Abstract
Background
It has been suggested that, during primary shoulder arthroplasty, surgeons should identify the axillary nerve through direct visualization, palpation, or the “tug test” to prevent iatrogenic nerve injury. Our goal was to document the rate of isolated axillary nerve injury (IANI) in patients who had undergone primary anatomic total shoulder arthroplasty (TSA) or reverse total shoulder arthroplasty (RTSA) without routine identification of the axillary nerve.
Methods
Data on 869 cases of primary shoulder arthroplasty (338 TSAs and 531 RTSAs) performed by 1 surgeon between 2003 and 2017 were reviewed. Neither the tug test nor identification of the axillary nerve through palpation or visualization was used in any case. The primary outcome was new IANI documented within 3 months after arthroplasty. The frequency of IANI was summarized using point estimates and 95% confidence intervals (CIs).
Results
Six cases met the criteria for IANI. The overall incidence of IANI was 0.7% (95% CI, 0.3%-1.4%). The incidence of IANI was 0.3% (95% CI, 0%-1.6%) after TSA and 0.9% (95% CI, 0.3%-2.1%) after RTSA. All IANIs were cases of neurapraxia, and all patients had experienced complete neurologic recovery at last follow-up.
Conclusion
Complete, permanent IANI resulting from direct surgical trauma during primary shoulder arthroplasty can be avoided without using the tug test or routine identification of the nerve. A low incidence of partial temporary IANI can be expected, which may be related to indirect traction injuries.
Keywords: Axillary nerve lesion, complications, neurologic complications, reverse total shoulder arthroplasty, anatomic total shoulder arthroplasty, primary shoulder arthroplasty
One of the major complications of shoulder arthroplasty is nerve injury. For anatomic total shoulder arthroplasty (TSA), the prevalence of nerve injury ranges from 1% to 4%.5, 16 The rate of nerve injury for primary reverse total shoulder arthroplasty (RTSA) ranges from 2% to 8%.3, 7, 16, 28 The most commonly reported pattern of nerve injury after primary TSA or RTSA is brachial plexopathy affecting 1 or more cords.5, 16, 20, 26, 27 The second most common pattern of nerve injury is injury to individual peripheral nerves, of which the axillary nerve is the most frequently affected.2, 16, 19, 20 Axillary nerve injuries can be devastating if they are permanent because the deltoid muscle is the primary elevator of the arm.
There are many potential causes of nerve injury after shoulder arthroplasty. The most common cause is stretching of the plexus during surgery by positioning of the arm.14, 34 Intraoperatively, excessive time with the operative arm in external rotation and extension, with either abduction or adduction, particularly during glenoid and humeral preparation, increases the risk of nerve injury.20, 26, 27 A second possible cause is a stretch injury to the nerves caused by retractors used for visualization. A third mechanism is direct injury to the nerves caused by a retractor, saw, or scalpel. Other possible causes include peripheral nerve block, vascular injuries, and arm lengthening.17, 18, 20, 27, 35, 38
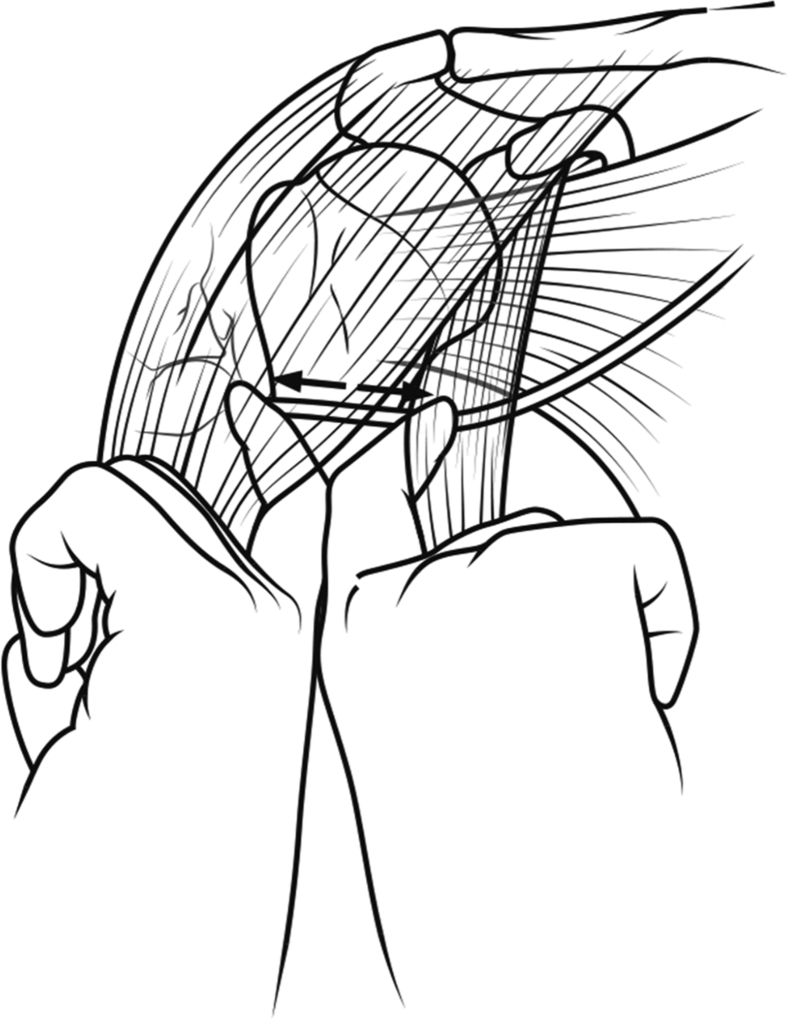
