ABSTRACT
Purpose: Surveys are needed to guide trachoma control efforts in Mozambique, with WHO guidelines for intervention based on the prevalence of trachomatous inflammation–follicular (TF) in children aged 1–9 years and the prevalence of trichiasis in adults aged 15 years and above. We conducted surveys to complete the map of trachoma prevalence in Mozambique.
Methods: Between July 2012 and May 2015, we carried out cross-sectional surveys in 96 evaluation units (EUs) covering 137 districts.
Results: A total of 269,217 individuals were enumerated and 249,318 people were examined using the WHO simplified trachoma grading system. Overall, 102,641 children aged 1–9 years, and 122,689 individuals aged 15 years and above were examined. The prevalence of TF in children aged 1–9 years was ≥10% in 12 EUs, composed of 20 districts, covering an estimated total population of 2,455,852. These districts require mass distribution of azithromycin for at least 3 years before re-survey. The TF prevalence in children was 5.0–9.9% in 17 EUs (28 districts, total population 3,753,039). 22 EUs (34 districts) had trichiasis prevalences ≥0.2% in adults 15 years and above, and will require public health action to provide surgical services addressing the backlog of trichiasis. Younger age, more children resident in the household, and living in a household that had an unimproved latrine or no latrine facility, were independently associated with an increased odds of TF in children aged 1–9 years.
Conclusions: Trachoma represents a significant public health problem in many areas of Mozambique.
KEYWORDS: Trachoma, prevalence, survey, Mozambique
Introduction
Trachoma is an infectious eye disease caused by repeated conjunctival infection with the bacterium Chlamydia trachomatis. Recurrent infections lead to chronic inflammation and scarring of the eyelid, altering the eyelid morphology and, in some people, causing an in-turning of the eyelashes. When eyelashes rub on the globe of the eye, trachomatous trichiasis is said to be present. This process of abrasion can ultimately lead to corneal opacification with visual impairment or blindness.1,2
Trachoma is the leading infectious cause of blindness worldwide, with the global focus in Sub-Saharan Africa, particularly its poorest and most isolated rural communities. In 2013, it was estimated that the African continent was host to 4 million cases of trichiasis (47% of all cases globally), with 33 of 56 African countries thought to be endemic.3 The disease is associated with low levels of sanitation and inadequate water access.4–6 In general, transmission occurs by close contact within the home, both directly (via contaminated hands) and indirectly (on cloths, or on the bodies of eye-seeking flies).1,7
The World Health Organization (WHO) has targeted trachoma for elimination as a public health problem by the year 2020, using the SAFE strategy of Surgery for trachomatous trichiasis; and Antibiotic distribution, Facial cleanliness and Environmental improvement in areas where the prevalence of the disease “trachomatous inflammation—follicular” (TF) in 1–9-year-olds is greater than 5%.
Mozambique, with a population of over 25 million people, was thought to have trachoma-endemic districts in many provinces, but there were few data to guide elimination efforts. Therefore the Mozambique Ministry of Health, in collaboration with partners, undertook population-based surveys to determine the prevalence of trachoma across all 11 provinces.
Methods
The suspected-trachoma-endemic area of Mozambique was mapped after dividing it into 96 evaluation units (EUs). To create these divisions, administrative districts with relatively low populations were combined with neighbouring districts, such that each EU had a population of approximately 100,000–250,000 people.8 The surveys were conducted in two phases – a first phase of five trachoma prevalence surveys in Erati, Memba, Nacala-a-Velha, Ribaue, and Malema of Nampula Province, which took place prior to the launch of the Global Trachoma Mapping Project (GTMP), and a second phase of 91 surveys conducted with the support of the GTMP.9
In each EU, a two-stage cluster random sampling methodology was used. The primary sampling unit was the village or enumeration area, selected with probability proportional to size, where the measure of size was the total number of households from the 2007 population census (data provided by the Mozambique National Statistics Institute).10 In the second stage, households were selected, on the day of the survey, either quasi-randomly using the random walk (first phase) or randomly using compact segment sampling (second phase). A list was kept of households from which some or all inhabitants were absent at the time of survey, with teams encouraged to return to those locations later in the day. In keeping with the known epidemiology of trachoma, urban areas (defined nationally as communities with more than 5000 inhabitants) were excluded from the sampling frame.
At sampled households, all residents aged 1 year and above were eligible for inclusion. In all 96 EUs, participants were examined for clinical signs of trachoma using the WHO simplified trachoma grading system, including TF and trichiasis.1,11 In the five first-phase EUs (Nampula Province), and in nine of the 91 second-phase EUs (those in Niassa Province, which used version 3 of the GTMP training system), data on the presence or absence of trachomatous scarring (TS)11 were collected either for all subjects (Nampula) or in eyes with trichiasis (Niassa). (The remaining 82 EUs were mapped following version 1 of the GTMP training system, which did not include collection of data on TS.) Field teams were trained on data collection and (in the second phase) were required to pass an examination using the standard GTMP procedures, following a 5-day training program led by a GTMP-certified master grader and a GTMP-certified grader trainer; prospective trachoma graders had to achieve kappa scores of at least 0.7 in an inter-grader agreement exercise with the grader trainer, in order to work on a survey.9 Data recorders were trained to conduct household interviews and to capture data using Android smartphones enabled with standard survey forms outlined in the GTMP protocol. In both phases, survey teams were taught to establish contact with and obtain permission from traditional leaders prior to data collection.
Water, Sanitation and Hygiene (WASH) factors associated elsewhere with trachoma were collected at household-level by focused interview of household heads, and by direct observation by trained data recorders. Variables collected were categorized according to WHO/UNICEF Joint Monitoring Programme standards, in which an “improved” sanitation facility is one that hygienically separates excreta from human contact, and an “improved” water source is one that is constructed to protect the source from outside contamination.9,12,13 Due to slight wording differences in the WASH-related questions used in the two phases of work, only the 91 second-phase surveys were included in the risk factor analysis.
