Abstract
Background
Antibody-mediated targeting of regulatory T cell receptors such as CTLA-4 enhances antitumor immune responses against several cancer entities including malignant melanoma. Yet, therapeutic success in patients remains variable underscoring the need for novel combinatorial approaches.
Methods
Here we established a vaccination strategy that combines engagement of the nucleic acid-sensing pattern recognition receptor RIG-I, antigen and CTLA-4 blockade. We used in vitro transcribed 5′-triphosphorylated RNA (3pRNA) to therapeutically target the RIG-I pathway. We performed in vitro functional analysis in bone-marrow derived dendritic cells and investigated RIG-I-enhanced vaccines in different murine melanoma models.
Findings
We found that protein vaccination together with RIG-I ligation via 3pRNA strongly synergizes with CTLA-4 blockade to induce expansion and activation of antigen-specific CD8+ T cells that translates into potent antitumor immunity. RIG-I-induced cross-priming of cytotoxic T cells as well as antitumor immunity were dependent on the host adapter protein MAVS and type I interferon (IFN-I) signaling and were mediated by dendritic cells.
Interpretation
Overall, our data demonstrate the potency of a novel combinatorial vaccination strategy combining RIG-I-driven immunization with CTLA-4 blockade to prevent and treat experimental melanoma.
Fund
German Research Foundation (SFB 1335, SFB 1371), EMBO, Else Kröner-Fresenius-Foundation, German Cancer Aid, European Hematology Association, DKMS Foundation for Giving Life, Dres. Carl Maximilian and Carl Manfred Bayer-Foundation.
Keywords: Immuno-oncology, Innate immunity, RIG-I, Immune checkpoint inhibitors, Anti-cancer vaccine, Dendritic cells
Research in context.
Evidence before this study
Immune checkpoint blockade is arguably the most promising approach for activating endogenous antitumor immunity. However, many patients do not respond to monotherapy. Combining immune checkpoint inhibitors with additional therapies such as cancer vaccines will be needed to overcome resistance and broaden the clinical utility of immunotherapy. Targeting pro-inflammatory innate nuclear acid receptor systems in addition to inhibition of the immune checkpoints PD-1 and CTLA-4 has shown promising results in preclinical tumor models. Therapeutic targeting of the cytosolic RNA receptor RIG-I has been shown to exert anti-tumor activity and to mediate cross-priming of cytotoxic T cells in a murine model of virus infection. However, the contribution of RIG-I signaling on the generation of T cell-based antitumor responses and its possible synergism with immune checkpoint blockade have not been investigated.
Added value of this study
Based on the selective activation of the nucleic acid-sensing pattern recognition receptor RIG-I, we established a combinatorial approach consisting of 5′-triphosphate-RNA (3pRNA) and anti-CTLA-4-based cancer vaccination, which could be rapidly translated into the clinic. Using two murine melanoma models, we provide preclinical evidence that both prophylactic and therapeutic protein vaccination in combination with selective activation of RIG-I by 3pRNA synergizes with anti-CTLA-4 checkpoint blockade to induce robust expansion of antigen-specific, cytotoxic T cells and associated antitumor immunity. Mechanistically, we identify MAVS signaling and type I interferons but not ASC-dependent inflammasome formation as essential prerequisites for cross-presentation of tumor-associated antigens, the induction of antigen-specific CTLs and anti-tumor immunity by this combinatorial approach.
Implications of all the available evidence
We established a novel RIG-I-based combinatorial strategy for generation of antigen-specific, cytotoxic T cells and antitumor immunity and characterized the respective signaling pathways and cell types responsible for this beneficial response. Given the recent genetic and molecular insights that govern the therapeutic success of checkpoint blockade in cancer patients and evolving techniques to identify patient-specific immunogenic cancer neoantigens, our results have the potential to form the basis for the design of new combinatorial approaches that may enhance personalized anticancer vaccines.
Alt-text: Unlabelled Box
1. Introduction
T cells express a variety of regulatory receptors, which serve as physiological checkpoints to prevent uncontrolled activity and T cell-mediated toxicity. Inhibitory cytotoxic T-cell associated antigen-4 (CTLA-4) competes with CD28 for binding of costimulatory B7 molecules on antigen presenting cells (APCs) to dampen activation and limit the amplitude of early T cell responses [1]. Blockade of CTLA-4 with a specific antibody (first-in-class ipilimumab) can release the brakes on antineoplastic T cell responses and has proven a successful tumor therapy in both mice and man [2,3]. Antibody mediated targeting of alternative checkpoint molecules such as programmed cell death receptor (PD)-1 on T cells or its ligand (PDL)-1 on tumor tissue also showed promising clinical results in patients with diverse advanced cancers [[4], [5], [6]]. Whereas both CTLA-4- and PD1-targeted therapy can induce long lasting complete remissions in a subset of patients, many others do not respond [2,6].
Several recent publications have begun to shed light on cellular and molecular factors that determine a positive clinical response to immune checkpoint blockade. Specifically, larger quantities of tumor-infiltrating T cells have long been known to be predictive for a favorable outcome in colon cancer and several other entities [7]. Based on the relationship between pre-therapy CD8+ T cell infiltrates and response to PD-1 blockade in melanoma [8], cytotoxic T play a central role in checkpoint blockade-mediated cancer immunotherapy. In addition, cancer patients with a high mutational load in tumor tissue and specific mutation-derived neoantigens show a long-term clinical benefit from checkpoint inhibitor treatment [9,10], presumably in part by reactivating T-cell responses against those neoantigens. These findings, in addition to experimental data that anti-CTLA-4 is inefficient against poorly-immunogenic tumors in mice [11], support the hypothesis that checkpoint inhibitors can potently enhance preexisting immune responses but cannot induce de novo T-cell immunity. Consequently, spurring a basal antitumor T cell response could bolster anti-CTLA-4 efficiency. However, the failure of a monovalent gp100 vaccine to improve ipilimumab-mediated tumor immunity in the phase III trial that led to its approval for metastatic melanoma [2] highlights the significant challenges faced on the way to develop an efficient tumor vaccine for combination with checkpoint blockade.
Type I interferon (IFN-I), originally defined by its ability to induce resistance against viral infection, has been shown to be of particular importance for the spontaneous generation of antigen-specific T-cell responses against growing tumors [12,13]. Two independent studies in mice have demonstrated that IFN-I is critical for intratumoral accumulation of CD8α+ dendritic cells (DCs), cross-priming of cytotoxic T cells and, ultimately, tumor regression [14,15]. Inducers of IFN-I therefore are promising candidates for a combinatorial approach with checkpoint blockade in tumor prevention and therapy. Cytosolic retinoic acid inducible gene I (RIG-I)-like helicases (RLH) are a family of nucleic acid-sensing pattern recognition receptors that can be activated to induce pro-inflammatory cytokine production, ASC (apoptosis-associated speck-like protein containing a carboxy-terminal CARD)-dependent inflammasome activation [[16], [17], [18]] and IFN-I release [19,20] via the mitochondria-located adapter molecule MAVS (mitochondrial antiviral signaling protein). Furthermore, therapeutic targeting of RIG-I within tumor cells can induce an immunogenic variant of cancer cell death that activates innate and adaptive immune responses [[21], [22], [23]]. Consistent with this, selective activation of RIG-I has been shown to result in growth inhibition of pre-established tumors, presumably via induction of tumor cell-intrinsic programmed cell death pathways as well as IFN-I-mediated activation of innate immune mechanism and NK cells [23].
