Abstract
Point-of-care B-type natriuretic peptide (BNP) testing with adequate analytical performance has the potential to improve patient flow and provide primary care givers with easy-to-use advanced diagnostic tools in the management of heart failure. We present the analytical evaluation of the Minicare BNP immunoassay under development on the Minicare I-20 platform for point-of-care testing. Analytical performance was evaluated using EDTA venous whole blood, EDTA plasma and capillary whole blood. Method comparison with a lab-testing system was performed using samples from 187 patients. Normal values were determined based on 160 healthy adults, aging from 19 to 70 years. Limit of blank (LoB), limit of detection (LoD) were determined to be 3.3 ng/L, 5.8 ng/L. Limit of quantitation (LoQ) in whole blood at 20% and 10% coefficient of variation (CV) was found < 9 ng/L and <30 ng/L respectively without significant differences between EDTA whole blood and EDTA plasma. Total CV was found to be from 6.7% to 9.7% for BNP concentrations between 92.6 and 3984 ng/L. The sample type comparison study demonstrated correlation coefficients between 0.97 and 0.99 with slopes between 1.03 and 1.09 between the different samples. Method comparison between Minicare BNP and Siemens ADVIA Centaur BNP demonstrated a correlation coefficient of 0.92 with a slope of 1.06. The 97.5% URL of a healthy population was calculated to be 72.6 ng/L. The Minicare BNP assay is a robust, easy-to-use and sensitive test for rapid determination of BNP concentrations that can be used in a near-patient setting.
Keywords: Heart failure, Diagnosis, B-type natriuretic peptide, Point-of-care, Capillary blood, Analytical performance
Abbreviations: BNP, B-type Natriuretic Peptide; CI, confidence interval; CLSI, clinical laboratory standards institute; CV, coefficient of variation; EDTA, ethylene-diamine-tetraacetic acid; fTIR, frustrated total internal reflection; HAMA, human anti-mouse antibody; HF, heart failure; K2-EDTA, dipotassium ethylene-diamine-tetraacetic acid; Li-heparin, lithium heparin; LoB, limit of blank; LoD, limit of detection; LoQ, limit of quantitation; NP, Natriuretic Peptide; NYHA, New York Heart Association; POC, point-of-care; RF, rheumatoid factor; RFID, radiofrequency identification; RT, room temperature; SD, standard deviation; URL, upper reference limit
1. Introduction
Twenty-six million adults worldwide are estimated to have heart failure and this number is expected to rise [1]. All heart failure patients, even those who are considered asymptomatic (New York Heart Association, NYHA, class I) or mildly symptomatic (NYHA class II), have a poor prognosis with a high mortality during long-term follow-up (34%–42% for class I-II and class III-IV respectively) [2].
The measurement of circulating natriuretic peptides (NP) is considered to be a useful marker of myocardial function, and international guidelines recommend its use for the diagnosis of patients with heart failure (HF) [[3], [4], [5]], in particular ruling out the presence of HF. Point-of-Care (POC) systems for NP with a short turnaround time enable rapid clinical decision making and can thereby improve patients flow. Furthermore, such systems have the potential to provide primary care givers such as the general practitioner access to advanced diagnostic tools, but this requires POC systems to show lab-quality test performance, be robust and easy-to use [6].
Current commercial POC B-type Natriuretic Peptide (BNP) testing systems using only samples from venous puncture are difficult to handle for non-lab-trained personnel. In addition, stability of BNP especially in plasma samples has been a concern for the reliability of NP tests [7].
Here, we demonstrate the analytical evaluation of the POC Minicare BNP assay under development, which runs on the Minicare I-20 platform with a turnaround time of less than 10 min (Fig. 1a) [8]. Aside from quantifying BNP from venous whole blood/plasma samples, Minicare BNP also accepts capillary whole blood samples from finger prick. This should lower the barrier for BNP testing at primary care settings, but also avoid BNP stability concerns since capillary samples are directly applied to the test system.
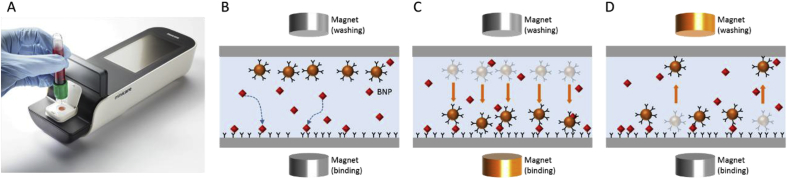
Fig. 1.
(a) Minicare I-20 platform consisting of the Minicare I-20 analyzer and a disposable plastic cartridge. (b–d) Schematic representation of the Magnotech single-epitope sandwich assay. First (b) the analyte, i.e. BNP, binds to the functionalized optical detection surface. Subsequently (c) magnetic particles are attracted to the surface and bind the complexes. Lastly (d) unbound particles are removed from the surface to enable detection of BNP-bound particle complexes via fTIR.
2. Materials and methods
2.1. Principle of the Minicare BNP test
Minicare BNP is an in vitro diagnostic POC test that is intended to be used as an aid in the diagnosis of heart failure. It uses a single test analyzer device (Minicare I-20 analyzer) and a plastic disposable cartridge containing the specific BNP assay reagents. Test results are generated within 10 min after application of the sample to the cartridge. The Minicare I-20 assays are based on proprietary Magnotech biosensor technology [9,10]. Briefly, Minicare I-20 assays are based on the precise controlled motion of magnetic nanoparticles functionalized with analyte-specific antibodies within a small reaction volume inside each cartridge. The same magnetic particles serve as labels that are detected using frustrated total internal reflection (fTIR) imaging. The system has been designed without moving parts to create a robust and stable handheld platform.
