Summary
Objective
Long intergenic non-coding RNAs (lincRNAs) are emerging as key regulators in gene expression; however, little is known about the lincRNA expression changes that occur in osteoarthritis (OA). Here we aimed to define a transcriptome of lncRNAs in OA cartilage, specifically comparing the lincRNA transcriptome of knee and hip cartilage.
Method
RNA-seq was performed on nucleic acid extracted from hip cartilage from patients undergoing joint replacement surgery because of either OA (n = 10) or because of a neck of femur fracture (NOF; n = 6). After transcript alignment, counts were performed using Salmon and differential expression for ENSEMBL lincRNAs determined using DESeq2. Hip RNA-seq lincRNA expression was compared to a knee dataset (ArrayExpress; E-MTAB-4304). ChIP-seq data from ENCODE was used to determine whether lincRNAs were associated with promoters (plncRNA) or unidirectional enhancer-like regulatory elements (elncRNAs).
Results
Our analysis of the hip transcriptome identified 1692 expressed Transcripts Per Million (TPM ≥1) Ensembl lincRNAs, of which 198 were significantly (FDR ≤0.05) differentially expressed in OA vs normal (NOF) cartilage. Similar analysis of knee cartilage transcriptome identified 648 Emsembl lincRNAs with 93 significantly (FDR ≤0.05) differentially expressed in intact vs damaged cartilage. In total, 1834 lincRNAs were expressed in both hip and knee cartilage, with a highly significant correlation in expression between the two cartilages.
Conclusion
This is the first study to use RNA-seq to map and compare the lincRNA transcriptomes of hip and knee cartilage. We propose that lincRNAs expressed selectively in cartilage, or showing differential expression in OA, will play a role in cartilage homoeostasis.
Keywords: Non-coding RNA, lincRNA, RNA-seq, Cartilage, Hip, Knee
Introduction
Osteoarthritis (OA) is a common debilitating musculoskeletal disease that affects the articulating joints and for which there remains no cure. The burden of OA continues to increase worldwide. In the United Kingdom, 89,288 hip and 98,591 knee primary replacements were recorded in the National Joint Registry in 2015, with the vast majority attributable to OA1. Clinically OA is characterised by pain, stiffness and deformity of the affected joint with loss of function. Macroscopically there is degradation and loss of articular cartilage with subsequent sclerosis of the underlying bone. At the molecular level, changes to chondrocytes and the composition of the extracellular matrix have been extensively investigated, with a maladaptive milieu of growth factors, inflammatory cytokines (e.g., Tumour Necrosis Factor α; TNFα) and degradative enzymes such as matrix metalloproteinases (MMPs) contributing to cartilage destruction2.
Gene expression changes in OA cartilage have been well characterised and indicate alterations in chondrocyte homoeostasis and activation in response to this maladaptive milieu3, 4. Long non-coding RNAs (lncRNAs) are emerging as key regulators of gene expression. They are conventionally defined as being over 200 nucleotides in length with many hallmarks of mRNA transcripts but an absence of coding ability. Many appear well conserved5, 6 though there is also evidence to the contrary6, 7 and they have been demonstrated to have tissue specificity, perhaps more so than protein-coding transcripts8, 9. Their mechanisms of action, although not fully characterised, are increasingly being recognised. They may act in cis or trans at the transcriptional and post-transcriptional level as guides or scaffolds for DNA, RNA and protein binding10, 11, manifesting – for example – in chromatin remodelling12. LncRNAs have key roles in development and many are proposed to have impact on disease, for example, the lncRNA HOTAIR is dysregulated in several cancers, and its overexpression has been shown to drive breast cancer metastasis13. Nevertheless, potential key regulatory roles for lncRNAs in both health and disease remain underexplored.
We have previously identified lncRNAs in human articular cartilage but little is known about the changes in lncRNA expression that occur in OA14. One recent study used RNA sequencing (RNA-seq) to look at IL-1β-induced lncRNAs in cultured chondrocytes isolated from human hip OA cartilage15. Other studies have used microarrays for transcriptome profiling of lncRNAs in knee OA cartilage3, 4, 16, and upon stimulation of isolated chondrocytes with key pro-inflammatory mediators IL-1β4, 15 and TNFα4, but such studies are limited by pre-existing probe design.
Whilst independent epidemiological risk factors have been established for both hip and knee OA, few studies have compared gene expression between the two. We have previously explored the gene expression profile of hip OA cartilage in comparison to age-matched neck of femur (NOF) fracture cartilage (NOF)17. Interestingly, when comparing our dataset to data from a similar study of knee OA cartilage we found only a small subset of differentially expressed genes in common but a large overlap in dysregulated canonical pathways. Other knee and hip OA comparison have included differential DNA methylation analysis of articular cartilage18, 19, identifying a large number of differentially methylated loci between both the joint site and disease-status.
Here we identify a repertoire of long intergenic non-coding RNAs (lincRNAs) in both hip and knee articular cartilage and the expression changes in OA progression. We highlight differential expression of lincRNAs between knee and hip to explore any pathological differences between the two at the molecular level.
Methods
Cartilage samples
Human hip articular cartilage samples were obtained from consented patients undergoing joint replacement surgery due to either end-stage OA or intracapsular NOF fracture with Ethical Committee approval from the Newcastle and North Tyneside Health Authority. Joints were inspected macroscopically and scored using a scheme adapted from Noyes classification20 to include the presence of osteophytes [Fig. 1(A) and (B)]. Samples used in this study included those previously scored and reported17. In addition, radiological grading of the pre-operative X-rays was carried out by two blinded orthopaedic trainees. The Kellgren and Lawrence grading system was used to score the latest pre-operative hip X-ray for the OA group. NOF hips were scored using the trauma admission X-rays. The Kellgren and Lawrence grading system is a five-tiered system (0- no arthritis to 5- severe arthritis) that takes into account joint space narrowing, osteophytes, sclerosis, presence of cysts and bone deformity. The grading system has been validated for different joints with good inter-observer reliability21, 22. Further to the overall grade, the observers assigned a binary scoring system for the presence of each specific feature of the grading system. Data was also collected from the contralateral side in order to avoid the potential confounding factor of displacement due to the fractured NOF [Fig. 1(C)]. For all joints, macroscopically normal cartilage was collected, snap frozen in liquid nitrogen within 2 h of surgery and stored at −80°C prior to RNA extraction.