However, strategies to target RIG-I for the development of (vaccination-induced) antitumor CD8+ T-cell responses in combination with checkpoint inhibition have not been established. We here demonstrate that protein vaccination together with RIG-I-activating immunostimulatory RNA foster MAVS- and IFN-I-dependent cross-priming of cytotoxic CD8+ T cells, which potently synergize with CTLA-4 blockade to induce antitumor immunity.
2. Materials and methods
2.1. Mice
Female C57Bl/6j mice were purchased from Janvier. Mice genetically deficient in Mavs, Asc and Ifnar1 and CD11c-DTR transgenic mice have been described [[24], [25], [26], [27]]. OT-I-transgenic mice with TCR specific for H-2Kb-OVA258–265 were purchased from Jackson Laboratories. Mice were at least 6 weeks of age at the onset of experiments and were maintained in specific pathogen free conditions. Animal studies were approved by the local regulatory agency (Regierung von Oberbayern, Munich, Germany).
2.2. Media and reagents
RPMI-1640 medium (Invitrogen) and DMEM (Invitrogen) were supplemented with 10% (vol/vol) FCS (Hyclone), 3 mM l-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin (all from Sigma-Aldrich). OptiMEM reduced serum medium was from Invitrogen. CpG 1826 and ultrapure LPS (from Escherichia coli strain K12) were from Invivogen. Double-stranded in vitro-transcribed 3pRNA (sense, 5′- UCA AAC AGU CCU CGC AUG CCU AUA GUG AGU CG -3′) was generated as described [23]. Synthetic dsRNA lacking the 5′-triphosphate (synRNA) was purchased from Eurofins (Ebersberg, Germany). ISD single strand oligonucleotides were purchased from Sigma-Aldrich (Munich, Germany) and were annealed by heating to 75 °C for 30 min and re-cooling to room temperature. CFSE and Celltracker violet were from Invitrogen. Endofit Ovalbumin for in vivo use was purchased from Invivogen (endotoxin level: <1 EU/mg).
2.3. Cell lines
The B16 murine melanoma cell line expressing the full-length chicken ovalbumin (here referred to as B16.OVA) was cultured in complete DMEM medium supplemented with 400 μg/mL G418 (from Sigma-Aldrich).
2.4. Cell purification and culture
Bone marrow-derived dendritic cells (BMDCs) were generated by culturing bone marrow cells in complete RPMI medium supplemented with 20 ng/ml GM-CSF (from Immunotools, Friesoythe, Germay). CD8+ T cells from OT-I splenocytes were purified with magnetic beads according to the manufacturer's protocol (Miltenyi Biotech, Bergisch Gladbach). In vitro, BMDCs were transfected with 3pRNA (1 μg/ml, if not indicated otherwise), ISD (2 μg/ml) or synRNA using Lipofectamin 2000 (Life Technologies, Darmstadt, Germany). Alternatively, DCs were stimulated with CpG ODN1826 (0.075 μM) or LPS (20 ng/ml). For maximal IL-1β release, DCs were primed with LPS overnight and ATP (5 mM, from Sigma-Aldrich) was added to the culture 2 h prior to the analysis.
2.5. Quantification of cytokines
Cell supernatants were analyzed for cytokine secretion by ELISA (BD, R&D Systems or eBioscience) according to the manufacturers' protocol.
2.6. Flow cytometry
Cell suspensions were stained in PBS with 1% FCS. Fluorochrome-coupled antibodies were purchased from eBioscience or BioLegend. The anti-mouse OVA257–264 (SIINFEKL) peptide bound to H-2Kb-antibody (clone 25-D1.16) was purchased from eBioscience. The iTAg MHC-I murine tetramers detecting SIINFEKL-specific CD8+ T cells were from MBL (Woburn, MA). For intracellular cytokine staining the Foxp3 Transcription Factor Fixation/Permeabilization Kit (eBioscience) was used. Data were acquired on a FACSCanto II (BD Biosciences) and analyzed using FlowJo software (TreeStar).
2.7. Immunization with OVA
For subcutaneous (sc) immunization, mice were injected sc in the hock of both hind legs with 50 μg OVA together with 25 μg 3pRNA complexed in 3.5 μl in vivo-jetPEI (Polyplus) or 25 μg CpG. The therapy was repeated on day 8. Alternatively, mice were intravenously (iv) injected once with 50 μg 3pRNA complexed in 3.5 μl in vivo-jetPEI and 100 μg OVA. In some experiments, mice were pre-treated intraperitoneally (ip) with 400 μg anti-murine IFNaR1 antibody (clone MAR1-5A3, BioXCell, West Lebanon, NH) one day prior to the above immunization. Administration of the anti-murine CTLA-4 antibody (clone 9H10, BioXCell) was performed ip on the day of immunization (200 μg) and was repeated 3 and 6 days later (100 μg each). For ex vivo restimulation, complete lymph node cells or splenocytes from immunized mice were stained with 3.6 μM CFSE and were cultured in the presence of 5 μg/ml OVA protein in complete RPMI medium. After, 48 and 72 h, proliferation was assessed by dye dilution. IFN-γ levels were analyzed by flow cytometry and ELISA from culture supernatant. For in vivo DC depletion, CD11c-DTR mice were injected with 500 ng diphtheria toxin (Sigma-Aldrich) ip on day 0 and 2 following vaccination.
2.8. In vivo cytotoxicity assay
In vivo cytotoxicity was evaluated 4 days after the final treatment as described previously [28]. In brief, splenocytes from naïve syngenic donor mice were pulsed with SIINFEKL peptide at different concentrations (0, 2, 20 and 200 nM) for 30–45 min at 37 °C. These four populations were then stained with different concentrations of Celltracker violet (0.1, 0.5, 2.5 and 5 μM), washed, mixed in a 1:1:1:1 ratio and were injected intravenously into the experimental animals. The next day, animals were sacrificed and the target cell frequency in the spleen was determined by flow cytometry. Specific killing was calculated using the formula 100-(1-%CFSE peptide/%CFSE no peptide) as described [28]. The frequency of antigen-specific CD8+ T cells in draining popliteal lymph nodes and spleen was analyzed with SIINFEKL-H-2Kb tetramers.
