Abstract
Disease-modifying treatment trials are increasingly advanced to the prodromal or preclinical phase of Alzheimer's disease (AD), and inclusion criteria are based on biomarkers rather than clinical symptoms. Therefore, it is of great interest to determine which biomarkers should be combined to accurately predict conversion from mild cognitive impairment (MCI) to AD dementia. However, up to date, only few studies performed a complete A/T/N subject characterization using each of the CSF and imaging markers, or they only investigated long-term (≥ 2 years) prognosis. This study aimed to investigate the association between cerebrospinal fluid (CSF), magnetic resonance imaging (MRI), amyloid- and 18F-FDG positron emission tomography (PET) measures at baseline, in relation to cognitive changes and conversion to AD dementia over a short-term (12-month) period. We included 13 healthy controls, 49 MCI and 16 AD dementia patients with a clinical-based diagnosis and a complete A/T/N characterization at baseline. Global cortical amyloid-β (Aβ) burden was quantified using the 18F-AV45 standardized uptake value ratio (SUVR) with two different reference regions (cerebellar grey and subcortical white matter), whereas metabolism was assessed based on 18F-FDG SUVR. CSF measures included Aβ1–42, Aβ1–40, T-tau, P-tau181, and their ratios, and MRI markers included hippocampal volumes (HV), white matter hyperintensities, and cortical grey matter volumes. Cognitive functioning was measured by MMSE and RBANS index scores. All statistical analyses were corrected for age, sex, education, and APOE ε4 genotype. As a result, faster cognitive decline was most strongly associated with hypometabolism (posterior cingulate) and smaller hippocampal volume (e.g., Δstory recall: β = +0.43 [p < 0.001] and + 0.37 [p = 0.005], resp.) at baseline. In addition, faster cognitive decline was significantly associated with higher baseline Aβ burden only if SUVR was referenced to the subcortical white matter (e.g., Δstory recall: β = −0.28 [p = 0.020]). Patients with MCI converted to AD dementia at an annual rate of 31%, which could be best predicted by combining neuropsychological testing (visuospatial construction skills) with either MRI-based HV or 18F-FDG-PET. Combining all three markers resulted in 96% specificity and 92% sensitivity. Neither amyloid-PET nor CSF biomarkers could discriminate short-term converters from non-converters.
Keywords: Mild cognitive impairment, Alzheimer's disease, Biomarkers, Positron emission tomography, Florbetapir, Cerebrospinal fluid, Hippocampal volume
Highlights
-
•
FDG-PET and MRI HV are the strongest predictors of cognitive decline and conversion to AD.
-
•
Combination of visuospatial construction testing with FDG-PET or MRI HV present high predicting power of conversion.
-
•
CSF and amyloid-PET seem less suitable markers of disease progression.
-
•
Increased AV45-PET predicts short-term cognitive decline if SUVR is referenced to WM instead of CB.
1. Introduction
Currently, the diagnosis of Alzheimer's disease (AD) combines clinical criteria with biological markers reflecting the pathological changes in the brain (Dubois et al., 2014; McKhann et al., 2011). Determination of biomarkers that can predict the clinical progression to AD is of utmost importance to accurately identify at-risk subjects for enrichment of disease-modifying treatment trials (Karran et al., 2011). In particular, the amnestic subtype of mild cognitive impairment (aMCI) is associated with an increased risk of developing AD, corresponding to an annualized conversion rate of 30% (Rozzini et al., 2007; Schmidtke and Hermeneit, 2007). In this respect, AD conversion prediction over a shorter time span, i.e. one or two years, can be considered clinically relevant, especially to identify rapid versus slow AD progressors.
The most accepted hypothetical model for the development of AD suggests a temporal order of the pathological changes in which amyloidosis triggers tau spreading outside of the medial temporal cortex, subsequently leading to the neurodegenerative processes and cognitive deterioration (Jack Jr and Holtzman, 2013). However, the causal role of amyloid in the etiology of AD pathogenesis (amyloid cascade hypothesis) remains elusive and some argue for a tau-centric pathway or a synergistic interaction between amyloid and tau (Jack Jr et al., 2016). In 2018, the NIA-AA research framework ‘A/T/N’ was established which avoids the assumptions of the temporal ordering of the biomarkers (Jack et al., 2018). Herein, AD is defined as a continuous process in both cognitive and biomarker domains rather than as three separate clinical entities (i.e., preclinical, MCI, and dementia). The ‘A+’ in A/T/N refers to fibrillary Aβ deposition quantified as high retention on amyloid-PET or low Aβ1–42/Aβ1–40 levels in the cerebrospinal fluid (CSF) (Blennow et al., 2010; Herholz and Ebmeier, 2011), ‘T+’ refers to tau pathology characterized by elevated CSF phosphorylated tau levels (P-tau181) or tau-PET uptake (Olsson et al., 2016; Villemagne et al., 2014), and ‘N+’ reflects synaptic dysfunction and neuronal degeneration based on decreased 18F-fluorodeoxyglucose (18F-FDG) PET uptake, atrophy on magnetic resonance imaging (MRI) or elevated CSF total tau (T-tau) (Jack Jr et al., 2011; Knopman, 2012). Thus, complete A/T/N characterization is possible using CSF or imaging biomarkers alone, as well as using a combination of both. However, the disagreement between biomarker modalities within the different A/T/N categories may introduce bias when individual subjects are classified as positive or negative (Illán-Gala et al., 2018). Moreover, these modalities (CSF/imaging) may not be fully interchangeable when predicting cognitive decline or MCI-to-AD conversion. Indeed, whereas amyloid-PET was found to be a predictor of future cognitive decline (Ben Bouallègue et al., 2017; Doraiswamy et al., 2014; Farrell et al., 2017; Landau et al., 2012), CSF Aβ had a rather limited prognostic value (Ben Bouallègue et al., 2017; De Vos et al., 2016; Walhovd et al., 2010a). Lastly, an extra level of variability may be introduced within one modality when using the CSF Aβ1–42/Aβ1–40 ratio instead of Aβ1–42 alone (Niemantsverdriet et al., 2017), or when selecting different reference regions for amyloid-PET standardized uptake value ratio (SUVR). For example, changes in 18F-florbetapir (18F-AV45) SUVR normalized to a white matter reference region were shown to be better associated with changes in cognition compared to applying a cerebellar or pontine reference region (Chen et al., 2015).
In contrast to the A+ and T+ biomarkers, the markers of neurodegeneration cannot be used to indicate Alzheimer's pathophysiologic processes (Jack et al., 2018) as they are known to show topographical overlap with non-AD pathologies (Wirth et al., 2013). However, structural and metabolic abnormalities correlate well with the symptom severity as they become present in closer proximity to the onset of cognitive decline (Chételat et al., 2005; Jack Jr and Holtzman, 2013; Landau et al., 2012; Landau et al., 2010; Prestia et al., 2015; Storandt et al., 2009; Walhovd et al., 2010a; Yu et al., 2012). Indeed previous literature have suggested their utility as predictors of short-term MCI-to-AD dementia conversion (Fellgiebel et al., 2007; Geroldi et al., 2006; Landau et al., 2010; Prestia et al., 2015; Visser et al., 2002; Yu et al., 2012), although with limited interchangeability (Illán-Gala et al., 2018).
Despite extensive literature on the associations between biomarkers and cognition, only few clinical trials and observational studies performed a complete A/T/N characterization of their participants included, using different biomarker modalities within the different categories (Dumurgier et al., 2017; Salloway et al., 2018; Wang et al., 2018). Therefore, in the present study, we aimed to investigate which A/T/N biomarkers (except for tau-PET) at baseline were associated with short-term cognitive decline in a population comprising the whole spectrum of AD, including cognitively healthy controls (HC), MCI, and AD dementia patients. Secondly, we determined which of the baseline biomarkers could discriminate MCI-to-AD dementia converters from non-converters.
2. Materials and methods
2.1. Study population
Seventy-eight subjects were enrolled in the study, including 13 HC, 49 MCI and 16 AD dementia patients. They underwent 18F-AV45 PET, 18F-FDG PET, lumbar puncture, MRI, and an extensive neuropsychological examination at baseline. 18F-AV45-PET was repeated after one year, whereas the cognitive battery was repeated after one and two years. The number of subjects per biomarker measure (both at baseline and follow-up) is listed in Table 1.
Table 1.