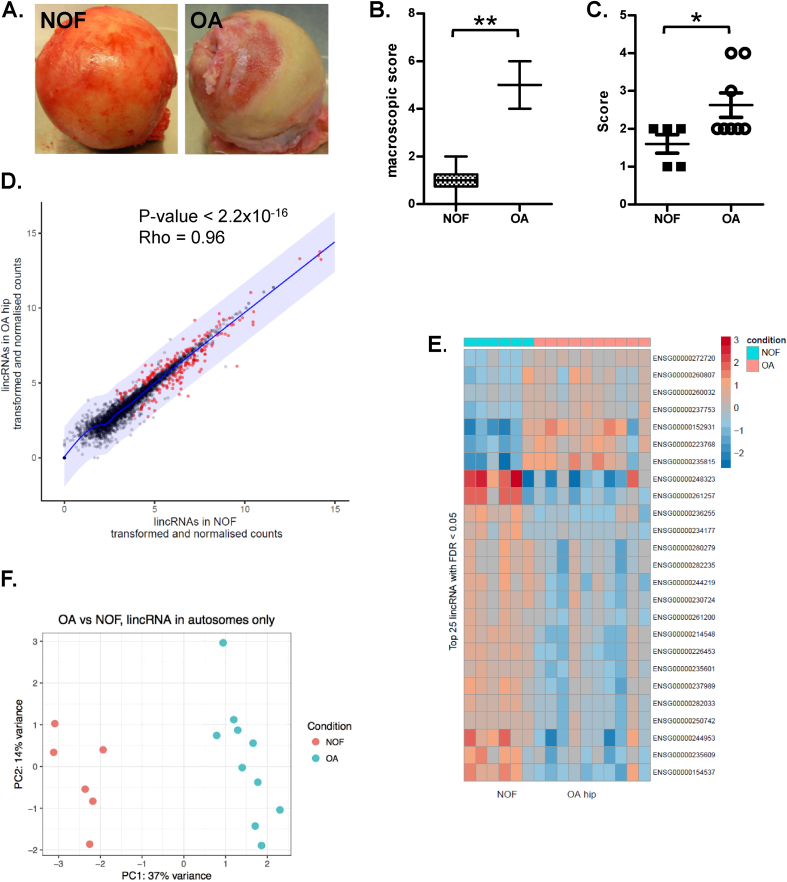
Fig. 1.
Differential lincRNA expression in NOF compared to OA hip cartilage. A. A NOF and OA femoral head used in this study. The OA femoral heads typically showed exposed bone and fibro-cartilage. B. Blinded cartilage macroscopic scores based on the Noyes classification20. Line mean, error bars are maximum and minimum, **represents P < 0.01. C. Hip osteoarthritis for NOF and OA patients were calculated from X-rays (as described in Methods) to give an overall grade (0–5) based on the Kellgren and Lawrence. Statistical significance was determined using a Mann Whitney U test where * P < 0.05. D. Scatter plot of lincRNA counts in OA vs NOF RNA-seq. Significant lincRNAs (DESeq2, FDR < 0.05) are coloured in red, the shading represents a log2 fold change of 2 from the regression line. A Spearman's rank test was used to assess the correlation between samples. E. Heatmap of top 25 differentially expressed lncRNAs in hip OA (peach) vs NOF (cyan). F. Principal component plot, hip OA vs NOF RNA-seq samples (autosomal lincRNAs only).
For knee, we used a pre-existing RNA-seq dataset from eight patients undergoing total knee arthroplasty for OA (age range 65–79 years, mean age 70.3; Supplementary Fig. 1(B)). The cartilage samples were from paired osteochondral specimens isolated from the ‘intact’ posterior lateral condyle (PLC) and the ‘damaged’ distal medial condyle (DMC) - sites OA23 (Supplementary Fig. 1(C)). To characterise the cartilage tissue, the PLC and DMC samples were graded histologically using a modified Mankin score24, which confirmed the DMC cartilage was significantly more damaged than that from the PLC (Supplementary Fig. 1(C) and (D)).
RNA extraction and sequencing
For the hip RNA-seq, total RNA was isolated directly from female articular cartilage samples (OA, n = 10; NOF, n = 6) as described25 and RNA integrity verified as previously described17. mRNA was purified and 78-base paired-end sequencing performed on an Illumina Genome Analyzer IIx (Illumina Inc., Saffron Walden, UK). Sequencing reads quality controls were as described26. Fastq files are available to download from the National Center for Biotechnology Information Expression Omnibus GSE111358. On average each sample contained >22 million mapped read counts (see below), which is sufficient, given the number of replicates to detect differentially expressed genes with an False Discovery Rate (FDR) <0.05 with a power close to 127. Knee RNA-seq data was from Dunn et al.23 ArrayExpress (E-MTAB-4304). As with the hip RNA, total knee RNA was extracted from liquid nitrogen frozen cartilage using TRIzol (LifeTechnologies) and homogenisation followed by a final purification using Qiagen RNeasy columns (Qiagen). Following sequencing on an Illumina HiSeq 2000 approximately 39 million reads per sample were generated23.
RNA-seq analysis
For both knee and hip datasets, transcript counts were estimated using Salmon (version 0.7.2) in quasi-mapping mode against human reference genome hg38 (release 87) with default settings. Salmon estimated counts were summarized to gene level using the tximport package in RStudio for use with DESeq2. The RUVg in silico approach was used to remove unwanted technical variation. RUVg variation factors were added into the design model of DESeq2 for differential expression testing. Differential expression analysis was performed paired and unpaired for OA knee and hip, respectively. All genes were included in DESeq2 analysis before filtering for genes designated as a “lincRNA” biotype by ENSEMBL. Heatmaps were generated with log2 transformed and normalised counts using the pheatmap() function in RStudio.
To generate scatterplots, the removeBatchEffect() function from limma was used with the DESeq2 normalised and log2 transformed counts, giving the RUVg unwanted variation factors as a co-variate. The ggplot2 package was used in Rstudio to build the scatterplots.