Sample size
Using 2007 census data, it was estimated that there would be 1.59 children aged 1–9 years per household. Therefore, if teams could visit 32 households per day, 24 clusters would be required per EU to achieve the sample size of 1019 children needed to estimate a TF prevalence of 10% with a precision of ±3% at the 95% confidence level, including a design effect of 2.65 to account for the clustered design.9
Data collection tools
In the five first-phase EUs, data collection was carried out using a paper-based questionnaire. In the 91 second-phase EUs, data collection was carried out using Android smartphones, with bespoke GTMP forms developed in open data kit (ODK) and available through LINKS (https://gtmp.linkssystem.org/).9
Statistical analysis
For the paper-based census questionnaires, all data were double-entered into a database using the Census and Survey Processing system (CSPro v5 United States Census Bureau, 2012). Adjustment was carried out using R (version 3.0.2, The R Foundation for Statistical Computing, 2013). The overall EU-level adjusted prevalence was calculated by adjusting the proportion of children with TF in each cluster, in 1-year age groups, using the 2007 Mozambique census data projected to 2010, and calculating the mean of all such clusters. The same method was used to calculate the overall adjusted trichiasis prevalence, but with adjustment for both sex and age in 5-year age groups. When data on TS were available, we report the prevalence of TT: this was possible for 14 EUs. (As noted above, in 82 of the 96 EUs, data on the presence or absence of TS in eyes with trichiasis were not collected.) Confidence intervals around prevalence estimates were calculated by bootstrapping the adjusted cluster proportions over 10,000 iterations and taking the 2.5th and 97.5th centiles. For the 91 second-phase EUs, analyses of associations of TF were carried out at individual level, accounting for clustering at EU, cluster, and household levels using mixed-effects logistic regression. Variables were considered for inclusion in the multivariable model if significant on univariable analysis at the p < 0.05 level. A step-wise inclusion approach was used to build the multivariable model, with variables retained if significant at the p < 0.05 level (Likelihood ratio test).
Ethical considerations
Approval for conducting the study was obtained from the National Committee on Bio-Ethics, the Provincial Directorates of Health in each province of Mozambique, and (for the second-phase, GTMP-supported EUs) the ethics committee of the London School of Hygiene & Tropical Medicine (references 6319 and 8355). In the paper-based, first-phase surveys, consent forms were signed or finger stamped by all participants before the start of the data collection process. For the second-phase, GTMP-supported surveys, consent was recorded electronically for all participants. Individuals with active trachoma and their family members received 1% tetracycline eye ointment, in accordance with Mozambique Ministry of Health guidelines. Each person who took part in the research was thanked and received a bar of soap plus information about the importance of daily face washing for the reduction of trachoma transmission. All patients found to have significant ocular pathology were referred to the nearest health centre.
Results
A total of 2378 clusters were visited within the 96 EUs, with 75,993 households enrolled between July 2012 and May 2015. In total, 249,318 people aged 1 year or older were examined from the 269,217 residents living in those households, representing a coverage of 92.6%. A total of 138,210 (55.4%) women were examined. A total of 102,641 children aged 1–9 years and 122,689 individuals aged 15 years or greater were examined. A greater proportion (60.8%) of examined subjects aged 15 years or greater were female. Among the 102,641 children aged 1–9 years examined, 50,911 (49.6%) were male and 51,730 (50.4%) were female.
In the 91 second-phase EUs included in the risk factor analyses, a total of 2240 clusters were surveyed, covering 70,757 households. A household head was present and consented to the survey in 70,663 (99.8% of) households, with 248,494 individuals subsequently sampled for inclusion, and 228,595 (92.0%) consenting to examination. 19,325 (7.8%) individuals were resident in selected households but not present at the time of survey, and 516 (0.2%) did not consent to examination. In the five first-phase surveys, a total of 20,723 individuals provided consent to be examined and were examined over 138 clusters covering 5233 households.
Table 1 shows, for each EU, the adjusted TF prevalence in children aged 1–9 years, and the adjusted trichiasis prevalence (or TT prevalence, for those EUs in which TS data could be applied to eyes with trichiasis) for adults aged 15 years and above. There were 12 EUs (20 districts, 2,455,852 total population) with TF prevalences in 1–9-year-olds ≥10%, and 17 EUs (28 districts, 3,753,039 total population) with TF prevalences of 5.0–9.9%. In 22 EUs, the trichiasis prevalence in adults aged 15 years and above was ≥0.2%, the WHO trichiasis elimination threshold.14
Table 1.
Prevalence of trachomatous inflammation—follicular (TF) in children aged 1–9 years and trichiasis in adults aged 15 years and above, by evaluation unit, Mozambique, 2012–2015. The name given to each evaluation unit is a concatenation of the names of its constituent districts.