To protect the axillary nerve from direct injury during primary shoulder arthroplasty, some authors have suggested that surgeons should routinely palpate the axillary nerve, expose the axillary nerve, or perform the “tug test” to isolate the axillary nerve.9, 11, 12 The tug test was first described by Flatow and Bigliani,11 who recommended that the surgeon direct the index finger posteriorly in the subdeltoid space, feeling for the circumflex branch of the axillary nerve while placing the other index finger medially into the subcoracoid space and over the axillary nerve. A gentle tug on the nerve with the subdeltoid index finger can be transmitted across the nerve and felt with the other index finger, allowing the surgeon to identify the nerve location and thereby protect it (Fig. 1). Although there is support for the use of the tug test, it has not been studied extensively or validated.12, 30 Moreover, the risk of direct injury to the axillary nerve when performing primary shoulder arthroplasty without routine identification of the nerve is undetermined. A previous study of patients undergoing open capsular shifts for shoulder instability reported that neither isolating the nerve nor performing the tug test was necessary to protect the nerve from direct injury during the procedure.24
Figure 1.
Tug test ( ). The index finger is directed posteriorly in the subdeltoid space while the other index finger is placed medially into the subcoracoid space and over the axillary nerve. A gentle tug on the nerve with the subdeltoid index finger can be transmitted across the nerve and felt with the other index finger, confirming that the nerve has been correctly identified.
). The index finger is directed posteriorly in the subdeltoid space while the other index finger is placed medially into the subcoracoid space and over the axillary nerve. A gentle tug on the nerve with the subdeltoid index finger can be transmitted across the nerve and felt with the other index finger, confirming that the nerve has been correctly identified.
(Reproduced with permission from Chalmers PN, Van Thiel GS, Trenhaile SW. Surgical exposures of the shoulder. J Am Acad Orthop Surg 2016;24:250-8.)
The aim of this study was to document the rate and characteristics of clinically evident isolated axillary nerve injury (IANI) in patients who had undergone primary TSA or RTSA without axillary nerve identification through direct visualization, palpation of the nerve, or use of the tug test. We hypothesized that primary TSA and RTSA can be performed safely without isolating or palpating the axillary nerve or performing the tug test. The results of this study will help patients and surgeons understand the risks of clinically evident IANI after primary shoulder arthroplasty without routine identification of the axillary nerve.
Materials and methods
Patient selection
All patients aged 18 years or older who underwent elective shoulder arthroplasty at our institution performed by the senior author (E.G.M.) between January 1, 2003, and July 31, 2017, were identified using our shoulder arthroplasty database. We included patients who underwent primary shoulder arthroplasty (first elective TSA or RTSA). We excluded patients who underwent revision arthroplasty, which was defined as any procedure performed after failure of cup arthroplasty, hemiarthroplasty, TSA, or RTSA.
We identified 872 primary TSAs performed during the study period. Of these patients, 3 did not have at least 3 months of follow-up and were excluded, leaving 869 patients (99.7%) for analysis. Of these, 338 (39%) underwent primary TSA and 531 (61%) underwent primary RTSA. Our cohort consisted of 453 women and 416 men with a mean age (± standard deviation) of 67 ± 11 years.