Number (and percentage, %) of subjects per biomarker measure at baseline and follow-up, and amount of follow-up days.
| HC (N = 13) |
MCI (N = 49) |
AD dementia (N = 16) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker | BL | FU | days ± SD | BL | FU | days ± SD | BL | FU | days ± SD |
| Cognitiona | |||||||||
| year 1 | 13 (100) | 12 (92) | 417 ± 33 | 49 (100) | 37 (76) | 413 ± 114 | 16 (100) | 13 (81) | 407 ± 80 |
| year 2 | 10 (77) | 792 ± 40 | 17 (35) | 726 ± 78* | 6 (38) | 737 ± 40 | |||
| AV45-PET | 13 (100) | – | – | 49 (100) | 19 (39) | 373 ± 13 | 16 (100) | 4 (25) | 407 ± 52 |
| FDG-PET | 13 (100) | – | – | 48 (98) | – | – | 16 (100) | – | – |
| CSF | 7 (54) | – | – | 45 (92) | – | – | 15 (94) | – | – |
| MRI | 13 (100) | – | – | 47 (96) | – | – | 16 (100) | – | – |
Abbreviations: AD Alzheimer's disease, BL baseline, CSF cerebrospinal fluid, FU follow-up, HC cognitively healthy control, MCI mild cognitive impairment, MRI magnetic resonance imaging, PET positron emission tomography, SD standard deviation.
*p < 0.05 vs control subjects via Kruskal-Wallis corrected for multiple comparisons via Bonferroni.
Cognition, i.e. at least MMSE measurement.
Categorization into diagnostic groups was made on a demographical and clinical basis (not biomarker-based) by consensus of three expert medical doctors (SE, TVDB, SVM). The panel made a consensus clinical diagnosis of ‘dementia due to AD’ (AD dementia) by applying the NIA-AA criteria (McKhann et al., 2011). A consensus diagnosis of ‘MCI due to AD’ (MCI) was based on the NIA-AA criteria (Albert et al., 2011), i.e. (1) cognitive complaint, preferably corroborated by an informant; (2) objective cognitive impairment, quantified as performance of >1.5 standard deviation (SD) below the appropriate mean on the neuropsychological subtests; (3) largely normal general cognitive functioning; (4) essentially intact activities of daily living (basic and instrumental activities of daily living were determined by a clinical interview with the patient and an informant); and (5) not demented. The inclusion criteria for HC were: (1) no neurological or psychiatric antecedents; (2) no organic disease involving the central nervous system following extensive clinical examination; and (3) normal neuropsychological exam. Exclusion criteria for the total population consisted of brain tumors, large cerebral infarction/bleeding, strategic infarctions, other neurodegenerative diseases, severe head trauma, epilepsy, brain infections, severe depression, unregulated diabetes mellitus, untreated thyroid disorders, or any severe somatic co-morbidity that interferes with study participation.
HC consisted of volunteers, mainly spouses of patients who visited the memory clinic. Patients were recruited through the Memory Clinic of ZNA Middelheim and Hoge Beuken, Antwerp, Belgium. They were clinically followed-up by the neurologist every six months. The conversion from MCI to AD dementia was based on the clinical non-biomarker-based diagnoses and was established using the clinical diagnostic dementia criteria (as described above). This included a decline in instrumental activities of daily living (IADL; lower scores indicate more problems with daily life activity) questionnaire, preferable reported by the caregiver.
Approval for the study was obtained from the Committee for Medical Ethics of the University of Antwerp/University Hospital Antwerp (14/12/130) and of Hospital Network Antwerp (ZNA) (4310), and all participants or their representatives provided informed consent.
2.2. Neuropsychological investigation
All patients underwent a neuropsychological examination, including the Mini-Mental State Examination (MMSE) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) which is composed of the immediate memory, delayed memory (subtests: word list recall, story recall, figure recall, word list recognition), language, attention, and visuospatial construction (figure copy and judgment of line orientation) index scores. The raw scores of the RBANS were converted to z-scores based on the norm scores. These normative z-scores of the separate RBANS domains were dichotomized by using a − 1.5 SD cut-off (no cognitive deficit versus cognitive deficit).
2.3. CSF biomarkers
CSF collection and processing methods are described elsewhere (Niemantsverdriet et al., 2017). CSF concentrations of Aβ1–42, Aβ1–40, T-tau, and P-tau181 were determined with commercially available single-analyte ELISA (INNOTEST® β-AMYLOID(1–42), β-AMYLOID(1–40), hTAU-Ag, and PHOSPHO-TAU(181P), respectively; Fujirebio Europe) as routinely performed in the BIODEM lab (Somers et al., 2016). The laboratory technician performing the biomarker analyses was blinded to clinical diagnosis. In addition, the Aβ1–42/Aβ1–40, Aβ1–42/T-tau, and Aβ1–42/P-tau181 ratios were calculated. CSF biomarker results were not included in the consensus clinical diagnosis made by the panel.
2.4. Imaging biomarkers
Procedures for acquisition of PET and MRI images are described in (Ottoy et al., 2017). Baseline and follow-up PET scans were coregistered to baseline MRIs for each subject using PMOD v3.6 (PMOD Technologies Ltd., Zurich, Switzerland). Regions of interest were delineated based on brain parcellation of the anatomic MRIs using automated knowledge-based grey matter/white matter/CSF segmentation in PMOD. 18F-FDG SUVRs were derived from a static scan at 30–40 min post-injection and calculated in the precuneus and posterior cingulate cortex (PCC) target regions with a cerebellar grey matter reference region. Regional 18F-AV45 SUVRs were derived at 50–60 min post-injection and normalized to either the cerebellar grey (SUVRCB) or the whole subcortical white matter (SUVRWM). A global cortical 18F-AV45 SUVR was calculated as the volume-weighted average uptake in the frontal, temporal and parietal lobe. The longitudinal change in SUVR was computed as ΔSUVR = (SUVRFU-SUVRBL)/SUVRBL⁎100 (with BL: baseline; FU: follow-up) and therefore expressed as a percentage, enabling comparison between different reference regions despite their different scales. All data was corrected for partial volume effects based on the region-based geometric transfer matrix method (GTM as described by (Rousset et al., 1998)) and a 5x5x5mm PET scanner resolution.
MRI volumes, including hippocampal volume (HV), cortical grey matter volume (CGM), and white matter hyperintensity volume (WMH), were extracted by icometrix, using the CE-labelled and FDA-cleared software IcoBrain. The processing method is described elsewhere (Niemantsverdriet et al., 2018; Smeets et al., 2016). All volumes were normalized to total head size.
2.5. Biomarker positivity
All continuous biomarker values at baseline were also dichotomized as positive (i.e., suggestive for AD) or negative.
Two dichotomous measures of CSF status were generated, one based on the Aβ1–42/Aβ1–40 ratio and one based on the Aβ1–42/T-tau ratio. The CSF biomarker profile was considered to be positive if the Aβ1–42/Aβ1–40 value was below the cut-off (<0.067 pg/mL) or the Aβ1–42/T-tau value was below the cut-off (<2.153 pg/mL). The cut-off values were in-house validated in autopsy-confirmed AD versus HC (Somers et al., 2016).
18F-FDG-PET biomarker positivity was defined using the automated quantitative analysis software MIMneuro (MIM Software Inc. Cleveland, OH), comparing each subject's scan on a voxel-by-voxel basis to a normal database comprising 43 HC. All images were normalized to the mean activity in the whole brain, pons, and cerebellum.
Two dichotomous measures of amyloid status were generated, one based on the visual PET reading and the other one using a quantitative cortical SUVR threshold. Both were described previously (Niemantsverdriet et al., 2017). The first measure used a database-assisted analysis in which the 18F-AV45 uptake pattern of all subjects was compared to a normal pattern on a voxel-by-voxel basis allowing for calculation of z-scores. The brain norm was created from a set of SUVR images acquired with Aβ-negative HC (N = 9) and normalized to the CB. The second measure used a receiver operating characteristic (ROC) area under the curve (AUC) analysis using the SUVRCB and SUVRWM values of HC and AD dementia patients (cortical SUVRCB threshold = 1.203; SUVRWM threshold = 0.485).
MRI biomarker positivity was visually assessed by consensus of the clinical panel and was based on the presence or absence of hippocampal atrophy using the axial T1 image (Scheltens et al., 1992).
2.6. Statistical analysis
Statistical analyses were performed in IBM SPSS Statistics v24 and parameters were described as mean ± SD. Significance was determined at p < 0.05. Differences among diagnostic groups were assessed using χ2 tests for categorical variables and Kruskal-Wallis tests with Bonferroni correction for continuous variables. The difference in cortical 18F-AV45 uptake between baseline and follow-up was tested using a Wilcoxon signed rank test.