2.9. Tumor challenge and treatment
In the prophylactic setting, mice were immunized iv with 50 μg 3pRNA and 100 μg OVA protein as described above. Anti-CTLA-4 was applied on day 0, 3 and 6 following vaccination as described. One week later, mice were injected iv with 105 B16.OVA melanoma cells. 19 days later, mice were sacrificed and superficial pulmonary pseudo-metastases were enumerated. T-cell analysis was performed as described. In the therapeutic setting, mice were injected sc with 105 B16.OVA cells. Vaccination (100 μg OVA +50 μg 3pRNA iv, 200 μg anti-CTLA-4 ip) was performed on day 6 after B16.OVA inoculation when tumors were readily visible; anti-CTLA-4 administration was repeated on day 9 and 12 (100 μg). Optional treatment with anti-CD8a (clone 2.43, BioXCell), anti-CD4 (clone GK1.5) or anti-NK1.1 (clone PK136) depleting antibodies was initiated one day prior to vaccination (100 μg i.p.) and was repeated twice weekly (50 μg i.p.). Tumor growth was monitored daily. Mice were euthanized when the maximum tumor diameter exceeded 15 mm according to standard legal procedure (responsible state office Regierung von Oberbayern).
2.10. Statistics
All data are presented as mean ± S.E.M. Statistical significance of single experimental findings was assessed with the independent two-tailed Student's t-test. For multiple statistical comparison of a data set, the one-way ANOVA test with Bonferroni post-test was used. Overall survival was analyzed using the Log-rank test. Significance was set at P values <0.05, P < 0.01 and P < 0.001 and was then indicated with an asterisk (*, ** and ***). All statistical calculations were performed using Prism (GraphPad Software).
3. Results
3.1. DC activation via RIG-I is mediated by the adapter protein MAVS and type I IFN signaling
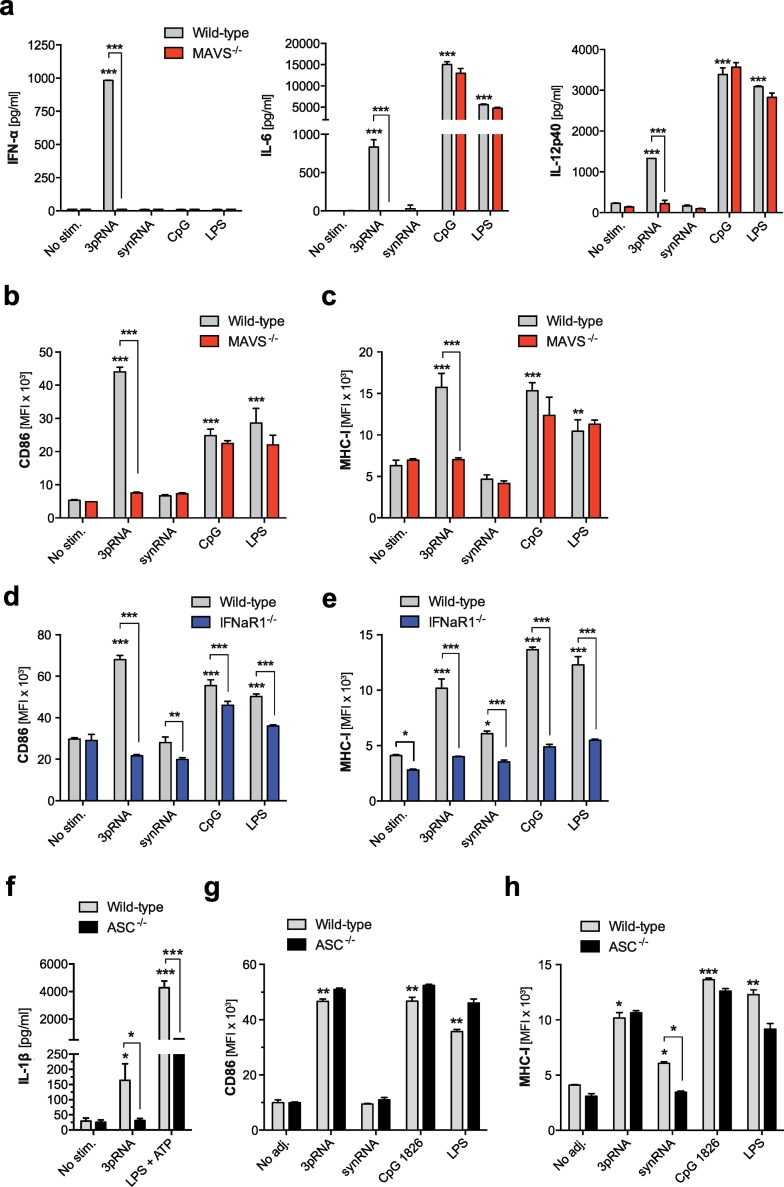
A prerequisite for cross-priming of CD8+ cytotoxic T cells and subsequent antitumor adaptive immunity is the efficient activation of APCs such as dendritic cells (DC) [29]. To investigate the effect of RIG-I ligation on DC activation and underlying signaling pathways, bone marrow-derived dendritic cells (BMDC) were transfected with the RIG-I ligand 5′-triphosphorylated-RNA (3pRNA) [20] or a synthetic, non-triphosphate-RNA with identical sequence (synRNA). As partly described previously [23], RIG-I ligation by 3pRNA resulted in activation of BMDCs associated with release of IFN-α and pro-inflammatory cytokines such as IL-6 and IL-12p40 (Fig. 1a). RIG-I ligation also triggered BMDC maturation with up-regulation of the costimulatory molecule CD86 and increased expression of MHC class I (Fig. 1b-c). Cytokine release and up-regulation of maturational markers in response to RIG-I ligation was totally abrogated in MAVS-deficient BMDCs (Fig. 1a-c). Furthermore, IFN-I signaling was critical, as DCs that lack the common interferon-α receptor subunit 1 (IFNaR1) did not mature in response to RIG-I stimulation (Fig. 1d-e). As described previously [16], BMDCs deficient for the inflammasome adaptor protein ASC (ASC−/−) failed to release bio-active IL-1β after RIG-I ligation (Fig. 1f). We now found that ASC−/− DCs showed unimpaired up-regulation of both CD86 and MHC-I (Fig. 1g-h), indicating that ASC-dependent IL-1β release - in contrast to MAVS-mediated IFN-I - is dispensable for DC maturation in response to RIG-I ligation.
Fig. 1.
DC activation via RIG-I is mediated by the adapter protein MAVS and type I IFN signaling. Bone marrow-derived dendritic cells (BMDCs) from wild-type (WT) and indicated genetically deficient mice were transfected with 3pRNA or synthetic non-triphosphorylated RNA of the same sequence (synRNA) or were stimulated with the TLR ligands CpG or LPS. (a-c) Levels of IFN-α, IL-6 and IL-12p40 in culture supernatants were determined by ELISA. (b-e) CD86 and MHC class I expression on BMDCs from wild-type, MAVS (MAVS−/−)- and IFNaR1 (IFNaR1−/−)-deficient mice were analyzed by flow cytometry. (f-h) BMDCs from ASC-deficient (ASC−/−) animals were stimulated identically. (f) Levels of IL-1β, (g) expression of CD86 and (h) MHC-I were determined. All data give mean ± S.E.M. of at least triplicate samples representative of three independent experiments. An asterisk without brackets indicates comparison to the WT unstimulated control group (*P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA) for multiple comparisons). MFI, mean fluorescence intensity.
