Abstract
Background
Controversy exists in reverse total shoulder arthroplasty in regard to variability in the center of rotation (COR), which modifies the superior-inferior position of the humerus to affect the acromiohumeral interval (AHI), and its effect on the deltoid lever arm (DLA), acromial index (AI), and critical shoulder angle (CSA). The purpose of this study was to investigate the variation in biomechanics and the association with patient-reported outcomes (PROs) and range of motion (ROM) measurements.
Methods
Radiographs, ROM, and 2-year PRO scores were retrospectively reviewed for 108 patients.
Results
There was large variability in preoperative and postoperative biomechanics. The COR was medialized 12.01 ± 4.8 mm. The CSA increased 2.64° ± 12.45°. The AHI increased 20.6 ± 9.80 mm. The DLA lengthened 21.21 ± 10.15 mm. The AI increased 0.009 ± 0.3. Postoperative AI positively correlated with American Shoulder and Elbow Surgeons score and Penn Shoulder Score (P = .03). Specifically, a postoperative AI of 0.62 corresponded to American Shoulder and Elbow Surgeons score (72.5 ± 18.3 vs. 62.3 ± 24.7; P = .02) and Penn Shoulder Score (71.2 ± 21.4 vs. 61.8 ± 25.6; P = .05), an average 10 points higher than AI of <0.6. Also, a smaller postoperative CSA (<25°) correlated with improved forward elevation (P = .02).
Conclusions
This is the first study that evaluates the variability of biomechanical factors and their impact on postoperative ROM and PROs. An increased AI and decreased CSA are associated with improved PROs in this study, and a smaller CSA is associated with better forward elevation. Change in the COR, AHI, or DLA, however, did not affect patient outcomes or ROM. Further study is warranted to determine the optimal position.
Keywords: Biomechanics, reverse shoulder arthroplasty, outcomes, range of motion, acromial index, critical shoulder angle
The increased use of reverse shoulder arthroplasty (RSA) for the treatment of a variety of conditions has led to a point equaling anatomic total shoulder arthroplasty in the United states.12 Whereas use has increased, there continues to be considerable controversy about the ideal design and positioning of components. Although early attempts at RSA were met with limited success2, the introduction of the Grammont-style prosthesis in the late 1980s began a process from which many of the modern prostheses have evolved. This design medialized the center of rotation (COR) and relatively lengthened the humerus, with a resultant theoretical increase in deltoid tension and more efficient deltoid lever arm (DLA).37 This modification did improve clinical outcomes,9, 17, 19 but new issues of scapular notching8, 33, 41 and limited external rotation requiring muscle transfers were encountered.4, 15, 17 In addition, as the humerus is lengthened, the force vector at the glenohumeral joint changes,7 creating concern about stability, particularly in the setting of subscapularis insufficiency in a medialized design.14
In response to these concerns, an alternative concept with a relatively lateralized COR compared with the Grammont-style prostheses has been advocated by some.21, 46 Gutiérrez et al25 have shown that one of the primary keys in increasing overall arc of motion is lateralizing the COR. Also, the lateralization retensions the remaining rotator cuff musculature, restoring a more normal moment arm for these muscles22, 44 as well as a more anatomic force vector for the deltoid as it wraps around the humeral component. With an improved ability of the remaining infraspinatus and teres minor to act as well as the posterior deltoid, this is the theoretical basis for the improved range of motion (ROM), particularly in external rotation, seen in lateralized designs.6, 11, 21, 36, 44
Several authors have studied the biomechanics of component positioning with focus on stability,16 scapular notching,10, 24 ROM,24, 27 and joint reactive forces.18, 26, 27, 43 Combining many of the individually assessed factors in these studies, Henninger et al28 directly compared biomechanical characteristics of a representative lateralized RSA vs. medialized RSA. Overall, these authors found no difference in the ROM allowed between designs and comparable generalized results between the designs. However, the lateralized design did allow slightly more adduction and resulted in a more lateralized humeral position, and less force was needed to initiate abduction compared with the medialized design. In contrast, medialization of the COR has been shown to decrease the deltoid moment arm by a factor of 5 with the arm at 90° of abduction. These seemingly competing interests raise the question of which combination of these factors produces the best clinical results with the fewest complications.