2.6.1. Regression analyses
Associations between cognition and the other biomarkers at baseline were assessed for each biomarker separately, using general linear modeling corrected for the covariates age, sex, education, APOE ε4 genotype, and baseline clinical diagnosis. For the association of Δcognition with baseline biomarkers, the covariates baseline cognition score and cognition time interval were added to the model. Z-scores were used to calculate standardized regression coefficients (β), allowing to compare the strength of the associations between (change in) cognition and the other biomarkers at baseline. The Benjamini-Hochberg procedure was used as False Discovery Rate (FDR) multiple comparisons correction for p-values across the 8 cognitions, using an FDR adjusted p-value of 0.05. In addition, model robustness was confirmed based on nonparametric bootstrapping with 1000 resamples and the 95% confidence intervals (C.I.) of the standardized βs that excluded zero (JMP Pro v14, SAS Institute Inc., USA). Regression analyses between ΔSUVR and SUVR at baseline were likewise assessed using general linear modeling corrected for the covariates age, sex, education, APOE ε4 genotype, baseline diagnosis, and PET time interval.
Lastly, linear mixed-effects regression analyses were conducted with the dichotomous 18F-FDG, MRI, CSF (Aβ1–42/Aβ1–40, Aβ1–42/T-tau) or 18F-AV45 status at baseline, time (baseline and 2 follow-up cognition time points) and the time-by-status interaction entered, to determine whether biomarker positivity was associated with changes in each of the cognitive measures over 1 and 2 years. The significance of the interaction term entailed whether biomarker positivity had a significant effect on cognitive change over time. All models included age, sex, education, and APOE ε4 genotype as covariates, as well as a random intercept to account for individual differences in cognition at baseline.
2.6.2. MCI-to-AD dementia conversion
18F-AV45-PET, 18F-FDG-PET, MRI (CGM, HV, WMH), CSF (all continuous measures), and cognition scores as well as demographic data obtained in MCI patients were used to determine conversion to AD dementia after 1 year. The Mann-Whitney U test with FDR multiple comparisons correction was used to assess differences between converters and non-converters on each of the biomarker measures at baseline. In addition, binary logistic regression was conducted to determine the most optimal combination of variables to discriminate converters from non-converters, and was conducted as following. First, regression analyses were conducted separately for each of the variables (one-variable model). Next, two-variable models were created by adding a significant single variable to the one-variable models. Only those combinations that could significantly improve the discriminative ability of the one-variable model were considered (χ2 test between log-likelihoods). Lastly, a third variable was added to the two-variable models in a similar way to build three-variable models. AUCs between models were compared using the AUC comparison function in JMP. Assumptions of the multiple variable models were met: 1) no collinearity between variables (e.g., three-variable model: variance inflation factor < 2, Pearson's r < 0.6), and 2) linear relationship between the variables and their log odds (Box-Tidwell test). For all analyses, the predictive power was evaluated through ROC analysis (probability cut-off = 50%). Sensitivity and specificity of the models were calculated. Sample sizes varied slightly between the different regression models due to missing data.
3. Results
3.1. Demographics
Table 2 represents the demographic and biomarker data at baseline, grouped by clinical diagnosis. No differences between diagnostic groups were found in sex and APOE ε4 genotype. The MCI and AD dementia group showed significantly higher 18F-AV45 and lower 18F-FDG PET uptake, more cognitive impairment, and smaller HV compared to the HC group. Additionally, the AD dementia group showed significantly higher CSF T-tau, lower Aβ1–42/T-tau and Aβ1–42/P-tau181, as well as smaller CGM and more WMH compared to the HC group. Differences in CSF P-tau181 and Aβ1–42/Aβ1–40 between HC and AD dementia showed a trend towards statistical significance. The percentage of Aβ-PET positive subjects increased across diagnostic groups (31% HC, 80% MCI, 100% AD dementia based on the cortical SUVRCB cut-off). Similarly, the percentage of CSF and MRI positive subjects also increased (CSF: 43% HC, 78% MCI, 93% AD dementia based on the Aβ1–42/Aβ1–40 cut-off, and 29% HC, 64% MCI, 93% AD dementia based on the Aβ1–42/T-tau cut-off; MRI: 15% HC, 71% MCI, 75% AD dementia based on visual reading).
Table 2.
Demographics at baseline.
| Variable | HC (N = 13) | MCI (N = 49) | AD dementia (N = 16) |
|---|---|---|---|
| Female sex, % | 62 | 43 | 50 |
| Age at baseline PET, years | 67.18 ± 7 | 72.74 ± 8 * | 73.54 ± 7 |
| Education, years | 18.23 ± 4 | 15.63 ± 5 | 14.44 ± 3 * |
| APOE ε4 carriers, % (N) | 45 (5/11) | 66 (27/41) | 79 (11/14) |
| Amyloid-PET | |||
| Cortical SUVRCB (%Aβ+) | 1.31 ± 0.4 (31) | 1.77 ± 0.6 * (80) | 1.75 ± 0.3 * (100) |
| Cortical SUVRWM (%Aβ+) | 0.58 ± 0.2 (38) | 0.76 ± 0.2 * (83) | 0.79 ± 0.2 * (100) |
| 18F-FDG-PET | |||
| Precuneus SUVR (N) | 1.37 ± 0.09 (13) | 1.31 ± 0.17 (47) | 1.27 ± 0.17 (16) |
| PCC SUVR (N) | 1.56 ± 0.15 (13) | 1.37 ± 0.29 * (48) | 1.28 ± 0.16 * (16) |
| Cognition | |||
| MMSE (N) | 28.85 ± 1.72 (13) | 25.18 ± 2.75 * (49) | 22.38 ± 4.30 * (16) |
| RBANS, z (N) | |||
| Delayed memory | +0.54 ± 1.07 (13) | −2.26 ± 1.11 * (48) | −3.32 ± 0.55 *,$ (14) |
| List recall | +0.45 ± 1.10 (13) | −1.59 ± 0.84 * (44) | −2.05 ± 0.46 * (15) |
| Story recall | +0.82 ± 1.05 (13) | −2.17 ± 1.41 * (44) | −2.86 ± 1.26 * (15) |
| Figure recall | +0.35 ± 1.20 (13) | −1.67 ± 1.23 * (44) | −2.55 ± 0.69 * (15) |
| Immediate memory | +0.74 ± 1.20 (13) | −1.61 ± 0.96 * (48) | −2.29 ± 0.69 * (14) |
| Language | +0.03 ± 0.71 (13) | −0.97 ± 0.85 * (46) | −1.75 ± 0.95 *,$ (14) |
| Visuospatial construction | +0.79 ± 0.88 (13) | +0.05 ± 1.13 (44) | −0.71 ± 1.40 * (13) |
| CSF | |||
| N subjects | 7 | 45 | 15 |
| Aβ1–42/Aβ1–40, pg/mL | 0.07 ± 0.03 | 0.06 ± 0.03 | 0.04 ± 0.02 |
| Aβ1–42, pg/mL | 1114.06 ± 440 | 771.34 ± 356 | 643.47 ± 122 |
| Aβ1–40, pg/mL | 16,291.06 ± 4320 | 14,338.88 ± 3911 | 16,131.00 ± 4864 |
| T-tau, pg/mL | 329.14 ± 118 | 451.67 ± 188 | 710.80 ± 372 * |
| P-Tau181, pg/mL | 61.87 ± 18 | 73.40 ± 24 | 93.14 ± 37 |
| Aβ1–42/T-tau, pg/mL | 3.95 ± 2.30 | 2.24 ± 1.82 | 1.32 ± 1.19 * |
| Aβ1–42/P-tau181, pg/mL | 19.89 ± 9.94 | 12.41 ± 8.79 | 8.68 ± 6.29 * |
| MRI | |||
| N subjects | 13 | 47 | 16 |
| Hippocampal volume, mm3 | 8422.51 ± 43 | 7136.66 ± 1074 * | 6666.81 ± 899 * |
| Cortical GM volume, ml | 767.85 ± 43 | 735.46 ± 38 | 723.64 ± 44 * |
| WMH, ml | 5.03 ± 5 | 13.95 ± 17 | 14.34 ± 13 * |
Values are given as mean ± standard deviation.
* p < 0.05 vs control subjects; $p < 0.05 vs MCI subjects. Categorical variables (sex, APOE ε4) via Fisher's Exact test; continuous variables via Kruskal-Wallis corrected for multiple comparisons via Bonferroni. Significant p-values are shown in bold typeface.
Abbreviations: Aβ + amyloid-beta positive, AD Alzheimer's disease, APOE gene encoding for apolipoprotein E, CSF cerebrospinal fluid, GM grey matter, HC cognitively healthy control, MCI mild cognitive impairment, MMSE Mini-Mental state examination, MRI magnetic resonance imaging, PCC posterior cingulate cortex, PET positron emission tomography, P-tau181 phosphorylated tau181, SUVRCB standardized uptake value ratio normalized to cerebellar grey matter, SUVRWM standardized uptake value ratio normalized to subcortical white matter, T-tau total tau, WMH white matter hyperintensities.
