Abstract
West Nile Virus (WNV) is the most widely distributed flavivirus worldwide. It is a mosquito-borne virus, and birds constitute its natural reservoir. Humans and equines are considered accidental hosts. Human WNV infections are usually asymptomatic or express as a mild febrile syndrome; however, in around 1% of cases they are responsible for more serious neurological diseases with a potentially lethal outcome. In the Mediterranean basin the virus circulation is regarded as endemic. Outbreaks of WNV meningoencephalitis are regularly notified, especially during summer and autumn seasons. In Algeria, although some surveys have reported WNV activity in the Sahara, to date few data are available about virus circulation in the northern part of the country. We conducted this study to detect possible WNV activity in this part of Algeria. For this purpose, in 2010 a total of 164 human sera were collected from native patients of the Algiers district and surrounding areas, then tested retrospectively for IgG anti-WNV by ELISA. Plaque reduction neutralization technique (PRNT) was used for result confirmation. In this cohort, 9.8% of the 164 collected sera returned positive for anti-WNV IgG; after confirmation by PRNT; 6.7% had specific neutralizing antibodies. No statistically significant difference was observed according to the sex or transfusion status of the patients. In conclusion, these data show for the first time serological evidence of WNV circulation in Algiers and its surrounding areas. They also highlight the need for implementing an integrated surveillance programme covering all aspects of WNV disease in order to better understand the circulation dynamics of WNV in this region. Other flaviviruses antigenically related to WNV should be investigated, given the evidence of serological cross-reaction, as specific IgG antibodies decrease after PRNT confirmation.
Keywords: Algeria, circulation, ELISA, north central, sero-neutralization (PRNT), West Nile virus
Introduction
West Nile virus (WNV) or West Nile fever virus is an arbovirus with a worldwide distribution. It is classified in the Japanese encephalitis virus complex, genus Flavivirus, family Flaviviridae [1]. Its natural transmission cycle involves birds (as an amplifying reservoir and disseminator of the virus) and mosquito vectors, particularly mosquitoes of the genus Culex [2]. Two types of cycle are described [3]; a sylvatic, rural cycle around wet and swampy areas, usually involving migratory birds and ornithophilic mosquitoes, and an urban cycle, involving domestic birds and both ornithophilic and anthropophilic mosquitoes [4]. Mammals; mainly humans and equidae; are considered as accidental hosts [5]. In addition to this mode of transmission to humans by arthropods, there are other modes of contamination such as blood transfusion and organ transplants [6], [7]. Clinically, most infections are asymptomatic or manifest as a mild influenza-like syndrome, but in 1% of cases the infection may be life-threatening, presenting as meningitis or meningoencephalitis [5]. Elderly and immunocompromised patients are the most at risk of developing such neurological disorders [1].
Formerly restricted to sub-Saharan Africa, with very few cases of neuromeningeal expression [5], WNV has now progressively spread to all continents since the 1990s, in particular with its introduction and massive spread in the United States and throughout America, with an increased frequency of neuro-invasive cases [5]. In the Mediterranean basin, WNV circulation is considered as endemic [9], [10]. Indeed, meningoencephalitis outbreaks are regularly reported by several countries. Similarly, seroprevalence surveys in many countries in this region have reported permanent viral activity. In Tunisia in 2007, for example, a seroprevalence of 27.7% of anti-WNV IgG was found in the human population residing in the governorate of Kairouan [11]. Also, it has been shown that seroprevalence increases with age, with a higher frequency in people over 40 years old. This age-dependent difference in seroprevalence was also found in other studies; in 2003 in Madagascar [12], and in 2010 in Gabon [8]. A greater risk of exposure of people over 40 years of age to mosquito bites could be the cause of this difference.
Studies carried out so far on the West Nile fever virus in Algeria are disparate. Except for the sero-epidemiological surveys carried out during the 1970s revealing viral activity in southern Algeria [13], and the descriptive study of human meningoencephalitis outbreaks that occurred in 1994 in the south-east of Algeria in the Timimoun region [14], very few data are available on the presence of WNV in the northern part of the country. A fatal case of WNV meningoencephalitis was reported in 2012 in the province of Jijel in north-eastern Algeria, affecting a 74-year-old person of Algerian origin residing in France [15]. Another study on equids in 2014 reported a seroprevalence of 17.4% in the El Kala Lake area, a wetland in the far east of the country [16]. The only data available for the central northern region of Algeria date back to 1965, when a serological survey of 281 human samples found no evidence of anti-WNV antibodies [13]. This study was conducted in order to investigate the possible circulation of WNV in the central northern part of Algeria by the demonstration of the presence of specific anti-WNV IgG antibodies in the serum of people aged ≥40 in the general population.