2.2. Selection of antibodies
The Minicare BNP assay uses proprietary “Single Epitope Sandwich” (“SES”) BNP antibodies (Hytest, Turku, Finland) which recognize a single epitope within the ring structure of BNP (1−32) [11]. Hence, SES-BNP assays are less affected by proteolytic degradation.
2.3. Assay format
After application of the blood sample onto the cartridge, blood cells are trapped within a semipermeable filter membrane while plasma containing BNP enters the microfluidic system and is transported to the reaction chambers by capillary forces. Within the reaction chambers, the first step of the SES assay is executed as anti-BNP capture antibodies printed onto the optical imaging surface bind to the analyte molecules; see Fig. 1b. At the same time, dried magnetic particles within the reaction chamber disperse into the sample volume. The magnetic particles are attracted to the imaging surface and bind to the complex of BNP molecules and anti-BNP-antibody via the application of magnetic fields to create a magnetic field gradient towards the imaging surface; see Fig. 1c. This latter step is effectively the second step of the SES assay, i.e. formation of the complete complex. After a limited amount of time to form the complex, unbound or weakly (non-specifically) bound particles are washed from the detection surface using a magnetic field gradient in the opposite direction. This leaves the particles which have specifically formed the complex with BNP at the detection surface to be optically detected via fTIR; see Fig. 1d.
2.4. Standardization
Due to the lack of a suitable commutable primary calibrator for BNP immunoassay standardization, new BNP standards were developed consisting of defibrinated, delipidated and charcoal-treated human plasma (Seracon II, SeraCare) and recombinant proBNP (catalogue number 8GOB2; Hytest). These standards were dose-assigned such that all sample types achieve a correlation with a target slope of 1.0 with a reference system, i.e. the Siemens ADVIA Centaur BNP. In particular for capillary whole blood, a compensation factor of 1.48 was established with respect to EDTA whole blood as determined from a paired comparison study on 117 samples reported here. Cartridge-lot specific calibration parameters were obtained from dose-response curves generated using these calibrators and programmed in the radio-frequency identification (RFID) of each cartridge.
2.5. Study samples
Samples for testing precision, detection capability, linearity, high-dose hook effect, cross-reactivity, interferences, and normal values studies were (a) left-over K2-EDTA venous blood samples from patients of the Canisius-Wilhelmina hospital (CWZ) in Nijmegen, The Netherlands, and (b) K2-EDTA venous blood samples from healthy volunteers (informed consent obtained) from Sanquin Blood bank in Nijmegen, The Netherlands. Negative and positive BNP EDTA plasma samples were selected to prepare sample pools with different levels of BNP as specified for each study.
For the sample stability and the method comparison studies, two sample types (K2-EDTA venous whole blood and K2-EDTA venous plasma) were collected for each patient at Diagnostiek voor U facilities, respectively of Sint Jans Gasthuis in Weert, The Netherlands, and central laboratory in Eindhoven, The Netherlands. Patients were selected to represent the range of BNP concentrations likely to be encountered in clinical practice, covering the measurement range of the Minicare BNP assay. Left-over samples from eligible patients were used for the study and the study was waived by local ethics committees. For the sample stability study both sample types were analyzed on Minicare BNP within 1 h after blood collection (reference time), stored at room temperature (RT, 18–26 °C) and tested again after 16 h. Another set of EDTA plasma samples was frozen at < -55 °C and tested after 2 months. EDTA plasma samples submitted to 4 freeze-thaw cycles were also tested. For the method comparison study EDTA whole blood and EDTA plasma samples were analyzed on Minicare BNP within 6 h after blood collection. EDTA plasma samples were analyzed on Siemens ADVIA Centaur BNP, Alere Triage BNP and Abbott I-STAT BNP according to manufacturer's instructions.
While the method comparison enabled a comparison between venous whole blood and venous plasma samples, a broader sample comparison study was set up to compare these sample types to capillary whole blood, as well as the anticoagulants used (K2-EDTA, Li-heparin, or none). For the broader sample type comparison study, the following sample types were collected for each patient at the Medical University Hospital in Innsbruck, Austria [1]: venous whole blood [2], venous plasma, and [3] capillary whole blood. For venous whole blood and plasma, both K2-EDTA and Li-heparin were used as anticoagulants. For capillary whole blood, samples without anticoagulants and containing K3-EDTA were tested. Samples covering the whole measurement range were measured within 2 h after sample draw. The Minicare BNP tests were executed by study nurses of the clinical trials unit of the Department of Internal Medicine III (Cardiology and Angiology), Medical University of Innsbruck. This study has been approved by all local ethical committees and written informed consent was obtained from patients prior to their participation in the study.
2.6. Precision
Assay precision was established across the dynamic range using pools of patient EDTA plasma samples in accordance with CLSI EP05-A3 [12]. Two replicates of each sample were tested twice per day in separate runs (at least 2 h apart), for 20 non-consecutive days, on two lots of Minicare BNP cartridges and using four Minicare I-20 instruments.
2.7. Detection capability
Limit of blank (LoB), limit of detection (LoD) and limit of quantitation (LoQ) for the Minicare BNP were established in accordance with CLSI EP17-A2 [13]. Four stripped human plasma pools (i.e. 4 different lots of SeraCon I, from SeraCare) were used to establish the LoB. Four low-concentration plasma pool samples derived from multiple donors targeting a concentration between 5 and 20 ng/L were used to establish the LoD. LoB and LoD samples were tested on five days, with three replicates per day, on two Minicare BNP cartridge lots, using five analyzers. The LoB was calculated non-parametrically, i.e. the interpolated numerical result at rank 57 and rank 58 was determined to be the LoB value.