3.2. Enhanced cross-presentation upon RIG-I activation mediates efficient cross-priming of cytotoxic T cells in vitro
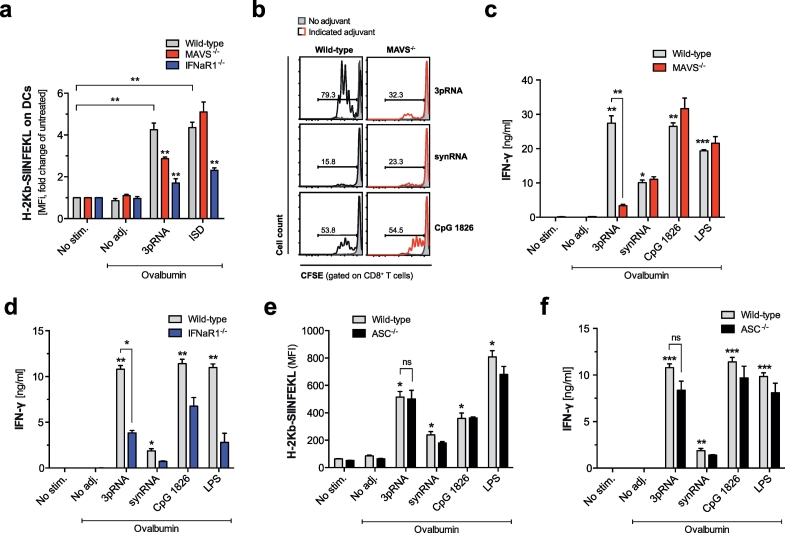
DC-mediated cross-presentation of ingested antigen on MHC-I is crucial for generating CD8 T-cell responses against exogenous antigens such as tumor cell-derived proteins, that are not expressed by APCs themselves. To investigate whether RIG-I activation has direct effects on cross-presentation, we transfected BMDCs with 3pRNA in the presence of the model antigen chicken egg ovalbumin (OVA). Using a conformation dependent antibody directed against pre-formed peptide (SIINFEKL)-MHC I complexes on the surface of the DC, we found that RIG-I activation resulted in significantly increased cross-presentation of the processed, immune-dominant OVA peptide epitope SIINFEKL (Fig. 2a). Consistent with RIG-I-dependent DC maturation described above, this effect required DC-intrinsic IFN-I and MAVS signaling (Fig. 2a). We could exclude a global defect in the cross-presentation machinery in MAVS-deficient DCs, as a 45-base pair non-CpG double-stranded DNA oligonucleotide (interferon stimulatory DNA, ISD) [30], which is an agonist for the cGAS-STING pathway, showed impaired cross-presentation in IFNaR1- but not MAVS-deficient DCs.
Fig. 2.
Enhanced cross-presentation upon RIG-I activation mediates efficient cross-priming of cytotoxic T cells in vitro. BMDCs were stimulated as described for Fig. 1 and were then cultured in the presence of OVA protein. (a) Cross-presentation of the processed peptide-epitope SIINFEKL in the context of MHC-I was analyzed by flow cytometry 18 h later. Data show H-2Kb-SIINFEKL expression on wild-type, IFNaR1- and MAVS-deficient cells. Data show mean fold change in comparison to untreated cells ± S.E.M. of quadruplicate samples pooled from two independent experiments. (b-d) Stimulated DCs were co-cultured with magnetically purified, CFSE-labeled CD8+ OT-I T cells in the presence of OVA protein. (b) CD8 T cell proliferation by CFSE dye dilution as well as IFN-γ levels in the supernatant from co-cultures with MAVS- (c) and IFNaR1-deficient DCs (d) were analyzed by flow cytometry or ELISA, respectively. (e-f) BMDCs from ASC-deficient animals were stimulated as described. (e) H-2Kb-SIINFEKL expression on DCs and (f) IFN-γ levels in the supernatant from co-cultures with CD8+ OT-I T cells were analyzed as described. All data give mean ± S.E.M. of at least triplicate samples representative of three independent experiments. (*P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA) for multiple comparisons). ns, not significant.
To investigate subsequent cross-priming of CD8+ cytotoxic T cells in vitro, BMDCs were transfected with 3pRNA in the presence of OVA protein and were cultured with OVA-specific CD8+ T cells from transgenic OT-I mice. As for cross-presentation, we found that RIG-I activation enhanced cross-priming resulting in more proliferation and IFN-γ production by specific CD8+ T cells (Fig. 2b-d). Both MAVS- and IFNaR1-deficient DCs, in line with their defect in cross-presentation, failed to induce cytotoxic T cell effector differentiation upon 3pRNA treatment. In contrast, cross-presentation and -priming were intact in ASC-deficient DCs after RIG-I activation (Fig. 2e-f). Thus, our results suggest that both MAVS and IFNaR1 signaling but not ASC-mediated inflammasome formation are critical for RIG-I-mediated cross-presentation of exogenous antigen by DCs and subsequent cross-priming of cytotoxic T cells.
However, we observed that 3pRNA-induced effects in DCs and co-cultured T cells were not completely abrogated in MAVS-deficient cells (Fig. 2a-c). The 3pRNA sequence used in these experiments has been designed not to include Toll-like receptor (TLR)-7-activating motifs. We have previously shown, that its in vivo immunostimulatory effects are independent of TLR7 [23]. To address possible “off-target” effects of our in vitro transcribed 3pRNA, we assessed activation of dendritic cells derived from Myd88- and Tlr3-deficient mice. MyD88 is an important adapter protein used by all TLRs except TLR3 to activate the pro-inflammatory transcription factor NF-κB. We found that the 3pRNA-induced maturation of DCs with upregulation of the co-stimulatory molecule CD86 and release of IFN-α were not dependent on MyD88 or TLR3 (Fig. S1a + b). However, due to possible redundancy in RNA recognition mechanisms, these data cannot rule out that TLR activation contributes to the immunostimulatory effects of in vitro transcribed 3pRNA in the absence of MAVS signaling.