To generate genome browser tracks, reads were aligned to hg38 using the HISAT2 aligner. Alignment files were converted into bigwig format first by converting into bedgraph format using BEDTools genomecov and then the bedGraphToBigWig utility from UCSC.
Enhancer and promoter lincRNA derivation
Histone ChIP-seq read alignment tracks for H3K4me3, H3K4me1 and H3K27ac for Roadmap cell type E049 (bone marrow-derived chondrocytes) accessed from NCBI GEO database, accession GSE19465. Bed tracks were converted to hg38 using the UCSC liftover tool and converted to bam format using the bedtobam utility in bedtools. Sorted bam files were used as input into ngs.plot.r28 to plot read coverage around the transcription start sites (TSS) of lincRNAs (±2000 bp). LincRNAs with a mean Transcripts Per Million (TPM) > 1 in all hip and knee samples were included. H3K4me1 and H3K4me3 coverages were plotted as bam pairs. The gene order was extracted using R from H3K4me1/H3K4me3 heatmaps and used to plot H3K27ac read coverage.
Results
For hip, we performed RNA-seq on cartilage RNA extracted from OA and NOF patients undergoing total hip arthroplasty. The NOF samples had a significantly lower level of OA than the OA samples, both macroscopically17 and by X-ray analysis [Fig. 1(A)–(C)]. There was no significant difference in the ages of the consented patients in each grouping (Supplementary Fig. 1(A)). RNA-seq analysis of all hip cartilage samples identified 1692 Ensembl lincRNAs with an average TPM ≥1. Ensembl lincRNA classification is based on cDNA alignments overlapping chromatin methylation regions (H3K4me3 or H3K36me3) outside of protein-coding loci. LincRNAs (long intergenic noncoding RNAs) are better described as long intervening non-coding RNAs and analysis of the this portion of lncRNAs avoids the complication arising from overlap with coding genes7.
When stratifying these hip cartilage samples into OA and NOF, DESeq2 analysis identified 198 lincRNAs as differentially expressed (FDR ≤0.05) with 28 of these showing a fold change ≥2 (log2 fold ≥1) (Fig. 1(D) and (E); Supplementary Table 1). Of these 198 and 28, 89 and 7 were increased, respectively, in OA hip cartilage compared to NOF. Importantly, when using just the expression data from these 1692 lincRNAs principal component analysis (PCA) segregated the OA and NOF samples, suggesting lincRNA expression alone is enough to define disease severity [Fig. 1(F)]. The most upregulated lincRNAs in hip OA included PART1, LINC01139 and NORAD, while the most downregulated included LUCAT1, MEG3 and LINC01679. LincRNAs have been proposed to regulate local gene expression29 but we could find no correlation between OA severity-associated changes in lincRNA expression with those of the neighbouring protein-coding genes (data not shown).
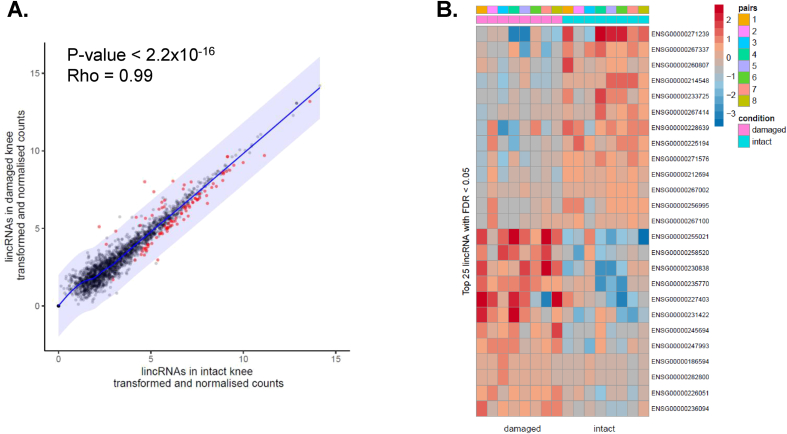
Next we performed a similar RNA-seq analysis pipeline for a previously published knee OA RNA-seq dataset23. Cartilage for the knee study was taken from eight patients and contained paired osteochondral samples isolated from both the intact PLC and the damaged DMC for each donor (Supplementary Fig. 1(C) and (D)). This analysis identified a total of 648 Ensembl lincRNAs with a TPM ≥1 in all damaged DMC and intact PLC OA knee cartilage samples. In the knee data, 93 lincRNAs were differentially expressed (FDR ≤0.05) between the damaged (DMC) and intact (PLC) cartilage groups with 13 of these showing a fold change ≥2 (log2 fold ≥1) (Fig. 2(A) and (B); Supplementary Table 2). Similarly, of these 93 and 13, 20 and 8 respectively were upregulated in the damaged knee cartilage, the remainder downregulated. The most upregulated lincRNAs in damaged knee cartilage were CRNDE, MIR22HG and LINC01614. The most downregulated lincRNAs included MEG3, ILF3-AS1 and LINC01089.
Fig. 2.
Differential lincRNA expression in paired OA-intact (PLC) compared to OA-damaged DMC) knee cartilage. A. Scatter plot of lincRNA counts in OA knee intact vs damaged RNA-seq. Significant lincRNAs (DESeq2, FDR < 0.05) are coloured in red, the shading represents a log2 fold change of 2 from the regression line. A Spearmans rank test was used to assess the correlation between samples. B. Heatmap of top 25 differentially expressed lncRNAs in damaged (pink) vs intact (cyan) knee OA RNA-seq samples. Patient pairings are indicated (1–8).