Whereas biomechanical studies suggesting the merits of different component positions are plentiful, few studies assess the actual radiographic positioning in vivo with corresponding clinical outcomes. Using Grammont-style prostheses, Jobin et al29 found deltoid lengthening to correlate with superior forward elevation, but degree of medialization did not correlate with ROM or outcome scores. One other small study38 showed improved outcomes with increased “acromioepiphyseal distance” compared with the other side, whereas another similar study found no difference in clinical outcomes in regard to humeral lengthening.20
There are clear differences between Grammont-style prostheses and more lateralized designs both in specific aspects of clinical outcomes and in biomechanical rationale. What is less clear is how often the proposed advantages of either are achieved in vivo as evidenced by radiographic measures and whether these measures have a direct effect on clinical outcomes. Little exists in the current literature evaluating these radiographic measures, and what does exist is inconclusive. Therefore, the primary objective of this study was to provide a comprehensive evaluation of the relationship of objective and subjective clinical outcomes to these measurements, including implant COR, acromiohumeral interval (AHI), DLA, acromial index (AI), and critical shoulder angle (CSA). The secondary objective was to assess the variability in implant positioning in vivo by these radiographic measurements.
Methods
A retrospective review was performed as part of an Institutional Review Board–approved ongoing outcomes database at a single institution. A total of 134 RSAs performed by 4 fellowship-trained shoulder surgeons with >2-year outcomes were identified. Revisions, proximal humerus fractures, and irreparable rotator cuff tears were excluded, leaving 108 primary RSAs for rotator cuff arthropathy to be included in the study group for further evaluation. Presence of preoperative and postoperative radiographs for analysis was confirmed for all patients. The majority of implant designs were lateralized on the glenoid but in select cases may have included combined glenoid and humeral lateralization.
Medical records referable to age, sex, confirmation of preoperative diagnosis, and preoperative ROM were reviewed. All procedures were performed through a standard deltopectoral approach. Patients were progressed postoperatively under a standardized and supervised physical therapy program with gradual progression to active and passive ROM during the first 6 weeks. Strengthening was then initiated, and all patients were returned to full activity without restriction at 3 months. Postoperative records were reviewed for ROM measurements. At >2 years, patient-reported outcomes (PROs) were obtained, including American Shoulder and Elbow Surgeons, visual analog scale, Penn, and Single Assessment Numeric Evaluation scores.
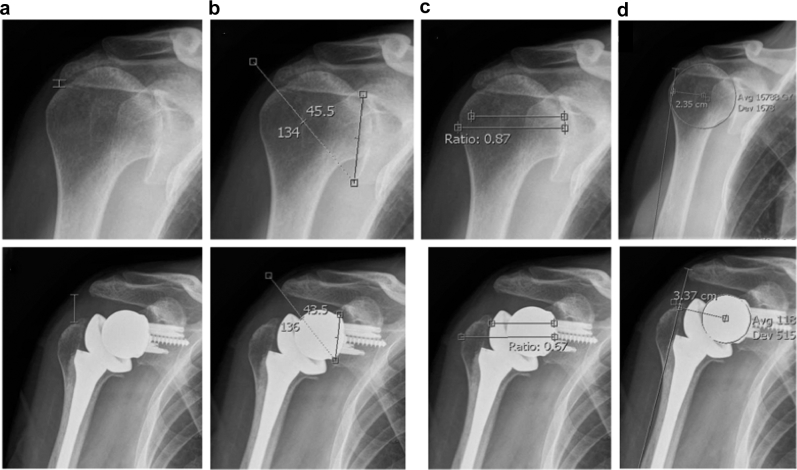
Preoperative and postoperative radiographs were reviewed independently by 2 orthopedic surgeons blinded to patient outcomes. Radiographic assessment was standardized to the anteroposterior plain radiographs taken at the last preoperative visit and the first visit in the office postoperatively. The initial 50 radiographs were reviewed by both surgeons to ensure standardization of measurements, with inter-rater reliability measured at >0.8 for all measurements. The remainder of the radiographs were then measured by 1 of the 2 reviewers. Radiographic measurements included CSA,34 AHI, COR, AI, and DLA (Fig. 1). Radiographs were accessed and analyzed by IMPAX (Agfa HealthCare, Mortsel, Belgium) viewing and measurement software. CSA was measured by a line from the superior to the inferior pole of the glenoid and a line from the inferior pole to the lateral edge of the acromion. Postoperatively, this was measured by the superior to inferior line of the base of the glenosphere with the corresponding line to the edge of the acromion. The AHI was measured and standardized from preoperative to postoperative measurements by calculating the distance from the undersurface of the acromion to the greater tuberosity perpendicular to the long axis of the acromial body. COR was measured preoperatively starting with a perfect circle of the humeral head defining the center and measurement of the perpendicular from the center of the glenoid. Postoperatively, a similar method was used, with the COR defined as the center glenosphere with measurement from the middle of the native glenoid. As a measure of the lateralization of the humerus, the AI was measured both preoperatively and postoperatively as a ratio of the distance from the glenoid to the lateral edge of the acromion over the distance from the glenoid to the lateral edge of the humeral head. To measure the DLA, a line was drawn from the lateral edge of the acromion to the deltoid tuberosity on the humerus. From this line, a perpendicular was drawn and measured to the respective preoperative and postoperative CORs.