The LoQ at 10% CV and 20% CV in EDTA plasma and EDTA whole blood was established using 15 EDTA whole blood and their corresponding EDTA plasma samples targeting a range from 0 to 400 ng/L. LoQ EDTA plasma samples were tested on 5 days, in four-fold, whereas LoQ EDTA whole blood samples were each tested on a single day (different samples over multiple days), in twenty-fold. Both EDTA plasma and EDTA whole blood samples were tested on two cartridge lots, using five analyzers. The LoQ was derived from a non-weighted second-order polynomial fit of the resulting precision profile.
2.8. Linearity on dilution
According to CLSI EP06-A recommendation [14], 11 EDTA plasma pools with different levels of BNP were prepared from a high and a low BNP EDTA plasma pool with steps of 10% dilution. The pool with the highest BNP level and the pool with the lowest BNP level were measured in 20-fold. All the other pools were measured in 5-fold. All tests were performed on one cartridge lot. A polynomial analysis method was applied [14].
2.9. High-dose hook effect
Eleven dilution blends prepared from pure recombinant BNP protein and a negative plasma pool were tested. All high-dose hook samples were measured in duplicate on one cartridge lot.
2.10. Cross-reactivity and interferences
Various blood components (hemoglobin, human albumin, bilirubin, triglycerides, human anti-mouse antibodies (HAMA), rheumatoid factor (RF), and total protein) and drugs (allopurinol, acetaminophen, ampicillin, ascorbic acid, acetylsalicylic acid, atenolol, caffeine, captopril, diclofenac, digoxin, dopamine, enalapril maleate, erythromycin, furosemide, heparin, ibuprofen, isosorbide dinitrate, methyldopa, niphedipine, phenytoin, propranolol, theophylline, verapamil, warfarin) were tested for interference and α-atrial natriuretic polypeptide 1–28, arg vasopressin, C-type natriuretic peptide and urodilatin (CCD/ANP) for cross-reactivity according to CLSI EP07-A2 recommendations [15] in an EDTA plasma pool with a BNP concentration between 80 and 120 ng/L. As a reference, only the dilution matrix of the substances was tested in the EDTA plasma pool. All samples were tested in 5-fold on one cartridge lot, divided over 18 analyzers.
2.11. Sample stability
A total of 36 EDTA whole blood and EDTA plasma samples covering the dynamic range were used to establish the room temperature (18–26 °C) stability of samples on the Minicare BNP assay. A total of 44 and 45 EDTA plasma samples covering the dynamic range were used to establish respectively the long-term stability (at < −55 °C) and freeze-thaw stability (4 cycles). Every sample was measured at least twice using four analyzers. The reference concentration (t = 0) was measured within 1 h after blood draw. Stability was assessed by comparing results to the reference (t = 0) using Passing-Bablok regression.
2.12. Method comparison
The Minicare BNP was compared with the Siemens ADVIA Centaur BNP, Alere Triage BNP and Abbott I-STAT BNP by testing and comparing EDTA whole blood samples (on Minicare, Triage and I-STAT) and corresponding EDTA plasma samples (on Minicare and Centaur) from respectively 187 to 216 individuals with a BNP concentration range as measured using Minicare from 10 to 2890 ng/L in accordance with CLSI EP09-A3 [16]. EDTA whole blood samples were applied to the Minicare BNP test both from blood tubes using SmearSafe (Typenex Medical) blood dispensers and using a pipette. Analysis was done using Passing-Bablok regression.
2.13. Sample type comparison
Sample type comparison was done in accordance with CLSI EP09-A3 recommendations [5]. The sample type comparison performed at the Medical University Hospital in Innsbruck, Austria, was done in two parts, I and II. Part I aimed to compare (a) different sample types (venous whole blood/venous plasma/capillary whole blood), (b) different anticoagulants (K2-EDTA/Li-Heparin/none), and (c) different blood tube sampling devices (blood-tube dispenser/pipette). Part I data was analyzed with an assay standardization (see section 2.4) that did not include a compensation for capillary whole blood samples. Part II aimed to confirm the compensation for capillary whole blood samples in the assay standardization and the use of different sampling devices (capillary tubes/blood-tube dispenser/pipette). Li-Heparin samples were excluded in part II. In each part at least 100 patient samples were collected for each comparison. Additional comparison data, i.e. 232 paired patient samples, between EDTA venous whole blood and EDTA venous plasma was also generated at the Eindhoven site during the method comparison studies. Results were analyzed using Passing-Bablok regression.
EDTA whole blood samples were applied to the Minicare BNP test from blood tubes (BD Vacutainer tubes and S-Monovette tubes from Sarstedt) using blood dispensers (SmearSafe, Typenex Medical/Haemo-Diff, Sarstedt) and/or via a pipette. Capillary samples were transferred using capillary tubes (Microsafe, Safe-Tec, uncoated; and POCT Minivette, Sarstedt, uncoated and EDTA-coated).
Excluded from the data sets were samples with observed concentrations below the LoQ with 20%CV (i.e. 10 ng/L).
2.14. Normal values
Samples from blood donors, presenting voluntarily at the Eindhoven MMC Sanquin post in the Netherlands, were used to determine normal values. Healthy adults were selected based on Sanquin standard questionnaire and were invited to take part in this study. All subjects enrolled signed an informed consent form.