Surgical technique
All patients underwent general anesthesia with or without a single-injection interscalene block or interscalene catheter. Patients underwent RTSA or TSA by a deltopectoral approach in a semi-sitting beach-chair position. The Solar Total Shoulder System (Stryker, Mahwah, NJ, USA) was used for all TSAs. RTSAs were performed using the Encore Reverse Shoulder Prosthesis (DJO Surgical, Austin, TX, USA) or the Reunion RTSA system (Stryker). When the subscapularis tendon was present, the interval between it and the conjoint tendon was developed. A knee retractor was placed between the 2 muscles to protect the brachial plexus. The subscapularis tendon and capsule were then released as a single unit from the lesser tuberosity and from the junction of the humeral head and the proximal humeral shaft. A blunt Hohmann retractor was placed along the axillary pouch to protect the axillary nerve while the capsule was released medially.
The rotator cuff interval was released, and the arm was then extended, adducted, and externally rotated to allow the humeral head to dislocate (Fig. 2). This arm position has been associated with nerve alerts related to brachial plexus traction.26, 27 Although external rotation alone may be helpful during subscapularis and/or capsule peel off to draw the lesser tuberosity away from the axillary nerve,29 care was taken not to leave the arm in the extended, adducted, and externally rotated position for prolonged periods. Retractors were then placed circumferentially around the humeral head, with one placed inferiorly to protect the axillary nerve and one placed medially to protect the glenoid and brachial plexus. After the humeral head was removed, the glenoid was exposed. The labrum was resected circumferentially by releasing it from its capsular attachments. The capsule was released from the glenoid rim by use of electrocautery with care taken to stay directly on bone. We did not perform a 360° subscapularis release in any case.22, 25 The described exposure allowed direct access to the glenoid without palpating or exposing the axillary nerve or using the tug test. Video 1 shows performance of this technique.
Figure 2.
Position of arm during humeral preparation in extension, adduction, and external rotation.
Study outcome
The primary outcome variable, IANI, was defined as the presence of an isolated new sensory or sensorimotor axillary nerve deficit documented in the medical record within 3 months of shoulder arthroplasty. The medical records of all included patients were reviewed in detail by 2 of the authors (C.L.L. and J.R.). Data on neurologic findings based on patient reports, physical examination findings, and electrodiagnostic studies were collected using a standard data collection form.
All physical examinations were performed by the senior author (E.G.M.) using the same examination protocol preoperatively and postoperatively. Preoperatively and postoperatively, the upper extremities were evaluated for sensation using light touch for the axillary, ulnar, radial, and median nerves.32 Sensation for all nerves was compared meticulously side to side, and any differences preoperatively or postoperatively were noted. Motor testing of the axillary nerve consisted of resisted abduction of the deltoid muscle at 90° of abduction preoperatively and postoperatively; this was compared with the contralateral side. All patients were evaluated each day while hospitalized and at 8 to 10 days, 6 weeks, and 12 weeks postoperatively. Indications for electromyography (EMG) included signs of brachial plexopathy or individual nerve injury with neurologic deficits that did not improve during the first 6 weeks after arthroplasty.
All cases of IANI were followed until complete resolution of neurologic symptoms or the date of the last documented follow-up. Documentation of neurologic status was recorded within defined periods (<3, 3-6, 7-12, or >12 months) to assess the timing of neurologic recovery. The extent of neurologic recovery was defined clinically as complete (ie, neurologic status returned to baseline), partial (ie, symptoms improved but residual deficit remained), or none (ie, deficit was unchanged from initial description), consistent with criteria used in prior studies.8, 31
Statistical analyses
The incidence of IANI was estimated for the whole cohort and for the TSA and RTSA subgroups using point estimates with their corresponding 95% confidence intervals (CIs). CIs were calculated using the Jeffreys interval method considering the low rate of events. A 2-tailed Z test was used to compare the incidence of IANI between primary TSA and RTSA. Continuous variables are reported as means and standard deviations, and categorical variables are reported as frequencies and percentages. P < .05 was considered statistically significant. All analyses were performed using Stata software (version 14; StataCorp, College Station, TX, USA).