| 1–9 years |
≥15 years |
||||
|---|---|---|---|---|---|
| Province | Evaluation unit | Examined (n) | Adjusted TFa (%, 95%CIb) | Examined (n) | Adjusted trichiasisc prevalence (%, 95%CIb) |
| Cabo Delgado | Ibo-Meluco-Macomia | 727 | 6.6 (3.7–10.5) | 1183 | 0.8 (0.2–1.2) |
| Cabo Delgado | Muidumbe-Mocimboa Praia | 769 | 8.8 (5.3–13.4) | 1254 | 0.7 (0.3–1.2) |
| Cabo Delgado | Pemba Metuge-Mecufi-Quissanga | 753 | 8.4 (5–12.4) | 1164 | 0.4 (0.1–0.7) |
| Cabo Delgado | Nangade-Palma | 908 | 15.7 (10.6–19.8) | 1480 | 1.2 (0.7–1.7) |
| Cabo Delgado | Ancuabe | 845 | 11.6 (7.5–16.8) | 1200 | 0.4 (0.1–0.6) |
| Cabo Delgado | Balama | 1019 | 5.3 (3.7–7.3) | 1275 | 0.8 (0.4–1.1) |
| Cabo Delgado | Chiure | 798 | 13.9 (8.7–19.4) | 1181 | 0.2 (0–0.6) |
| Cabo Delgado | Montepuez | 820 | 7.8 (5–11.5) | 1233 | 0.6 (0.4–1) |
| Cabo Delgado | Mueda | 744 | 12.2 (8.6–16.8) | 1313 | 0.7 (0.3–1.2) |
| Cabo Delgado | Namuno | 835 | 4.8 (2.9–6.6) | 1106 | 0.6 (0.2–1.1) |
| Gaza | Macia | 1043 | 0.3 (0–0.6) | 1112 | 0 (0–0.3) |
| Gaza | Chibuto | 866 | 0.1 (0–0.2) | 1088 | 0.1 (0–0.3) |
| Gaza | Guija-Chigubo-Mabalane | 868 | 0.9 (0–2.4) | 1055 | 0 (0–0.4) |
| Gaza | Chokwe | 862 | 0.2 (0–0.6) | 1086 | 0.2 (0–0.4) |
| Gaza | Manjacaze | 935 | 0 (0–0) | 1214 | 0.3 (0–0.6) |
| Gaza | Massingir-Massangena-Chicualacuala | 1042 | 1.2 (0.3–2.5) | 1026 | 0 (0–0.4) |
| Gaza | Xai-Xai | 925 | 0.1 (0–0.3) | 1114 | 0 (0–0.3) |
| Inhambane | Inhassoro-Govuro-Mabote | 1095 | 1.4 (0.7–2.5) | 1342 | 0.1 (0–0.4) |
| Inhambane | Funhalouro-Panda | 879 | 0.4 (0–1) | 1133 | 0 (0–0.3) |
| Inhambane | Homoine | 857 | 0.1 (0–0.2) | 1195 | 0.2 (0.1–0.4) |
| Inhambane | Inharrime | 925 | 0 (0–0) | 1236 | 0.1 (0.1–0.2) |
| Inhambane | Jangamo | 936 | 0 (0–0) | 1213 | 0.1 (0–0.3) |
| Inhambane | Massinga | 819 | 0.4 (0–1) | 1183 | 0.3 (0–0.5) |
| Inhambane | Morrumbene | 894 | 0.3 (0–0.6) | 1222 | 0.1 (0–0.2) |
| Inhambane | Vilankulo | 904 | 0.9 (0.3–1.4) | 1233 | 0.2 (0–0.4) |
| Inhambane | Zavala | 963 | 0.7 (0.1–1.3) | 1318 | 0.2 (0.1–0.5) |
| Manica | Macossa-Guro-Tambara | 1693 | 10.7 (7.2–14.3) | 1280 | 0.3 (0.1–0.5) |
| Manica | Barue | 1344 | 1.6 (0.5–2.8) | 1321 | 0 (0–0.3) |
| Manica | Macate-Gondola | 1394 | 0.3 (0–0.7) | 1240 | 0.1 (0–0.2) |
| Manica | Machaze | 1273 | 2.6 (1.2–3.9) | 1085 | 0.1 (0–0.3) |
| Manica | Vanduzi-Manica | 1432 | 0.3 (0.1–0.6) | 1219 | 0 (0–0.3) |
| Manica | Mossurize | 1218 | 0.1 (0–0.4) | 1110 | 0 (0–0.3) |
| Manica | Sussundenga | 1293 | 0.4 (0.1–0.8) | 1181 | 0.1 (0–0.3) |
| Maputo | Boane | 760 | 0.6 (0.1–1.3) | 1245 | 0.1 (0–0.3) |
| Maputo | Manhica | 1294 | 1.1 (0.2–2.4) | 1452 | 0 (0–0.3) |
| Maputo | Marracuene | 1211 | 0.6 (0.2–1) | 1582 | 0 (0–0.2) |
| Maputo | Moamba-Magude | 768 | 1 (0.2–2) | 1161 | 0 (0–0.3) |
| Maputo | Matutuine-Namaacha | 1125 | 0.5 (0.1–1.1) | 1447 | 0 (0–0.3) |
| Nampula | Ilha Mozambique-Mossuril | 1192 | 14.5 (9.1–20) | 1262 | 0.1 (0–0.1) |
| Nampula | Mecuburi-Lalaua | 1137 | 3.7 (2.2–5.2) | 1212 | 0.1 (0–0.3) |
| Nampula | Nacaroa-Muecate | 1049 | 6.2 (3.6–9.2) | 1211 | 0.3 (0–0.7) |
| Nampula | Angoche | 1193 | 10.3 (6.1–14.9) | 1291 | 0 (0–0.3) |
| Nampula | Meconta | 1000 | 3.7 (1.7–6.4) | 1239 | 0.1 (0–0.1) |
| Nampula | Mogincual-Liupo | 1159 | 17.1 (11.6–22) | 1249 | 0.1 (0–0.1) |
| Nampula | Mogovolas | 1084 | 3.6 (1.8–5.3) | 1273 | 0.1 (0–0.2) |
| Nampula | Moma-Larde | 1209 | 8.3 (6.3–10.4) | 1288 | 0.8 (0.2–1.9) |
| Nampula | Monapo | 1114 | 7.6 (4.5–10.7) | 1265 | 0 (0–3) |
| Nampula | Murrupula | 1035 | 2.7 (1.3–4.4) | 1222 | 0 (0–0.3) |
| Nampula | Nampula | 984 | 2.9 (1.3–4.8) | 1198 | 0 (0–0.3) |
| Nampula | Ribauee | 1964 | 1.3 (0.7–1.7) | 2335 | 0 (0–0.2)d |
| Nampula | Malemae | 1680 | 1.1 (0.5–1.8) | 2227 | 0 (0–0.2)d |
| Nampula | Membae | 1846 | 11.9 (9–16) | 2300 | 0 (0–0.2)d |
