Highlights
-
•
To avoid L-dopa side effects it’s administration is delayed.
-
•
Natural antioxidants have wide range of medicinal properties.
-
•
The natural antioxidants might inhibit oxidation and toxin formation.
-
•
The L-dopa use with antioxidants, may be a necessary approach in modern PD therapy.
Keywords: Parkinson disease, Oxidative stress, L-dopa, Antioxidants, PCC, AGEs
Abstract
Parkinson disease (PD) is a multifactorial disease that takes a leading place among contemporary frequent diseases of the central nervous system (CNS) with not well-established mechanism. One of the most popular and effective therapy for patients with PD is Levodopa (L-dopa), but clinical effect of the drug diminished by motor complications resulting from prolonged treatment. Due to the L-dopa neurotoxic effect in the disease treatment, the L-dopa administration is delayed as long as possible in order to avoid side effects. In addition, combining L-dopa therapy with antioxidants, may decrease side-effects and provide symptomatic relief. The aim of the current research was to explore the possibility to reduce the oxidative stress (OS) induced by the L-dopa after its combining with two different antioxidants an essential oil isolated from Rosa damascena Mill., and vitamin C through experimental model of healthy mice. For this purpose, some oxidative stress indicators were evaluated - the lipid and protein oxidation end products – such as lipid peroxidation products measured as malondialdehyde (MDA) levels, protein carbonyl content (PCC), and advanced glycation end products (AGEs) in blood plasma of the experimental mice. For this purpose, was studied blood isolated from healthy mice after i.p. treatment with L-dopa (100 mg/kg). The groups with combining therapy were pre-treated first with Ascorbic acid (400 mg/kg), Rose oil (400 mg/kg). Statistically significant increased MDA levels, PCC and AGEs were found in the blood L-dopa treated mice compared to the controls, while the same parameters were significantly decreased in group pre-treated with antioxidants compared to the same controls. As a conclusion, the studied antioxidants can protect organisms from induced L-dopa oxidative toxicity and may play a key role in end products protection.
1. Introduction
Parkinson disease (PD) is a multifactorial disease that takes a leading place among contemporary frequent diseases of the central nervous system (CNS) with not well-established mechanism [1]. The major clinical disturbances in PD are dopamine depletion consequence in the neostriatum, due to dopaminergic neurons’ degeneration [2]. The drug therapy depends on disease severity, remains relatively nonspecific and limited long term efficacy. In the early disease stages, is used a monoamine oxidase β-inhibitor. Initially, the drug is used to inhibit the dopamine degradation [3]. In later disease phases, the patients are treated with Levodopa (L-dopa) a dopamine precursor [4]. The dihydroxyphenylalanine (Levodopa, L-dopa) - is a "gold standard" and the most effective symptomatic treatment for PD, against which new drugs are compared [5]. A number of therapies have been developed in an attempt to improve the disease treatment, such as dopaminergic agonists and inhibitors COMT and MAO-B, but most patients still depend on L-dopa because it is the most able to control the PD symptoms [6]. The L-dopa clinical effect is diminished by motor complications resulting from prolonged treatment. Due to the L-dopa neurotoxic effect in the disease treatment, the L-dopa administration is delayed as long as possible in order to avoid side effects [7].
Over the last decade, many different hypothesis, including oxidant-antioxidant theory, have been suggested to explain the etiology of PD. Local and systemic oxidative injuries, triggered by free radicals and reactive oxygen species (ROS), may be a reason for dopaminergic neurons’ degeneration [8]. Oxidative stress is a consequence of overproducing or inadequate removal of ROS in the cells, causing impairment of the oxidant-antioxidant balance in the body and may lead to non-specific damages of different biomolecules such as proteins, lipids, and nucleic acids. In addition, combining L-dopa therapy with antioxidants (from natural or synthetic origin), may reduce the oxidative processes, decrease side-effects and provide symptomatic relief [9].
The vitamin C possesses the strong antioxidant properties, and interacts with H2O2, hydroxyl radical (•OH) and •O2−, and turning them into non-radical products, providing cells with a free radical “neutralizer” [10,11]. In addition, the Vitamin C exhibit pro-oxidant properties in the presence of free transition metals, because it reduces ferrions to ferro-ions in a Fenton-like reaction and in the H2O2 presence stimulate the hydroxyl radicals formation [12]. Whether the ultimate effect will be a pro-oxidant or an antioxidant depends on the ratio of the ascorbic acid concentration to the available ferrions [13]. At sufficiently high concentrations, ascorbic acid reduces and destroys the formed radicals [14,15]. Plasma antioxidants traceability under oxidative stress indicates that the ascorbic acid level has been lowered at the earliest, and after depletion, lipid peroxidation has developed, even if the plasma Tocopherol is in normal concentrations [16].