In total 160 blood donors (94 males and 66 females; with 84 aged more than 55 years and 76 aged 55 years or less) qualified as final study population for the study. Normal values were determined by testing EDTA whole blood and EDTA plasma samples. Reference intervals were estimated using a non-parametric method [17].
3. Results
3.1. Precision
Total imprecision and within-run imprecision expressed as CV were calculated at various BNP levels for the Minicare BNP assay; see Table 1. Total imprecision of 7% and within-run imprecision of 6% was obtained for BNP concentrations around 100 and 300 ng/L. For high BNP levels (i.e. around 3000 ng/L) a total imprecision of less than 10% and within-run imprecision of 7% was obtained.
Table 1.
Total imprecision and within-run imprecision for measurements (with n repeats) of BNP on pooled EDTA plasma samples. Total imprecision contains within-run, run-to-run and day-to-day variability.
| EDTA plasma sample | n | Mean conc |
Within-run imprecision |
Total imprecision |
|---|---|---|---|---|
| ng/L | CV | CV | ||
| Pool I | 80 | 92.6 | 6.1% | 6.7% |
| Pool 2 | 80 | 327 | 5.8% | 7.0% |
| Pool 3 | 80 | 3984 | 7.3% | 9.7% |
3.2. Detection capability
The LoB for lot 1 and lot 2 was found to be respectively 3.3 ng/L and 2.5 ng/L.
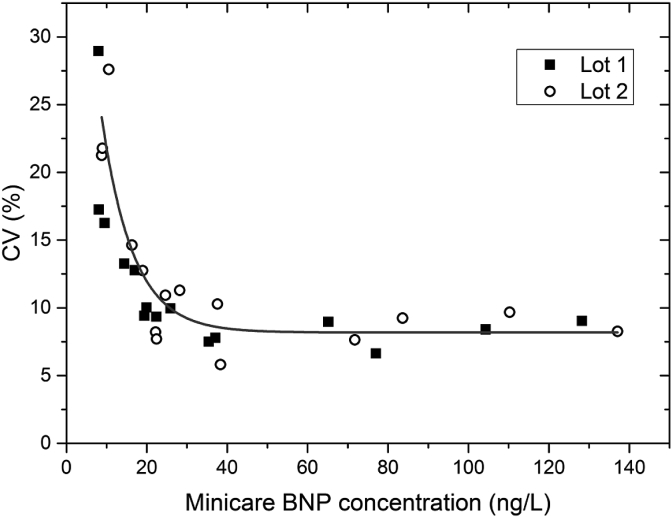
The highest value of LoB was used to calculate the LoD. The LoD was determined to be 5.6 ng/L and 5.8 ng/L for respectively lot 1 and lot 2. For EDTA whole blood the CV profile is shown in Fig. 2. For EDTA whole blood, the LoQ at 10%CV was below 30 ng/L and the LoQ at 20%CV was below 9 ng/L for both cartridge lots; see Table 2.
Fig. 2.
CV profile for EDTA whole blood. On the horizontal axis the average Minicare BNP concentration in ng/L is depicted. The line is a guide to the eye.
Table 2.
Limit of Quantitation (LoQ) for whole blood and plasma.
| Lot | Whole blood LoQ (ng/L) |
Plasma LoQ (ng/L) |
||
|---|---|---|---|---|
| 10% CV | 20%CV | 10% CV | 20% CV | |
| Lot 1 | 22 | 8.4 | 39 | 4.3 |
| Lot 2 | 30 | 8.8 | 22 | 7.6 |
3.3. Linearity
Weighted linear regression between observed and expected concentrations, resulted in sustained linearity over the 9.3–5766 ng/L tested range, with recovery within ± 15% of expected values.
3.4. High-dose hook effect
No High-dose hook effect was found for samples up to a BNP concentration of 20,000 ng/L.
3.5. Cross-reactivity and interference
Results were considered acceptable if samples enriched with the possible interferents were within 10% of the results for the control samples. None of the tested blood components and/or drugs showed interference beyond 90% and 110%. The observed percentage of cross-reactivity of Minicare BNP was <0.2% for all tested cross-reactants.
3.6. Sample stability
Samples were considered stable if a bias smaller than 10% was observed. Results are shown in Table 3. It was found that Minicare BNP can measure BNP concentrations in EDTA whole blood and EDTA plasma samples stored at room temperature for 16 h (95% CI of Passing-Bablok slope for all cases within 0.92 and 1.02). Frozen EDTA plasma samples showed stable results up to 2 months (95% CI of Passing-Bablok slope within 0.96 and 1.02). EDTA plasma samples showed no instability after 4 freeze-thaw cycles (95% CI of Passing-Bablok slope within 0.97–1.02).
Table 3.
Sample stability data from comparison of measured concentrations from stored samples to reference measurements done within 1 h after blood drawn. Passing-Bablok slopes including 95% confidence intervals were computed.