3.2. Cognition and longitudinal cognitive decline
Table 3 shows the effect sizes of the associations between cognition and the other biomarkers, all at baseline. Higher cortical amyloid deposition, hypometabolism in the PCC, and smaller HV are significantly associated with more cognitive impairment. Lower CSF Aβ1–42 and Aβ1–42/P-tau181 levels were associated with reduced RBANS delayed memory (figure recall) index score, whereas the other CSF measures did not survive FDR correction.
Table 3.
Associations of baseline cognition with other biomarkers (18F-AV45-PET, 18F-FDG-PET, MRI, CSF) at baseline.
| Baseline cognition | AV45 |
FDG |
MRI |
CSF |
||
|---|---|---|---|---|---|---|
| Cortical SUVRCB | Cortical SUVRWM | PCC SUVR | HV | Aβ1–42 | Aβ1–42/P-tau181 | |
| MMSE | −0.21 (0.099) | −0.15 (0.235) | +0.34 (0.004) | +0.16 (0.258) | −0.03 (0.866) | +0.10 (0.433) |
| Delayed memory | −0.23 (0.017) | −0.26 (0.005) | +0.33 (<0.001) | +0.38 (<0.001) | +0.32 (0.005) | +0.25 (0.019) |
| List recall | −0.29 (0.004) | −0.23 (0.016) | +0.27 (0.004) | +0.28 (0.010) | +0.24 (0.026) | +0.13 (0.188) |
| Story recall | −0.34 (0.002) | −0.25 (0.022) | +0.12 (0.255) | +0.21 (0.100) | +0.23 (0.070) | +0.12 (0.328) |
| Figure recall | −0.36 (0.002) | −0.40 (<0.001) | +0.32 (0.004) | +0.33 (0.011) | +0.36 (0.008) | +0.40 (0.001) |
| Immediate memory | −0.29 (0.002) | −0.34 (<0.001) | +0.19 (0.042) | +0.11 (0.310) | +0.20 (0.084) | +0.16 (0.125) |
| Language | −0.15 (0.258) | −0.13 (0.319) | +0.02 (0.872) | −0.11 (0.459) | +0.23 (0.105) | +0.21 (0.103) |
| Visuospatial construction | −0.31 (0.038) | −0.18 (0.221) | +0.20 (0.163) | +0.37 (0.022) | −0.02 (0.889) | −0.12 (0.455) |
Standardized regression coefficients β (and p-values) were retrieved from linear regression adjusted for age, sex, APOE ε4, education, and baseline clinical diagnosis. Significant associations that survived FDR-correction are shown in bold typeface. All biomarkers that only showed non-significant associations were omitted from the Table.
Abbreviations: CSF cerebrospinal fluid, HV hippocampal volume, MMSE Mini-Mental state examination, MRI magnetic resonance imaging, PCC posterior cingulate cortex, PET positron emission tomography, P-tau181 phosphorylated tau181, SUVRCB standardized uptake value ratio normalized to cerebellar grey matter, SUVRWM standardized uptake value ratio normalized to subcortical white matter.
Table 4 shows the effect sizes of the associations between change in cognition and the other biomarkers at baseline. Hypometabolism in the PCC and smaller HV at baseline are significantly associated with more cognitive decline after 1 year.
Table 4.
Associations of 1-year cognitive decline (Δ) with other biomarkers (18F-FDG-PET, MRI) at baseline.
| Δcognition | FDG |
MRI |
|---|---|---|
| PCC SUVR | HV | |
| ΔMMSE | +0.19 (0.286) | +0.47 (0.010) |
| ΔDelayed memory | +0.55 (0.003) | +0.46 (0.044) |
| ΔList recall | +0.31 (0.020) | +0.27 (0.076) |
| ΔStory recall | +0.43 (<0.001) | +0.37 (0.005) |
| ΔFigure recall | +0.37 (0.008) | +0.31 (0.052) |
| ΔImmediate memory | +0.26 (0.120) | +0.14 (0.439) |
| ΔLanguage | +0.08 (0.563) | +0.36 (0.019) |
| ΔVisuospatial construction | +0.09 (0.601) | −0.07 (0.711) |
Standardized regression coefficients β (and p-values) were retrieved from linear regression adjusted for age, sex, APOE ε4, education, baseline clinical diagnosis, baseline cognition, and cognition interval. Significant associations that survived FDR-correction are shown in bold typeface. All biomarkers that only showed non-significant associations were omitted from the Table
Δcognition = ZFU – ZBL.
Abbreviations: HV hippocampal volume, MMSE Mini-Mental state examination, MRI magnetic resonance imaging, PCC posterior cingulate cortex, SUVR standardized uptake value ratio.
3.2.1. Influence of reference region selection for AV45 SUVR
When Aβ deposition was quantified as cortical SUVR referenced to WM, higher baseline SUVRWM was significantly associated with more cognitive decline over time (Δ delayed recall β = −0.40 (p = 0.029); Δ list recall β = −0.29 (p = 0.028); Δ story recall β = −0.28 (p = 0.020)), though none of the p-values survived FDR-correction. No associations were found for reference CB. The superior performance of WM normalization compared to CB normalization became also clear when 1-year changes in SUVR were investigated in a subset of our MCI and AD dementia patients with a follow-up PET scan (N = 23). Firstly, global cortical 18F-AV45 SUVRWM was significantly increased at 1-year follow-up compared to baseline (+2.61 ± 4% on average over all subjects, p = 0.015), in contrast to SUVRCB (+2.33 ± 9%, p = 0.412). Secondly, the number of subjects that showed a (contra-intuitively) SUVR decline over time (ΔSUVR < −0.5%) was smaller for reference WM compared to CB (17% versus 35% of the cases). Lastly, higher baseline 18F-AV45 SUVRWM was significantly (p = 0.05) associated with smaller increases in SUVRWM (i.e., ΔSUVRWM) over time. This most likely indicates that amyloid accumulation slowed down in the clinical phase (Supplementary Fig. 1). There was no such association for reference CB (p = 0.38).
3.2.2. Influence of biomarker positivity on longitudinal cognitive changes
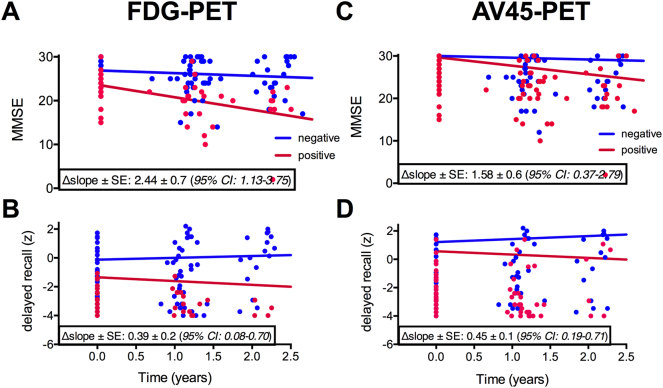
All biomarker positive groups (except CSF) at baseline showed significantly faster decline (time-by-status interaction) in MMSE and RBANS delayed memory index score over 2 years compared to their corresponding negative group (Table 5 and Fig. 1). The 18F-FDG- and 18F-AV45-positive group (based on visual reading) also showed a faster 1-year decline in MMSE and delayed memory index score, respectively, compared to the negative group.
Table 5.
Difference in the rate of cognitive change over 2 years between positive and negative 18F-FDG-, 18F-AV45-, or MRI-groups at baseline.
| FDG |
AV45 |
MRI |
|||
|---|---|---|---|---|---|
| Read |
Quantitative |
Read |
Read |
||
| SUVRCB | SUVRWM | ||||
| MMSE | +0.42 (<0.001) | +0.33 (0.004) | +0.34 (0.005) | +0.27 (0.011) | +0.27 (0.012) |
| Delayed memory | +0.17 (0.014) | +0.14 (0.024) | +0.16 (0.011) | +0.19 (0.001) | +0.14 (0.022) |
| List recall | +0.12 (0.222) | +0.24 (0.004) | +0.31 (<0.001) | +0.21 (0.012) | +0.12 (0.172) |
| Story recall | +0.06 (0.589) | +0.15 (0.093) | +0.02 (0.801) | +0.12 (0.197) | +0.23 (0.012) |
| Figure recall | +0.09 (0.388) | +0.11 (0.231) | +0.14 (0.137) | +0.02 (0.820) | +0.06 (0.542) |
| Immediate memory | +0.19 (0.058) | +0.17 (0.051) | +0.16 (0.079) | +0.11 (0.233) | +0.18 (0.040) |
| Language | +0.27 (0.013) | −0.006 (0.954) | −0.02 (0.883) | −0.01 (0.900) | +0.26 (0.009) |
| Visuospatial construction | +0.01 (0.925) | +0.10 (0.456) | +0.03 (0.794) | +0.08 (0.509) | +0.11 (0.373) |
Standardized regression coefficients β (and p-values) were retrieved from linear mixed model analysis, adjusted for the covariates. Values in bold typeface represent a significant time-by-status interaction that survived FDR-correction, i.e. a significant faster cognitive decline in the biomarker positive versus negative group at baseline.