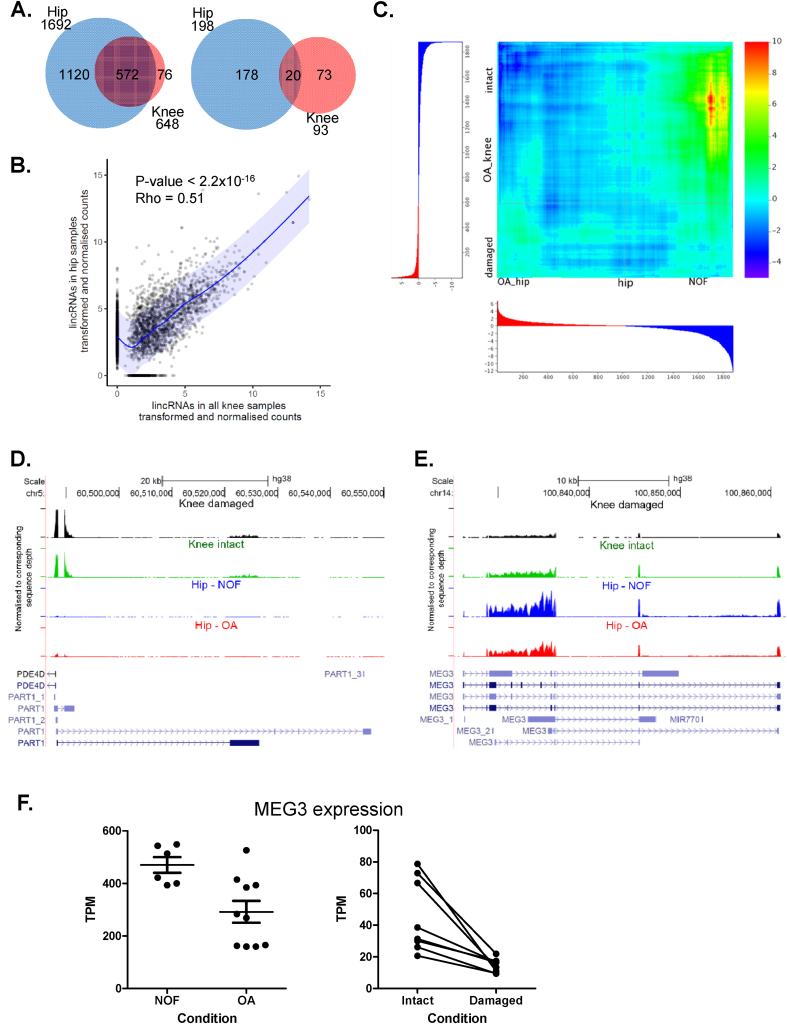
When comparing lincRNAs between hip and knee 572 were detected in common between the cartilages from both joints [Fig. 3(A)]. Furthermore, the average expression level of all lincRNAs between the two cartilages showed a good degree of correlation [Fig. 3(B)], especially when considering the known variability of lincRNA expression levels between tissues7. Of the differentially expressed lincRNAs from hip or knee only 20 lincRNAs were in common including established lincRNAs such as NEAT1 and MEG3 (Table I). However, when using the whole lincRNA dataset for each joint ranked by degree of differential expression using the Rank–rank Hypergeometric Overlap (RRHO) threshold-free algorithm30 a robust correlation was observed in lincRNAs with reduced expression in intact knee and NOF cartilage [Fig. 3(C)]. Of course, one important caveat is that neither dataset contains a true non-OA control.
Fig. 3.
Joint site comparisons in lincRNA expression. A. Venn diagrams showing the 572 lincRNAs in common between hip and knee and those differentially expressed between the tissues and disease status at both joint sites (20). B. Scatter plot of mean lincRNA counts (TPM) for all hip samples (NOF and OA hip) vs all knee (intact and damaged) samples. C. Rank–rank hypergeometric overlap representation of lincRNA lists ranked by the degree of differential expression observed in the two profiling experiments (positive values indicate an enrichment of overlapping lincRNAs). Key depicts –log10 P value. Axis graphs depict ranked gene regulation with red and blue highlighting upregulated and downregulated lincRNAs, respectively, in damaged knee or OA hip datasets. D. Total read coverages of each sample group on UCSC Browser hg38. PART1 expression in damaged & intact knee OA, and hip OA & NOF. E. Read coverage plot on UCSC Browser hg38. MEG3 expression in damaged & intact knee OA, and hip OA & NOF. F. MEG3 expression in individual NOF vs hip OA and intact vs damaged paired knee OA samples. TPM for each experimental dataset are presented.
Table I.
Differentially expressed LincRNAs in common between OA hip vs NOF and intact vs damage knee cartilage
| Ensembl ID | lincRNA name | Hip_Av_TPM | Knee_Av TPM | Hip log2 Fold Change | Knee log2 Fold Change | Hip adjusted pvalue | Knee adjusted pvalue |
|---|---|---|---|---|---|---|---|
| ENSG00000260807 | RP11-161M6.2 | 73.26 | 21.41 | 0.669 | 0.692 | 3.17E-04 | 3.05E-06 |
| ENSG00000230724 | LINC01001 | 40.41 | 4.61 | −0.735 | 0.659 | 4.91E-04 | 6.60E-03 |
| ENSG00000214548 | MEG3 | 381.13 | 30.04 | −0.864 | 1.350 | 1.25E-03 | 4.23E-11 |
| ENSG00000261200 | RP11-989E6.10 | 5.91 | 0.55 | −0.629 | 0.719 | 1.43E-03 | 1.25E-02 |
| ENSG00000235815 | RP1-125N5.2 | 8.95 | 9.94 | 1.220 | 0.684 | 1.56E-03 | 3.14E-03 |
| ENSG00000230606 | AC159540.1 | 32.76 | 14.93 | 0.504 | 0.646 | 1.72E-03 | 5.21E-03 |
| ENSG00000179523 | EIF3J-AS1 | 21.25 | 17.74 | −0.405 | 0.322 | 2.01E-03 | 6.31E-03 |
| ENSG00000212694 | LINC01089 | 20.08 | 14.49 | 0.421 | 0.792 | 2.21E-03 | 3.01E-06 |
| ENSG00000272374 | RP3-329A5.8 | 0.61 | 0.23 | −0.746 | 0.849 | 3.07E-03 | 1.29E-02 |
| ENSG00000203709 | C1orf132 | 8.65 | 0.65 | −1.058 | 0.462 | 3.28E-03 | 1.88E-02 |
| ENSG00000224652 | LINC00885 | 0.99 | 0.94 | 0.887 | 0.713 | 5.05E-03 | 8.52E-03 |
| ENSG00000230487 | PSMG3-AS1 | 4.77 | 2.51 | 0.625 | 0.504 | 7.08E-03 | 7.18E-03 |
| ENSG00000245532 | NEAT1 | 1149.80 | 250.31 | −0.865 | 0.407 | 9.60E-03 | 4.22E-02 |
| ENSG00000255135 | RP11-111M22.3 | 11.10 | 12.76 | −0.389 | 0.390 | 1.20E-02 | 4.41E-02 |