| Nampula | Eratie | 1581 | 8.4 (6.3–11.4) | 2285 | 0 (0–0.2)d |
| Nampula | Nacala-A-Velhae | 1858 | 9.4 (7.3–11.5) | 2647 | 0 (0–0.1)d |
| Niassa | Cuamba | 1094 | 2.1 (0.9–3) | 1119 | 0 (0–0.3)d |
| Niassa | Lichinga | 1016 | 1.2 (0.4–2) | 978 | 0 (0–0.4)d |
| Niassa | Mandimba | 1395 | 1.4 (0.6–2.5) | 1142 | 0 (0–0.3)d |
| Niassa | Mecanelhas | 1308 | 1.9 (1–2.6) | 1194 | 0.1 (0–0.4)d |
| Niassa | Sanga-Lago | 1120 | 0.5 (0.2–0.9) | 1106 | 0 (0–0.3)d |
| Niassa | Magune-Marupa | 985 | 2.4 (1.1–4.1) | 1047 | 0.1 (0–0.2)d |
| Niassa | N’gauma | 1139 | 3.3 (1.7–5.3) | 1086 | 0 (0–0.4)d |
| Niassa | Maúa-Nipepe-Metarica | 1245 | 1.4 (0.7–2.4) | 1206 | 0 (0–0.3)d |
| Niassa | Muembe -Mecula-Mavago | 1172 | 0.7 (0.1–1.5) | 1113 | 0 (0–0.3)d |
| Sofala | Buzi | 1063 | 0 (0–0) | 1086 | 0 (0–0.4) |
| Sofala | Caia | 1449 | 2.1 (1.3–3.3) | 1224 | 0.2 (0–0.5) |
| Sofala | Machanga-Chibabava | 979 | 0.8 (0.2–1.5) | 1111 | 0 (0–0.3) |
| Sofala | Dondo | 1128 | 0.4 (0.1–0.7) | 998 | 0 (0–0.4) |
| Sofala | Gorongoza | 1292 | 1.2 (0.4–2.4) | 1173 | 0 (0–0.3) |
| Sofala | Cheringoma-Muanza-Marromeu | 1510 | 4.1 (2.8–5.7) | 1306 | 0.1 (0–0.3) |
| Sofala | Chemba-Maringue | 1391 | 6.9 (4.8–9.5) | 1139 | 0.2 (0–0.4) |
| Sofala | Nhamatanda | 1197 | 1 (0.3–1.8) | 1081 | 0.4 (0–1.1) |
| Tete | Angonia | 834 | 3 (1.2–5.6) | 1272 | 0.2 (0–0.5) |
| Tete | Chiuta-Cahora Bassa | 892 | 5.7 (3.7–8.3) | 1210 | 0.1 (0–0.1) |
| Tete | Changara | 903 | 9.9 (6.9–12.9) | 1163 | 0.2 (0–0.5) |
| Tete | Chifunde | 1042 | 4.1 (2.5–6) | 1228 | 0.1 (0–0.3) |
| Tete | Macanga | 1063 | 3.6 (2.1–5.2) | 1269 | 0 (0–0.3) |
| Tete | Magoe-Zumbu | 845 | 5.2 (2.9–8.3) | 1253 | 0.2 (0–0.4) |
| Tete | Maravia | 879 | 1.9 (0.8–3.1) | 1224 | 0 (0–0.3) |
| Tete | Moatize | 1006 | 4.7 (2.8–6.8) | 1200 | 0.2 (0–0.5) |
| Tete | Doa-Mutarara | 1073 | 9.7 (7.2–13) | 1248 | 0.1 (0–0.2) |
| Tete | Tsangano | 836 | 1.7 (0.8–2.9) | 1193 | 0 (0–3) |
| Zambezia | Chinde-Luabo-Inhassunge | 1613 | 19.8 (15.6–24.1) | 2200 | 0.2 (0–0.5) |
| Zambezia | Gile | 935 | 2.3 (0.9–3.4) | 1217 | 0 (0–3) |
| Zambezia | Gurue | 797 | 3.3 (1.9–4.7) | 1098 | 0 (0–0.4) |
| Zambezia | Ile-Mulevala | 829 | 1.2 (0.3–2.3) | 1150 | 0.3 (0–0.7) |
| Zambezia | Lugela | 794 | 2 (0.6–3.3) | 1231 | 0.1 (0–0.3) |
| Zambezia | Mocubela-Maganja da Costa | 856 | 10.2 (6.5–13.3) | 1188 | 0.2 (0–0.4) |
| Zambezia | Milange | 872 | 3.7 (1.9–5.9) | 1169 | 0 (0–0.3) |
| Zambezia | Mocuba | 896 | 1.6 (0.8–2.7) | 1214 | 0 (0–0.3) |
| Zambezia | Molocue | 821 | 1.8 (0.9–2.5) | 1170 | 0 (0–0.3) |
| Zambezia | Mopeia | 853 | 6.9 (4.4–10.3) | 1196 | 0 (0–0.3) |
| Zambezia | Morrumbala | 865 | 7 (3.9–11.1) | 1168 | 0.1 (0–0.3) |
| Zambezia | Namacurra | 770 | 3.4 (1.6–5.7) | 1263 | 0.2 (0–0.4) |
| Zambezia | Namaroi | 774 | 2.7 (1–4.4) | 1147 | 0 (0–0.3) |
| Zambezia | Nicoadala | 1438 | 3.9 (2.2–6) | 1713 | 0.1 (0–0.1) |
| Zambezia | Pebane | 751 | 14.1 (9.9–19.7) | 1210 | 0.3 (0–0.9) |
aPopulation-based trachomatous inflammation–follicular (TF) prevalence derived from cluster-level proportions adjusted for age in 1 year agebands using the latest available census data
b95% Confidence intervals estimated by bootstrapping adjusted cluster-level proportions over 10,000 replicates. Zero-count estimate upper confidence interval limits estimated as a one-sided 97.5% exact binomial confidence interval.
cPopulation-based trichiasis prevalence derived from cluster-level proportions adjusted for sex and age in 5 year agebands using the latest available census data
dData on trachomatous scarring (TS) recorded for all subjects, or for eyes with trichiasis (see text), in these surveys, so the trichiasis prevalence estimates here may be accurately referred to as trachomatous trichiasis (TT) prevalence estimates.
eFirst-phase, paper-based surveys.