Results
Six cases met our criteria for IANI, and all were neurapraxia lesions that resolved with no sequelae. Of the 6 IANIs, 5 occurred after RTSA and 1 occurred after TSA. The overall incidence of IANI for all patients was 0.7% (95% CI, 0.3%-1.4%). For TSA, the incidence of IANI was 0.3% (95% CI, 0%-1.6%). For RTSA, the incidence of IANI was 0.9% (95% CI, 0.3%-2.1%). Although RTSA was associated with a higher incidence of IANI than was TSA, this difference was not significant (P = .26).
The characteristics and clinical course of patients who experienced IANI are summarized in Table I. In the patient with an IANI after TSA, the deficit was both sensory and motor. Of the 5 patients with IANI after RTSA, 2 had both motor and sensory deficits whereas 3 had isolated sensory deficits. In the 3 patients with only sensory deficits, EMG was not performed and all deficits resolved within 6 months. In the 3 patients with sensory and motor changes (1 TSA and 2 RTSA patients), EMG confirmed partial IANI. All 3 patients experienced clinically complete recovery by 9 months. No patient had residual clinical sensory or motor changes at last follow-up.
Table I.
Characteristics and clinical course of axillary nerve injuries
| Case no. | Procedure | Sex | Age, yr | Diagnosis | Type of neurologic deficit | Time until neurologic deficit, wk | Subjective findings | Objective findings | EMG findings | Time until complete recovery, mo |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TSA | F | 57 | Primary OA | Sensorimotor | 12 | Weakness, numbness | Weakness in elevation, drop-arm sign | Partial axillary neuropathy∗ | 9 |
| 2 | RTSA | M | 63 | OA with bone loss | Sensory | 11 | Dysesthesia, numbness | Decreased sensation | Not performed | 6 |
| 3 | RTSA | F | 53 | IMRCT with pseudoparalysis | Sensorimotor | 2 | Dysesthesia, numbness | Decreased sensation, weakness in elevation, drop-arm sign | Partial axillary neuropathy∗ | 10 |
| 4 | RTSA | M | 85 | CTA | Sensory | 2 | Numbness | Decreased sensation | Not performed | 1 |
| 5 | RTSA | F | 70 | RA | Sensorimotor | 8 | Weakness, numbness | Decreased sensation, weakness in elevation, drop-arm sign | Partial axillary neuropathy∗ | 6 |
| 6 | RTSA | M | 59 | CTA | Sensory | 7 | None | Decreased sensation | Not performed | 1 |
EMG, electromyography; TSA, anatomic total shoulder arthroplasty; F, female; OA, osteoarthritis; RTSA, reverse total shoulder arthroplasty; M, male; IMRCT, irreparable massive rotator cuff tear; CTA, cuff tear arthropathy; RA, rheumatoid arthritis.
Abnormal spontaneous activity of the deltoid with fast-firing voluntary motor units of increased duration and amplitude.
Discussion
In our patients, the incidence of IANI after primary TSA or RTSA was less than 1% and all isolated injuries were cases of neurapraxia that resolved completely. These findings suggest that to prevent iatrogenic direct complete injury to the axillary nerve during primary shoulder arthroplasty, routine identification of the nerve by performing palpation, visualization, or the tug test is unnecessary if precautions are taken to protect the nerve. Despite not palpating the axillary nerve, directly observing the axillary nerve, or performing the tug test on the axillary nerve, neurapraxia lesions were uncommon. This study supports the safety of not routinely identifying the axillary nerve during primary shoulder arthroplasty using the described surgical technique, but it does not show whether routine identification of the axillary nerve might reduce or prevent IANIs.
The incidence of IANI in our study (0.7%) is within the range of previously reported rates (0%-16%; Table II).1, 6, 15, 16, 18, 20, 21, 22, 26, 37 Our reported incidence of IANI after TSA of 0.3% is also comparable to previously reported rates of 0.72% to 1.8%.10, 13, 23, 33 Similarly, our reported incidence of IANI after primary RTSA of 0.9% is within the previously reported range of 0.42% to 16%.6, 15, 16, 18, 37 Although we found a higher incidence of IANI after RTSA (0.9%) than after TSA (0.3%), this difference was not significant. RTSA has been associated with a higher risk of nerve injury compared with TSA. Lädermann et al16 compared the incidence of nerve injury in TSA vs. RTSA using postoperative EMG and nerve conduction studies (NCS) and found that RTSA was associated with a 10.9-fold greater risk of postoperative nerve injury than TSA. They reported 10 isolated nerve injuries, 6 of which were in the axillary nerve and all of which were in the RTSA group. They speculated that the greater risk of any neurologic injury during RTSA compared with TSA was attributable to excessive arm lengthening.