| ENSG00000232931 | LINC00342 | 6.02 | 2.93 | 0.284 | 0.566 | 2.93E-02 | 2.41E-02 |
| ENSG00000283445 | RP11-63G10.4 | 1.90 | 0.79 | 0.406 | 0.758 | 2.99E-02 | 2.84E-03 |
| ENSG00000261888 | AC144831.1 | 1.34 | 1.14 | −0.599 | −0.727 | 3.00E-02 | 2.63E-03 |
| ENSG00000255284 | AP006621.5 | 4.79 | 4.29 | 0.451 | 0.531 | 3.39E-02 | 4.87E-02 |
| ENSG00000265485 | RP11-449D8.1 | 2.07 | 4.83 | 0.638 | 0.419 | 4.93E-02 | 3.64E-02 |
| ENSG00000256321 | RP11-153K16.1 | 3.02 | 2.05 | −0.496 | 0.794 | 4.99E-02 | 3.83E-03 |
Further to identifying the lincRNAs altered during OA progression and cartilage damage we also sought to determine whether any lincRNAs exhibit joint site selective expression levels. Initially we examined the relative expression of all coding transcripts (after removing the mitochondrial encoded genes) between the differing cartilage joint sites. Correlation analysis of the expression level of all coding mRNAs gave a Spearman's Rank of 0.87 (P < 0.00001) (Supplementary Fig. 1). 18 of the 25 most abundant mRNAs were common between the two joint sites, with the remainder found in the top 100 expressed transcripts, the only exception being superoxide dismutase 3 (SOD3) which was expressed higher in hip. Patterning differences between the two joint sites were reflected by differential expression of HOX genes with more 3′ HOX cluster genes (HOXA4-6 and HOXB1-3) showing higher expression in hip, with more 5′ genes (HOXD8-10) enriched in knee – possibly demonstrating maintenance of the temporal sequential expression pattern observed during development31. Correlation analysis of the expression level of all the lincRNAs gave a Spearman's Rank of 0.51 (P < 0.00001) [Fig. 3(B)]. Of the 10 most abundant lincRNAs in each cartilage type, 8 were in common, with the remainder being found in the top 100 expressed lincRNAs. Some lincRNA showed joint site differential expression. For example, in knee cartilage the lincRNA PART1 was more abundant than in hip, yet was only significantly upregulated in OA hip cartilage vs NOF [Fig. 3(D)]. On the contrary MEG3 was more abundant in hip compared to knee cartilage [Fig. 3(E)] but differentially expressed in both [Fig. 3(E) and (F)].
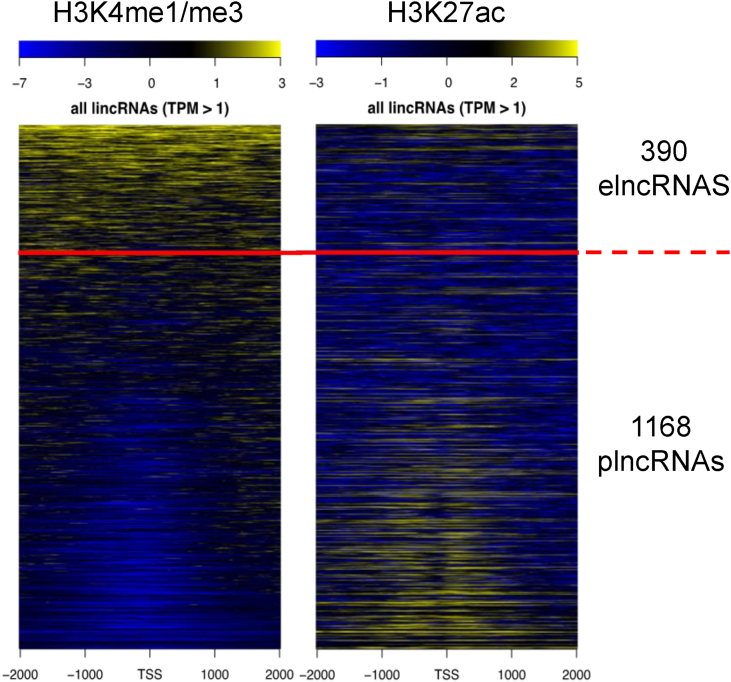
Numerous studies have identified a large number of lincRNAs, far outstripping the number of coding transcripts. However, the bottleneck in research has been in determining which lincRNAs are functional. Several studies have attempted to address how to identify functional lincRNAs. We used a modification of the method defined by Marques et al.32 which divides lincRNAs as either promoter–associated lncRNAs (plncRNA) or unidirectional enhancer-like regulatory element associated lncRNAs (elncRNAs) based on their overlying chromatin status. plncRNAs and elncRNAs are defined as those with an enriched H3K4me1 or H3K4me3 mark respectively at or around their TSS. We used histone ChIP-seq data from bone marrow derived chondrocytes generated as part of Roadmap33 to define the H3K4Me1 to H3K4Me3 ratio around (+/− 2 kbp) the TSS of the lincRNAs expressed in both hip and knee cartilage (Fig. 4). We also included H3K27Ac as a marker of biological activity. Approximately 75% of these cartilage lincRNAs were defined as plncRNAs; those which have been proposed have a function beyond regulation of the nearest gene32. plncRNAs had a slightly higher, but non-significant, expression levels compared to elncRNAs (plncRNA mean TPM of 6.82 and 6.35 for hip and knee respectively vs an elncRNA mean TPM of 4.37 for hip and knee respectively). plncRNAs included PART1 and MEG3.
Fig. 4.