18F-FDG and MRI dichotomization (positive or negative) was based on visual reading, whereas 18F-AV45 dichotomization was based on either visual reading or a quantitative cut-off (SUVRCB or SUVRWM). The CSF Aβ1–42/Aβ1–40 and Aβ1–42/T-tau ratios were omitted from the Table, as there were no significant interactions.
Abbreviations: MMSE Mini-Mental state examination, MRI magnetic resonance imaging, SUVRCB standardized uptake value ratio normalized to cerebellar grey matter, SUVRWM standardized uptake value ratio normalized to subcortical white matter.
Fig. 1.
Trajectories of MMSE and RBANS delayed memory index score over 2 years grouped by 18F-FDG (A, B) and 18F-AV45 (C, D) positivity based on visual reading at baseline. The Δslope (non-standardized β) indicates the difference in change in cognitive score per year between PET+ and PET- subjects, adjusted for covariates. Abbreviations: CI confidence interval, MMSE Mini-Mental state examination, PET positron emission tomography, SE standard error.
3.3. MCI-to-AD dementia conversion
3.3.1. Baseline characteristics of converters versus non-converters
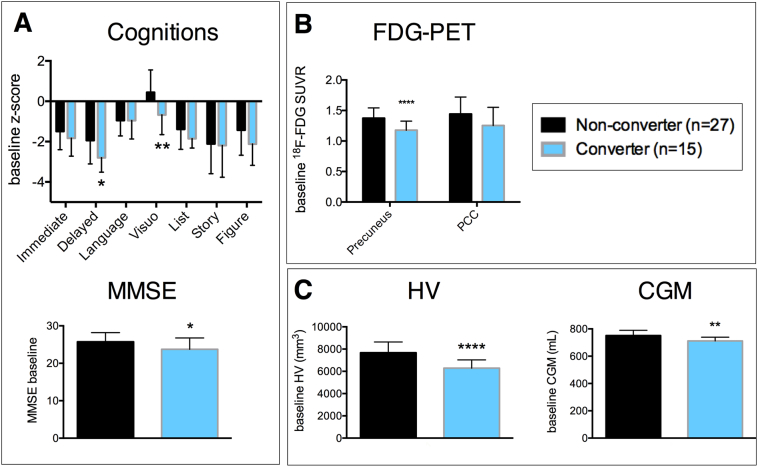
Of the 42 MCI patients with a follow-up clinical diagnosis, 15 patients progressed to AD dementia at the time of the first follow-up (mean follow-up time was 415 ± 18 days), corresponding to an annual conversion rate of 31%. The groups of converters versus non-converters were not different with respect to age, sex, APOE ε4 genotype, education, 18F-AV45-PET, and CSF levels. There was a significant difference in baseline MMSE (p = 0.023), RBANS delayed memory index score (p = 0.022), RBANS visuospatial construction index score (p = 0.007), HV (p < 0.0001), CGM volume (p = 0.002), and 18F-FDG-PET in the precuneus (p < 0.0001) between both groups (Fig. 2). However, after FDR-correction, only HV, CGM, and 18F-FDG-PET remained significant.
Fig. 2.
Baseline cognitive scores (A), regional 18F-FDG uptake (B), and MRI-based HV and CGM (C) in MCI-to-AD dementia converters (blue) versus non-converters (black) after 1 year follow-up. Error bars correspond to standard deviation. * p < 0.05, ** p < 0.01, **** p < 0.0001. Abbreviations: CGM cortical grey matter volume, HV hippocampal volume, MMSE Mini-Mental state examination, PCC posterior cingulate cortex, PET positron emission tomography, SUVR standardized uptake value ratio. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3.2. Prediction of MCI-to-AD dementia conversion
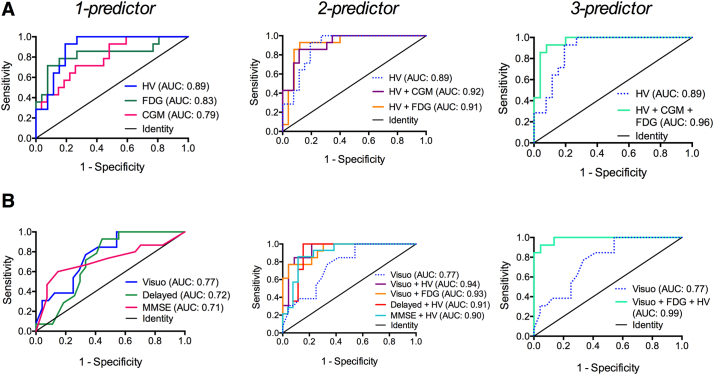
Based on binary logistic regression (dependent variable: conversion within 1 year), significant single independent variables that could predict conversion included HV (odds ratio (OR) = 0.107, 95% C.I.: [0.025–0.453], p = 0.002), CGM (OR = 0.198, 95% C.I.: [0.062–0.632], p = 0.006), 18F-FDG-PET (OR = 0.162, 95% C.I.: [0.048–0.547], p = 0.003), and cognition (RBANS visuospatial construction index score: OR = 0.389, 95% C.I.: [0.186–0.812], p = 0.012; RBANS delayed memory index score: OR = 0.434, 95% C.I.: [0.211–0.896], p = 0.024; MMSE: OR = 0.748, 95% C.I.: [0.573–0.977], p = 0.033).
HV was the strongest single variable based on its highest AUC of 0.89 (95% C.I.: [0.80–0.99], sensitivity: 64%, specificity: 89%), and approximately 51.7% explained variance (Nagelkerke R2) (Fig. 3A). Addition of CGM or 18F-FDG-PET to the single-variable model of HV resulted in an AUC of 0.92 (95% C.I.: [0.83–1.00], sensitivity: 71%, specificity: 89%) and 0.91 (95% C.I.: [0.82–1.00], sensitivity: 86%, specificity: 92%), respectively (Fig. 3A). However, the increases in AUC of the two- versus one-variable models were non-significant. The highest 2-variable AUC was reached for a combination of the RBANS visuospatial construction index score and either HV or 18F-FDG-PET (Fig. 3B). Their AUCs significantly increased (p = 0.023 and p = 0.030, resp.) compared to the one-variable model with visuospatial construction skills only. The combination of HV, 18F-FDG-PET and visuospatial construction skills resulted in the strongest three-variable model with an AUC of 0.99 (95% C.I.: [0.96–1.00], sensitivity: 92%, specificity: 96%) (Fig. 3B). However, the increase in AUC compared to the two-variable models was non-significant. All significant one-, two- and three-variable(s) combinations are reported in Supplementary Table 1, together with their AUC, accuracy, sensitivity and specificity.
Fig. 3.
Receiver operating characteristic curves of the strongest (i.e., highest AUC) one-, two- and three- variable models describing MCI-to-AD dementia conversion over 1 year (N = 42). Panel A (left column) shows the strongest single imaging biomarker variables, whereas panel B (left column) shows the strongest single cognitive variables. The middle and right columns show the two- and three-variable models for which the discriminating ability significantly improved after addition of an extra biomarker. Abbreviations: AUC area under the curve, CGM cortical grey matter volume, Delayed RBANS delayed memory index score, FDG 18F-fluorodeoxyglucose PET in the precuneus, HV hippocampal volume, MMSE Mini-Mental state examination, Visuo RBANS visuospatial construction index score.