Natural antioxidants are isolated from different parts of plants, like flowers, seeds, leaves, roots, bark and their phenolic extracts can slow the lipid oxidation [17]. Essential Rose oil (Rosa damascena Mill. f. Trigintipetala Dieck) is an integral part of traditional homeopathic medicine, because its antioxidant properties [18], neuropharmacological effects [19], and relaxant effects [20]. Bulgarian rose oil is a mixture of over 300 components belonging to the terpene and non-terpene hydrocarbons, glycosides, flavonoids [21], citronellol (20–34%), Geraniol (15–22%), Farnesol (2.35%), Neroll (5–12%), linalool and esters [21]. In addition, some components of rose oil as anthocyanins, flavonoids, terpenes, glycosides, kaempferol and quercetin, have antioxidant activity [22,23]. Moreover, Ulusoy et al. [23]. has evaluated the chemical compositions of rose oil isolated from Rose Damascena Mill. by GC–MS and found the main constituents were citronellol and geraniol (>55%). Nevertheless, phenylpropanoid such as eugenol is also an important component in rose essential oil [24]. Several studies indicate that the rose oil has a wide range of biochemical activities, such as scavenging free radicals, antimutagenic and antidepressant effects which may partly be due to the phenolics abundance [16,25]. Oil polar phenolics have a scavenging activity against superoxide anion (•O2‾) and hydrogen peroxide (H2O2), also a capability to prevent the ROS generation. In vitro inhibitory the effect on eicosanoids production and on platelet aggregation have also indicated their capacity to scavenge nitrogen reactive species (RNS) such as peroxynitrite (NOO‾) suggests a protective effect against nitration of tyrosine and DNA and protein damage [26].
The aim of the present study was to investigate whether the pretreatment with antioxidants would decrease oxidative stress induced by the L-dopa. To achieve the ultimate goal of this research, we investigated the levels of lipid peroxidation measured as MDA, protein carbonyl content (PCC), and advanced glycation end products (AGEs) in blood plasma of experimental model of healthy mice.
2. Materials and methods
2.1. Chemicals
2-Thiobarbituric acid (TBA), L-3,4 dihydroxyphenyl alanine (L-dopa), L-Ascorbic acid were purchased from Sigma-Aldrich Chemie GmbH (Germany). All other chemicals that were used in the study were analytical grade. Deionized and distilled water were used for all experiments. Essential Rose oil was provided from the Institute for Roses and Aromatic Plants, Kazanluk, Bulgaria.
2.2. Animals
In the experiment were used male non-inbred albino mice (25–40 g). The experimental animals were housed in polycarbonate cages in controlled conditions: 12 h light/dark cycles, 18–23 °C and humidity 55%, with free access to tap water, provided by the Trakia University vivarium, Stara Zagora, Bulgaria. The animal procedures were in accordance with Directive 2010/63/EU on the protection of animals used for experimental and other scientific work, and approved by the Ethical Committee for Animals of BABH and Trakia University, Stara Zagora, Bulgaria (131/ 6000-0333/ 09.12.2015).
Mice were divided into four groups (12 animals in each group). The control group of mice was inoculated two i.p. injections with solvent, only. The second injection was administered 45 min after the first. To study the L-dopa effect we used the acute model [27]. The mice from all tested groups (except controls) received either two i.p. injections of L-dopa (100 mg/kg) followed by benserazide (10 mg/kg). The second injection was administered 45 min after the first. The groups undergoing combination therapy were pre-treated first for 1 h with i.p. injections with Ascorbic acid (400 mg/kg), Rose oil (400 mg/kg) and after that received L-dopa and benserazide. All mice were sacrificed by light anesthesia (Nembutal 50 mg/kg i.p.) after 30 min. Fresh blood (1-2 cm3) was collected directly from the heart in cold EDTA-containers (5 cm3 Monovette, Germany), and centrifuged at 4000 rpm, 10 min, 4 °C and plasma samples were carefully separated.
2.3. Ex vivo spectrophotometry assay for evaluation the levels of MDA
To evaluate the levels of lipid peroxidation was used Thiobarbituric acid reactive substances (TBARS) assay, which measures MDA reactive substances [28]. The spectrophotometric measurements were performed on a Thermo Scientific spectrophotometer.