Figure 1.
Representative preoperative and postoperative measurements. (a) Acromiohumeral interval. (b) Critical shoulder angle. (c) Acromial index. (d) Deltoid lever arm measured to center of rotation.
Statistical analysis was performed to assess the effect of these radiographic measurements on ROM and PROs. The variation in ROM and outcome scores was examined using receiver operating characteristic (ROC) curves to establish cut scores for each radiologic measurement that influenced the recorded outcomes (Fig. 2). Based on groups established by the ROC, a one-way analysis of variance was performed to compare ROM and PROs on the basis of the cut score for each radiologic parameter. All statistical analyses were conducted with SPSS Statistics 22.0 software (IBM Corp., Armonk, NY, USA). Statistical significance was set a priori at P < .05 for all statistical comparisons and additionally for the ROC curve analysis of a minimum area under the curve (AUC) >0.60.
Figure 2.
Receiver operating characteristic (ROC) curve for forward elevation and critical shoulder angle (CSA).
Results
There were 108 patients who fully met criteria for analysis at an average of 68 ± 8 months of follow-up and 69 ± 8 years of age. There were 38 men and 70 women with an overall average body mass index of 31 kg/m2; the Charlson Comorbidity Index was low at an average of 0.9. For the overall group, ROM improved from 77° of forward elevation and 20° of external rotation preoperatively to 125° of forward elevation and 31° of external rotation postoperatively; the visual analog scale score improved from 6.3 to 2.9. Outcome scores for the overall group at >5½ years from surgery are included in Table I.
Table I.
Overall outcomes after reverse shoulder arthroplasty
| Preoperative ROM | |
| Forward flexion | 77° |
| External rotation | 20° |
| Postoperative ROM | |
| Forward flexion | 125° |
| External rotation | 31° |
| Outcome scores | |
| Penn Shoulder Score | 68 |
| ASES | 69 |
| VAS | 2.9 |
| SANE | 72 |
ROM, range of motion; ASES, American Shoulder and Elbow Surgeons; VAS, visual analog scale; SANE, Single Assessment Numeric Evaluation.
There was large variability in the preoperative and postoperative biomechanical determinants measured in each patient. The COR of the shoulder was medialized an average of 12.01 ± 4.8 mm (variable range, 28 mm). The CSA was increased an average of 2.64° ± 12.45° (variable range, 59°). The AHI was increased an average of 20.6 ± 9.80 mm (variable range, 6.05 cm). The DLA was lengthened by an average of 21.21 ± 10.15 mm (variable range, 5.0 cm). Finally, the AI was increased 0.009 ± 0.3 (variable range, 1.45%) (Table II).
Table II.