| Sample type | Storage time | Storage temperature | N freeze-thaw cycles | Passing-Bablok slope | 95% CI |
|---|---|---|---|---|---|
| EDTA Whole blood | 2 h | Room temperature | NA | 0.98 | [0.95–1.00] |
| EDTA Whole blood | >16 h | Room temperature | NA | 0.96 | [0.92–0.99] |
| EDTA Plasma | 4 h | Room temperature | NA | 0.98 | [0.97–1.00] |
| EDTA Plasma | >16 h | Room temperature | NA | 1.00 | [0.97–1.02] |
| EDTA Plasma | NA | < −55 °C | 4 | 1.00 | [0.97–1.02] |
| EDTA Plasma | 1 month | < −55 °C | 1 | 0.99 | [0.97–1.02] |
| EDTA Plasma | 2 months | < −55 °C | 1 | 0.97 | [0.96–0.99] |
3.7. Method comparison
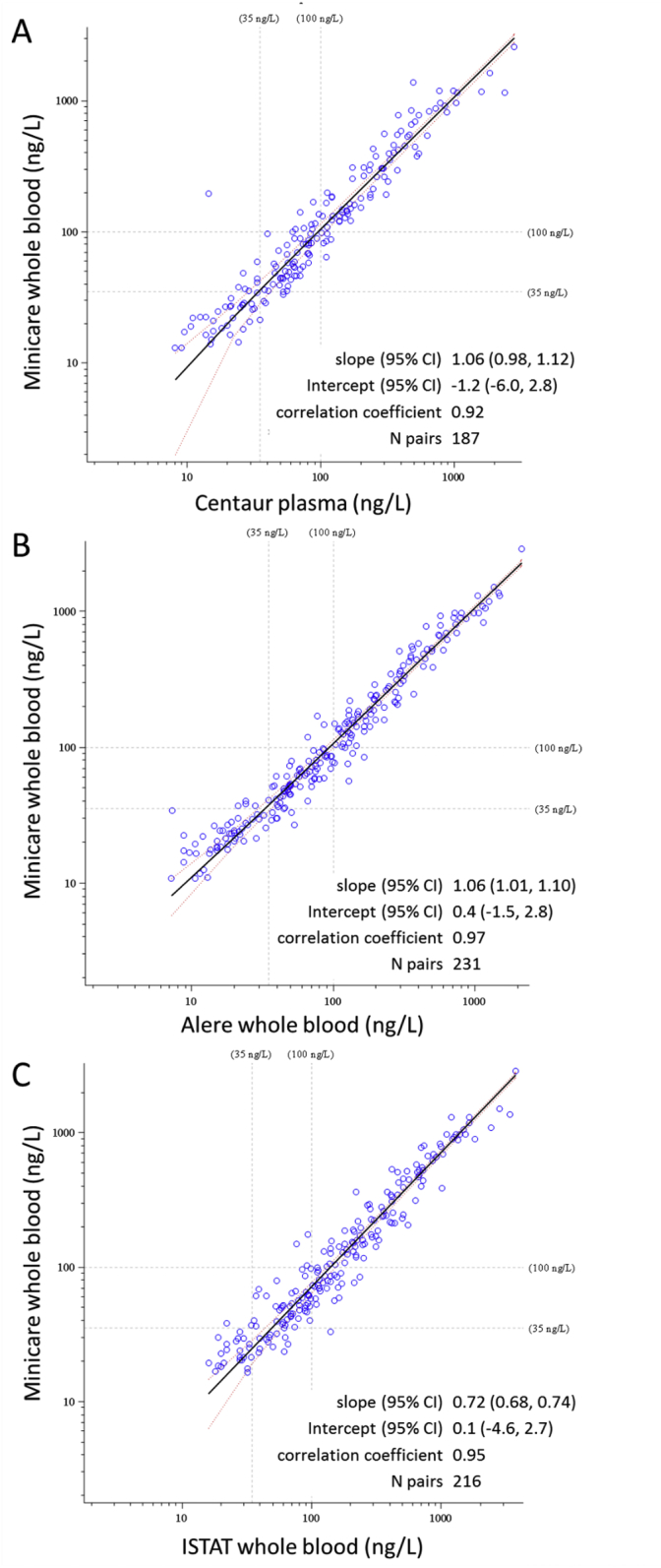
Fig. 3 shows the method comparison results for the different methods tested. In Table 4 the estimated bias based on Blant-Altman analysis is shown for the different method comparisons. Comparing Minicare BNP to the Siemens Centaur BNP, a slope was found of 1.06 (95% CI of 0.98–1.12) and a Pearson correlation coefficient was found of 0.92. Compared to the Alere Triage BNP, the slope was 1.06 (95% CI of 1.01–1.10) and the Pearson correlation coefficient was 0.97. Compared to the Abbott I-STAT BNP, the slope was 0.72 (95% CI of 0.68–0.74) and the Pearson correlation coefficient was 0.95. No significant differences were observed between Minicare BNP whole blood measurements using a pipette or SmearSafe dispenser: a Passing-Bablok slope of 1.00 (95% CI of 0.98–1.02) with a Pearson correlation coefficient of 1.00 based on 245 measurement pairs.
Fig. 3.
Method comparison between Minicare BNP whole blood (WB) and (A) Siemens ADVIA Centaur BNP, (B) Alere Triage BNP, and (C) Abbott I-STAT BNP. Data is shown on a logarithmic scale. Passing-Bablok regression results are shown in the inset. The solid black line corresponds to the regression line.
Table 4.
Blant-Altman analysis of mean differences of method comparison results.
| Method A | Method B | mean difference | 95% C.I. |
|---|---|---|---|
| Minicare BNP | Centaur BNP | 6.5% | 1.0% to 12.0% |
| Minicare BNP | Biosite BNP | 9.2% | 5.0% to 13.3% |
| Minicare BNP | I-stat BNP | −30% | −34% to −25% |
3.8. Sample type comparison
Sample type comparison studies were done to establish and confirm the assay standardization for all sample types and transfer devices. Studies were done in 2 parts, a first part to establish possible systematic biases and a second study to verify the results. Data in Table 5 shows the sample types where systematic biases (slopes of the Passing-Bablok regression outside the range of 0.9–1.1) were observed. Table 6 shows sample type comparisons without systematic biases when including a compensation factor of 1.48 for capillary samples in the assay standardization.