2.4. Enzyme-linked immunosorbent assay
Preliminarily were determined the total protein amount in the test sample with the Total Protein Kit, Human. Each protein sample were diluted to 10 μg/mL in 1X PBS, pH = 7.4 prior to use in the assay. Protein carbonyl content was measured using an OxiSelect protein carbonyl ELISA kit (Cell Biolabs). Briefly, the BSA standard (reduced/oxidized) and the assayed samples (10 μg/mL) were adsorbed on a 96-well plate for 2 h at 37 °C. Protein carbonyl found in samples and standard reacted with DNPH to form DNP hydrazone, treated with anti-DNP antibody and HRP conjugated secondary antibody. Protein carbon content in the samples was determined by a standard curve prepared from the absorbance obtained on the basis of the oxidized/reduced BSA standards and the protein carbonyl content in the samples were calculated in nmol/mg.
The AGEs level was similarly assessed with an OxiSelect AGE competitive ELISA kit (Cell Biolabs). an AGE conjugate is coated on an ELISA plate. The unknown AGE protein samples or AGEBSA standards are then added to the AGE conjugate preabsorbed ELISA plate. After a brief incubation, an anti-AGE polyclonal antibody is added, followed by an HRP conjugated secondary antibody. The content of AGE protein adducts in unknown samples is determined by comparison with a predetermined AGE-BSA standard curve.
2.5. Statistical analysis
Statistical analysis was performed with Statistica 7, StaSoft, Inc. and the results were expressed as means ± S.E. All data were expressed as mean ± SE and obtained by one-way ANOVA. p > 0.05 was considered statistically significant. To define which groups are different from each other were used LSD post hoc tests.
3. Results
In the current manuscript was investigated the possibilities of reducing the oxidative stress induced by L-dopa by combining with liposoluble Rose oil [29]. The results were compared with animals treated with L-dopa alone and with a reference antioxidant –vitamin C (ascorbic acid). Based on literature data [30], we selected the 400 mg/kg dose as "protector" for essential oils and the referent antioxidants –ascorbic acid.
3.1. MDA levels in blood plasma
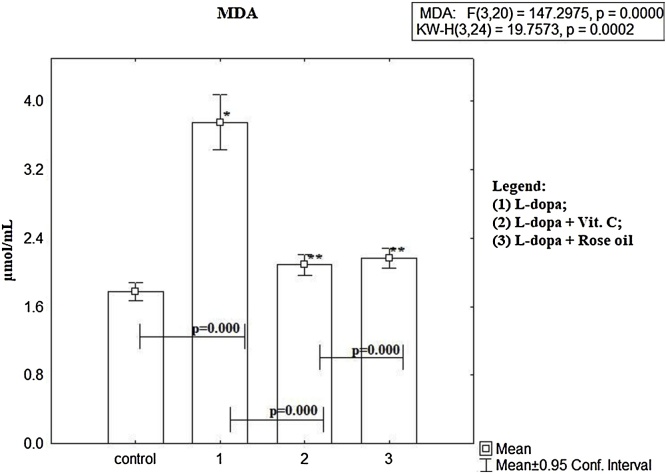
Fig. 1 shows the plasma MDA level in healthy untreated mice and mice treated with L-dopa and combinations of L-dopa with Rose oil.
Fig. 1.
The MDA level in mice treated with L-dopa alone and in combination of L-dopa + SLENU/ Ascorbic acid/Rose oil, were statistically significant higher vs controls, p < 0.00, t-test. To define which groups are different from each other have used LSD post hoc test. The results are presented as mean ± S.E. p < 0.01; (*) vs to controls; (**) vs L-dopa.
The lipid peroxidation products measured as MDA level from mice treated with L-dopa alone was statistically higher compared to controls (mean 3.75 ± 0.12 μmol/mL, vs mean 1.77 ± 0.03 μmol/mL, p < 0.00, t –test). The MDA level in the combination L-dopa + Ascorbic acid is statistically significantly higher than in controls (mean 2.08 ± 0.04 μmol/mL vs mean 1.77 ± 0.03 μmol/mL, p < 0.00, t –test) and statistically significantly lower than in mice treated with L-dopa alone (mean 2.08 ± 0.04 μmol/mL vs mean3.75 ± 0.12 μmol/mL, р <0.00, t–test). MDA level in combination L-dopa + Rose oil is statistically significantly higher than controls (mean 2.16 ± 0.05 μmol/mL vs mean 1.77 ± 0.03 μmol/mL, p < 0.00, t-test), and statistically lower than samples treated with L-dopa alone (mean 2.16 ± 0.05 μmol/mL vs mean 23.75 ± 0.12 μmol/mL, p < 0.00, t-test).