Radiographic variables: preoperative, postoperative, and change
| Preoperative | Postoperative | Change | |
|---|---|---|---|
| Critical shoulder angle (CSA) | 23.3° ± 6.6° | 25.9° ± 6.7° | 2.64° ± 12.5° |
| Acromial index (AI) | 0.72 ± 0.13 | 0.62 ± 0.14 | 0.009 ± 0.30 |
| Deltoid lever arm (DLA), mm | 17.5 ± 6.3 | 37.5 ± 8.6 | 21.2 ± 10.2 |
| Acromiohumeral interval (AHI), mm | 11.6 ± 6.5 | 32.2 ± 9.2 | 20.6 ± 9.8 |
| Center of rotation (COR), mm | 24.0 ± 3.5 | 11.6 ± 3.3 | 12.0 ± 4.8 |
ROCs demonstrated significant cut scores for CSA of 25° (AUC = 0.65; P = .03) and AI of 0.62 (AUC = 0.62; P = .05). Postoperative AI positively correlated with American Shoulder and Elbow Surgeons score and Penn Shoulder Score (P = .03). Specifically, a postoperative AI of >0.62 corresponded to American Shoulder and Elbow Surgeons score (72.5 ± 18.3 vs. 62.3 ± 24.7; P = .02) and Penn Shoulder Score (71. 2 ± 21.4 vs. 61.8 ± 25.6; P = .05) that were on average 10 points higher than for RSA patients with an AI of <0.6. In addition, a smaller postoperative CSA correlated with improved forward elevation (P = .02). Those patients with a postoperative CSA of <25° had forward elevation of 131°, which was significantly greater than that of patients with a CSA ≥25° (forward elevation of 112°).
Discussion
Considerable debate persists in the ideal placement of RSA components to optimally restore function while maintaining longevity. In this study, we aimed to evaluate the impact of a number of radiographic biomechanical markers on objective and subjective patient outcomes along with variability of implant positioning. Of the studied radiographic markers, only the CSA and AI showed association with outcomes, whereas considerable variation exists in the final position of the implant in vivo. One possible explanation of this is that among experienced surgeons, the implant is being used in a patient-specific way to restore soft tissue tension in the hope of restoring function, and a great deal of intraoperative modularity exists to achieve this. There still remains considerable confusion in how to best achieve this goal.
Therefore, the results of this study should be examined in the context of the specifics of the measurements recorded and how they relate to our current understanding of the literature. A medialized COR after RSA is one of the primary tenets of RSA. Although clinical results of medialized implant4, 5, 9, 15, 17 and lateralized implant11, 21, 36 designs are widely reported in the literature, only 2 studies29, 39 currently reported on outcomes with radiographically defined COR. Each of these studies was performed with a Grammont-style prosthesis, and neither found the COR to correlate with functional outcomes, similar to our findings with predominantly lateralized glenoid implants. Both studies, however, reported the COR as the perpendicular from the line of deltoid pull, whereas our measurements were taken from the face of the glenoid with no influence from the position of the arm.
Whereas COR did not correlate with outcomes, the overall lateralization as measured by the AI showed improved PROs with an increased AI. The components of the AI are the distance from the glenoid to acromion edge (numerator) over the distance to the lateral edge of the humerus (denominator). With a relatively fixed distance to the edge of the acromion, the primary variable of change is the lateralization of the humerus, and thus a larger AI would be representative of a relatively more medial humerus. No previous studies reported on the effect of AI after RSA, but previous studies have suggested that higher AI may be a risk factor for rotator cuff tear progression31 as well as for increased disability after rotator cuff repair.1 These findings in the context of the results of this study suggest that although relative lateralization of RSA components has a proposed advantage of retensioning the remaining rotator cuff muscles, it may be possible to overlateralize to a deleterious degree. This suggests that although a current trend toward lateralization may be advantageous, we must further define at which point we may achieve the desired middle ground of lateralization while maintaining the biomechanical advantage of the relatively medialized COR.
In addition to the medial-lateral dimension, deltoid lengthening has been thought to be a key component of RSA design, although clinical results on this variable have been mixed. Of studies using a Grammont-style implant, 2 studies29, 32 have shown deltoid lengthening to be associated with an increase in forward elevation without associated improved overall outcomes, whereas another reported no correlation of deltoid lengthening with ROM.39 Deltoid lengthening was measured by the AHI in this study, and our results agree with a previous report in a lateralized design,40 indicating no correlation of deltoid lengthening with ROM.