In the 82 surveys in which trachomatous scarring (TS) did not form part of the examination, a total of 100,904 individuals aged 15 years or greater consented to examination and were examined. Altogether, 38,522 (38.2%) males aged 15 years or greater were examined, and 87 were found to have trichiasis (0.22%). Among the 62,382 (61.8%) females aged 15 years or greater examined in these surveys, 276 had trichiasis (0.44%).
In the 14 surveys in which TS was included in the examination, a total of 21,753 individuals aged 15 years or greater were examined. Of these, 9517 (43.8%) of those examined aged 15 years or greater were male, with one case of TT identified (0.01%). A total of 12,236 (56.2%) of those examined aged 15 years or greater were female, with five cases of TT identified (0.04%).
EU-level prevalences of TF in children aged 1–9 years and trichiasis (or TT) in adults aged 15 years and above are shown in Figures 1 and 2, respectively. In 74 EUs, the trichiasis (or TT) prevalence was less than the WHO elimination threshold of <0.2% of the population aged 15 years and above, and in 67 EUs, the TF prevalence in children aged 1–9 years was less than the 5% threshold for elimination. Overall, 59 EUs had both a TF prevalence <5% in children aged 1–9 years and a trichiasis prevalence of <0.2% of those aged 15 years and above. Among the 22 EUs with trichiasis prevalences in adults ≥0.2%, seven had TF prevalence >10% and seven had TF prevalence between 5.0 and 9.9%.
Figure 1.
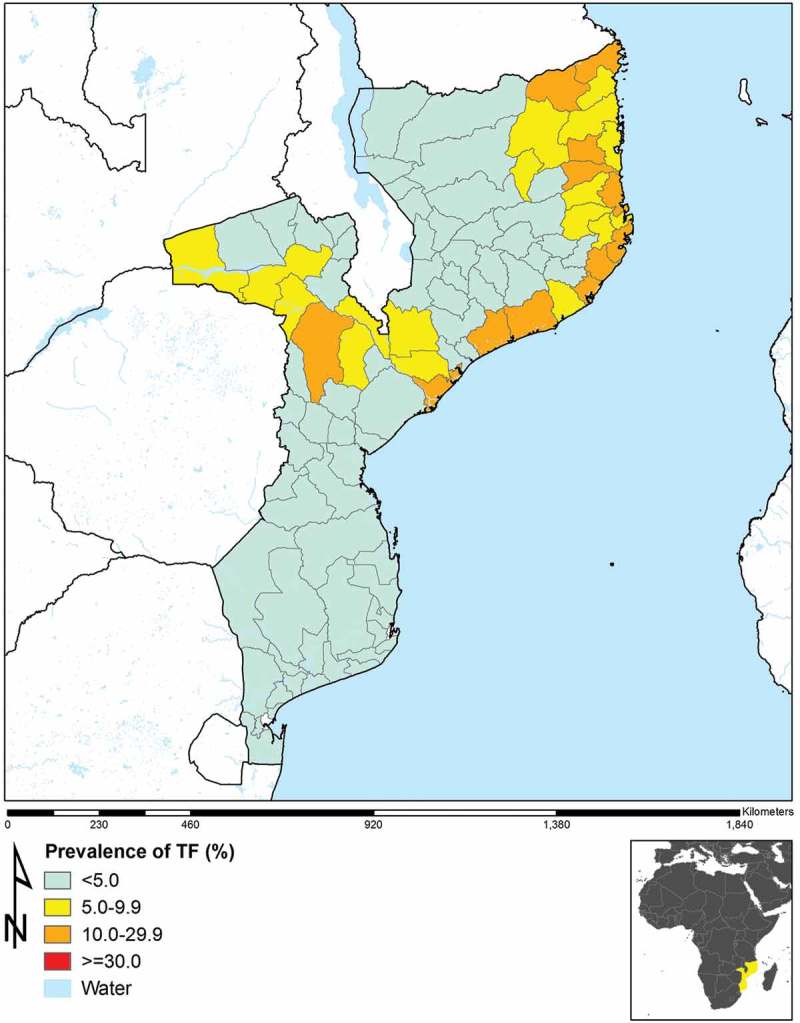
Prevalence of trachomatous inflammation–follicular (TF) in those aged 1–9 years in 96 population-based prevalence surveys, Mozambique, 2012–2015.
Figure 2.
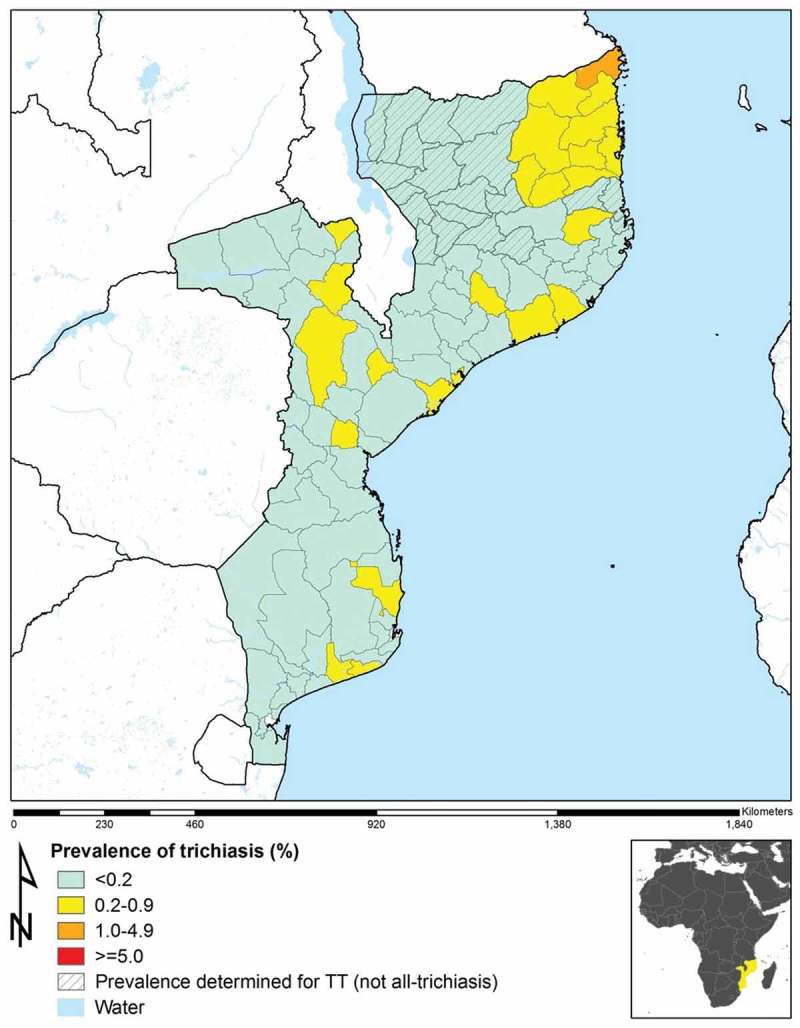
Prevalence of trichiasis in those aged 15 years and above in 96 population-based prevalence surveys, Mozambique, 2012–2015. In 14 evaluation units (hatched), prevalence categories shown are for trachomatous trichiasis (TT), defined as trichiasis and (in the same eye) either (a) trachomatous conjunctival scarring (TS); or (b) the examiner’s inability to evert the eyelid to look for TS (with the difficulty in eversion presumed to be due to TS). In the other 82 evaluation units, prevalence categories shown are for all-trichiasis, regardless of the presence or absence of TS.