3.2. Protein carbonyl content (PCC) determination in blood plasma
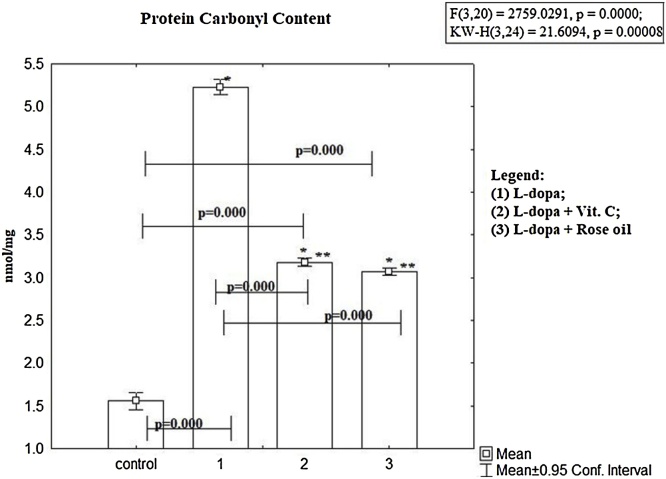
Fig. 2 shows the PCC in blood plasma from mice treated with L-dopa alone and in combination with antioxidants. Compared to controls the PCC in L-dopa group is statistically significant higher then controls (mean 5.23 ± 0.01 nmol/mg vs mean 1.55 ± 0.04 nmol/mg, p < 0.00, t–test), and all group pretreated with combination L-dopa + antioxidants. Statistically significant increase in PCC is observed in group treated with L-dopa + Ascorbic acid versus controls (mean 3.18 ± 0.09 nmol/mg vs mean 1.55 ± 0.04 nmol /mg, p < 0.00, t-test), but compared with group treated with L-dopa alone, the PCC level was statistically significant lower (mean 3.18 ± 0.09 nmol/mg vs. 5.23 ± 0.01 nmol/mg, p < 0.00, t-test). The L-dopa + Rose oil combination compared to controls was statistically significantly higher (mean 3.07 ± 0.02 nmol/mg vs. mean 1.55 ± 0.04 nmol/mg, p < 0.00, t-test), and statistically significantly lower in samples treated with L-dopa alone (mean 3.07 ± 0.02 nmol/mg vs. mean 5.23 ± 0.35 nmol/mg, p < 0.00, t-test).
Fig. 2.
The protein carbonyl content (PCC) in mice treated with L-dopa alone and in combination of L-dopa + SLENU/ Ascorbic acid/ Rose oil were statistically significant higher than controls, p < 0.00, t-test. To define which groups are different from each other have used LSD post hoc test. The results are presented as mean ± S.E. p < 0.01; (*) vs to controls; (**) vs L-dopa.
3.3. Advanced glycation end products (ages) blood plasma
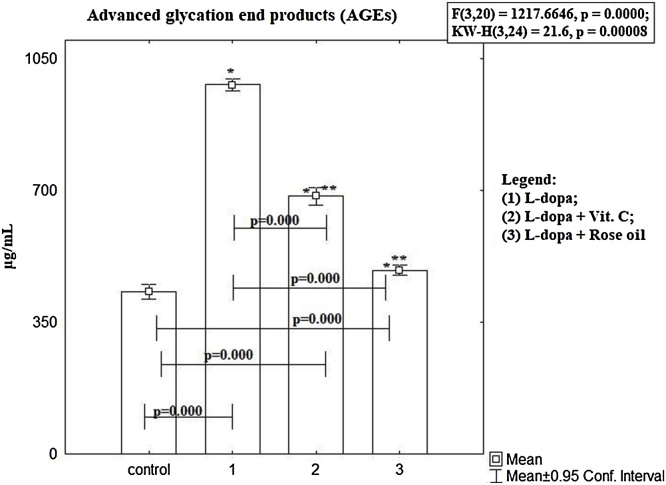
The AGEs (Fig. 3) in blood plasma in group treated with L-dopa alone was statistically significantly higher compared to control group (982 ± 14.69 μg/ml vs 432 ± 19.05 μg/ml, p < 0.00, t-test). Statistically significantly increase was observed in pretreated with antioxidants groups compared to controls: Ascorbic acid + L-dopa (686 ± 21.6 μg/ml, vs 432 ± 19.05 μg/ml, p < 0.00, t-test), and Rose oil + L-dopa (489 ± 13.1 μg/ml, vs 432 ± 19.05 μg/ml, p < 0.000, t-test). Both groups pretreated with antioxidants show statistically significant decrease compared to group treated with L-dopa alone (p < 0.00).