Table II.
Axillary nerve mononeuropathy reported in the literature after primary shoulder arthroplasty
| Author (year) | Procedure | No. of shoulders | No. of axillary nerve injuries (%) | Diagnostic basis | Type of neurologic deficit | Recovery (time until recovery, mo) |
|---|---|---|---|---|---|---|
| Torchia et al33 (1997) | Primary TSA | 113 | 1 (0.88) | NR | NR | NR (NR) |
| Edwards et al10 (2002) | Primary HA and TSA | 555 | 4 (0.72) | NR | NR | Complete in 2 patients (NR), none in 2 patients (NA) |
| Godenèche et al13 (2002) | Primary TSA | 268 | 2 (0.75) | Clinical | NR | None (NA) |
| Matsoukis et al23 (2003) | Primary HA and TSA | 55 | 1 (1.8) | NR | NR | Complete (NR) |
| Werner et al37 (2005) | Primary RTSA | 58 | 1 (1.7) | NR | NR | Complete (NR) |
| Boileau et al7 (2006) | Primary RTSA | 45 | 1 (2.2) | NR | NR | Partial (36) |
| Nagda et al26 (2007) | Primary HA and TSA, revision TSA | 30 | 3 (10) | EMG∗ | NR | NR (NR) |
| Lädermann et al18 (2009) | Primary and revision RTSA | 199 | 1 (0.50) | NR | NR | Complete (12) |
| Lädermann et al16 (2011) | Primary TSA | 23 | 0 (0) | NA | NA | NA |
| Primary RTSA | 19 | 3 (16) | EMG† | Sensorimotor | Complete (≤6) | |
| Walch et al36 (2012) | Primary and revision RTSA | 240 | 1 (0.42) | Clinical, EMG‡ | Sensorimotor | Complete (5) |
TSA, anatomic total shoulder arthroplasty; NR, not reported; HA, hemiarthroplasty; NA, not applicable; RTSA, reverse total shoulder arthroplasty; EMG, electromyography.
All patients underwent intraoperative neurologic monitoring. Patients with intraoperative nerve alerts underwent diagnostic EMG at least 4 weeks postoperatively.
All patients underwent EMG 3 weeks after surgery.
Patients did not undergo EMG routinely. EMG was performed after clinical diagnosis.
We defined nerve injury as a clinically evident sensory and/or motor deficit consistent with an axillary nerve injury. The use of clinical detection to define the incidence of nerve injury may lead to underdetection of subclinical axillary nerve injury, but the use of clinical detection alone has precedence in the literature.1, 4, 20, 31, 32 Although electrodiagnostic studies can often detect subclinical nerve injury, this was not a prospective study to determine subclinical axillary nerve dysfunction. Our results are consistent with what one would expect when performing an examination for nerve injury alone without routine EMG-NCS, and this practice represents the usual standard of care for detecting nerve injury after primary shoulder arthroplasty. Intraoperative neuromonitoring and postoperative electrodiagnostic studies have suggested that there are more subclinical injuries to the brachial plexus and individual nerves than commonly appreciated.1, 21, 26, 27 Nagda et al26 documented episodes of nerve dysfunction in up to 57% of patients undergoing intraoperative neuromonitoring during shoulder hemiarthroplasty and TSA. Of these episodes, 16.7% involved the axillary nerve alone. In most cases, nerve dysfunction alerts occurred during humeral and glenoid preparation and returned to baseline after the arm was returned to a neutral position. These authors found that the most common arm position creating stress on the nerves was extension and external rotation with abduction or adduction. In a study of 36 patients undergoing TSA or RTSA with intraoperative neuromonitoring, Parisien et al27 reported 203 nerve events in the 2 cohorts. Most nerve alerts in the brachial plexus occurred during humeral and glenoid preparation in both cohorts. The axillary nerve was the most frequently affected (27%) of all the peripheral nerves in both cohorts. Aleem et al1 used continuous intraoperative neuromonitoring in 282 patients who underwent TSA, RTSA, or hemiarthroplasty to determine whether nerve alerts were associated with postoperative peripheral nerve injury. The greatest frequency of nerve alerts was noted for the axillary nerve, but no axillary nerve injuries were detected clinically after surgery. Two postoperative, clinically detectable peripheral nerve injuries occurred (0.7%), but both were radial nerve injuries. Lädermann et al16 documented subclinical electromyographic changes involving mainly the axillary nerve in 47% of patients who underwent routine EMG-NCS after RTSA. However, all were partial injuries that resolved completely in less than 6 months.