Risk factor analysis
TF risk factors
Full univariable results for the outcome of TF in children aged 1–9 years are shown in Table 2. Among the 91 surveys conducted in the second phase, TF prevalence was 4% among boys and 4% among girls. A multivariable analysis found that younger age, living with more children aged 1–9 years in the household, and living in a household with unimproved or no latrine facilities (including use of the bush or field) was independently associated with TF in the same age group (Table 3).
Table 2.
Univariable mixed-effects logistic regression analysis of the outcome trachomatous inflammation–follicular (TF) in children aged 1–9 years against putative risk factors, using data from 91 population-based prevalence surveys, Global Trachoma Mapping Project, Mozambique, 2012–2015.
| Variable | Examined (n) | TF cases (%) | Odds ratio (95% CI)a | p-valueb |
|---|---|---|---|---|
| Age | ||||
| 1–4 | 42,919 | 2377 (5.5) | 2.16 (2.01–2.33) | <0.0001 |
| 5–9 | 50,793 | 1386 (2.7) | 1 | |
| Sex | ||||
| Male | 46,532 | 1859 (4.0) | 1 | |
| Female | 47,180 | 1904 (4.0) | 1.03 (0.96–1.10) | 0.43 |
| Number of children aged 1–9 years in household | ||||
| 1–3 | 76,899 | 2900 (3.8) | 1 | |
| ≥4 | 16,813 | 863 (5.1) | 1.44 (1.31–1.57) | <0.0001 |
| Latrine typec | ||||
| Improved | 16,040 | 382 (2.4) | 1 | <0.0001 |
| Unimproved | 36,684 | 1160 (3.2) | 1.21 (1.07–1.37) | |
| No facilities, bush or field | 40,988 | 2221 (5.4) | 1.46 (1.30–1.64) | |
| Latrine accessd | ||||
| Shared latrine | 6681 | 172 (2.6) | 1.05 (0.86–1.29) | <0.0001 |
| Private latrine | 42,920 | 1182 (2.8) | 1 | |
| No structure, outside near the house, in the bush or field | 44,111 | 2409 (5.5) | 1.25 (1.13–1.38) | |
| Time to source of water for face-washingd | ||||
| All face-washing done at source | 307 | 6 (1.9) | 1.51 (0.60–3.78) | 0.2226 |
| <30 mins round-trip | 43,251 | 1486 (3.4) | 1 | |
| ≥30 mins round-trip | 50,154 | 2271 (4.5) | 1.09 (0.98–1.22) | |
| Time to source of water for drinkingd | ||||
| <30 mins round-trip | 43,084 | 1485 (3.4) | 1 | |
| ≥30 mins round-trip | 50,628 | 2278 (4.5) | 1.08 (0.97–1.21) | 0.1576 |
| Source of water for drinking | ||||
| Improved water source | 53,561 | 1911 (3.6) | 1 | 0.112 |
| Unimproved water source | 17,930 | 934 (5.2) | 1.11 (0.95–1.30) | |
| Surface water (river, lake, dam) | 22,221 | 918 (4.1) | 1.16 (0.99–1.36) | |
| Source of water for face-washing | ||||
| Improved water source | 52,367 | 1872 (3.6) | 1 | 0.0944 |
| Unimproved water source | 18,240 | 949 (5.2) | 1.12 (0.96–1.31) | |
| Surface water (river, lake, dam) | 22,835 | 942 (4.1) | 1.17 (1.00–1.35) |
aUnivariable odds ratio and 95% confidence interval against the outcome trachomatous inflammation—follicular in children aged 1–9 years. Analysis carried out at individual level.
bWald’s test
cDirect observation by recorders
dSelf-reported estimates from household head.
Table 3.
Multivariable mixed-effects logistic regression analysis of the outcome trachomatous inflammation–follicular (TF) in children aged 1–9 years against putative risk factors, using data from 91 population-based prevalence surveys, Global Trachoma Mapping Project, Mozambique, 2012–2015.
| Variable | Odds ratio (95% CI)a | p-valueb |
|---|---|---|
| Age | ||
| 1–4 | 2.19 (2.03–2.36) | <0.0001 |
| 5–9 | 1 | |
| Number of children aged1–9 years in household | ||
| 1–3 | 1 | |
| ≥4 | 1.51 (1.38–1.65) | <0.0001 |
| Latrine typec | ||
| Improved | 1 | <0.0001 |
| Unimproved | 1.24 (1.03–1.49) | |
| No facilities, bush or field | 1.45 (1.22–1.73) | |
aMultivariable odds ratio and 95% confidence interval against the outcome trachomatous inflammation–follicular in children aged 1–9 years. Analysis carried out at individual level.
bLikelihood ratio test of inclusion/exclusion in the full multivariable model.
cDirect observation by recorders.