Fig. 3.
Advanced glycation end products (AGEs) in blood plasma in mice treated with L-dopa alone and in combination of L-dopa + SLENU/Ascorbic acid/Rose oil were statistically significant higher than controls, p < 0.00, t-test. To define which groups are different from each other have used LSD post hoc test. The results are presented as mean ± S.E. p < 0.01; (*) vs to controls; (**) vs L-dopa.
3.4. Correlations between parameters
All parameters show positive correlation MDA vs AGEs show r = 0.91, p = 0.00; MDA vs PCC r = 0.92, p = 0.00; PCC vs AGEs show r = 0.93, p = 0.00.
4. Discussion
L-dopa therapy is the most efficient method of Parkinson Disease treatment [31]. Although L-dopa has a relatively short half-life and is rapidly metabolized in plasma, the result of treatment is only symptomatic relief, and moreover, there is development of dyskinesia and ultimately worsening of the disease. In the current research, to evaluate whether OS is involved in L-dopa -induced toxicity was studied the influence of acute treatment with L-dopa on the OS biomarkers MDA, PCC and AGEs in experimental model of healthy mice. The protective effects of two different antioxidants an essential oil isolated from Rosa damascena Mill., and vitamin C was also investigated.
The demand for natural antioxidants, especially with plant origin, has increased in recent years [32]. The effectiveness of antioxidants is probably limited by their bioavailability and the fact that they would have to be present in high concentrations to be able to compete with endogenous targets. Current result provides evidence that MDA level in plasma of acute model of mice treated with L-dopa alone was significantly higher than controls (Fig. 1), meaning that L-dopa treatment induced OS in mice. Another indirect proof for OS involvement in drug-induced toxicity is the overcome by adding typical antioxidants [31,33]. Antioxidants are needed to prevent the formation and counteract the action of free radicals. In the present study MDA results obtained from a blood after treatment with the combinations L-dopa + Ascorbic acid and L-dopa + Rose oil shows levels commensurate with the controls and statistically decreased from the L-dopa group, only. This appears to confirm that the antioxidants can successfully limit peroxidation processes in lipids under the described experimental conditions. The intake of vitamin C and Rose oil is associated with a reduced risk of degenerative or chronic disease, possibly through antioxidant mechanisms [16]. Lipid peroxidation induced by OS and oxidants, generates a variety of lipid peroxidation products, including reactive proteins and more stable products as ketones, alkanes and malondialdehydes. These products react on cellular and tissular proteins and form adducts, which induced protein dysfunction and alter cellular response, and increase with aging together with antioxidant defense. MDA is formed during lipid peroxidation and binds to nucleophiles, is one of the most common aldehydes. In addition, MDA reacts with Lys residues, creating Schiff bases and plays an important role in the modulation of low density lipoproteins. The protein residues formed during oxidation of polyunsaturated fatty acid, leads to formation of stable Michael adducts with hemiacetal structure [34]. This reaction involve in fatty acid interaction with proteins, and their modification. As it seen in our model the improved antioxidant status helps to minimize oxidative damage, and thus can delay or prevent pathological changes. This suggests the potential benefits of antioxidant dietary strategies for reducing the amount of free radicals and their complications [35,36].
The presented results show that the blood after treatment with L-dopa, compared with the controls, has a significant increase in the proteins (PCC, p < 0.00). PCC assays involve interaction between the carbonyl group and 2, 4-dinitrophenylhydrazine (DNPH), which leads to formation of a stable dinitrophenyl (DNP) hydrazone product. The last is detected by enzyme -linked Immunosorbent assay (ELISA). DNPH method is useful to quantify carbonyl content in the mixture of proteins, such as plasma, tissue homogenates, cellular extracts, or in isolated proteins. Current results show a significant increase in the PCC level in the L-dopa treated group, compared with the control, which again clearly emphasizes that the oxidative stress caused by drug therapy is induced in the mice plasma. In addition, the groups treated with antioxidants show statistically significant decrease compared to L-dopa treated group (p < 0.00, Fig. 2). These results may be related to the presence of oxidative stress after treatment with L-dopa and show that antioxidants can slowly migrate proteins under oxidative conditions and protect them from oxidation [37].