Table 5.
Summary of the sample type comparison results for assay standardization without compensation for capillary samples. C.I.: 95% confidence interval. Different comparison types are labeled: A: anticoagulants, B: sample types, and C: transfer devices.
| Result “X” | Result “Y” | Type comparison | N | Passing-Bablok regression |
Pearson correlation (r) | |||
|---|---|---|---|---|---|---|---|---|
| Slope |
Intercept |
|||||||
| estimate | C.I. | estimate | C.I. | |||||
| EDTAwhole blood pipette |
Capillary whole blood Sarstedt (uncoated) |
ABC | 117 | 1.48 | 1.38 1.55 |
0.4 | −6.9 6.1 |
0.98 |
| EDTA whole blood pipette |
EDTA capillary whole blood Sarstedt |
BC | 117 | 1.32 | 1.25 1.43 |
−2.6 | −12 3.9 |
0.93 |
| EDTAwhole blood pipette |
LiHepwhole blood pipette |
A | 117 | 1.44 | 1.37 1.51 |
−1.9 | −7.2 2.0 |
0.97 |
| EDTAplasma pipette |
LiHepplasma pipette |
A | 119 | 1.40 | 1.33 1.47 |
−1.1 | −5.7 5.3 |
0.98 |
| EDTAwhole blood pipette |
EDTAwhole blood Haemodiff |
C | 116 | 0.929 | 0.895 0.982 |
1.3 | −2.1 3.7 |
0.98 |
Table 6.
Summary of the sample type comparison results for assay standardization with compensation of capillary samples with a factor 1.48 as found in the first part of the Innsbruck study (see Table 5). C.I.: 95% confidence interval. Different comparison types are labeled: A: anticoagulants, B: sample types, and C: transfer devices.
| Result “X” | Result “Y” | Type comparison | N | Passing-Bablok regression |
Pearson correlation (r) | |||
|---|---|---|---|---|---|---|---|---|
| Slope |
Intercept |
|||||||
| estimate | C.I. | estimate | C.I. | |||||
| EDTA whole blood pipette | Capillary whole blood Safe-Tec (uncoated) | ABC | 150 | 1.05a | 0.991 1.09 |
2.1 | −2.6 6.2 |
0.98 |
| EDTA whole blood pipette | Capillary whole blood Sarstedt (uncoated) | ABC | 148 | 1.03a | 0.990 1.07 |
0.3 | −3.7 4.6 |
0.98 |
| Capillary whole blood Sarstedt (uncoated) | Capillary whole blood Safe-Tec (uncoated) | C | 150 | 1.01 | 0.992 1.04 |
1.7 | −1.0 4.2 |
0.99 |
| EDTA whole blood pipette | EDTA whole blood Haemodiff | C | 116 | 0.93 | 0.895 0.982 |
1.3 | −2.1 3.7 |
0.98 |
| EDTA whole blood Pipetteb | EDTA whole blood SmearSafe | C | 245 | 1.00 | 0.98 1.02 |
0.2 | −0.9 1.5 |
1.00 |
| EDTA whole blood pipette | EDTA plasma pipette | B | 151 | 1.09 | 1.06 1.12 |
−2.0 | −5.6 0.9 |
0.98 |
| EDTA whole blood pipetteb | EDTA plasma pipetteb | B | 232 | 1.04 | 1.02 1.06 |
−0.2 | −1.8 1.3 |
0.99 |
Corrected for the systematic bias of 1.48 as found in a preceding sample type comparison study.
Measured at the Eindhoven site.
3.9. Normal values
The primary objective of this study was to establish the reference ranges for Minicare BNP in healthy volunteers EDTA plasma and EDTA whole blood samples.
Results in Table 7 show that the distribution of the BNP values measured in EDTA whole blood and EDTA plasma are similar for the two sample types. One sample yielded invalid results for both EDTA whole blood and EDTA plasma, and another one for EDTA plasma only.
Table 7.
Descriptive statistics of the Minicare BNP normal values.
| K2-EDTA whole blood | K2-EDTA plasma | |
|---|---|---|
| N | 159 | 158 |
| Median Age, years (Min-Max) | 56 (19–70) | 56 (19–70) |
| Male/Female | 94/65 | 93/65 |
| Median BNP level, ng/L (Min-Max) | 15.8 (0–101) | 16.7 (0.6–109) |
| 2.5th percentile, ng/L (95% CI) | 4.4 (0.0–5.5) | 2.8 (0.6–5.2) |
| 95th percentile, ng/L (95% CI) | 54.5 (34.8–90.7) | 59.8 (35.7–81.5) |
| 97.5th percentile, ng/L (95% CI) | 72.6 (43.1–101) | 71.6 (52.1–109) |
The 97.5th percentile for EDTA whole blood and EDTA plasma were 72.6 ng/L and 71.6 ng/L respectively. The reference ranges for the two sample types were comparable (overlapping confidence intervals).
4. Discussion
The Minicare BNP detection capability (LoQ 20%CV < 9 ng/L and LoQ 10% CV < 30 ng/L for whole blood) was tested both on whole blood samples and plasma samples, and showed no significant difference. In relation to the normal values study, at least 80% of the healthy population quantified above the LoQ 20%CV = 30 ng/L. At least 99% of healthy donors quantified below the 100 ng/L cut-off classically used for the rule out of acute HF [3].
Sample stability data show that whole blood can be reliably used up to at least 16 h at room temperature conditions, in agreement with earlier reports [18].