Materials and methods
Samples
The samples consisted of 164 human sera collected after centrifugation of whole blood obtained by venepuncture from patients consulting at the virology laboratory of the Pasteur Institute in Algeria for various serological check-ups in 2010. Sera were stored at –20°C before being analysed. For each subject included in this study, information on transfusion history and sociodemographic characteristics was collected from their medical records. The average age of this cohort—consisting of 100 men (61%) and 64 women (39%)—was 53.7 years, with extremes ranging from 40 to 81 years. Of the 116 individuals for whom transfusion history information was available, 32 had had at least one blood transfusion.
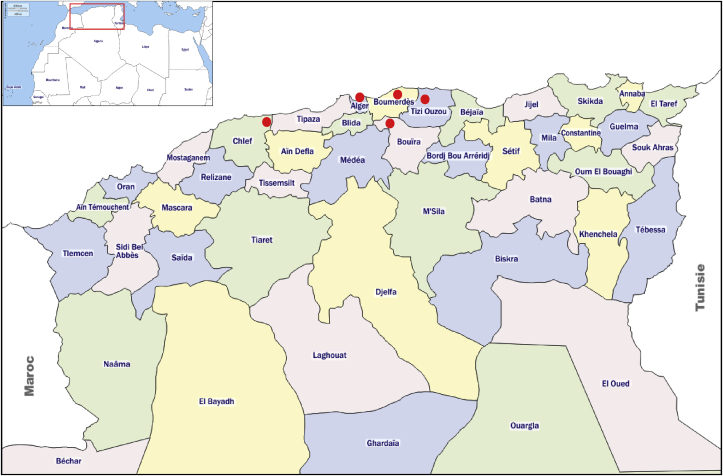
All subjects lived in the region of Algiers and its surroundings (Fig. 1). This region, located in the centre of northern Algeria, is one of the densest in terms of population. This Mediterranean climate region is also characterized by the presence of several natural and artificial water reservoirs.
Fig. 1.
Geographical origin of the subjects included from North central of Algeria.
Detection of anti-WNV antibodies
Sera were tested for WNV-specific IgG-class antibodies by indirect ELISA test (Anti-WNV IgG ELISA, Euroimmun, Lubeck, Germany) according to the manufacturer's instructions. Because of cross-reactions with other flaviviruses, ELISA-positive samples were confirmed by plaque reduction neutralization test (PRNT) for the detection of specific neutralizing antibodies anti-WNV. The test was performed as described previously [17]. Serial dilutions (1:20 to 1: 640) of each ELISA-positive serum were mixed with a viral suspension of the Egyptian strain Eg101 to detect a reduction in the number of cytopathic effect plaques formed. The dilution resulting in at least a 90% reduction in the number of plaques formed by the standard dose of virus is defined as the PRNT90. Sera with PRNT90 at a dilution ≥1:20 were considered positive.
Statistical analysis
The χ2 statistical test was used to compare the percentages with a risk of error α equal to 0.05; p < 0.05 indicates a statistically significant difference.
Results
Sixteen (9.8%) out of 164 sera tested were positive for anti-WNV IgG. After confirmation by PNRT, 11 sera (6.7%) were positive, with antibody titres ranging from 1:20 to 1: 640 (Table 1). No significant difference in the frequency of anti-WNV IgG was observed between male and female subjects (7% versus 6.25%, p > 0.05) (Table 2). Also, among the 116 subjects for whom information was available, the study of the frequency of anti-WNV IgG based on the notion of prior transfusion found no statistically significant difference between the two groups (9.4% versus 7.15%, p > 0.05) (Table 2).
Table 1.
Frequencies and titres of anti-West Nile virus (WNV) IgG antibodies
| Effective (n) | Positive in IgG anti-WNV |
Titre in PRNT90 |
||||||
|---|---|---|---|---|---|---|---|---|
| ELISA | PRNT90 | 1:20 | 1:80 | 1:160 | 1:320 | ≥1:640 | ||
| Sample | 164 | 16 (9.8%) | 11 (6.7%) | 2 (18.2%) | 3 (27.3%) | 3 (27.3%) | 1 (9%) | 2 (18.2%) |
PRNT, plaque reduction neutralization technique.