Discussion
The GTMP may be the largest field-based infectious disease mapping project ever attempted, and the work conducted in Mozambique was its largest single constituent project. Altogether, 91 separate standardised population-based trachoma prevalence surveys, involving the individual examination of a total of more than 200,000 people of all ages, were carried out by trained and certified field teams; complementing five trachoma prevalence surveys previously carried out in 2012. The successful completion of this work is an excellent indication of the Mozambique programme’s readiness to proceed to national elimination of trachoma as a public health problem.
Slightly more women than men were examined. This reflects the demographic structure of the Mozambican population, in which there are more women than men, but also a higher probability of women being at home at the time of data collection. (In the areas surveyed, during the day, the majority of men work in the fields.)
Most of the surveys reported in this paper were baseline surveys, i.e., conducted prior to the initiation of any community-based interventions against trachoma. The exceptions were the nine surveys done in Niassa Province, where a previous (July 2011) province-level survey had estimated the TF prevalence in 1–9-year-olds to be 32% [unpublished Ministry of Health data]. On the basis of that estimate, mass treatment with azithromycin was undertaken in 10 of Niassa’s 16 districts in 2013 (with 9/10 districts achieving >80% coverage), and in all 16 districts in 2014 (with 6/16 districts achieving >80% coverage). Whether the 2011 estimates were overestimates or the effect of those rounds of mass treatment was large – or both – is difficult to say, but it is notable that each of the Niassa EUs for which we conducted impact surveys in 2015 (this study) returned estimated TF prevalences of <5%. Surveillance surveys should be undertaken in these EUs in 2017.
In total, of 96 EUs, 12 had TF prevalences ≥10% among children aged 1–9 years. These 12 EUs, composed of 20 districts, should have mass treatment with azithromycin annually for the next 3 years.15 In addition, 17 EUs, composed of 28 districts, had TF prevalences between 5.0 and 9.9% and may be considered for one round of azithromycin treatment before re-survey, following informal recommendations from the International Trachoma Initiative. All 29 of these EUs also need implementation of the F&E components of the SAFE strategy. Overall, 67 EUs had a TF prevalence in children aged 1–9 years below the WHO elimination threshold, yet eight of these EUs had a trichiasis prevalence ≥0.2% of those aged 15 years and above, suggesting that active trachoma is likely to have been a public health problem there in the past.
A total of 77% of the EUs surveyed (74/96) had a trichiasis (or TT) prevalence below the threshold defined by WHO to indicate a public health problem: 0.2% in adults aged 15 years and above.14 The EU comprising Nangade and Palma districts in Cabo Delgado Province had a trichiasis prevalence in those aged 15 years and above of >1%. The reason for the much higher burden of trichiasis in this area is unclear. In the 22 EUs with trichiasis prevalences ≥0.2% in adults, resources should be made available to ensure that individuals who need surgery have access to a high quality service provided, if possible, at no cost to the recipient.
We found that younger children, and children sharing a household with larger numbers of children had higher odds of TF in this population. This is in keeping with the belief that younger children act as the reservoir of infection for ocular C. trachomatis,16 and that close contact with more children is likely to facilitate spread.17–20 In addition, we found that unimproved latrine facilities, or living in a household with no latrine facilities, was an independent association of TF in children. Similar associations have been noted elsewhere, both prior to21–24 and within the GTMP,20,25–27 and are in keeping with the belief that latrine facilities of a given standard limit the reproductive potential of the flies associated with transmission.5,28,29
At larger scale, it is noted that active trachoma exists as a public health problem in Mozambique in an arc of endemicity stretching from Cabo Delgado in the country’s north-east to Tete in the west. We are unsure as to the reasons for this phenomenon, and will work with local epidemiologists and international experts to try to generate an explanation.
Active trachoma is a public health concern for 48 districts of Mozambique. 6.2 million people live in those districts. It is essential to undertake distribution of azithromycin, improve sanitation services, push the importance of facial cleanliness and improve water access in these areas in order to eliminate trachoma. In addition, there is a significant backlog of individuals with trichiasis who urgently need corrective eyelid surgery in order to preserve their remaining vision.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: 1. Advisory Committee, 2. Information Technology, Geographical Information Systems, and Data Processing, 3. Epidemiological Support, 4. Ethiopia Pilot Team, 5. Master Grader Trainers, 6. Methodologies Working Group, 7. Prioritisation Working Group, 8. Proposal Development, Finances and Logistics, 9. Statistics and Data Analysis, 10. Tools Working Group, 11. Training Working Group.
Funding Statement
This study was made possible thanks to funding from USAID and the ENVISION project led by RTI International under cooperative agreement number AID-OAA-A-11-00048. Core support to the GTMP was provided by a grant from the United Kingdom’s Department for International Development (DFID; ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organisations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. The views expressed in this article are the views of the authors alone and do not necessarily reflect the decisions, policies or views of USAID, DFID, or the Governments of the USA or United Kingdom. None of the funders had any role in project design, project implementation, analysis or interpretation of data, or in the decisions on where, how or when to publish in the peer-reviewed press, or in preparation of the manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the writing and content of this article.
Funding
This study was made possible thanks to funding from USAID and the ENVISION project led by RTI International under cooperative agreement number AID-OAA-A-11-00048. Core support to the GTMP was provided by a grant from the United Kingdom’s Department for International Development (DFID; ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organisations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. The views expressed in this article are the views of the authors alone and do not necessarily reflect the decisions, policies or views of USAID, DFID, or the Governments of the USA or United Kingdom. None of the funders had any role in project design, project implementation, analysis or interpretation of data, or in the decisions on where, how or when to publish in the peer-reviewed press, or in preparation of the manuscript.