3.3. The RIG-I/MAVS/IFN-I pathway augments vaccine-induced cross-priming of cytotoxic T cells in vivo
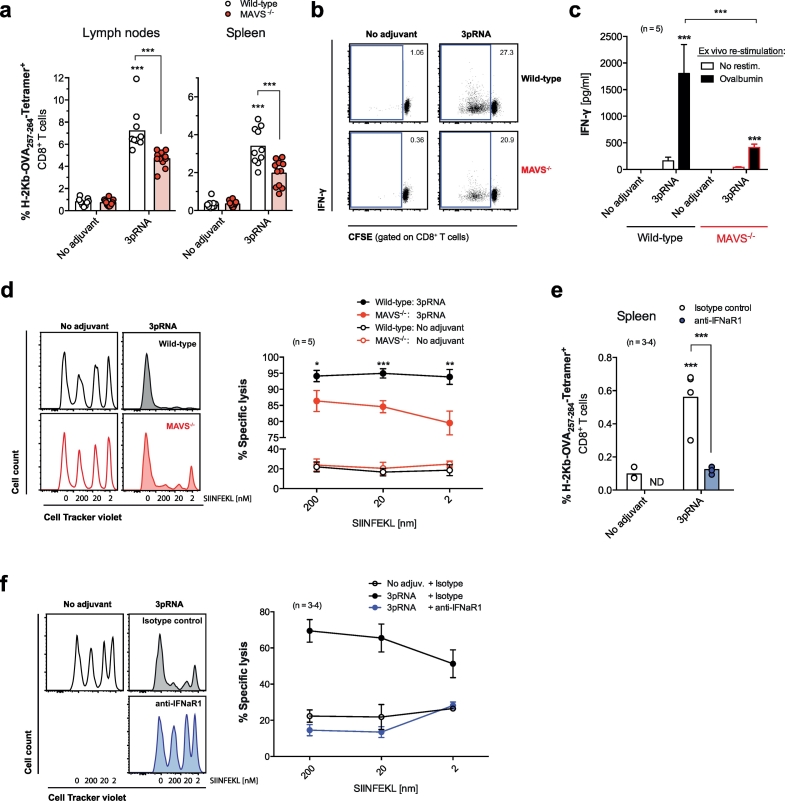
We next investigated the effect of RIG-I activation on cross-priming of cytotoxic T cells in vivo. Boosted subcutaneous protein vaccination with OVA and RIG-I ligand but not OVA alone resulted in strong, MAVS-dependent expansion of MHC-I-SIINFEKL Tetramer+ CD8+ T cells in local draining lymph nodes and the spleen (Fig. 3a). Consistently, CD8+ T cell proliferation and IFN-γ production in restimulated ex vivo cultured lymph node cell suspensions from 3pRNA-vaccinated mice were largely diminished in cells from MAVS-deficient animals (Fig. 3b-c). In addition, we observed strong cytolytic activity towards SIINFEKL-pulsed target cells in 3pRNA-vaccinated wild-type mice, which was significantly reduced in MAVS-deficient animals (Fig. 3d). Of note, modifying the vaccination strategy to a single systemic intravenous administration of OVA and low-dose 3pRNA (25 μg) still resulted in the expansion of OVA-specific cytotoxic T cells in the spleen and robust elimination of SIINFEKL-pulsed target cells (Fig. 3e-f), although the cytolytic activity was less efficient when compared to the boosted subcutaneous immunization. Consistent with impaired DC maturation under absent IFN-I signaling, antibody-mediated blockade of IFN-I signaling resulted in a complete loss of OVA-specific T-cell expansion and cytolytic activity in response to 3pRNA-based immunization in vivo (Fig. 3e-f). To investigate the role of DCs for CD8 T-cell cross-priming in vivo, we used CD11c DTR-transgenic mice [27] to deplete CD11c+ DCs before vaccination. Depletion of DCs by diphtheria toxin (Fig. S2a) resulted in complete abolishment of MHC-I-SIINFEKL Tetramer+ T cell expansion and IFN-γ production after subcutaneous administration of OVA and 3pRNA (Fig. S2b-c). Taken together, our data highlight that activation of RIG-I strongly promotes vaccine-induced cross-priming of cytotoxic T cells by DCs in vivo and that this requires intact MAVS and type I IFN signaling for optimal T-cell expansion and cytolytic activity.
Fig. 3.
The RIG-I / MAVS / IFN-I pathway induces robust cross-priming of cytotoxic T cells in vivo. WT and MAVS-deficient mice were injected sc with OVA +3pRNA twice. (a) Frequency of H-2Kb-SIINFEKL Tetramer+ cytotoxic T cells in draining lymph nodes (LN) and spleen. Each data point represents one individual of at least n = 10 mice and the mean per group is depicted as a bar. (b) Representative blots are gated on CD8+ T cells from ex vivo OVA restimulated LN cell cultures and give the percentage of proliferating cells. (c) IFN-γ levels from the above cultures. Data give the mean ± S.E.M. of n = 5 independent cell cultures per group each derived from individual mice. (d) In vivo cytotoxic activity was measured by target cell elimination of fluorescently labeled, SIINFEKL peptide-pulsed syngenic splenocytes. Histograms show the frequency of transferred target cells in the spleen of a representative recipient mouse. Numbers give the concentration [nm] of SIINFEKL-pulsing and thus the immunogenicity of the indicated target cell population. The graph shows mean specific lysis ± S.E.M. of n = 5 individual mice. (e-f) WT mice were vaccinated iv with OVA +3pRNA once and were additionally treated with anti-IFNaR1 blocking antibody one day prior to vaccination. (e) Frequency of H-2Kb-SIINFEKL Tetramer+ cytotoxic T cells in the spleen and (f) cytolytic activity were analyzed as described above (individual mice, n = 3 for the ‘no adjuvant’ and n = 4 for the ‘3pRNA’ group). All data are representative of at least two independent experiments. (*P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA) for multiple comparisons). ND, not determined.