DLA was intended to account for the overall combined medialization and inferiorization of the reconstructed glenohumeral joint relative to the deltoid. Previous studies have used a measurement similar to the COR,29, 39 but the true COR as is understood by shoulder surgeons in discussing RSA is most commonly referenced in the medial-lateral dimension only with superior-inferior dimension assessed separately. As such, the DLA provides a combined analysis of these 2 dimensions as the perpendicular to the anatomic line of the deltoid. With the combination of medialization and inferiorization being one of the theoretical and foundational elements of RSA, the results of this study surprisingly showed no correlation of DLA with ROM or any outcome measure.
CSA has not yet been reported in relation to RSA, but it has been a point of interest in recent literature in rotator cuff tears,3, 34, 42 as has its role in glenohumeral joint loading.35, 45 Although much remains to be learned about the significance of this measurement, these studies suggest that a larger CSA results in a more superiorly directed force and therefore more stress on the superior rotator cuff and superior glenoid. This study showed improved forward elevation with a lower CSA. In considering the previous literature on the CSA, one theory to explain these findings may be that the CSA is a marker for inclination of the glenosphere. A more inferiorly inclined glenosphere results in a lower CSA against a fixed lateral edge of the acromion with a more compressive force of the deltoid rather than a shear force of a less inclined glenosphere. Previous biomechanical studies have suggested increased ROM with inferior inclination,23, 24, 37 but clinical studies have generally yet to show correlation of ROM to glenosphere inclination.13, 30 However, neither of these clinical studies used in vivo postoperative radiographs to assess inclination, instead relying on operative technique assumptions. This study suggests that there is considerable variability in the actual implantation of components, and therefore relying on operative technique rather than on radiographic measurement may be insufficient to reliably draw conclusions. Therefore, the findings of increased forward elevation with a lower CSA in this study may serve as a stimulus for further study of the role of inferior inclination in a lateralized design.
A primary strength of this study is the comprehensive and carefully considered approach to preoperative and postoperative measurements affecting the biomechanics and outcomes after RSA. Little exists in the literature about some of these measurements, and what little does exist is often reported in a Grammont-style design. We are the first to report the influence of AI and CSA on outcomes in RSA and to provide a framework from which further study may be generated on these parameters. A limitation is the reliance on the quality of existing radiographs in a retrospective fashion. Other limitations include the single-center design and, given the number of parameters measured, lack of robust sample size in addition to variability between 4 different surgeons. In addition, although most cases were performed with a lateralized system on the glenoid, select cases may have been impacted on both the humeral and glenoid. The small changes in these radiographic measurements also provide possibility of the differences being within measurement error.
Conclusions
There was a surprising amount of variability in radiographic measurements after implantation. An increased AI and decreased CSA are associated with improved outcomes scores within the range implanted in this study, and a smaller CSA is associated with better forward elevation. Change in the COR, AHI, or DLA, often indicated as critical measures in RSA, did not affect patient outcomes or ROM, however. Further study is warranted to determine the optimal position of components in RSA.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
This work was completed at Steadman Hawkins Clinic of the Carolinas, Greenville Health System, Greenville, South Carolina.
Institutional Review Board approval for this project is from Greenville Health System, #Pro00040469.