Table 2.
Proportion of anti-West Nile virus (WNV) IgG antibodies according to sex and transfusion
| Characteristics | Variable | Effective (n) | Positive in IgG anti-WNV by PRNT |
p | |
|---|---|---|---|---|---|
| n | % | ||||
| Sex | Male | 100 | 7/100 | 7 | >0.05 |
| Female | 64 | 4/64 | 6.25 | ||
| Transfusion | Yes | 32 | 3/32 | 9.4 | >0.05 |
| No | 84 | 6/84 | 7.15 | ||
Discussion
To date, epidemiological data regarding WNV circulation in Algeria are limited. The virus was isolated from mosquitoes of the genus Culex in the extreme south-east (in the region of Djanet) in 1968 [18]. During the 1970s, serological surveys in humans detected anti-WNV IgG in 1973 and 1975 in the same region (14.6% and 58.3% positive sera, respectively). In 1976, in the city of Biskra (northern Sahara region), 37.3% of 24 sera tested were positive for anti-WNV antibodies [13]. In 1994, a WNV meningoencephalitis outbreak was described in the region of Timimoun located in the south-east of the country [14]. These were the only symptomatic human cases so far described in the country. Eighteen years later, one fatal meningoencephalitis case due to WNV was reported in 2012 in the province of Jijel (north-east of Algeria), affecting a 74-year-old man of Algerian origin living in France and passing through in the region [15]. Another study on equids in 2014 reported a seroprevalence of 17.4% in the El Kala Lake area, a wetland in the far east of the country [16].
There are no data available on the circulation of this virus in central Algeria. The only work that has been undertaken in this part of the country dates back to 1965, when the 281 human sera tested for specific antibodies against arbovirus antigens, including WNV, were found negative [13]. The results of the present study show that WNV is present in this part of the country. Indeed, of the 164 human sera from individuals originating from Algiers and its surroundings, 11 (6.7%) had specific anti-WNV IgG antibodies, witnessing a previous infection with this virus.
Our results are in agreement with many arguments for active circulation of WNV in central Algeria, that are: mosquitoes of the genus Culex (mainly Culex pipiens), the vectors of this infection, are widespread in this part of the country [19], [20], and their ability to transmit the virus efficiently has been experimentally proven [21]; the geographical location on the way of migratory birds between Africa and Europe [2]; the presence of and there are several natural and artificial water stretches such as dams all around the region (unpublished data). These water reservoirs would constitute ecological niches conducive to contact between the domestic or migratory birds which periodically cross these areas and the mosquitoes present in great abundance, thus allowing the amplification of the virus in an enzootic cycle [4]; the mediterranean climate, characterized by hot, arid summers and mild, temperate winters, is favourable for mosquito breeding and increasing their vectoral capacity [22], as well as for resting migratory birds hibernation and rest; the endemic circulation of WNV in the Mediterranean, in areas with similar climatic and ecological conditions to that of Algiers region [9], [10], [11], [24].
Although our results provide serological evidence of WNV circulation in this part of Algeria, to our knowledge no human or veterinary clinical case has been reported to date. Several hypotheses can be considered; the circulating virus may be a less virulent strain, or there may be a lack of declaration or laboratory capacities for the diagnosis of neuromeningeal infections of viral origin, especially those occurring during the summer/autumn season (the period of arbovirus activity) [5], particularly since Algeria records annually from May to September a peak in the incidence of aseptic meningitis of unknown aetiology (unpublished data).
As already described by several authors [23], [24], [25], the results of this study also agree on the lack of specificity of ELISA tests in the diagnosis of recent or past WNV infections. Of the 16 sera tested positive for anti-WNV IgG by ELISA, only 11 had specific neutralizing antibodies (Table 1). This can be explained by serological cross-reactions with other flaviviruses antigenically related to WNV [1]. However, in our series none of the subjects with ELISA-positive anti-WNV IgG had been vaccinated against yellow fever virus or travelled to countries where dengue fever or yellow fever are endemic. In addition, Algeria, like all countries around the Mediterranean Sea, is located outside the circulation zones of yellow fever [13]. Therefore, further studies are needed to investigate the possible circulation of other flaviviruses in Algeria, including the dengue virus; whose vector (Aedes albopictus) was recently identified for the first time in northern Algeria [26], [27]; and the Usutu virus, the only flavivirus of the Japanese encephalitis virus serogroup closely related to WNV circulating in Europe and the Mediterranean [28], [29].