3.4. RIG-I activation synergizes with anti-CTLA-4 blockade to enhance the efficacy of anticancer vaccines
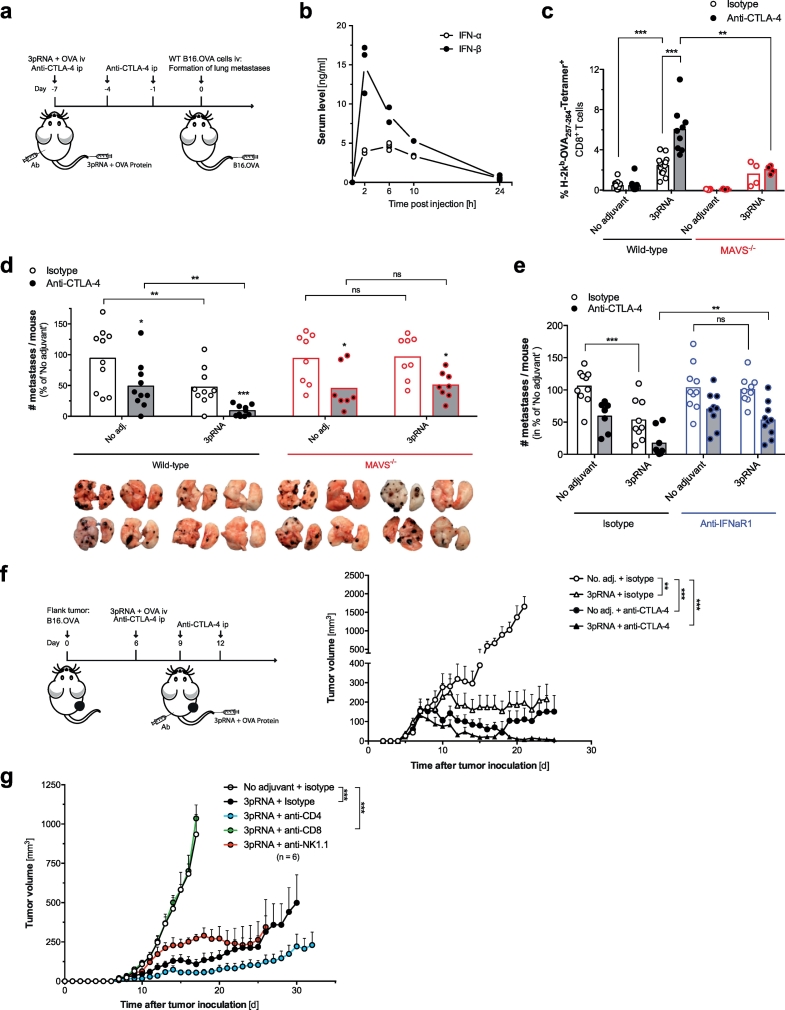
Next, we investigated whether RIG-I-enhanced vaccination and subsequent cross-priming of cytotoxic T cells would translate into antitumor immunity. Tumor cell intrinsic RIG-I ligation can result in the induction of programmed tumor cell death as a form of immunogenic cell death (ICD) with release of damage-associated molecular patterns (DAMPs) like nucleic acids and other soluble factors [21,22]. Therefore, we opted to exclude this bias by choosing a model of prophylactic vaccination that allows targeting of RIG-I in host cells prior to tumor induction (Fig. 4a). Following intravenous (iv) RIG-I-based protein vaccination, mice were iv injected with B16.OVA cells and the formation of lung metastases was later analyzed. Intravenous injection of 3pRNA and OVA protein rapidly resulted in a short peak of serum IFN-I (Fig. 4b), followed by robust expansion of OVA-specific cytotoxic T cells in peripheral blood (Fig. 4c). Albeit this expansion, we only observed partial protection from the development of lung metastases in 3pRNA-vaccinated animals (Fig. 4d). CTLA-4 blockade in combination with RIG-I-assisted OVA vaccination significantly enhanced expansion of antigen-specific CD8+ T cells (Fig. 4c), which translated into improved antitumor immunity with reduction of metastatic tumor burden (Fig. 4d). Interestingly, mice that were treated with anti-CTLA-4 and OVA protein alone also showed some degree of protection against metastases, despite undetectable levels of specific MHC-I-SIINFEKL Tetramer+ T cells in their peripheral blood.
Fig. 4.
RIG-I activation synergizes with anti-CTLA-4 blockade to enhance the efficacy of anticancer vaccines. (a) ‘Prophylactic’ vaccination scheme: WT and MAVS−/− mice were vaccinated with OVA and 3pRNA iv. Anti-CTLA-4 antibody was administered ip B16.OVA tumor cells were injected iv on day 7. (b) Serum levels of IFN-I after a single 3pRNA injection. (c) Frequency of H-2Kb-SIINFEKL Tetramer+ cytotoxic T cells in peripheral blood of WT and MAVS−/− mice on day 7 after vaccination. (d) Number of macroscopically visible pseudo-metastases in the lung at day 19 after tumor induction. Data are pooled from two independent experiments and are presented as percentage of the ‘No adjuvant’ control group. (e) Number of pseudo-metastases in WT animals that were additionally injected with anti-IFNaR1 or isotype control antibody one day prior to vaccination. All data are representative of at least two independent experiments. (f) ‘Therapeutic’ vaccination scheme: WT mice were injected sc with B16.OVA cells. Intravenous vaccination with OVA and 3pRNA was performed on day 6 after tumor inoculation; anti-CTLA-4 was administered ip on day 6, 9 and 12. Tumor growth in mice was analyzed. Data show mean tumor growth ± S.E.M. from n = 5 individual mice. (g) Tumor-bearing mice were vaccinated with OVA +3pRNA as described for Fig. 4f. Some mice were additionally treated with CD4+ T-cell, CD8+ T cell or NK-cell depleting antibodies. Data give mean tumor growth ± S.E.M. of n = 6 individual mice per group. All data are representative of at least two independent experiments. (*P < 0.05, **P < 0.01, ***P < 0.001, ordinary one-way ANOVA for multiple comparisons or two-tailed unpaired t-test.)
In line with our cross-priming data, MAVS-deficient mice showed strongly reduced frequency of OVA-specific cytotoxic T cells in the peripheral blood (Fig. 4c) and were unable to mount tumor immunity in response to vaccination with OVA and 3pRNA (Fig. 4d). The effect of OVA vaccination and anti-CTLA-4 treatment alone was intact in MAVS-deficient mice, whereas the synergistic antitumor effect of CTLA-4 blockade and RIG-I activation was completely lost. Furthermore, mice failed to induce antitumor immunity when treated with anti-IFNaR1 neutralizing antibodies one day prior to immunization with OVA and 3pRNA, while the effects of CTLA-4 blockade were unaltered (Fig. 4e).
To test whether our combined vaccination approach was also effective in a therapeutic setting in which treatment was initiated after tumor induction, we used a subcutaneous tumor model that facilitates objective size measurement of pre-established tumors before therapy onset (Fig. 4f). Tumor-bearing animals were intravenously injected with a 3pRNA/OVA vaccine in combination with intraperitoneal application of anti-CTLA-4. Although vaccination with OVA together with either 3pRNA or anti-CTLA-4 resulted in a significant delay in subsequent tumor growth, only the combination of 3pRNA, protein vaccine and anti-CTLA-4 led to a complete regression of pre-established tumors associated with long-term survival in all animals. Using antibodies to deplete specific immune cell subsets, we found that antitumor immunity following iv administration of a RIG-I-enhanced protein vaccine was mediated by CD8+ cytotoxic T cells, but was independent of NK cells and CD4+ T cells (Fig. 4g). In sum, our data show that RIG-I activation synergizes with protein vaccination and checkpoint blockade to induce strong T-cell antitumor immunity and that this combination requires MAVS and IFN-I signaling in host cells.