Histone ChIP-seq analysis of chondrocyte lincRNA. Histone ChIP-seq read alignment tracks for H3K4me3, H3K4me1 and H3K27ac were analysed from Roadmap cell type E049 (bone marrow-derived chondrocytes). Data presented are from the 1558 lincRNAs with a mean TPM > 1 in all hip and knee samples. Read coverage density is plotted around the TSS of lincRNAs (±2000 bp). H3K4me1 and H3K4me3 coverages were plotted as bam pairs using ngs.plot, with the same lincRNA order used to subsequently plot the H3K27ac read coverage. To define enhancer-associated (elncRNA) and promoter-associated (plncRNA) lincRNAs a threshold of 0.5 of H3K4me1/H3K4me3 was defined (red line).
Discussion
Current non-coding RNA research in OA has generally focused on microRNAs. Here for the first time we have used RNA sequencing to define lincRNA expression in OA in both hip and knee cartilage. We identified 198 lincRNAs altered in hip OA progression and 93 in damaged knee cartilage of which 20 were in common. We additionally identified lincRNAs with joint site selectivity and further assessed their potential functionality by interrogating their chromatin status. Pearson et al., have also used RNA-Seq to profile lncRNAs in chondrocytes, although their focus was on those lncRNAs upregulated by IL-115. In contrast to our RNA extracted from cartilage, their study makes use of isolated chondrocytes. It is known that chondrocytes that are isolated dedifferentiate, adopting a more fibroblastic phenotype34, 35, 36. The authors also derived some of their non-OA cartilage from post-mortem donors, but only for real-time PCR analysis of a subset of IL-1-induced lncRNAs. Conversely the hip and knee OA samples used for RNA-seq in our study were obtained from live tissue donors and processed within 2 h post-surgery.
Interestingly, lincRNAs can be detected within exosomes present in extra-cellular fluid such as serum and therefore could represent biomarkers of disease status, as suggested for cancer37. Recently for OA, exosomal levels of lncRNA PCGEM1 in synovial fluid has been proposed as a biomarker for different stages of disease38.
Importantly, we have highlighted lincRNAs that are differentially expressed in damaged OA cartilage. Of interest, we observed that the lincRNA ROCR (ENSG00000228639), which we identified and characterised as a critical regulator of MSC chondrogenesis, is significantly down-regulated in damaged knee cartilage, but not differentially expressed in the hip dataset14, 39. Further, our results show that MEG3 is significantly downregulated in both OA hip and damaged knee OA cartilage, confirming knee cartilage data from Su et al.40 and data from a rodent OA-model41. However, this somewhat contrasts with the findings of Fu and colleagues where MEG3 transcript isoforms were both down or upregulated in knee OA cartilage compared to age-matched non-OA controls3. MEG3 has been principally identified as an influential tumour suppressor, whereby it negatively regulates cell proliferation and induces apoptosis42, 43, 44, 45. Downregulation of this imprinted lincRNA has been associated with multiple cancers43, 44, 45, 46, 47. In a rat OA model MEG3 has been proposed to regulate miR-16 expression, thus indirectly regulating Smad741. Indeed, others have shown that MEG3 may negatively regulate the Transforming growth factor (TGF)β signalling pathway48, with isolated fibroblasts overexpressing MEG3 unable to proliferate when incubated with TGFβ49. TGFβ plays a somewhat pleiotropic role in both normal and OA joints. In normal tissue it is active only transiently following joint loading while in OA joints high TGFβ activity is detected50. MEG3 may additionally have a role in the osteogenic differentiation of MSCs. Zhuang et al., examined MEG3 and BMP4 expression in isolated MSCs. In these cells the overexpression of MEG3 resulted in greater expression of osteogenic markers such as RUNX2, whilst reducing MEG3 expression had the opposite effect51.
We have also highlighted lincRNAs that may be selective for knee (e.g., PART1) and hip (e.g., MEG3), respectively. Interestingly, though many lincRNAs were shared between the two joints only 20 lincRNAs were differentially expressed in common between the disease states of both joint sites. When we previously used microarray analysis to explore gene expression changes in normal and OA cartilage of hip and knee, we similarly found a limited overlap (approximately 10%) of differentially expressed genes17. The two joints in question do have independent risk factors for OA such as gender, ethnicity and occupation52, 53, 54, the molecular and pathophysiological basis of which remain elusive. Further, although highly correlative, the two joint sites do have characteristic gene expression programmes, potentially regulated by differential Hox gene expression31. Interestingly, the Hox gene cluster is well known to include a number of well-studied non-coding transcripts55. Of particular interest, lncRNA-HIT appears a critical regulator of early limb development56.
The challenge remains to determine which of the lincRNAs we and others have identified are functional and which lack a specific biological function. Although lincRNA loci may contain active promoters and associated chromatin marks and the transcripts themselves can be capped, spliced and polyadenylated, none of these features offer an informative indicator of function. Marques et al., describe a multifaceted approach to discerning the biological relevance of lincRNAs32. Using their criteria, we classified the cartilage expressed lincRNAs into plncRNAs and elncRNAs, with the majority in our analysis being plncRNAs. The association of plncRNAs with their own promoter suggests a potentially more evolutionary conserved role than their elncRNA counterparts. Testing the function of lincRNAs still remains a challenge. Gene function is often initially sought using experimental animals, most commonly the genetically tractable mouse. However, determining the function of a lncRNA by its deletion in mice can also be fraught since removing the genetic loci can also disrupt important underlying DNA elements such as enhancers57. Moreover, using comparative genomics to understand the function of lincRNAs is not straightforward since the majority show little sequence or splicing pattern conservation. There are notable exceptions, but these tend to be those lncRNAs that are relatively highly expressed58. With these caveats in mind, much recent work has described interactions between short and lncRNAs59. In OA, several lncRNAs have been shown act as potential ‘sponges’ to regulate microRNA function60, 61.
In summary, we have outlined lincRNA expression patterns in both hip and knee OA cartilage. We have highlighted differential expression between hip OA and NOF and between intact and damaged knee OA (sites of low and high mechanical loading, respectively). We have additionally highlighted significantly changing lincRNAs that are common to both, namely the tumour suppressor MEG3, which is downregulated. Mondal et al. identified MEG3 as forming DNA-RNA triplex complexes with chromatin, targeting constituents of the TGFβ signalling pathway; indeed48, fully characterising the mechanisms of action of lincRNAs in general remains another key area for development.