Advanced glycation end products (AGEs) are a heterogeneous, complex group of compounds that are formed mainly via the Maillard reaction. This reaction become as the reducing sugar reacts in a non-enzymatic way with amino acids in proteins, lipids or DNA. Protein carbonyl formation is an important marker for protein oxidation that results from free radical attack on amino acid side chains [38,39]. The PCC increases in stress and accumulated carbonyls cause protein damage and dysfunction. Augmented accumulation of AGEs may indicate an accelerated process of aging-related deterioration that likely accompanies neurodegeneration. However, AGEs mainly accumulate on long-lived proteins, and analysis of AGEs in blood may therefore not necessarily reflect their tissue levels, yet circulating AGEs keep their biomarker potential [39]. In vivo study the Maillard reaction products have received increasing attention due to AGEs association with certain chronic diseases, such as diabetes mellitus, Parkinson’s disease and Alzheimer’s disease, as well as during the aging process [40].
Carbonyl stress induced progressively protein dysfunctions and harmed all tissues, with pathological consequences such as inflammation and apoptosis contributing to the diseases progress [35]. This proteins inhibiting could stop pathological consequences of carbonyl stress. Moreover, carbonyl stress is the accumulation of reactive di-carbonyls, whether due to an increase in substrate content or a decrease in detoxification. Such intermediate products can continue to form AGEs or, during accumulation, as in carbonyl stress, can only cause damage [6]. Reactive carbonyl compound (RCC) is formed endogenously during lipid peroxidation and the glycation of carbohydrates are precursors of advanced glycation products (AGEs), which form cross-links on tissular proteins (carbonyl stress) and accumulate during aging in chronic disease [35]. Various research groups investigated in vitro the antioxidant and free radical scavenging activities of ethanol and acetone extracts of fresh Rosa damascene flower petals. It was established a good expression of DPPH· and ABTS+ radical scavenging activity and compared with standard ascorbic acid [34]. Another research team studied methanol extracts obtained from various plants, including Rosa Damascena extract, containing significant amounts of polyphenols, and reported excellent antioxidant activity for this extract, as evidenced by the scavenging of DPPH·, ABTS+, •NO, •OH, O2 and ONOO‾ [40]. In the same study, Rosa damascena extract shows significant potential for preventing oxidative end-products and radical activity [41]. Phenols and flavonoids are the keys ingredients indicated in most essential plants (rose oil), which have been reported by a number of researchers to have antioxidant and free radical activity [42]. Natural polyphenols and terpenes contained in essential oil shows a protective effect on neurodegenerative diseases [43], which is due to their ability to cross the blood-brain barrier and directly to capture the chelated transition metal ions and ROS/RNS in pathological concentrations [44] and to exert antioxidant effects into brain [45,46]. Essential oils as antioxidants have been studied in detail to investigate their protective role for highly unsaturated lipids in animal tissues [47]. They shown their actions as hepatoprotective agents [48] and coincide with our results. Furthermore, oils possess antioxidant properties at extremely low dilution rates administered either by inhalation or by lipophilic fractions, interacting with the lipid parts of the cell membranes, thereby altering the calcium ion channels activity and in some dosas saturated the membranes [49].
5. Conclusion
This study shows that L-dopa induces oxidative toxicity proved by the increased levels of OS biomarkers MDA, PCC and AGEs tested in experimental model of healthy mice. The antioxidants – Vitamin C and Rose oil, exhibit pronounced interfering and antioxidant properties against the acute oxidative toxicity of L-dopa. Furthermore, obviously the studied classic antioxidant, and essential oil can protect organisms from L-dopa oxidative toxicity induced and may play a key role in end products disarm. It must be emphasized, that Rosa damascena oil exhibited behavior very similar to the classic antioxidants –vitamin C, making it potential candidates for extensive experimental research to their possible use as protectors against oxidative toxicity triggered by drug therapy of neurodegenerative diseases.
Transparency document
Acknowledgment
This work was supported by the scientific project № 1/2018 Trakia University, Stara Zagora, Bulgaria.