4. Discussion
The current study investigated the utility of the A/T/N biomarkers (A: CSF Aβ and 18F-AV45-PET; T: CSF P-tau181; N: CSF T-tau, MRI atrophy and 18F-FDG-PET) with respect to concurrent cognitive function, cognitive decline and MCI-to-AD dementia conversion over 1 year. In line with literature (Chételat et al., 2005; Landau et al., 2012; Landau et al., 2010; Prestia et al., 2015; Storandt et al., 2009; Walhovd et al., 2010a; Yu et al., 2012) we found that the neurodegenerative markers, including MRI-based HV and FDG-based hypometabolism in the posterior cingulate, were most strongly associated with baseline cognition and short-term cognitive decline. Moreover, HV and 18F-FDG-PET were the strongest single determinants of short-term conversion to AD dementia in the MCI group, corresponding to an accuracy of 80 and 83%, respectively. This is in line with accuracies of 66 to 83% that have been reported previously (Fellgiebel et al., 2007; Landau et al., 2010; Prestia et al., 2015; Visser et al., 2002; Yu et al., 2012). The combination of 18F-FDG-PET and HV resulted in an accuracy of 90% in our study. This implies that both imaging modalities are yielding complementary information when interpreted together (Walhovd et al., 2010b). The AD-like patterns of 18F-FDG hypometabolism and MRI atrophy develop according to a similar anatomic pattern and extent as the clinical features, explaining their direct relationship with cognition. However in clinical practice, it may be of greater interest to combine an imaging modality with a cognitive marker to predict conversion to AD dementia. In this respect, we found that the combination of a visuospatial construction score with either 18F-FDG-PET or MRI HV significantly improved AUCs and resulted in a predictive accuracy of 89% and 86%, respectively. A combination of all three markers resulted in the highest accuracy of 94% (96% specificity, 92% sensitivity). Research on visuospatial abilities is relatively scarce, though it has shown significant diagnostic and prognostic potential in dementia as concluded by a recent review paper (Salimi et al., 2018). Unlike memory deficits, visuospatial deficits rely on the dysfunctioning of the parietal lobe, which is among the earliest manifestations of AD. The parietal lobe is involved in higher cognitive functions and highly vulnerable to metabolic impairment. Hence, cognitive testing for visuospatial functioning may be applied for differential diagnosis (discriminate AD dementia from non-AD dementias such as frontotemporal dementia) as well as for the prediction of MCI-to-AD dementia conversion. In addition, it seems possible that different subtypes in prodromal AD are related to other cognitive areas, and thus show different patterns of cognitive decline (Kate et al., 2018). Visuospatial functioning is associated with two subtypes of prodromal AD; the diffuse subtype in which cortical atrophy with intermediate clinical, cognitive and biological features are found, and also the subtype with pronounced parieto-occipital atrophy in combination with high CSF tau levels. On the other hand, combining MRI or 18F-FDG-PET with either RBANS delayed memory index score or MMSE corresponded to diagnostic accuracies between 76 and 80%, however the sensitivity was relatively low in our study (57–71%).
Biomarkers related to the early events of the pathological cascade (referring to A+ of the A/T/N model) showed that higher baseline cortical 18F-AV45 SUVRWM, notably in the parietal lobe and precuneus (data not shown), was associated with faster cognitive decline, irrespective of baseline clinical diagnosis. However, when conducted separately in the Aβ-PET positive group, we could not find a significant association between amyloid SUVR at baseline and cognitive decline (data not shown). These findings are in line with a previous study in a combined cohort of Aβ-PET positive and negative MCI patients (Doraiswamy et al., 2012), indicating that the presence of Aβ could be an important predictor of cognitive decline. On the other hand, the extent and anatomical pattern of Aβ deposition are known to show poor correlations to cognitive severity or disease progression (Vandenberghe et al., 2013). Therefore, the association between Aβ and cognitive deficit is most likely of an indirect origin, in which abnormal Aβ accumulation triggers downstream neurodegenerative processes that, on their turn, exert direct effect on cognition. This might also explain why amyloid-PET was significantly associated with short-term cognitive decline in our whole cohort, but could not discriminate short-term MCI-to-AD dementia converters from non-converters. Due to small sample sizes, we could however not perform within-diagnostic group analyses to test this hypothesis.
The association between baseline 18F-AV45 SUVR and ΔSUVR as well as Δcognition was only significant when a WM reference region was considered. Moreover, regional SUVRWM but not SUVRCB was significantly increased at follow-up in a subset of our clinical cohort, corresponding to +2–3% yearly. In contrast, Aβ deposition quantified by SUVRCB was (unexpectedly) decreased over time in 35% of the cases. In 4 out of the 8 subjects with longitudinal declining cortical SUVRCB, SUV was more increased in the reference region than in the target region, questioning the use of CB as a reference region for longitudinal purposes. Several recent studies have suggested the superior performance of a WM reference region for the 18F-AV45 ligand, both in cross-sectional and longitudinal designs (Brendel et al., 2015; Chen et al., 2015; Landau et al., 2015; Ottoy et al., 2017; Shokouhi et al., 2016). This could be related to its more favourable location in the same axial plane as the neocortical target areas (Blautzik et al., 2018). However, a recent 11C-PiB study by (Lowe et al., 2018) found WM uptake to be dependent on age and cortical Aβ deposition, thereby questioning its validity as a reference region. In our study, WM SUVRCB changes were small (−0.1 ± 10%), most likely because of the relatively advanced stage of our clinical cohort with high amyloid load at baseline and the short follow-up time (1 year).
Considered as neurodegenerative markers, CSF T-tau and Aβ1–42/T-tau were not associated with cognitive decline nor were they predictors of conversion in our study. Previous studies found an association with cognitive decline (Ben Bouallègue et al., 2017; Dumurgier et al., 2017; Kester et al., 2009) or conversion (Bouwman et al., 2007; Mattsson et al., 2009; Vemuri et al., 2009; Vos et al., 2012), whereas others did not (De Vos et al., 2016; Walhovd et al., 2010a). In line with (Vos et al., 2016), we found only a weak correlation between the CSF tau and MRI-based HV markers of neuronal injury (data not shown). Vemuri et al. (Vemuri et al., 2009) reported that MRI could be closer related to disease progression than CSF T-tau as the latter may be more prone to diurnal physiologic variations, therefore reflecting transient rather than cumulative damage. In general, we could not find good relationships between any of the CSF markers and cognition at baseline. It appeared from our analysis that the prediction of cognition by CSF markers could be explained by the link with disease state, as CSF (in particular T-tau and P-tau markers) disappeared from the model after we additionally corrected for clinical diagnosis. In contrast, amyloid-PET/CSF, 18F-FDG-PET, and HV retained an association with cognition even when diagnosis was considered. These findings were in line with the study by (Mattsson et al., 2017). Cognitive function is, by clinical diagnostic definition, lower for subjects with MCI/AD than for controls. Therefore, in an attempt to attend this potential confound and investigate the independent and complementary information of a single biomarker on cognitive function and cognitive decline in a larger cohort, we corrected for baseline diagnosis. Models without clinical diagnosis as a covariate can be found in Supplementary Table 2 and 3.
This study has a number of limitations. In our 1-year follow-up study, 15 out of 42 MCI patients (31%) converted to AD dementia, which is higher than the 8–16% expected annual risk of conversion (Landau et al., 2010; Mitchell and Shiri-Feshki, 2009). This can be explained by a high percentage of Aβ-PET positive (85%) and amnestic MCI subtypes (86%) in our cohort. Moreover, our MCI participants were recruited from a memory clinic rather than through community screening. Secondly, due to small sample size, we could not perform within-group regression analyses (i.e., investigate the effect of clinical phenotype [non-amnestic vs amnestic MCI] or clinical diagnostic group [HC vs MCI vs AD dementia] or A/T/N criteria). It is likely that for example CSF T-tau levels were associated with cognitive decline in the (non-converting) MCI subjects, but have reached a plateau in the later MCI/AD dementia phase. Thirdly, MCI subjects with clinical progression to unspecified dementias were included as it was not always possible to make the diagnosis of dementia due to AD solely on a clinical basis. A non-biomarker based diagnosis was performed in this study to avoid circular reasoning (i.e., the same tests are used to diagnose a disease and then to predict the diagnosis). However, the inclusion of unspecified dementia subjects may have decreased the predictive power of the biomarkers that were analyzed and may have increased the contribution of visuospatial construction score as a cognitive measure. Next, while we found that short-term (1 year) prognosis of AD conversion relates strongly to neurodegenerative markers, we could not find such association for amyloid-PET or -CSF biomarkers. Based on earlier findings (Dickerson and Wolk, 2013), it is likely that longer-term prognosis (> 2 years) in these individuals would be associated with a marker of amyloidosis. Lastly, 18F-FDG SUVR was normalized to a cerebellar reference region, although pons normalization has been applied in most of the previous literature. In our study however, automatic segmentation processes tend to fail in reliably separating the pons from the whole brainstem, most likely because the posterior border of this region was not well delineated on MRI (Bauer et al., 2013). Moreover, cerebellar normalization has been recommended for 18F-FDG-PET by (Bauer et al., 2013) as this region was least affected by metabolic changes in AD. Lastly, the pons may be more susceptible to noise and thus longitudinal variability, owing to its small size and sensitivity to head motion.
