‐diastolic diameter, and pulmonary arterial systolic pressure,5, 19, 20 parameters associated with overall survival of patients with cirrhosis.21 Moreover, diastolic dysfunction is frequent in advanced cirrhosis and is associated with failure to resolve ascites and increased mortality after TIPS.11 However, no correlation between diastolic dysfunction and severity of liver disease was shown.12, 13 In previous animal and human studies on cirrhosis, unimpaired resting systolic function decompensated after several precipitating factors, such as physical stress and exercise.22 These apparently contradictive findings can explain whether TIPS is regarded as the factor that unmasks a preexisting subclinical cardiomyopathy in patients with cirrhosis. Our findings underline this hypothesis and show that patients with better cardiac contractility develop less ACLF and death after TIPS. This effect was mainly observed in the patients with ascites of our cohort, but when all patients were analyzed, the effect was still statistically significant.
The question remains as to what technique should be used for the detection of circulatory dysfunction or predisposition to it. Conventional echocardiography shows several limitations, as outlined above, whereas TTE could assess early and subclinical myocardial dysfunction when enhanced by speckle tracking.23, 24, 25, 26 Speckle tracking is independent of volume status, hyperdynamic situation, and as shown by us previously,15 may give a more objective view on the estimation of potential subclinical cardiomyopathy in patients with cirrhosis. As already mentioned, LV contractility appears to be decreased in patients with cirrhosis,14 whereas the lack of response of LV contractility to hemodynamic changes might be the reason for development of ascites and decompensation.13 In one elaborate, invasive, pathophysiologic, but small study in patients with TIPS, profound changes were induced in cardiac contractility after TIPS, which could only be reflected noninvasively by speckle tracking.4 Our present study confirms that the most suitable, noninvasive, and less biased technique seems to be STE, while it describes for the first time LV‐GLS as the best predictor of ACLF development and mortality.
Although the patients were well characterized, the conclusions of the present study are limited due to its retrospective nature. A selection bias cannot be excluded because only patients with good quality TTE were included. Moreover, the subgroup of patients with ascites in our cohort was predominant, while patients receiving TIPS for bleeding and hepatorenal syndrome were fewer; therefore this might have introduced another bias. However, the results were similar when controlling for the indication of TIPS. Independent validation of these findings and prospective stratification of management based on LV‐GLS are tasks for future studies.
Summarizing, LV‐GLS may identify patients with latent cardiac dysfunction, who, following stress, such as TIPS insertion, are then more predisposed to deterioration, development of ACLF, and death. Our results suggest a more meticulous patient selection for TIPS and a more personalized management approach to react to decompensating events. Finally, this study suggests a cutoff for LV‐GLS to identify patients at risk of developing ACLF and death and thereby to stratify management of patients receiving TIPS.
Supporting information
Acknowledgment
We thank Kristin Gehrmann, Nadine Koestlmeier, Gudrun Hack, and Silke Bellinghausen for excellent technical support.
Supported by the Deutsche Forschungsgemeinschaft (SFB TRR57 to P18 to J.T.), the European Union's Horizon 2020 Research and Innovation Program (No. 668031 to J.T.) and Societal Challenges (Health, demographic change, and well‐being, No. 731875 to J.T.), and the Cellex Foundation (PREDICT to V.A., J.T.).