References
- 1.Mabey DCW, Solomon AW, Foster A.. Trachoma. Lancet (London, England). 2003;362(9379):223–229. doi: 10.1016/S0140-6736(03)13914-1. [DOI] [PubMed] [Google Scholar]
- 2.Mabey D, Fraser-Hurt N. Trachoma. Clin Evid (Online). 2004;(11):880–891. [PubMed] [Google Scholar]
- 3.Smith JL, Flueckiger RM, Hooper PJ, et al. The geographical distribution and burden of trachoma in Africa. PLoS Negl Trop Dis. 2013;7(8). doi: 10.1371/journal.pntd.0002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocks ME, Ogden S, Haddad D, Addiss DG, McGuire C, Freeman MC. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med. 2014;11(2):e1001605. doi: 10.1371/journal.pmed.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson PM, Burton M, Solomon AW, Bailey R, Mabey D. The SAFE strategy for trachoma control: using operational research for policy, planning and implementation. Bull World Health Organ. 2006;84(8):613–619. doi: 10.2471/BLT.05.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Last AR, Burr SE, Weiss HA, et al. Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naïve trachoma-hyperendemic communities of the Bijagós Archipelago, Guinea Bissau. PLoS Negl Trop Dis. 2014;8(6). doi: 10.1371/journal.pntd.0002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright HR, Turner A, Taylor HR. Trachoma. Lancet (London, England). 2008;371(9628):1945–1954. doi: 10.1016/S0140-6736(08)60836-3. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Report of the 3rd Global Scientific Meeting on Trachoma, Johns Hopkins University, Baltimore, MA. 19-20July2010. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 9.Solomon AW, Pavluck A, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi: 10.3109/09286586.2015.1037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Instituto Nacional de Estatistica (INE) Manual De Cartografia Do III Recenseamento Geral De Populacao De Habitacao. Maputo, Mozambique: INE; 2007. [Google Scholar]
- 11.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65(4):477–483. [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation(WHO)/UNICEF Improved and Unimproved Water Sources and Sanitation Facilities. WHO/UNICEF Joint Monitoring Programme(JMP) for Water Supply and Sanitation; World Health Organization; UNICEF March 2012. [Google Scholar]
- 13.Exley JLR, Liseka B, Cumming O, Ensink JHJ. The sanitation ladder, what constitutes an improved form of sanitation? Environ Sci Technol. 2015;49(2):1086–1094. doi: 10.1021/es503945x. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Validation of Elimination of Trachoma as a Public Health Problem (WHO/HTM/NTD/2016.8). Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 15.Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW FA. Trachoma Control: A Guide for Programme Managers. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 16.Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362(9379):198–204. doi: 10.1016/S0140-6736(03)13909-8. [DOI] [PubMed] [Google Scholar]
- 17.Bailey R, Duong T, Carpenter R, Whittle H, Mabey D. The duration of human ocular Chlamydia trachomatis infection is age dependent. Epidemiol Infect. 1999;123(3):479–486. doi: 10.1017/S0950268899003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards T, Harding-Esch EM, Hailu G, et al. Risk factors for active trachoma and Chlamydia trachomatis infection in rural Ethiopia after mass treatment with azithromycin. Trop Med Int Health. 2008;13(4):556–565. doi: 10.1111/j.1365-3156.2008.02034.x. [DOI] [PubMed] [Google Scholar]
- 19.Hägi M, Schémann J-F, Mauny F, et al. Active trachoma among Children in Mali: clustering and environmental risk factors. PLoS Negl Trop Dis. 2010;4(1). doi: 10.1371/journal.pntd.0000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elshafie BE, Osman KH, Macleod C, et al. The epidemiology of trachoma in Darfur States and Khartoum State, Sudan: results of 32 population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(6):381–391. doi: 10.1080/09286586.2016.1243718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courtright P, Sheppard J, Lane S, Sadek A, Schachter J, Dawson CR. Latrine ownership as a protective factor in inflammatory trachoma in Egypt. Br J Ophthalmol. 1991;75(6):322–325. doi: 10.1136/bjo.75.6.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schemann JF, Guinot C, Ilboudo L, et al. Trachoma, flies and environmental factors in Burkina Faso. Trans R Soc Trop Med Hyg. 2003;97(1):63–68. doi: 10.1016/S0035-9203(03)90025-3. [DOI] [PubMed] [Google Scholar]
- 23.Ngondi J, Matthews F, Reacher M, Baba S, Brayne C, Emerson P. Associations between active trachoma and community intervention with antibiotics, facial cleanliness, and environmental improvement (A,F,E). PLoS Negl Trop Dis. 2008;2(4):e229. doi: 10.1371/journal.pntd.0000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallam TA, Raja’a YA, Al-Zubiery TK, et al. Chlamydia trachomatis infections among Yemeni school pupils in relation to environmental conditions. Saudi Med J. 2003;24(1):84–87. [PubMed] [Google Scholar]
- 25.Bero B, Macleod C, Alemayehu W, et al. Prevalence of and risk factors for trachoma in oromia regional state of Ethiopia: results of 79 population-based prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(6):392–405. doi: 10.1080/09286586.2016.1243717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko R, Macleod C, Pahau D, et al. Population-based trachoma mapping in six evaluation units of Papua New Guinea. Ophthalmic Epidemiol. 2016;23(Suppl. 1):22–31. doi: 10.1080/09286586.2016.1235715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adera TH, Macleod C, Endriyas M, et al. Prevalence of and risk factors for trachoma in Southern nations, nationalities, and peoples’ region, Ethiopia: results of 40 population-based prevalence surveys carried out with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(sup1):84–93. doi: 10.1080/09286586.2016.1247876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emerson PM, Simms VM, Makalo P, Bailey RL. Household pit latrines as a potential source of the fly Musca sorbens–a one year longitudinal study from The Gambia. Trop Med Int Health. 2005;10(7):706–709. doi: 10.1111/j.1365-3156.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- 29.Rabiu M, Alhassan MB, Ejere HOD, Evans JR. Environmental sanitary interventions for preventing active trachoma. Cochrane Database Syst Rev. 2012;2. doi: 10.1002/14651858.CD004003.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]