Other factors have been suggested to contribute to the incidence of nerve injury after TSA or RTSA. In an anatomic study, Lädermann et al17 suggested that the axillary nerve may also be at risk at the junction of the humeral head and humeral shaft in the posterior metaphyseal area. They recommended that care be taken when reaming the metaphysis to avoid posterior humeral cortical violation, particularly when having a low humeral cut and using a large reamer. It has been suggested that patients with decreased range of motion (<10° of passive external rotation with the arm at the side) or a history of open shoulder surgery26 may be at increased risk of nerve injuries. Similarly, patients with pre-existing peripheral neuropathy or cervical radiculopathy may be at risk of increased neurologic symptoms postoperatively.16 In our study, the small number of patients with IANI prohibited evaluation of these variables and their possible contributions to IANI.
To our knowledge, ours is the first study to report the incidence of clinically evident IANI after primary shoulder arthroplasty performed without routine identification of the axillary nerve. However, the study has limitations that should be considered when interpreting the results. First, the retrospective method of data collection introduces the possibility of missing transient yet clinically relevant events that may not have been reported by the patient or documented by the surgical team in the patient records. Second, without a control group in which the routine identification of the axillary nerve was performed, we cannot conclude that the presented technique is equivalent in, better at, or worse at preventing partial or complete IANI compared with routine performance of the tug test or palpation or visualization of the axial nerve. Third, our results are those of a high-volume, fellowship-trained shoulder and elbow surgeon who performs many primary and complex revision shoulder procedures.
The surgical technique described in this study may differ from the techniques used by other surgeons, who may resect the anterior capsule or release the subscapularis inferiorly to increase excursion of this muscle. Similarly, the presented surgical technique may differ from that required to expose the glenoid in patients with major scarring in the anterior-inferior glenoid. Consequently, our conclusions cannot be extrapolated to primary shoulder arthroplasty performed with a more extensive capsular release, such as that performed with the 360° subscapularis release,22, 25 or to patients undergoing revision arthroplasty.
Other factors may have influenced our results. Prosthetic designs changed during the study period, which may have affected the axillary nerve position or tension. The retractors we used around the shoulder may not be the same as those used by other surgeons. During humeral and glenoid preparation, the arm was purposely kept out of an abducted and externally rotated position as much as possible to minimize traction on the brachial plexus and the peripheral nerves. In our study, surgical exposure of the glenoid did not include a 360° release of the subscapularis tendon, so it is possible that in the cohort studied, the final range of motion postoperatively, especially external rotation, might be less than that in other studies in which a 360° release was performed.
Conclusion
Our results suggest that when performing primary TSA or RTSA, the tug test or palpation or visualization of the axillary nerve may not be necessary to prevent complete, permanent IANI resulting from direct surgical trauma if proper precautions are taken to protect the nerve. When IANIs do occur, they are typically cases of neurapraxia that resolve within a year. This study is limited to 1 surgical technique for exposing the glenohumeral joint and should not be extrapolated to other techniques.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Funded in part by the Wayne H Lewis Endowed Professorship and the Bill and Donna Marriott Research Fund.
Footnotes
This study was approved by the Johns Hopkins Medicine Institutional Review Board (IRB00133656).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jses.2018.12.002.
Supplementary data
Surgical steps to perform glenoid exposure without identification of axillary nerve through palpation, visualization, or tug test.