References
- 1.Silva B., Breydo L., Fink A. Agrochemicals, α-synuclein, and Parkinson’s disease. Molec Neurobiol. 2013;47:598–612. doi: 10.1007/s12035-012-8333-2. [DOI] [PubMed] [Google Scholar]
- 2.Petzinger G., Holschneider D., Fisher B. The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast. 2015;1:29–39. doi: 10.3233/BPL-150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández B., Ferrer I., Gil F. Biomonitorization of iron accumulation in the substantia nigra from Lewy body disease patients. Toxicol. Rep. 2017;4:188–193. doi: 10.1016/j.toxrep.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies S., Kennewell P., Russell A. Wynne Stemistry: the control of stem cells in situ using chemistry. J. Med. Chem. 2015;58:2863–2894. doi: 10.1021/jm500838d. [DOI] [PubMed] [Google Scholar]
- 5.Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov. Disord. 2015;30:4–18. doi: 10.1002/mds.26102. [DOI] [PubMed] [Google Scholar]
- 6.Fox S., Katzenschlager R., Lim S. Movement Disorder Society Evidence Based Medicine Committee, International Parkinson and movement disorder society evidence based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018 doi: 10.1002/mds.27372. [DOI] [PubMed] [Google Scholar]
- 7.Brott D., Andersson H., Stewart J. A peripherally restricted P2Y12 receptor antagonist altered rat tumor incidences with no human relevance: mode of action consistent with dopamine agonism. Toxicol. Rep. 2014;1:1202–1212. doi: 10.1016/j.toxrep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh R., Barden A., Mori T. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 9.Roede J., Uppal K., Park Y. Transcriptome–metabolome wide association study (TMWAS) of maneb and paraquat neurotoxicity reveals network level interactions in toxicologic mechanism. Toxicol. Rep. 2014;1:435–444. doi: 10.1016/j.toxrep.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valnet C. Edizioni REI.; 2015. Essential Oils and Aromatherapy. [Google Scholar]
- 11.Lei W. University of Missouri-Columbia; 2015. Modulation of Inflammatory Responses by Sutherlandia frutescens. [Google Scholar]
- 12.Smirnoff N. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Rad. Biol. Med. 2018 doi: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahal A., Kumar V., Singh B. Oxidative stress, prooxidants, and antioxidants: the interplay. Comput. Biomed. Res. 2014 doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliwell J. Oxford University Press; USA: 2015. Gutteridge Free Radicals in Biology and Medicine. [Google Scholar]
- 15.Jaiswal S., Gupta V., Ansari M. Vitamin C acts as a hepatoprotectant in carbofuran treated rat liver slices in vitro. Toxicol. Rep. 2017;1(4):265–273. doi: 10.1016/j.toxrep.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolova G., Karamalakova Y., Kovacheva N. Protective effect of two essential oils isolated from Rosa damascena Mill. And Lavandula angustifolia Mill, and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. Regul. Toxicol. Pharmacol. 2016;81:1–7. doi: 10.1016/j.yrtph.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Sagdiç O., Özkan G., Baydar N. Note: antioxidant and antibacterial activities of Rosa damascena flower extracts. Rev. Agaroquimica Y Tecnol. Aliment. 2004;10(4):277–281. [Google Scholar]
- 18.Nyeem M., Alam M., Awal M. CNS depressant effect of the crude ethanolic extract of the flowering tops of Rosa damascena. Iran. J. Pharm. Res. 2007;5:171–174. [Google Scholar]
- 19.Boskabady M., Kiani S., Rakhshandah H. Relaxant effects of Rosa damascena on guinea pig tracheal chains and its possible mechanism (s) J. Ethnopharm. 2006;106:377–382. doi: 10.1016/j.jep.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Rusanov K., Kovacheva N., Atanassov A. Rosa damascena Mill., the oil-bearing Damask rose: genetic resources, diversity and perspectives for molecular breeding. Floricult. Ornamental Biotechnol. 2009;3:14–20. [Google Scholar]
- 21.Duke J., Cseke L., Warber S. CRC press; 2016. Natural Products From Plants. [Google Scholar]
- 22.Slavov A., Vasileva I., Stefanov L. Valorization of wastes from the rose oil industry. Rev. Environ. Sci. Biotechnol. 2017;16:309–325. [Google Scholar]
- 23.Ulusoy S., Bosgelmez-Tmaz G., Secilmis-Canbay H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr. Microbiol. 2009;59:554. doi: 10.1007/s00284-009-9475-y. [DOI] [PubMed] [Google Scholar]
- 24.Mahboubi M. Rosa damascena as holy ancient herb with novel applications. Afr. J. Tradit. Complement. Altern. Med. 2016;6:10–16. doi: 10.1016/j.jtcme.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hongratanaworakit T. Relaxing effect of rose oil on humans. Nat. Prod. Commun. 2009;4:291–296. [PubMed] [Google Scholar]