Declaration of contributions
DAY and MJB were involved in concept and study design. BA, KC, YX, AP, AJS, MJB and DAY were involved in collection, assembly and analysis and interpretation of the data. YX and MJB processed patient material. KC and AJS also provided statistical expertise. DJD was involved in the provision of study materials/patient samples. JS and TEH provided the knee data, histology and patient demographic data. DAY, MJB and DJD obtained the funding. All authors were involved in drafting the manuscript and gave final approval of the version submitted.
Competing interest statement
All authors declare no competing interests.
Acknowledgements
This work was supported by the Medical Research Council and Arthritis Research UK as part of the MRC-Arthritis Research UK Centre for Integrated Research into Musculoskeletal Ageing (CIMA, grant references JXR 10641 and MR/P020941/1); Arthritis Research UK [grant number 19424]; the JGW Patterson Foundation; The Dunhill Medical Trust; and the NIHR Newcastle Biomedical Research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2018.12.015.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.National Joint Registry . Northgate Public Services (UK) Ltd; Hemel Hempstead: 2016. 13th Annual Report 2016. [Google Scholar]
- 2.Pap T., Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis – two unequal siblings. Nat Rev Rheumatol. 2015;11:606–615. doi: 10.1038/nrrheum.2015.95. [DOI] [PubMed] [Google Scholar]
- 3.Fu M., Huang G., Zhang Z., Liu J., Zhang Z., Huang Z. Expression profile of long noncoding RNAs in cartilage from knee osteoarthritis patients. Osteoarthritis Cartilage. 2015;23:423–432. doi: 10.1016/j.joca.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q., Zhang X., Dai L., Hu X., Zhu J., Li L. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Chondrocyte Biol. 2014;66:969–978. doi: 10.1002/art.38309. [DOI] [PubMed] [Google Scholar]
- 5.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercer T.R., Dinger M.E., Mattick J.S. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 7.Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guil S., Esteller M. Cis-acting noncoding RNAs: friends and foes. Nat Struct Mol Biol. 2012;19:1068–1075. doi: 10.1038/nsmb.2428. [DOI] [PubMed] [Google Scholar]
- 12.Khalil A., Guttman M., Huarte M., Garber M., Raj A., Rivea Morales D. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barter M.J., Gomez R., Hyatt S., Cheung K., Skelton A.J., Xu Y. The long non-coding RNA ROCR contributes to SOX9 expression and chondrogenic differentiation of human mesenchymal stem cells. Development. 2017;144:4510–4521. doi: 10.1242/dev.152504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson M.J., Philp A.M., Heward J.A., Roux B.T., Walsh D.A., Davis E.T. Long intergenic noncoding RNAs mediate the human chondrocyte inflammatory response and are differentially expressed in osteoarthritis cartilage. Arthritis Rheumatol. 2016;68:845–856. doi: 10.1002/art.39520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing D., Liang J.Q., Li Y., Lu J., Jia H.B., Xu L.Y. Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop Surg. 2014;6:288–293. doi: 10.1111/os.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Barter M.J., Swan D.C., Rankin K.S., Rowan A.D., Santibanez-Koref M. Identification of the pathogenic pathways in osteoarthritic hip cartilage: commonality and discord between hip and knee OA. Osteoarthritis Cartilage. 2012;20:1029–1038. doi: 10.1016/j.joca.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Rushton M.D., Reynard L.N., Barter M.J., Refaie R., Rankin K.S., Young D.A. Characterization of the cartilage DNA methylome in knee and hip osteoarthritis. Arthritis Rheumatol. 2014;66:2450–2460. doi: 10.1002/art.38713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.den Hollander W., Ramos Y.F., Bos S.D., Bomer N., van der Breggen R., Lakenberg N. Knee and hip articular cartilage have distinct epigenomic landscapes: implications for future cartilage regeneration approaches. Ann Rheum Dis. 2014;73:2208–2212. doi: 10.1136/annrheumdis-2014-205980. [DOI] [PubMed] [Google Scholar]
- 20.Kijowski R., Blankenbaker D., Stanton P., Fine J., De Smet A. Arthroscopic validation of radiographic grading scales of osteoarthritis of the tibiofemoral joint. Am J Roentgenol. 2006;187:794–799. doi: 10.2214/AJR.05.1123. [DOI] [PubMed] [Google Scholar]
- 21.Altman R.D., Gold G.E. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl. A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn S.L., Soul J., Anand S., Schwartz J.M., Boot-Handford R.P., Hardingham T.E. Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthritis Cartilage. 2016;24:1431–1440. doi: 10.1016/j.joca.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little C.B., Smith M.M., Cake M.A., Read R.A., Murphy M.J., Barry F.P. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthritis Cartilage. 2010;18(Suppl. 3):S80–S92. doi: 10.1016/j.joca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Kevorkian L., Young D.A., Darrah C., Donell S.T., Shepstone L., Porter S. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 26.Bui C., Barter M.J., Scott J.L., Xu Y., Galler M., Reynard L.N. cAMP response element-binding (CREB) recruitment following a specific CpG demethylation leads to the elevated expression of the matrix metalloproteinase 13 in human articular chondrocytes and osteoarthritis. FASEB J. 2012;26:3000–3011. doi: 10.1096/fj.12-206367. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Zhou J., White K.P. RNA-seq differential expression studies: more sequence or more replication? Bioinformatics. 2014;30:301–304. doi: 10.1093/bioinformatics/btt688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen L., Shao N., Liu X., Nestler E. ngs.plot: Quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics. 2014;15:284. doi: 10.1186/1471-2164-15-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisler S., Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plaisier S.B., Taschereau R., Wong J.A., Graeber T.G. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38:e169. doi: 10.1093/nar/gkq636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallo M. Reassessing the role of Hox genes during vertebrate development and evolution. Trends Genet. 2018;34:209–217. doi: 10.1016/j.tig.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Marques A.C., Hughes J., Graham B., Kowalczyk M.S., Higgs D.R., Ponting C.P. Chromatin signatures at transcriptional start sites separate two equally populated yet distinct classes of intergenic long noncoding RNAs. Genome Biol. 2013;14:R131. doi: 10.1186/gb-2013-14-11-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roadmap Epigenomics C., Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caron M.M., Emans P.J., Coolsen M.M., Voss L., Surtel D.A., Cremers A. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Duan L., Ma B., Liang Y., Chen J., Zhu W., Li M. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am J Transl Res. 2015;7:194–208. [PMC free article] [PubMed] [Google Scholar]