4. Discussion
Combined genomics and bioinformatics approaches now allow the identification of tumor-specific mutated proteins and associated neoantigens that can serve as a basis for the development of personalized anticancer vaccines [31,32]. Our results highlight that targeted RIG-I activation in non-malignant host cells can strongly improve the efficacy of such anticancer vaccines. Mechanistically, the RIG-I-activating ligand 3pRNA, a synthetic “mimicry” of viral and bacterial RNA, enhanced cross-presentation and cross-priming of specific cytotoxic T cells by activation of DCs. These effects required the RIG-I adapter MAVS and DC-intrinsic IFN-I signaling. Yet, MAVS- or IFNaR1-deficient DCs were not completely devoid of cross-presentation or -priming, suggesting that additional RNA sensing pathways systems may synergize with the RIG-I/MAVS pathway in the regulation of cross-presentation and -priming. Non-TLR RNA sensors that might be involved in the recognition of in vitro transcribed 3pRNA may include protein kinase R (PKR), interferon-induced protein with tetratricopeptide repeats 1 (IFIT1), 2ʹ-5ʹ-oligoadenylate synthetase 1 (OAS1) and RNaseL [33]. Chemically synthesized, highly purified 3pRNAs as now being used in phase I/II clinical trials in patients with advanced solid tumors and lymphomas (RGT100-PEI; ClinicalTrials.gov Identifier: NCT03065023) are likely to have less “off-target” effects.
Despite potent expansion of tumor antigen-specific CTLs, RIG-I ligands induced only partial regression of melanoma growth in two distinct models. We think this is most likely due to interferon-mediated upregulation of immune checkpoints (and other immunosuppressive factors in the tumor microenvironment) [34]. Along these lines, our data show that combination of RIG-I-enhanced vaccines and anti-CTLA-4 immune checkpoint inhibitors synergistically boost antitumor immunity with almost complete regression of all melanoma formations. The importance of cross-priming of CTLs in our in vivo models was further strengthened by the experimental finding, that RIG-I-induced antitumor immunity is abrogated following depletion of CD8+ T cells.
The crucial role of DCs in mediating RIG-I/vaccine-induced T-cell priming was emphasized as targeted depletion of CD11c+ DCs in vivo completely abolished 3pRNA/OVA-induced cross-priming of OVA specific CD8 T cells. This is in line with previous reports showing that RIG-I signaling and DC-intrinsic IFN-I signaling is crucial for antigen cross-presentation and induction of antiviral and antitumor cytotoxic T [14,15,35]. As for the source of IFN-I, macrophages and to some extent plasmacytoid dendritic cells have been identified to be the most important producers of IFN-I in response to RIG-I activation in vivo [35].
Using a prophylactic melanoma treatment model, we found that targeted RIG-I activation during protein-based vaccination potently synergized with anti-CTLA-4 blockade for strong expansion of tumor antigen-specific CD8+ T cells and subsequent tumor regression. These processes were dependent on MAVS and IFN-I signaling. Unexpectedly, in this model, treatment with a protein vaccine and anti-CTLA-4 in the absence of adjuvant (such as 3pRNA) resulted in delayed tumor progression, despite (un-)detectable levels of antigen-specific CD8+ T cells. These data might be explained by previous findings that anti-CTLA-4 antibodies can mediate antitumor activity by Fc receptor dependent depletion of regulatory T cells (Treg) in the tumor milieu, thereby increasing the endogenous CD8+ effector T cell to Treg ratio [36].
Importantly, the combination of RIG-I-stimulated protein vaccination and anti-CTLA-4 checkpoint blockade also showed high effectiveness in pre-established tumors. By subcutaneous and intravenous delivery, 3pRNA stimulated RIG-I signaling in host cells to augment the efficacy of anti-CTLA-4. In a different approach, therapeutic targeting of RIG-I within tumor cells has been shown to induce an immunogenic variant of cancer cell death that can also activate innate and adaptive immune responses [[21], [22], [23]]. The jetPEI-RNA complexes used in this study to facilitate in vivo delivery of 3pRNA, are known to predominantly accumulate in the lung, liver and spleen rather than the tumor microenvironment when injected intravenously [23,37]. Thus, different application routes (i.e. intratumorally) may allow for additional therapeutic benefit from RIG-I-mediated programmed tumor cell death as well as local NK cell activation when applying protein-based vaccines in combination with a RIG-I ligand [23].
Our data are in line with a recent report which shows that a combination therapy of localized Newcastle disease virus (NDV) and systemic CTLA-4 blockade can lead to rejection of pre-established distant tumors and induction of tumor-specific T cells [38]. Given that NDV activates both the RIG-I / MAVS and the TLR7 / MyD88 pathway for potent IFN-I production, it is tempting to speculate that these pathways are required for the observed therapeutic efficacy. Several preclinical studies suggest that therapeutic efficacy of such oncolytic viruses is not only due to direct infection-mediated tumor cell cytotoxicity but also critically depends on subsequent activation of innate and adaptive antitumor immunity [39]. However, neither the relevance of the RIG-I / MAVS pathway nor a prophylactic vaccination approach was investigated in the above-mentioned study.
In sum, our study provides the first experimental evidence that immune checkpoint blockade with anti-CTLA-4 potentiates specific cytotoxic T cell responses and subsequent tumor immunity initiated by protein vaccination and RIG-I-activating immunostimulatory RNA ligands. Given that checkpoint blockade acts at least in part through neoantigen-specific T cell reactivity [40], our combined vaccination approach may provide a novel strategy to enhance the activity of neoantigen-specific T cells induced by personalized anticancer vaccines.
The following are the supplementary data related to this article.
Bone marrow-derived dendritic cells (BMDCs) from wild-type (WT), MyD88 (Myd88-/-)- and TLR3-deficient (Tlr3-/-) mice were transfected with 3pRNA or interferon stimulatory DNA (ISD). (a) Levels of IFN-α in culture supernatants were determined by ELISA. (b) CD86 expression on BMDCs was analyzed by flow cytometry. All data give mean ± S.E.M. of triplicate samples that are representative of two independent experiments. An asterisk without brackets indicates comparison to the WT unstimulated control group (***P < 0.001, one-way analysis of variance (ANOVA) for multiple comparisons). MFI, mean fluorescence intensity. Unstim, unstimulated.
CD11c-DTR/GFP mice were treated ip with diphtheria toxin to deplete dendritic cells (DCs) and were subsequently vaccinated with OVA + 3pRNA as described for Fig. 3. (a) Flow cytometric analysis of spleen cells shows the frequency of GFP+ DTR-transgenic DCs in individual mice +/- application of diphtheria toxin. (b) Expansion of H-2Kb-SIINFEKL Tetramer+ cytotoxic T cells in draining LN. (c) Triplicate samples of the frequency of IFN-γ+ cytotoxic T cells in ex vivo restimulated, complete LN cells from these animals. Data is expressed as fold change against untreated wild-type mice (marked as dotted line). Data give the mean of n = 2 individual mice ± S.E.M.
Author contributions
A.W., D.K., T.H., S.H. and H·P. designed the research, analyzed and interpreted the results. A.W., D.K., T.N., F.S., S.B., J.F. and S.H. did the experiments. S.H. and H.P. prepared the manuscript. M.B., T.G., U.K. and F.B. gave methodological support and conceptual advice. T.H. and H.P. guided the study.