Several studies have described a difference in the frequency of distribution of anti-WNV IgG according to sex [11], [12], but no significant difference was found in our series (p > 0.05). The authors of these studies explained this difference by a higher risk of exposure to WNV in male subjects.
A link between blood transfusion and the risk of WNV infection could not be established in our cohort (Table 2), although blood transfusion has been recognized as one of the transmission routes for this virus [6], [7]. This discrepancy could be explained by the small number of subjects analysed in this work. A study of the seroprevalence of WNV infection in blood donors is recommended in order to assess the transfusion risk inherent for this virus.
In conclusion, the results of our study report for the first time the circulation of West Nile virus in Algiers and its surroundings. These preliminary data confirm that, as in the south and east of the country, the central part of Algeria is also affected by this virus.
The potential impact of WNV infection on human and animal health, as well as the evidence of endemic circulation in the Mediterranean region, requires the establishment of an integrated surveillance programme in Algeria and in the Mediterranean region. This will provide a better understanding of the circulation dynamics of WNV in this region of the world which is a crossroads between three continents (Africa, Europe and Asia) and very favourable for trade and movement. Further investigations are therefore essential for a better understanding of the epidemiological situation of this disease at the entomological, veterinary and medical levels.
The evidence of cross-serological reactions, observed by the decrease in the proportion of anti-VWN IgG after confirmation by PRNT, also highlights the need to investigate the possible circulation of other flaviviruses.
Ethical statement
Not applicable, the samples were tested retrospectively.
Transparency declaration
The authors declare that they have no conflicts of interest.
References
- 1.Monath T.P., Heinz F.X. Flavivirus. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields virology. 3rd ed. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 961–1034. [Google Scholar]
- 2.Jourdain E., Gauthier-Clerc M., Bicout D.J. Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg Infect Dis. 2007;13(3):365–372. doi: 10.3201/eid1303.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubálek Z., Halouzka J. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5(5):643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeller H.G., Murgue B. Rôle des oiseaux migrateurs dans l'épidémiologie du virus West Nile. Méd Mal Infect. 2001;31(Suppl. 2):168–174. [Google Scholar]
- 5.Kramer L.D., Li J., Shi P.Y. West Nile virus. Lancet Neurol. 2007;6:171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- 6.Biggerstaff B.J., Petersen L.R. Estimated risk of transmission of West Nile virus through blood transfusion in the US. Transfusion. 2002;43:1007–1017. doi: 10.1046/j.1537-2995.2003.00480.x. 2003. [DOI] [PubMed] [Google Scholar]
- 7.Pealer L.N., Marfin A.A., Petersen L.R., Lanciotti R.S., Page P.L., Stramer S.L. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 8.Pourrut X., Dieudonné N., Janusz P., Leroy E. First serological evidence of West Nile virus in human rural populations of Gabon. Virol J. 2010;7:132. doi: 10.1186/1743-422X-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calistri P., Giovannini A., Hubalek Z., Ionescu A., Monaco F., Savini G. Epidemiology of West Nile in Europe and in the Mediterranean basin. Open Virol J. 2010;4:29–37. doi: 10.2174/1874357901004010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muegue B., Murri S., Triki H. West Nile in the Mediterranean basin: 1950–2000. Ann N Y Acad Sci. 2001;951:117–126. doi: 10.1111/j.1749-6632.2001.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 11.Bahri O., Dhifallah I., Ben Alaya-Bouafif N., Fekih H., Gargouri J., Triki H. Étude séroépidémiologique de la circulation du virus West Nile chez l’homme en Tunisie. Bull Soc Pathol Exot. 2010;104(4):272–276. doi: 10.1007/s13149-010-0100-x. [DOI] [PubMed] [Google Scholar]
- 12.Lonchampt C., Migliani R., Ratsitorahina M. Persistance d’une circulation endémique du virus West Nile à Madagascar. Arch Inst Pasteur de Madagascar. 2003;69:33–36. [PubMed] [Google Scholar]
- 13.Bouguermouh A., Bouslama Z., Bitam I. Ces arbovirus qui menacent l’Algérie. La Revue Medico-Pharmaceutique. 2008;48(3):46–52. [Google Scholar]
- 14.Le Guenno B., Bougermouh A., Azzam T., Bouakaz R. West Nile: a deadly virus? Lancet. 1996;348:1315. doi: 10.1016/s0140-6736(05)65799-6. [DOI] [PubMed] [Google Scholar]
- 15.Institut National de Veille Sanitaire. Bulletin hebdomadaire international N°369.[http://invs.santepubliquefrance.fr/fr/Publications-et outils/Bulletin-hebdomadaire-international/Tous lesnumeros/2012/Bulletin-hebdomadaire-international-du-10-au-16-octobre-2012.-N-369].