5. Conclusions
Our results show that the neurodegenerative markers (MRI-based HV and 18F-FDG-PET) were strongly associated to cognitive decline and short-term (1-year) MCI-to-AD dementia conversion. The use of a neurodegenerative imaging marker in addition to a cognitive marker such as a visuospatial construction score could be applied in clinical practice to predict short-term conversion to AD dementia and for enrichment of disease-modifying treatment trials in MCI patients. CSF and amyloid-PET markers were not associated with short-term conversion. However, increased amyloid-PET uptake at baseline was associated with faster cognitive decline over time, only if SUVR was referenced to a WM reference region.
Declaration of interest
The authors declare that they have no competing interests.
Funding
This study was in part funded by IWT TGO BIOADAPTAD and by the University of Antwerp and its University Hospital, under grant agreement n° 120835 A14/0161; University Hospital Antwerp; the University of Antwerp Research Fund; the Institute Born-Bunge (IBB, www.bornbunge.be); the Flanders Impulse Program on Networks for Dementia Research (VIND); and the Methusalem excellence grant (EWI, www.ewi-vlaanderen.be); Belgium.
Acknowledgements
The authors acknowledge the personnel of the VIB-Uantwerp Neuromics Support Facility for DNA sequencing, Femke Soetewey (BIODEM, UAntwerp) for starting up this study, Naomi De Roeck (BIODEM, UAntwerp) for CSF biomarker analyses, and Marleen Cauchie for the help with the PET acquisitions. Icobrain is proprietary software, developed by icometrix for the automated quantification of brain volumes and white matter hyperintensities.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101771.
Appendix A. Supplementary data
Supplementary material
References
- Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C.M., Cabral H.J., Greve D.N., Killiany R.J. Differentiating between normal aging, mild cognitive impairment, and Alzheimer's disease with FDG-PET: effects of normalization region and partial volume correction method. J. Alzheimers Dis. Parkinsonism. 2013;3:1. [Google Scholar]
- Ben Bouallègue F., Mariano-Goulart D., Payoux P. Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer's disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database. Alzheimers Res. Ther. 2017;9:1–13. doi: 10.1186/s13195-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blautzik J., Brendel M., Sauerbeck J., Kotz S., Scheiwein F., Bartenstein P., Seibyl J., Rominger A. Reference region selection and the association between the rate of amyloid accumulation over time and the baseline amyloid burden. Eur. J. Nucl. Med. Mol. Imaging. 2018;44:1364–1374. doi: 10.1007/s00259-017-3666-8. [DOI] [PubMed] [Google Scholar]
- Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- Bouwman F.H., Schoonenboom S.N.M., van der Flier W.M., van Elk E.J., Kok A., Barkhof F., Blankenstein M.A., Scheltens P. CSF biomarkers and medial temporal lobe atrophy predict dementia in mild cognitive impairment. Neurobiol. Aging. 2007;28:1070–1074. doi: 10.1016/j.neurobiolaging.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Brendel M., Högenauer M., Delker A., Sauerbeck J., Bartenstein P., Seibyl J., Rominger A., Initiative F.T.A.D.N. Improved longitudinal [18F]-AV45 amyloid PET by white matter reference and VOI-based partial volume effect correction. Neuroimage. 2015;108:450–459. doi: 10.1016/j.neuroimage.2014.11.055. [DOI] [PubMed] [Google Scholar]
- Chen K., Roontiva A., Thiyyagura P., Lee W., Liu X., Ayutyanont N., Protas H., Luo J.L., Bauer R., Reschke C., Bandy D., Koeppe R.A., Fleisher A.S., Caselli R.J., Landau S., Jagust W.J., Weiner M.W., Reiman E.M., for the Alzheimer's Disease Neuroimaging Initiative Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J. Nucl. Med. 2015;56:560–566. doi: 10.2967/jnumed.114.149732. [DOI] [PubMed] [Google Scholar]
- Chételat G., Eustache F., Viader F., Sayette La, Pélerin V., Mézenge F., Hannequin D., Dupuy B., Baron J.-C., Desgranges B. FDG-PET measurement is more accurate than neuropsychological assessments to predict global cognitive deterioration in patients with mild cognitive impairment. Neurocase. 2005;11:14–25. doi: 10.1080/13554790490896938. [DOI] [PubMed] [Google Scholar]
- De Vos A., Struyfs H., Jacobs D., Fransen E., Klewansky T., De Roeck E., Robberecht C., Van Broeckhoven C., Duyckaerts C., Engelborghs S., Vanmechelen E. The cerebrospinal fluid neurogranin/BACE1 ratio is a potential correlate of cognitive decline in Alzheimer's disease. J. Alzheimers Dis. 2016;53:1523–1538. doi: 10.3233/JAD-160227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Wolk D. Biomarker-based prediction of progression in MCI: comparison of AD-signature and hippocampal volume with spinal fluid amyloid-β and tau. Front. Aging Neurosci. 2013;5:1–9. doi: 10.3389/fnagi.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy P.M., Sperling R.A., Coleman R.E., Johnson K.A., Reiman E.M., Davis M.D., Grundman M., Sabbagh M.N., Sadowsky C.H., Fleisher A.S., Carpenter A., Clark C.M., Joshi A.D., Mintun M.A., Skovronsky D.M., Pontecorvo M.J. Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline. Neurology. 2012;79:1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy P.M., Sperling R.A., Johnson K., Reiman E.M., Wong T.Z., Sabbagh M.N., Sadowsky C.H., Fleisher A.S., Carpenter A., Joshi A.D., Lu M., Grundman M., Mintun M.A., Skovronsky D.M., Pontecorvo M.J. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol. Psychiatry. 2014;19:1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Feldman H.H., Jacova C., Hampel H., Molinueva J.L., Blennow K., DeKosky S.T., Gauthier S., Selkoe D., Bateman R., Cappa S., Crutch S., Engelborghs S., Frisoni P.G.B., Fox N.C., Galasko D., Habert M.-O., Jicha G.A., Nordberg A., Pasquier F., Rabinovici G., Robert P., Rowe C., Salloway S., Sarazin M., Epelbaum S., de Souza L.C., Vellas B., Visser P.J., Schneider L., Stern Y., Scheltens P., Cummings J.L. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Dumurgier J., Hanseeuw B.J., Hatling F.B., Judge K.A., Schultz A.P., Chhatwal J.P., Blacker D., Sperling R.A., Johnson K.A., Hyman B.T., Gómez-Isla T. Alzheimer's disease biomarkers and future decline in cognitive normal older adults. J. Alzheimers Dis. 2017;60:1451–1459. doi: 10.3233/JAD-170511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.E., Kennedy K.M., Rodrigue K.M., Wig G., Bischof G.N., Rieck J.R., Chen X., Festini S.B., Devous M.D., Sr., Park D.C. Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults. JAMA Neurol. 2017;74:830–838. doi: 10.1001/jamaneurol.2017.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellgiebel A., Scheurich A., Bartenstein P., Müller M.J. FDG-PET and CSF phospho-tau for prediction of cognitive decline in mild cognitive impairment. Psychiatry Res. 2007;155:167–171. doi: 10.1016/j.pscychresns.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Geroldi C., Rossi R., Calvagna C., Testa C., Bresciani L., Binetti G., Zanetti O., Frisoni G.B. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2006;77:1219–1222. doi: 10.1136/jnnp.2005.082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K., Ebmeier K. Clinical amyloid imaging in Alzheimer's disease. Lancet Neurol. 2011;10:667–670. doi: 10.1016/S1474-4422(11)70123-5. [DOI] [PubMed] [Google Scholar]
- Illán-Gala I., Pegueroles J., Montal V., Vilaplana E., Carmona-Iragui M., Alcolea D., Dickerson B.C., Sánchez-Valle R., de Leon M.J., Blesa R., Lleó A., Fortea J., for the Alzheimer's Disease Neuroimage Initiative Challenges associated with biomarker-based classification systems for Alzheimer's disease. Alzheimers Dement (AMST) 2018;10:346–357. doi: 10.1016/j.dadm.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Holtzman D.M. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Barkhof F., Bernstein M.A., Cantillon M., Cole P.E., DeCarli C., Dubois B., Duchesne S., Fox N.C., Frisoni G.B., Hampel H., Hill D.L.G., Johnson K., Mangin J.-F., Scheltens P., Schwarz A.J., Sperling R., Suhy J., Thompson P.M., Weiner M., Foster N.L. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer's disease. Alzheimers Dement. 2011;7:474–485. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B., Hampel H., Jagust W.J., Johnson K.A., Knopman D.S., Petersen R.C., Scheltens P., Sperling R.A., Dubois B. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neuroimage. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Elliott C., Haeberlein S.B., Holtzman D., Jagust W., Jessen F., Karlawish J., Liu E., Molinuevo J.L., Montine T., Phelps C., Rankin K.P., Rowe C., Ryan L., Scheltens P., Siemers E., Silverberg N., Snyder H.M., Sperling R. 2018 NIA-AA research framework to investigate the Alzheimer's disease continuum. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Kester M.I., van der Vlies A.E., Pijnenburg Y.A., van Elk E.J., Scheltens P., van der Flier W.M. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1353–1358. doi: 10.1212/WNL.0b013e3181bd8271. [DOI] [PubMed] [Google Scholar]