- 36.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullock M.D., Silva A.M., Kanlikilicer-Unaldi P., Filant J., Rashed M.H., Sood A.K. Exosomal non-coding RNAs: diagnostic, prognostic and therapeutic applications in cancer. Noncoding RNA. 2015;1:53–68. doi: 10.3390/ncrna1010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018 December;42(12):2865–2872. doi: 10.1007/s00264-018-4093-6. [DOI] [PubMed] [Google Scholar]
- 39.Barter M.J., Gomez R., Hyatt S., Cheung K., Skelton A., Xu Y. Long noncoding RNA ROCR contributes to SOX9 expression and chondrogenic differentiation of human mesenchymal stem cells. Development. 2017 Dec 15;144(24):4510–4521. doi: 10.1242/dev.152504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su W., Xie W., Shang Q., Su B. The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. Biomed Res Int. 2015;2015:356893. doi: 10.1155/2015/356893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J., Xu Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 2017;7:69. doi: 10.1186/s13578-017-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boon R.A., Hofmann P., Michalik K.M., Lozano-Vidal N., Berghauser D., Fischer A. Long noncoding RNA Meg3 controls endothelial cell aging and function: implications for regenerative angiogenesis. J Am Coll Cardiol. 2016;68:2589–2591. doi: 10.1016/j.jacc.2016.09.949. [DOI] [PubMed] [Google Scholar]
- 43.Chak W.P., Lung R.W., Tong J.H., Chan S.Y., Lun S.W., Tsao S.W. Downregulation of long non-coding RNA MEG3 in nasopharyngeal carcinoma. Mol Carcinog. 2017;56:1041–1054. doi: 10.1002/mc.22569. [DOI] [PubMed] [Google Scholar]
- 44.Peng W., Si S., Zhang Q., Li C., Zhao F., Wang F. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J Exp Clin Cancer Res. 2015;34:79. doi: 10.1186/s13046-015-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong G.F., Qin N., Sun L.W., Xu X.L. Long noncoding RNA MEG3 suppresses glioma cell proliferation, migration, and invasion by acting as competing endogenous RNA of MiR-19a. Oncol Res. 2017;25(9):1471–1478. doi: 10.3727/096504017X14886689179993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Lin Z., Gao Y., Yao T. Downregulation of long noncoding RNA MEG3 is associated with poor prognosis and promoter hypermethylation in cervical cancer. J Exp Clin Cancer Res. 2017;36:5. doi: 10.1186/s13046-016-0472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Wagner E.K., Guo X., May I., Cai Q., Zheng W. Long intergenic non-coding RNA expression signature in human breast cancer. Sci Rep. 2016;6:37821. doi: 10.1038/srep37821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mondal T., Subhash S., Vaid R., Enroth S., Uday S., Reinius B. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat Commun. 2015;6:7743. doi: 10.1038/ncomms8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Wang J., Wei L.J., Zhu D.M., Zhang J.S. Biological function and mechanism of lncRNA-MEG3 in Tenon's capsule fibroblasts proliferation: by MEG3-Nrf2 protein interaction. Biomed Pharmacother. 2017;87:548–554. doi: 10.1016/j.biopha.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 50.van der Kraan P.M. Differential role of transforming growth factor-beta in an osteoarthritic or a healthy joint. J Bone Metab. 2018;25:65–72. doi: 10.11005/jbm.2018.25.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang W., Ge X., Yang S., Huang M., Zhuang W., Chen P. Upregulation of lncRNA MEG3 promotes osteogenic differentiation of mesenchymal stem cells from multiple myeloma patients by targeting BMP4 transcription. Stem Cells. 2015;33:1985–1997. doi: 10.1002/stem.1989. [DOI] [PubMed] [Google Scholar]
- 52.Cooper C., Campbell L., Byng P., Croft P., Coggon D. Occupational activity and the risk of hip osteoarthritis. Ann Rheum Dis. 1996;55:680–682. doi: 10.1136/ard.55.9.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominick K.L., Baker T.A. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis. 2004;14:558–566. [PubMed] [Google Scholar]
- 54.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Kumar B., Krumlauf R. HOXs and lincRNAs: two sides of the same coin. Sci Adv. 2016;2:e1501402. doi: 10.1126/sciadv.1501402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson H.L., Quinn J.J., Yang Y.W., Thornburg C.K., Chang H.Y., Stadler H.S. LncRNA-hit functions as an epigenetic regulator of chondrogenesis through its recruitment of p100/CBP complexes. PLoS Genet. 2015;11:e1005680. doi: 10.1371/journal.pgen.1005680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bassett A.R., Akhtar A., Barlow D.P., Bird A.P., Brockdorff N., Duboule D. Considerations when investigating lncRNA function in vivo. Elife. 2014;3:e03058. doi: 10.7554/eLife.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulitsky I. Evolution to the rescue: using comparative genomics to understand long non-coding RNAs. Nat Rev Genet. 2016;17:601–614. doi: 10.1038/nrg.2016.85. [DOI] [PubMed] [Google Scholar]
- 59.Ulitsky I. Interactions between short and long noncoding RNAs. FEBS Lett. 2018 Sep;592(17):2874–2883. doi: 10.1002/1873-3468.13085. [DOI] [PubMed] [Google Scholar]
- 60.Li Y.F., Li S.H., Liu Y., Luo Y.T. Long noncoding RNA CIR promotes chondrocyte extracellular matrix degradation in osteoarthritis by acting as a sponge for Mir-27b. Cell Physiol Biochem. 2017;43:602–610. doi: 10.1159/000480532. [DOI] [PubMed] [Google Scholar]
- 61.Wang G., Bu X., Zhang Y., Zhao X., Kong Y., Ma L. LncRNA-UCA1 enhances MMP-13 expression by inhibiting miR-204-5p in human chondrocytes. Oncotarget. 2017;8:91281–91290. doi: 10.18632/oncotarget.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.