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Trebicka J. Predisposing factors in acute‐on‐chronic liver failure. Semin Liver Dis 2016;36:167‐173. [DOI] [PubMed] [Google Scholar]
- 2. Trebicka J. Emergency TIPS in a Child‐Pugh B patient: when does the window of opportunity open and close? J Hepatol 2017;66:442‐450. [DOI] [PubMed] [Google Scholar]
- 3. Trebicka J. Does transjugular intrahepatic portosystemic shunt stent differentially improve survival in a subset of cirrhotic patients? Semin Liver Dis 2018;38:87‐96. [DOI] [PubMed] [Google Scholar]
- 4. Busk TM, Bendtsen F, Poulsen JH, Clemmesen JO, Larsen FS, Goetze JP, et al. Transjugular intrahepatic portosystemic shunt: impact on systemic hemodynamics and renal and cardiac function in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol 2018;314:G275‐G286. [DOI] [PubMed] [Google Scholar]
- 5. Merli M, Valeriano V, Funaro S, Attili AF, Masini A, Efrati C, et al. Modifications of cardiac function in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt (TIPS). Am J Gastroenterol 2002;97:142‐148. [DOI] [PubMed] [Google Scholar]
- 6. Wiese S, Hove JD, Bendtsen F, Moller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 2014;11:177‐186. [DOI] [PubMed] [Google Scholar]
- 7. Zardi EM, Zardi DM, Chin D, Sonnino C, Dobrina A, Abbate A. Cirrhotic cardiomyopathy in the pre‐ and post‐liver transplantation phase. J Cardiol 2016;67:125‐130. [DOI] [PubMed] [Google Scholar]
- 8. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426‐1437, 1437.e1421–e1429. [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406‐460. Erratum. In: J Hepatol 2018;69:1207. [DOI] [PubMed] [Google Scholar]
- 10. Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, et al. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut 2007;56:869‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol 2009;104:2458‐2466. [DOI] [PubMed] [Google Scholar]
- 12. Merli M, Calicchia A, Ruffa A, Pellicori P, Riggio O, Giusto M, et al. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med 2013;24:172‐176. [DOI] [PubMed] [Google Scholar]
- 13. Nazar A, Guevara M, Sitges M, Terra C, Sola E, Guigou C, et al. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol 2013;58:51‐57. [DOI] [PubMed] [Google Scholar]
- 14. Pagourelias ED, Sotiriou P, Papadopoulos CE, Cholongitas E, Giouleme O, Vassilikos V. Left ventricular myocardial mechanics in cirrhosis: a speckle tracking echocardiographic study. Echocardiography 2016;33:223‐232. [DOI] [PubMed] [Google Scholar]
- 15. Jansen C, Cox A, Schueler R, Schneider M, Lehmann J, Praktiknjo M, et al. Increased myocardial contractility identifies patients with decompensated cirrhosis requiring liver transplantation. Liver Transpl 2018;24:15‐25. [DOI] [PubMed] [Google Scholar]
- 16. Macias‐Rodriguez RU, Duarte‐Rojo A, Cantu‐Brito C, Sauerbruch T, Ruiz‐Margain A, Trebicka J, et al. Cerebral haemodynamics in cirrhotic patients with hepatic encephalopathy. Liver Int 2015;35:344‐352. [DOI] [PubMed] [Google Scholar]
- 17. Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol 2012;9:382‐391. [DOI] [PubMed] [Google Scholar]
- 18. Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004;363:1461‐1468. [DOI] [PubMed] [Google Scholar]
- 19. Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rossle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut 1999;44:743‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wannhoff A, Hippchen T, Weiss CS, Friedrich K, Rupp C, Neumann‐Haefelin C, et al. Cardiac volume overload and pulmonary hypertension in long‐term follow‐up of patients with a transjugular intrahepatic portosystemic shunt. Aliment Pharmacol Ther 2016;43:955‐965. [DOI] [PubMed] [Google Scholar]
- 21. Merli M, Torromeo C, Giusto M, Iacovone G, Riggio O, Puddu PE. Survival at 2 years among liver cirrhotic patients is influenced by left atrial volume and left ventricular mass. Liver Int 2017;37:700‐706. [DOI] [PubMed] [Google Scholar]
- 22. Gaskari SA, Honar H, Lee SS. Therapy insight: cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol 2006;3:329‐337. [DOI] [PubMed] [Google Scholar]
- 23. Becker M, Bilke E, Kuhl H, Katoh M, Kramann R, Franke A, et al. Analysis of myocardial deformation based on pixel tracking in two dimensional echocardiographic images enables quantitative assessment of regional left ventricular function. Heart 2006;92:1102‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light‐chain amyloidosis. JACC Cardiovasc Imaging 2010;3:333‐342. [DOI] [PubMed] [Google Scholar]
- 25. Cameli M, Lisi M, Righini FM, Focardi M, Lunghetti S, Bernazzali S, et al. Speckle tracking echocardiography as a new technique to evaluate right ventricular function in patients with left ventricular assist device therapy. J Heart Lung Transplant 2013;32:424‐430. [DOI] [PubMed] [Google Scholar]
- 26. Sera F, Kato TS, Farr M, Russo C, Jin Z, Marboe CC, et al. Left ventricular longitudinal strain by speckle‐tracking echocardiography is associated with treatment‐requiring cardiac allograft rejection. J Card Fail 2014;20:359‐364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