The sample type comparison study between capillary whole blood, K2-EDTA venous whole blood and K2-EDTA venous plasma samples demonstrated good correlation. The data confirm that the standardization approach leads to a comparable test performance for the three sample types. This enables interchangeable use of the sample types, under the condition that the user pre-selects the sample type (venous/capillary) to be used before testing due to a correction factor of 1.48 between capillary and venous blood samples (based on Part I study data in Table 5). The origin of this difference is unknown.
In the part I study, the slope between Li-Heparin whole blood and EDTA whole blood was found to be 1.44 (95% CI of 1.37–1.51, N = 117). A bias between Li-Heparin and EDTA has been observed before. Santos et al. [19] found a mean bias of +65% for Li-Heparin BNP samples over EDTA samples on the Beckman Coulter Access 2 system. Others however [18] found that heparinized and non-anticoagulated samples quantify with a negative bias of −39% compared to EDTA samples on the Centaur system. The differences are generally attributed to the sample handling, and most importantly in relation to the sample device used [20]. In our studies, we find that for specific sample types, the use of different sampling devices does not lead to significant quantification differences when the same sample type is used. For EDTA venous whole blood, this concerned the use of different tubes (vacutainer and S-Monovette) in combination with different dispensers (SmearSafe and Haemodiff) and a pipette. For non-anticoagulated capillary whole blood, this concerned the use of two different capillary transfer devices (Safe-Tec and POCT Minivette).
Method comparison between Minicare BNP and the reference BNP assay (Siemens ADVIA Centaur BNP) demonstrated comparable BNP results. The Minicare assay uses a single epitope sandwich (SES) format, in contrast to the conventional assays used by the other systems in the method comparison, which bind to two epitopes of the BNP structure. With the SES assay targeting a single epitope within the ring structure of BNP(1–32), the SES assay is less affected by proteolytic degradation [11] and therefore differences should be observable compared to conventional assays. Systematic measurement differences as observed before [21], were not observed comparing Minicare to the Centaur BNP assay. However, any systematic difference would be masked in this study since the Minicare BNP was standardized to quantify with a slope of approximately one with respect to the Centaur BNP test. Method comparison results show that the I-STAT reports significantly higher BNP levels compared to the other three BNP systems. This possibly leads to higher incidence of false positive BNP outcomes when using the I-STAT device with the same cut-off (i.e. 100 ng/L). These differences are likely due to the absence of BNP standards or standard references for assay standardization. This also supports not using a single cut-off value for diagnosis without considering the method used.
Considering BNP as a marker for heart failure, the FDA approved heart failure drug LCZ696 (Entresto™, Novartis) has led to speculation about the reliability of BNP over NT-proBNP. LCZ696 combines a neprilysin inhibitor and an angiotensin II receptor, of which the former is known to inhibit BNP degradation and thereby expected to raise circulating BNP levels. It has however been argued that before concluding on the effects on circulating BNP levels, more understanding is needed on the effect of the drug on the complex biochemistry of proBNP-derived peptides [22]. For example, a recent study showed that the precursor proBNP is not degraded by neprilysin, while it is the major circulating BNP immunoreactive form [23]. The study showed that single epitope BNP assays are much less sensitive to BNP degradation by neprilysin than sandwich assays targeting the region 14–21 of BNP. Based on these data one may speculate that the Minicare BNP may be less susceptible to the use of LCZ696 than other BNP assays. This should be confirmed in further studies.
Minicare BNP is an easy-to-use POC test that can accurately measure BNP near the patient with a turnaround time of less than 10 min, using a single droplet of blood (30 μL). With a CV of 7% near the 100 ng/L cut-off recommended for rule out of acute HF [3], the test is competitive with state-of-the-art POC BNP tests. Next to EDTA whole blood and EDTA plasma, the test supports capillary samples which allows for simple and easy blood collection via finger prick that can be done by minimally trained care-givers, in contrast to whole blood/plasma sample collection procedures. Support of capillary samples also provides robustness against BNP instability since capillary samples are applied directly after finger prick, and hence avoid any instability effects. The absence of any required sample preparation steps reduces turnaround time and the possibility of user errors. Robustness is also improved via the selected single-epitope sandwich format which makes the assay less susceptible to degradation of BNP molecules. This makes the test suitable for use by healthcare professionals and/or healthcare facilities with limited/no access to advanced diagnostic tools such as BNP lab tests or echocardiography.
In conclusion, the Minicare BNP assay under development is a fast, robust and sensitive test that can be used in a near-patient setting on capillary or EDTA venous whole blood sample. This offers for the Minicare BNP test the potential for good clinical performance in the diagnosis of HF in various patient settings, which is to be evaluated in a large clinical study.
Acknowledgements
We thank Canisius Wilhelmina Ziekenhuis (CWZ), Nijmegen, The Netherlands and Sanquin Blood Bank, Nijmegen, The Netherlands for their support in the analytical studies. We thank the clinical trials unit (M. Spechtenhauser, G. Laschober, C. Mayerl) of the Department of Internal Medicine III (Medical University of Innsbruck, Innsbruck, Austria) for assistance in performing this study at this study site.
Contributor Information
Alexander van Reenen, Email: alexander.van.reenen@minicare.com.
Femke de Theije, Email: femke.de.theije@minicare.com.
Jeroen Nieuwenhuis, Email: jeroen.nieuwenhuis@minicare.com.
Veronique Semjonow, Email: veronique.semjonow@philips.com.