Acknowledgments
Acknowledgements
We thank Veit R. Buchholz and Dirk H. Busch (Technical University Munich) for kindly providing CD11c-DTR/GFP transgenic mice. Tlr3-/- and Myd88-/- mice were a generous gift from Klaus-Peter Janssen (Technical University Munich). This study was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 360372040 – SFB 1335, PO 1575/3-1 and SFB1371 (P04) to H.P., the Else-Kröner-Fresenius-Stiftung (2012_A61 and 2015_A06 to H.P.; EKFK to S.H), a Feodor-Lynen-Scholarship for Experienced Researchers by the Alexander von Humboldt Foundation (to H.P), the German Cancer Aid (111620 to H.P and S.H), the European Hematology Association (to H.P), Mechtild Harf Research Grant from the DKMS Foundation for Giving Life (to H·P) and Dres. Carl Maximilian and Carl Manfred Bayer-Foundation (to S.H.). H. P. is supported by the EMBO Young Investigator Program. This work is part of the thesis of D.K. and S.B. at the Technical University of Munich.
Declaration of interests
Dr. Bscheider reports personal fees from F Hoffmann La Roche AG, Basel, Switzerland, outside the submitted work. The other authors have nothing to disclose.
Contributor Information
Simon Heidegger, Email: simon.heidegger@tum.de.
Hendrik Poeck, Email: hendrik.poeck@tum.de.
References
- 1.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 4.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 6.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pages C. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 8.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A. Genetic basis for clinical response to CTLA-4 blockade in melanoma. New England J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Elsas A., Hurwitz A.A., Allison J.P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson L.J., Kolumam G.A., Thomas S., Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177(3):1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 13.Tovey M.G., Lallemand C., Meritet J.F., Maury C. Adjuvant activity of interferon alpha: mechanism(s) of action. Vaccine. 2006;24(Suppl. 2):S2. doi: 10.1016/j.vaccine.2005.01.117. 46-7. [DOI] [PubMed] [Google Scholar]
- 14.Fuertes M.B., Kacha A.K., Kline J., Woo S.R., Kranz D.M., Murphy K.M. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond M.S., Kinder M., Matsushita H., Mashayekhi M., Dunn G.P., Archambault J.M. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11(1):63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 17.Franchi L., Eigenbrod T., Munoz-Planillo R., Ozkurede U., Kim Y.G., Chakrabarti A. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol. 2014;193(8):4214–4222. doi: 10.4049/jimmunol.1400582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pothlichet J., Meunier I., Davis B.K., Ting J.P., Skamene E., von Messling V. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza a virus infected cells. PLoS Pathog. 2013;9(4) doi: 10.1371/journal.ppat.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goubau D., Schlee M., Deddouche S., Pruijssers A.J., Zillinger T., Goldeck M. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature. 2014 Oct 16;514(7522):372–375. doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H. 5′-triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 21.Besch R., Poeck H., Hohenauer T., Senft D., Hacker G., Berking C. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest. 2009;119(8):2399–2411. doi: 10.1172/JCI37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duewell P., Steger A., Lohr H., Bourhis H., Hoelz H., Kirchleitner S.V. RIG-I-like helicases induce immunogenic cell death of pancreatic cancer cells and sensitize tumors toward killing by CD8(+) T cells. Cell Death Differ. 2014;21(12):1825–1837. doi: 10.1038/cdd.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poeck H., Besch R., Maihoefer C., Renn M., Tormo D., Morskaya S.S. 5′-triphosphate-siRNA: turning gene silencing and rig-I activation against melanoma. Nat Med. 2008;14(11):1256–1263. doi: 10.1038/nm.1887. [DOI] [PubMed] [Google Scholar]
- 24.Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 25.Seth R.B., Sun L., Ea C.K., Chen Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Mariathasan S., Newton K., Monack D.M., Vucic D., French D.M., Lee W.P. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430(6996):213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 27.Jung S., Unutmaz D., Wong P., Sano G., De los Santos K., Sparwasser T. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leibundgut-Landmann S., Osorio F., Brown G.D. Reis e Sousa C. stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112(13):4971–4980. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 29.Dalod M., Chelbi R., Malissen B., Lawrence T. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014;33(10):1104–1116. doi: 10.1002/embj.201488027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stetson D.B., Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24(1):93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav M., Jhunjhunwala S., Phung Q.T., Lupardus P., Tanguay J., Bumbaca S. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 33.Schlee M., Hartmann G. Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol. 2016;16(9):566–580. doi: 10.1038/nri.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel S.A., Minn A.J. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48(3):417–433. doi: 10.1016/j.immuni.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochheiser K., Klein M., Gottschalk C., Hoss F., Scheu S., Coch C. Cutting edge: the RIG-I ligand 3pRNA potently improves CTL cross-priming and facilitates antiviral vaccination. J Immunol. 2016;196(6):2439–2443. doi: 10.4049/jimmunol.1501958. [DOI] [PubMed] [Google Scholar]
- 36.Simpson T.R., Li F., Montalvo-Ortiz W., Sepulveda M.A., Bergerhoff K., Arce F. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebert G., Poeck H., Lucifora J., Baschuk N., Esser K., Esposito I. 5′ Triphosphorylated small interfering RNAs control replication of hepatitis B virus and induce an interferon response in human liver cells and mice. Gastroenterology. 2011;141(2):696–706. doi: 10.1053/j.gastro.2011.05.001. e1–3. [DOI] [PubMed] [Google Scholar]
- 38.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226) doi: 10.1126/scitranslmed.3008095. 226ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbull S., West E.J., Scott K.J., Appleton E., Melcher A., Ralph C. Evidence for oncolytic virotherapy: where have we got to and where are we going? Viruses. 2015;7(12):6291–6312. doi: 10.3390/v7122938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bone marrow-derived dendritic cells (BMDCs) from wild-type (WT), MyD88 (Myd88-/-)- and TLR3-deficient (Tlr3-/-) mice were transfected with 3pRNA or interferon stimulatory DNA (ISD). (a) Levels of IFN-α in culture supernatants were determined by ELISA. (b) CD86 expression on BMDCs was analyzed by flow cytometry. All data give mean ± S.E.M. of triplicate samples that are representative of two independent experiments. An asterisk without brackets indicates comparison to the WT unstimulated control group (***P < 0.001, one-way analysis of variance (ANOVA) for multiple comparisons). MFI, mean fluorescence intensity. Unstim, unstimulated.
CD11c-DTR/GFP mice were treated ip with diphtheria toxin to deplete dendritic cells (DCs) and were subsequently vaccinated with OVA + 3pRNA as described for Fig. 3. (a) Flow cytometric analysis of spleen cells shows the frequency of GFP+ DTR-transgenic DCs in individual mice +/- application of diphtheria toxin. (b) Expansion of H-2Kb-SIINFEKL Tetramer+ cytotoxic T cells in draining LN. (c) Triplicate samples of the frequency of IFN-γ+ cytotoxic T cells in ex vivo restimulated, complete LN cells from these animals. Data is expressed as fold change against untreated wild-type mice (marked as dotted line). Data give the mean of n = 2 individual mice ± S.E.M.