References
- 1.Ames J.B., Horan M.P., Van der Meijden O.A., Leake M.J., Millett P.J. Association between acromial index and outcomes following arthroscopic repair of full-thickness rotator cuff tears. J Bone Joint Surg Am. 2012;94:1862–1869. doi: 10.2106/JBJS.K.01500. [DOI] [PubMed] [Google Scholar]
- 2.Bayley J., Kessel L. Springer-Verlag; New York: 1982. The Kessel total shoulder replacement. [Google Scholar]
- 3.Blonna D., Giani A., Bellato E., Mattei L., Caló M., Rossi R. Predominance of the critical shoulder angle in the pathogenesis of degenerative diseases of the shoulder. J Shoulder Elbow Surg. 2016;25:1328–1336. doi: 10.1016/j.jse.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P., Chuinard C., Roussanne Y., Bicknell R.T., Rochet N., Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466:584–593. doi: 10.1007/s11999-008-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boileau P., Gonzalez J.F., Chuinard C., Bicknell R., Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18:600–606. doi: 10.1016/j.jse.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P., Moineau G., Roussanne Y., O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469:2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boileau P., Watkinson D.J., Hatzidakis A.M., Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl S):147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P., Watkinson D., Hatzidakis A.M., Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Boulahia A., Edwards T.B., Walch G., Baratta R.V. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129–133. doi: 10.3928/0147-7447-20020201-16. [DOI] [PubMed] [Google Scholar]
- 10.Chou J., Malak S.F., Anderson I.A., Astley T., Poon P.C. Biomechanical evaluation of different designs of glenospheres in the SMR reverse total shoulder prosthesis: range of motion and risk of scapular notching. J Shoulder Elbow Surg. 2009;18:354–359. doi: 10.1016/j.jse.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Cuff D., Pupello D., Virani N., Levy J., Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 12.Day J.S., Paxton E.S., Lau E., Gordon V.A., Abboud J.A., Williams G.R. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24:766–772. doi: 10.1016/j.jse.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Edwards T.B., Trappey G.J., Riley C., O'Connor D.P., Elkousy H.A., Gartsman G.M. Inferior tilt of the glenoid component does not decrease scapular notching in reverse shoulder arthroplasty: results of a prospective randomized study. J Shoulder Elbow Surg. 2012;21:641–646. doi: 10.1016/j.jse.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 14.Edwards T.B., Williams M.D., Labriola J.E., Elkousy H.A., Gartsman G.M., O'Connor D.P. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Favre P., Loeb M.D., Helmy N., Gerber C. Latissimus dorsi transfer to restore external rotation with reverse shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2008;17:650–658. doi: 10.1016/j.jse.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Favre P., Sussmann P.S., Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:550–556. doi: 10.1016/j.jse.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Gerber C., Pennington S.D., Lingenfelter E.J., Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89:940–947. doi: 10.2106/JBJS.F.00955. [DOI] [PubMed] [Google Scholar]
- 18.Giles J.W., Langohr G.D., Johnson J.A., Athwal G.S. Implant design variations in reverse total shoulder arthroplasty influence the required deltoid force and resultant joint load. Clin Orthop Relat Res. 2015;473:3615–3626. doi: 10.1007/s11999-015-4526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grammont P.M., Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 20.Greiner S.H., Back D.A., Herrmann S., Perka C., Asbach P. Degenerative changes of the deltoid muscle have impact on clinical outcome after reversed total shoulder arthroplasty. Arch Orthop Trauma Surg. 2010;130:177–183. doi: 10.1007/s00402-009-1001-y. [DOI] [PubMed] [Google Scholar]
- 21.Greiner S., Schmidt C., Herrmann S., Pauly S., Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24:1397–1404. doi: 10.1016/j.jse.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 22.Greiner S., Schmidt C., Konig C., Perka C., Herrmann S. Lateralized reverse shoulder arthroplasty maintains rotational function of the remaining rotator cuff. Clin Orthop Relat Res. 2013;471:940–946. doi: 10.1007/s11999-012-2692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutiérrez S., Comiskey C.A., Luo Z.P., Pupello D.R., Frankle M.A. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90:2606–2615. doi: 10.2106/JBJS.H.00012. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez S., Levy J.C., Frankle M.A., Cuff D., Keller T.S., Pupello D.R. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17:608–615. doi: 10.1016/j.jse.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez S., Levy J.C., Lee W.E., Keller T.S., Maitland M.E. Center of rotation affects abduction range of motion of reverse shoulder arthroplasty. Clin Orthop Relat Res. 2007;458:78–82. doi: 10.1097/BLO.0b013e31803d0f57. [DOI] [PubMed] [Google Scholar]