References
- 1.Aleem A.W., Wilent W.B., Narzikul A.C., Kuntz A.F., Chang E.S., Williams G.R. Incidence of peripheral nerve injury during shoulder arthroplasty when motor evoked potentials are monitored. J Clin Monit Comput. 2018;32:897–906. doi: 10.1007/s10877-017-0080-5. [DOI] [PubMed] [Google Scholar]
- 2.Apaydin N., Uz A., Bozkurt M., Elhan A. The anatomic relationships of the axillary nerve and surgical landmarks for its localization from the anterior aspect of the shoulder. Clin Anat. 2007;20:273–277. doi: 10.1002/ca.20361. [DOI] [PubMed] [Google Scholar]
- 3.Bacle G., Nové-Josserand L., Garaud P., Walch G. Long-term outcomes of reverse total shoulder arthroplasty: a follow-up of a previous study. J Bone Joint Surg Am. 2017;99:454–461. doi: 10.2106/jbjs.16.00223. [DOI] [PubMed] [Google Scholar]
- 4.Ball C.M. Neurologic complications of shoulder joint replacement. J Shoulder Elbow Surg. 2017;26:2125–2132. doi: 10.1016/j.jse.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Bohsali K.I., Bois A.J., Wirth M.A. Complications of shoulder arthroplasty. J Bone Joint Surg Am. 2017;99:256–269. doi: 10.2106/jbjs.16.00935. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P., Watkinson D.J., Hatzidakis A.M., Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Boileau P., Watkinson D., Hatzidakis A.M., Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Borgeat A., Ekatodramis G., Kalberer F., Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology. 2001;95:875–880. doi: 10.1097/00000542-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers P.N., Van Thiel G.S., Trenhaile S.W. Surgical exposures of the shoulder. J Am Acad Orthop Surg. 2016;24:250–258. doi: 10.5435/jaaos-d-14-00342. [DOI] [PubMed] [Google Scholar]
- 10.Edwards T.B., Boulahia A., Kempf J.F., Boileau P., Nemoz C., Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84:2240–2248. doi: 10.2106/00004623-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Flatow E.L., Bigliani L.U. Tips of the trade. Locating and protecting the axillary nerve in shoulder surgery: the tug test. Orthop Rev. 1992;21:503–505. [PubMed] [Google Scholar]
- 12.Gill T.J., Hawkins R.J., editors. Complications of shoulder surgery: treatment and prevention. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- 13.Godenèche A., Boileau P., Favard L., Le Huec J.C., Lévigne C., Nové-Josserand L. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11:11–18. doi: 10.1067/mse.2002.120140. [DOI] [PubMed] [Google Scholar]
- 14.Kam A.W., Lam P.H., Murrell G.A.C. Brachial plexus injuries during shoulder arthroplasty: what causes them and how to prevent them. Tech Shoulder Elbow Surg. 2014;15:109–114. doi: 10.1097/BTE.0000000000000030. [DOI] [Google Scholar]
- 15.Kohan E.M., Chalmers P.N., Salazar D., Keener J.D., Yamaguchi K., Chamberlain A.M. Dislocation following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1238–1245. doi: 10.1016/j.jse.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 16.Lädermann A., Lübbeke A., Melis B., Stern R., Christofilopoulos P., Bacle G. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93:1288–1293. doi: 10.2106/jbjs.j.00369. [DOI] [PubMed] [Google Scholar]
- 17.Lädermann A., Stimec B.V., Denard P.J., Cunningham G., Collin P., Fasel J.H. Injury to the axillary nerve after reverse shoulder arthroplasty: an anatomical study. Orthop Traumatol Surg Res. 2014;100:105–108. doi: 10.1016/j.otsr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Lädermann A., Williams M.D., Melis B., Hoffmeyer P., Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:588–595. doi: 10.1016/j.jse.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Lenoir H., Dagneaux L., Canovas F., Waitzenegger T., Pham T.T., Chammas M. Nerve stress during reverse total shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg. 2017;26:323–330. doi: 10.1016/j.jse.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Lynch N.M., Cofield R.H., Silbert P.L., Hermann R.C. Neurologic complications after total shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5:53–61. doi: 10.1016/s1058-2746(96)80031-0. [DOI] [PubMed] [Google Scholar]
- 21.Malik A.A., Aresti N., Plumb K., Cowan J., Higgs D., Lambert S. Intraoperative nerve monitoring during total shoulder arthroplasty surgery. Shoulder Elbow. 2014;6:90–94. doi: 10.1177/1758573214526364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsen F.A., Lippitt S.B., Rockwood C.A., Wirth M.A. Glenohumeral arthritis and its management. In: Rockwood C.A., Matsen F.A., Wirth M.A., Lippitt S.B., Fehringer E.V., Sperling J.W., editors. Rockwood and Matsen's the shoulder. Elsevier; Philadelphia, PA: 2017. pp. 831–1042. [Google Scholar]