- 16.Lafri I., Prat C.M., Bitam I., Gravier P., Besbaci M., Zeroual F. Seroprevalence of West Nile virus antibodies in equids in the North-East of Algeria and detection of virus circulation in 2014. Comp Immunol Microbiol Infect Dis. 2017;50:8–12. doi: 10.1016/j.cimid.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 17.De Madrid A.T., Porterfield J.S. A simple micro-culture method for the study of group B arboviruses. Bull Org Mond Santé. 1969;40:113–121. [PMC free article] [PubMed] [Google Scholar]
- 18.Pilo-Moron E., Vincent J., Le Corroller Y. Isolation of West-Nile virus in the extreme south of the Algerian Sahara (Djanet) Arch Inst Pasteur Algerie. 1970;48:181–184. [PubMed] [Google Scholar]
- 19.Benallal K., Benbetka S., Tail G., Harrat Z. Molecular characterization of Culex pipiens (Diptera, Culicidae) in Reghaïa lake, Algeria. Ann Bio Sci. 2015;3(1):20–24. [Google Scholar]
- 20.Brunhes J., Hassaine K., Rhaim A., Hervy J.P. Les culicidés de l'Afrique méditerranéenne: espèces présentes et répartition (Diptera, Nematocera) Bull Soc Entomo Franc. 2000;105(2):195–204. [Google Scholar]
- 21.Amraoui F., Krida G., Bouattour A., Rhim A., Daaboub J., Harrat Z. Culex pipiens, an experimental efficient vector of West Nile and Rift Valley fever viruses in the Maghreb region. PLOS ONE. 2012;7(5) doi: 10.1371/journal.pone.0036757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paz S., Albersheim I. Influence of warming tendency on Culex pipiens population abundance and on the probability of West Nile Fever outbreaks (Israeli Case Study: 2001–2005) EcoHealth. 2008;5(1):40–48. doi: 10.1007/s10393-007-0150-0. [DOI] [PubMed] [Google Scholar]
- 23.Bernabeu-Wittel M., Ruiz-Pérez M., del Toro M.D. Seroprevalencia de infecciones por el virus del Nilo Occidental en la población general del sur de España. Enferm Infecc Microbiol Clin. 2007;25(9):561–565. doi: 10.1157/13111181. [DOI] [PubMed] [Google Scholar]
- 24.Holmes D.A., Purdy D.E., Chao D.Y., Noga A.J., Chang G.J. Comparative analysis of immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay using virus-like particles or virus-infected mouse brain antigens to detect IgM antibody in sera from patients with evident flaviviral infections. J Clin Microbiol. 2005;43:3227–3236. doi: 10.1128/JCM.43.7.3227-3236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tardei G., Ruta S., Chitu V. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J Clin Microbiol. 2000;38:2232–2239. doi: 10.1128/jcm.38.6.2232-2239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benallal K.E., Allal-Ikhlef A., Benhamouda K., Schaffner F., Harrat Z. First report of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Oran, West of Algeria. Acta Trop. 2016;164:411–413. doi: 10.1016/j.actatropica.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Izri A., Bitam I., Charrel R.N. First entomological documentation of Aedes (Stegomyia) albopictus (Skuse 1894) in Algeria. Clin Microbiol Infect. 2011;17(7):1116–1118. doi: 10.1111/j.1469-0691.2010.03443.x. [DOI] [PubMed] [Google Scholar]
- 28.Ben Hassine T., De Massis F., Calistri P., Savini G., BelHaj M.B., Ranen A. First detection of cocirculation of West Nile and Usutu viruses in equids in the south-west of Tunisia. Trans Bound Emerg Dis. 2014;61(5):385–389. doi: 10.1111/tbed.12259. [DOI] [PubMed] [Google Scholar]
- 29.Vázquez A., Jiménez-Clavero M., Franco L. Usutu virus – potential risk of human disease in Europe. Euro Surveill. 2011;16(31):1–30. [PubMed] [Google Scholar]