- 26.Gutiérrez S., Walker M., Willis M., Pupello D.R., Frankle M.A. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:732–739. doi: 10.1016/j.jse.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Henninger H.B., Barg A., Anderson A.E., Bachus K.N., Burks R.T., Tashjian R.Z. Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21:1128–1135. doi: 10.1016/j.jse.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Henninger H.B., King F.K., Tashjian R.Z., Burks R.T. Biomechanical comparison of reverse total shoulder arthroplasty systems in soft tissue–constrained shoulders. J Shoulder Elbow Surg. 2014;23:e108–e117. doi: 10.1016/j.jse.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Jobin C.M., Brown G.D., Bahu M.J., Gardner T.R., Bigliani L.U., Levine W.N. Reverse total shoulder arthroplasty for cuff tear arthropathy: the clinical effect of deltoid lengthening and center of rotation medialization. J Shoulder Elbow Surg. 2012;21:1269–1277. doi: 10.1016/j.jse.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 30.Kempton L.B., Balasubramaniam M., Ankerson E., Wiater J.M. A radiographic analysis of the effects of prosthesis design on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:571–576. doi: 10.1016/j.jse.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.R., Ryu K.J., Hong I.T., Kim B.K., Kim J.H. Can a high acromion index predict rotator cuff tears? Int Orthop. 2012;36:1019–1024. doi: 10.1007/s00264-012-1499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lädermann A., Walch G., Lubbeke A., Drake G.N., Melis B., Bacle G. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:336–341. doi: 10.1016/j.jse.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Lévigne C., Boileau P., Favard L., Garaud P., Molé D., Sirveaux F. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Moor B.K., Bouaicha S., Rothenfluh D.A., Sukthankar A., Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint? A radiological study of the critical shoulder angle. Bone Joint J. 2013;95-B:935–941. doi: 10.1302/0301-620X.95B7.31028. [DOI] [PubMed] [Google Scholar]
- 35.Moor B.K., Kuster R., Osterhoff G., Baumgartner D., Werner C.M., Zumstein M.A. Inclination-dependent changes of the critical shoulder angle significantly influence superior glenohumeral joint stability. Clin Biomech (Bristol, Avon) 2016;32:268–273. doi: 10.1016/j.clinbiomech.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Mulieri P., Dunning P., Klein S., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 37.Nyffeler R.W., Werner C.M., Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14:524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Renaud P., Wahab H., Bontoux L., Dauty M., Richard I., Brégeon C. Total inverted shoulder prosthesis and rotator cuff insufficiency: evaluation and determination of anatomical parameters predictive of good functional outcome in 21 shouldersAnn Readapt Med Phys. 2001;44:273–280. doi: 10.1016/s0168-6054(01)00102-7. [DOI] [PubMed] [Google Scholar]
- 39.Sabesan V.J., Lombardo D., Josserand D., Buzas D., Jelsema T., Petersen-Fitts G.R. The effect of deltoid lengthening on functional outcome for reverse shoulder arthroplasty. Musculoskelet Surg. 2016;100:127–132. doi: 10.1007/s12306-016-0400-9. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz D.G., Cottrell B.J., Teusink M.J., Clark R.E., Downes K.L., Tannenbaum R.S. Factors that predict postoperative motion in patients treated with reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1289–1295. doi: 10.1016/j.jse.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 41.Simovitch R.W., Zumstein M.A., Lohri E., Helmy N., Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 42.Spiegl U.J., Horan M.P., Smith S.W., Ho C.P., Millett P.J. The critical shoulder angle is associated with rotator cuff tears and shoulder osteoarthritis and is better assessed with radiographs over MRI. Knee Surg Sports Traumatol Arthrosc. 2016;24:2244–2251. doi: 10.1007/s00167-015-3587-7. [DOI] [PubMed] [Google Scholar]
- 43.Terrier A., Reist A., Merlini F., Farron A. Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. J Bone Joint Surg Br. 2008;90:751–756. doi: 10.1302/0301-620X.90B6.19708. [DOI] [PubMed] [Google Scholar]
- 44.Valenti P., Sauzieres P., Katz D., Kalouche I., Kilinc A.S. Do less medialized reverse shoulder prostheses increase motion and reduce notching? Clin Orthop Relat Res. 2011;469:2550–2557. doi: 10.1007/s11999-011-1844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viehöfer A.F., Gerber C., Favre P., Bachmann E., Snedeker J.G. A larger critical shoulder angle requires more rotator cuff activity to preserve joint stability. J Orthop Res. 2016;34:961–968. doi: 10.1002/jor.23104. [DOI] [PubMed] [Google Scholar]
- 46.Walker M., Brooks J., Willis M., Frankle M. How reverse shoulder arthroplasty works. Clin Orthop Relat Res. 2011;469:2440–2451. doi: 10.1007/s11999-011-1892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]