- 23.Matsoukis J., Tabib W., Guiffault P., Mandelbaum A., Walch G., Nemoz C. Shoulder arthroplasty in patients with a prior anterior shoulder dislocation. Results of a multicenter study. J Bone Joint Surg Am. 2003;85-A:1417–1424. doi: 10.2106/00004623-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 24.McFarland E.G., Caicedo J.C., Kim T.K., Banchasuek P. Prevention of axillary nerve injury in anterior shoulder reconstructions: use of a subscapularis muscle-splitting technique and a review of the literature. Am J Sports Med. 2002;30:601–606. doi: 10.1177/03635465020300042101. [DOI] [PubMed] [Google Scholar]
- 25.Mulieri P.J., Hussey M.M., Frankle M. DJO surgical reverse shoulder prosthesis (RSPTM) In: Frankle M., Marberry S., Pupello D., editors. Reverse shoulder arthroplasty: biomechanics, clinical techniques, and current technologies. Springer; Cham, Switzerland: 2016. pp. 343–356. [Google Scholar]
- 26.Nagda S.H., Rogers K.J., Sestokas A.K., Getz C.L., Ramsey M.L., Glaser D.L. Neer Award 2005: peripheral nerve function during shoulder arthroplasty using intraoperative nerve monitoring. J Shoulder Elbow Surg. 2007;16:S2–S8. doi: 10.1016/j.jse.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Parisien R.L., Yi P.H., Hou L., Li X., Jawa A. The risk of nerve injury during anatomical and reverse total shoulder arthroplasty: an intraoperative neuromonitoring study. J Shoulder Elbow Surg. 2016;25:1122–1127. doi: 10.1016/j.jse.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Saltzman B.M., Chalmers P.N., Gupta A.K., Romeo A.A., Nicholson G.P. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1647–1654. doi: 10.1016/j.jse.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Simone J.P., Streubel P.N., Sanchez-Sotelo J., Steinmann S.P., Adams J.E. Change in the distance from the axillary nerve to the glenohumeral joint with shoulder external rotation or abduction position. Hand (N Y) 2017;12:395–400. doi: 10.1177/1558944716668849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperling J.W., Cofield R.H. Complications of shoulder arthroplasty. In: Iannotti J.P., Williams G.R., editors. Disorders of the shoulder: diagnosis and management: shoulder reconstruction. Lippincott Williams & Wilkins; Baltimore, MD: 2014. pp. 451–470. [Google Scholar]
- 31.Sviggum H.P., Jacob A.K., Mantilla C.B., Schroeder D.R., Sperling J.W., Hebl J.R. Perioperative nerve injury after total shoulder arthroplasty: assessment of risk after regional anesthesia. Reg Anesth Pain Med. 2012;37:490–494. doi: 10.1097/AAP.0b013e31825c258b. [DOI] [PubMed] [Google Scholar]
- 32.Tan E.W., Ting B.L., Jia X., Skolasky R.L., McFarland E.G. Diagnostic errors in orthopedic surgery: evaluation of resident documentation of neurovascular examinations for orthopedic trauma patients. Am J Med Qual. 2013;28:60–68. doi: 10.1177/1062860612447856. [DOI] [PubMed] [Google Scholar]
- 33.Torchia M.E., Cofield R.H., Settergren C.R. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6:495–505. doi: 10.1016/s1058-2746(97)90081-1. [DOI] [PubMed] [Google Scholar]
- 34.Van Hoof T., Gomes G.T., Audenaert E., Verstraete K., Kerckaert I., D'Herde K. 3D computerized model for measuring strain and displacement of the brachial plexus following placement of reverse shoulder prosthesis. Anat Rec (Hoboken) 2008;291:1173–1185. doi: 10.1002/ar.20735. [DOI] [PubMed] [Google Scholar]
- 35.Villacis D., Sivasundaram L., Pannell W.C., Heckmann N., Omid R., Hatch G.F. Complication rate and implant survival for reverse shoulder arthroplasty versus total shoulder arthroplasty: results during the initial 2 years. J Shoulder Elbow Surg. 2016;25:927–935. doi: 10.1016/j.jse.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Walch G., Bacle G., Lädermann A., Nové-Josserand L., Smithers C.J. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon's experience? J Shoulder Elbow Surg. 2012;21:1470–1477. doi: 10.1016/j.jse.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Werner C.M., Steinmann P.A., Gilbart M., Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. doi: 10.2106/jbjs.d.02342. [DOI] [PubMed] [Google Scholar]
- 38.Zumstein M.A., Pinedo M., Old J., Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20:146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surgical steps to perform glenoid exposure without identification of axillary nerve through palpation, visualization, or tug test.