Johannes Mair, Email: johannes.mair@i-med.ac.at.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am. J. Cardiol. 2007;99(4):549–553. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., Gonzalez-Juanatey F.R. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. (2016) [DOI] [PubMed] [Google Scholar]
- 4.Hunt S.A., Abraham W.T., Chin M.H., Feldman A.M., Francis G.S., Ganiats T.G. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112:154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 5.Doust J.A., Glasziou P.P., Pietrzak E., Dobson A.J. A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch. Intern. Med. 2004;164:1978–1984. doi: 10.1001/archinte.164.18.1978. [DOI] [PubMed] [Google Scholar]
- 6.Maalouf R., Bailey S. A review on B-type natriuretic peptide monitoring: assays and biosensors. Heart Fail. Rev. 2016;21:567–578. doi: 10.1007/s10741-016-9544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murdoch D., Byrne J., Morton J., McDonagh T., Robb S., Clements S., Ford I., McMurray J., Dargie H. vol. 78. 1997. pp. 594–597. (Brain Natriuretic Peptide Is Stable in Whole Blood and Can Be Measured Using a Simple Rapid Assay: Implications for Clinical Practice Heart). (6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemper D.W.M., Semjonow V., de Theije F., Keizer D., van Lippen L., Mair J., Wille B., Christ M., Geier F., Hausfater P., Pariente D., Scharnhorst V., Curvers J., Nieuwenhuis J. Analytical evaluation of a new point of care system for measuring cardiac Troponin I. Clin. Biochem. 2017;50:174–180. doi: 10.1016/j.clinbiochem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Bruls D.M., Evers T.H., Kahlman J.A., van Lankvelt P.J., Ovsyanko M., Pelssers E.G. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip. 2009;9(24):3504–3510. doi: 10.1039/b913960e. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer W.U., Evers T.H., Hardeman W.M., Huijnen W., Kamps R., de Kievit P. Rapid, high sensitivity, point-of-care test for cardiac troponin based on optomagnetic biosensor. Clin. Chim. Acta. 2010;411:868–873. doi: 10.1016/j.cca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Rosjo H., Tamm N.N., Kravdal G., Seferian K.R., Hoiseth A.D., Nygard S., Badr P., Roysland R., Omland T. Diagnostic utility of a single-epitope sandwich B-type natriuretic peptide assay in stable coronary artery disease: data from the Akershus Cardiac Examination (ACE) 1 Study. Clin. Biochem. 2012;45:1269–1275. doi: 10.1016/j.clinbiochem.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Clinical Laboratory Standards Institute . CLSI; Wayne (PA): 2014. Evaluation of Precision of Quantitative Measurement Procedures; Approved Guideline—Third Edition. CLSI Document EP05-A3. [Google Scholar]
- 13.Clinical Laboratory Standards Institute . CLSI; Wayne (PA): 2012. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline—Second Edition. CLSI Document EP17-A2. [Google Scholar]
- 14.Clinical Laboratory Standards Institute . CLSI; Wayne (PA): 2003. Evaluation of the Linearity of Quantitative Measurement Procedure: A Statistical Approach; Approved Guideline. CLSI Document EP06-A. [Google Scholar]
- 15.Clinical Laboratory Standards Institute . CLSI; Wayne (PA): 2005. Interference Testing in Clinical Chemistry; Approved Guideline—Second Edition. CLSI Document EP07-A2. [Google Scholar]
- 16.Clinical Laboratory Standards Institute . CLSI; Wayne (PA): 2013. Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline—Third Edition. CLSI Document EP09-A3. [Google Scholar]
- 17.Clinical Laboratory Standards Institute . CLSI; Wayne (PA): 2010. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline—Third Edition. CLSI Document EP28-A3c. [Google Scholar]
- 18.Wu A.H.B., Packer M., Smith A., Bijou R., Fink D., Mair J. Analytical and clinical evaluation of the bayer ADVIA Centaur automated B-type natriuretic peptide assay in patients with heart failure: a multisite study. Clin. Chem. 2004;50(5):867–873. doi: 10.1373/clinchem.2003.026138. [DOI] [PubMed] [Google Scholar]
- 19.Santos H., Cauliez B., Tron C., Brunel V., Lavoinne A. Is heparin plasma suitable for the determination of B-type natriuretic peptide on the Beckman-Coulter Access 2? Clin. Chem. Lab. Med. 2010;48(3):399–401. doi: 10.1515/CCLM.2010.069. [DOI] [PubMed] [Google Scholar]
- 20.Cemin R., Daves M. Pre-analytic variability in cardiovascular biomarker testing. J. Thorac. Dis. 2015;7(10):E395–E401. doi: 10.3978/j.issn.2072-1439.2015.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamm N.N., Semenov A.G., Seferian K.R., Bereznikova A.V., Murakami M.M., Apple F.S., Koshkina E.V., Krasnoselsky M.I., Katrukha A.G. Measurement of B-type natriuretic peptide by two assays utilizing antibodies with different epitope specificity. Clin. Biochem. 2011;44:257–259. doi: 10.1016/j.clinbiochem.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 22.Semenov A.G., Katrukha A.G. Analytical issues with natriuretic peptides – has this been overly simplified? eJIFCC. 2016;27(3):189–207. [PMC free article] [PubMed] [Google Scholar]
- 23.Semenov A.G., Katrukha A.G. Different susceptibility of B-type natriuretic peptide (BNP) and BNP precursor (proBNP) to cleavage by neprilysin: the N-terminal Part Does matter. Clin. Chem. 2016;62(4):617–622. doi: 10.1373/clinchem.2016.254524. [DOI] [PubMed] [Google Scholar]