- 26.Dimitrios B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006;17:505–512. [Google Scholar]
- 27.Bottiglieri T., Arning E., Wasek B. Acute administration of L-DOPA induces changes in methylation metabolites, reduced protein phosphatase 2A methylation, and hyperphosphorylation of Tau protein in mouse brain. J. Neurosci. 2012;32:9173–9181. doi: 10.1523/JNEUROSCI.0125-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plaser Z., Cushman L., Jonson B. Estimation of product of lipid peroxidation (Malonyl Dialdehyde) in biochemical systems. Anal. Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 29.Umezu T., Nagano K., Ito H. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2006;85:713–721. doi: 10.1016/j.pbb.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Melo A., Monteiro L., Lima R. Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxidative Med. Cell Longev. 2011 doi: 10.1155/2011/467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang J., Xiong Y. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: a review. Meat Sci. Vet. Public Health. 2016;120:107–117. doi: 10.1016/j.meatsci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Alam Z., Jenner A., Daniel S. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8‐hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69(3):1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuo Y., Nussbaum R. Prolongation of chemically-induced methemoglobinemia in mice lacking α-synuclein: a novel pharmacologic and toxicologic phenotype. Toxicol. Rep. 2015;2:504–511. doi: 10.1016/j.toxrep.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negre A., Salvayre C., Coatrieux C. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Brit. J. Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uttara A., Singh P., Zamboni R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hondur G., Göktaş E., Yang X. Oxidative stress–related molecular biomarker candidates for Glaucoma. Investig. Ophthalmol. Visual Sci. 2017;58(10):4078–4088. doi: 10.1167/iovs.17-22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oscar A., Cherninkova S., Haykin V. Amblyopia screening in Bulgaria. J. Pediatr. Ophthalmol. Strabismus. 2014;51(5):284–288. doi: 10.3928/01913913-20140618-01. [DOI] [PubMed] [Google Scholar]
- 38.Tatke P., Patil P., Gabhe S. In vitro antioxidant and free radical scavenging activity of extracts of Rosa damascena flower petals. AJPCT. 2015;3:589–601. [Google Scholar]
- 39.Kalim M., Bhattacharyya D., Banerjee A. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Compl. Altern. Med. 2010;10:77. doi: 10.1186/1472-6882-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manjamalai A., Grace V. Antioxidant activity of essential oils from Wedelia chinensis (Osbeck) in vitro and in vivo lung cancer bearing C57BL/6 mice. Asian Pac. J. Cancer Prev. 2012;13:3065–3071. doi: 10.7314/apjcp.2012.13.7.3065. [DOI] [PubMed] [Google Scholar]
- 41.Mihaylova B., Petkova I., Ch Rankova-Yotova. Plasma endothelin-1 and endothelin-A receptor concentrations in patients with primary open-angle glaucoma. Biotechnol. Biotechnol. Eq. 2017;31(4):782–787. [Google Scholar]
- 42.Aquilano K., Baldelli S., Rotilio G. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem. Res. 2008;33(12):2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 43.Mokni M., Elkahoui S., Limam F. Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochem. Res. 2007;32:981–987. doi: 10.1007/s11064-006-9255-z. [DOI] [PubMed] [Google Scholar]
- 44.Komsiiska D., Nikolova G., Karamalakova Y. Real time oxidative stress markers of patients with post-stroke depression: EPR study. Bulg. Chem. Commun. 2018;50(Sp Iss C):64–68. [Google Scholar]
- 45.Komsiiska D. Oxydative stress and post-stroke depression. Tr J. Sci. 2018;3:249–253. [Google Scholar]
- 46.Dakov N., St Kostova, Tanev Iv. Selective laser trabeculoplasty in primary open angle glaucoma - efficiency and correlated parameters. Compt. rend Acad. Bulg. Sci. 2018;71(2):288–298. [Google Scholar]
- 47.Li T., Liu W., Zhao P. Evaluation of essential oil or/and emulsifier in low energy density diets on growth performance, nutrient digestibility, blood cholesterol and meat quality in finishing pigs. Ital. J. Anim. Sci. 2017;16(4):624–630. [Google Scholar]
- 48.Sharifi-Rad J., Sureda A., Tenore G. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules. 2017;22(1):70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stine K., Brown T. 3rd edition. Published CRC Press; 2015. Principles of Toxicology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.