- Knopman D.S. Diagnostic tests for Alzheimer disease: FDG-PET imaging is a player in search of a role. Neurol. Clin. Pract. 2012;2:151–153. doi: 10.1212/CPJ.0b013e31825a7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Harvey D., Madison C.M., Reiman E.M., Foster N.L., Aisen P.S., Petersen R.C., Shaw L.M., Trojanowski J.Q., Jack C.R.J., Weiner M.W., Jagust W.J. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S., Weiner M.W., Jagust W.J., for the Alzheimer's Disease Neuroimaging Initiative Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Fero A., Baker S.L., Koeppe R., Mintun M., Chen K., Reiman E.M., Jagust W.J. Measurement of longitudinal β-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J. Nucl. Med. 2015;56:567–574. doi: 10.2967/jnumed.114.148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe V.J., Lundt E.S., Senjem M.L., Schwarz C.G., Min H.-K., Przybelski S.A., Kantarci K., Knopman D., Petersen R.C., Jack C.R., Jr. White matter reference region in PET studies of 11C-Pittsburgh compound B uptake: effects of age and amyloid-β deposition. J. Nucl. Med. 2018;59:1583–1589. doi: 10.2967/jnumed.117.204271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N., Zetterberg H., Hansson O., Andreasen N., Parnetti L., Jonsson M., Herukka S.-K., van der Flier W.M., Blankenstein M.A., Ewers M., Rich K., Kaiser E., Verbeek M., Tsolaki M., Mulugeta E., Rosén E., Aarsland D., Visser P.J., Schröder J., Marcusson J., de Leon M., Hampel H., Scheltens P., Pirttila T., Wallin A., Jonhagen M.E., Minthon L., Winblad B., Blennow K. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA Neurol. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- Mattsson N., Schöll M., Strandberg O., Smith R., Palmqvist S., Insel P.S., Hägerström D., Ohlsson T., Zetterberg H., Jögi J., Blennow K., Hansson O. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer's disease. EMBO Mol. Med. 2017;9:1212–1223. doi: 10.15252/emmm.201707809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., Mohs R.C., Morris J.C., Rossor M.N., Scheltens P., Carrillo M.C., Thies B., Weintraub S., Phelps C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.J., Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia - meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- Niemantsverdriet E., Ottoy J., Somers C., De Roeck E., Struyfs H., Soetewey F., Verhaeghe J., Van den Bossche T., Van Mossevelde S., Goeman J., De Deyn P.-P., Mariën P., Versijpt J., Sleegers K., Van Broeckhoven C., Wyffels L., Albert A., Ceyssens S., Stroobants S., Staelens S., Bjerke M., Engelborghs S. The cerebrospinal fluid Aβ1–42/Aβ1–40 ratio improves concordance with amyloid-PET for diagnosing Alzheimer's disease in a clinical setting. J. Alzheimers Dis. 2017;60:561–576. doi: 10.3233/JAD-170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemantsverdriet E., Ribbens A., Bastin C., Benoit F., Bergmans B., Bier J.-C., Bladt R., Claes L., De Deyn P.-P., Deryck O., Hanseeuw B., Ivanoiu A., Lemper J.-C., Mormont E., Picard G., Salmon E., Segers K., Sieben A., Smeets D., Struyfs H., Thiery E., Tournoy J., Triau E., Vanbinst A.-M., Versijpt J., Bjerke M., Engelborghs S. A retrospective Belgian multi-Center MRI biomarker study in Alzheimer's disease (REMEMBER) J. Alzheimers Dis. 2018;63:1509–1522. doi: 10.3233/JAD-171140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Lautner R., Andreasson U., Öhrfelt A., Portelius E., Bjerke M., Hölttä M., Rosén C., Olsson C., Strobel G., Wu E., Dakin K., Petzold M., Blennow K., Zetterberg H. CSF and blood biomarkers for the diagnosis of Alzheimer'sdisease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- Ottoy J., Verhaeghe J., Niemantsverdriet E., Wyffels L., Somers C., De Roeck E., Struyfs H., Soetewey F., Deleye S., Van den Bossche T., Van Mossevelde S., Ceyssens S., Versijpt J., Stroobants S., Engelborghs S., Staelens S. Validation of the semiquantitative static SUVR method for 18F-AV45 PET by pharmacokinetic modeling with an arterial input function. J. Nucl. Med. 2017;58:1483–1489. doi: 10.2967/jnumed.116.184481. [DOI] [PubMed] [Google Scholar]
- Prestia A., Caroli A., Wade S.K., van der Flier W.M., Ossenkoppele R., Van Berckel B., Barkhof F., Teunissen C.E., Wall A., Carter S.F., Schöll M., Choo Han, Nordberg A., Scheltens P., Frisoni G.B. Prediction of AD dementia by biomarkers following the NIA-AA and IWG diagnostic criteria in MCI patients from three European memory clinics. Alzheimers Dement. 2015;11:1191–1201. doi: 10.1016/j.jalz.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Rousset O.G., Ma Y., Evans A.C. Correction for partial volume effects in PET: principle and validation. J. Nucl. Med. 1998;39:904–911. [PubMed] [Google Scholar]
- Rozzini L., Chilovi B.V., Conti M., Bertoletti E., Delrio I., Trabucchi M., Padovani A. Conversion of amnestic mild cognitive impairment to dementia of Alzheimer type is independent to memory deterioration. Int. J. Geriat. Psychiatry. 2007;22:1217–1222. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- Salimi S., Irish M., Foxe D., Hodges J.R., Piguet O., Burrell J.R. Can visuospatial measures improve the diagnosis of Alzheimer's disease? Alzheimers Dement. 2018;10:66–74. doi: 10.1016/j.dadm.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S., Honigberg L.A., Cho W., Ward M., Friesenhahn M., Brunstein F., Quartino A., Clayton D., Mortensen D., Bittner T., Ho C., Rabe C., Schauer S.P., Wildsmith K.R., Fuji R.N., Suliman S., Reiman E.M., Chen K., Paul R. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to- moderate Alzheimer's disease (BLAZE) Alzheimers Res. Ther. 2018;10:1–13. doi: 10.1186/s13195-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H.C., Vermersch P., Kuiper M., Steinling M., Wolters E.C., Valk J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke K., Hermeneit S. High rate of conversion to Alzheimer's disease in a cohort of amnestic MCI patients. Int. Psychogeriatr. 2007;20:96–108. doi: 10.1017/S1041610207005509. [DOI] [PubMed] [Google Scholar]
- Shokouhi S., Mckay J.W., Baker S.L., Kang H., Brill A.B., Gwirtsman H.E., Riddle W.R., Claassen D.O., Rogers B.P. Reference tissue normalization in longitudinal 18F-florbetapir positron emission tomography of late mild cognitive impairment. Alzheimers Res. Ther. 2016;8:1–12. doi: 10.1186/s13195-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets D., Ribbens A., Sima D.M., Cambron M., Horakova D., Jain S., Maertens A., Van Vlierberghe E., Terzopoulos V., Van Binst A.-M., Vaneckova M., Krasensky J., Uher T., Seidl Z., De Keyser J., Nagels G., De Mey J., Havrdova E., Van Hecke W. Reliable measurements of brain atrophy in individual patients with multiple sclerosis. Brain Behav. 2016;6 doi: 10.1002/brb3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers C., Struyfs H., Goossens J., Niemantsverdriet E., Luyckx J., De Roeck N., De Roeck E., De Vil B., Cras P., Martin J.-J., De Deyn P.-P., Bjerke M., Engelborghs S. A decade of cerebrospinal fluid biomarkers for Alzheimer's disease in Belgium. J. Alzheimers Dis. 2016;54:383–395. doi: 10.3233/JAD-151097. [DOI] [PubMed] [Google Scholar]
- Storandt M., Mintun M.A., Head D., Morris J.C. Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B. Arch. Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Kate M., Dicks E., Visser P.J., van der Flier W.M., Teunissen C.E., Barkhof F., Scheltens P., Tijms B.M., Alzheimer's Disease Neuroimaging Initiative Atrophy subtypes in prodromal Alzheimer's disease are associated with cognitive decline. Brain. 2018;141:3443–3456. doi: 10.1093/brain/awy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R., Adamczuk K., Dupont P., Van Laere K., Chételat G. Amyloid PET in clinical practice: its place in the multidimensional space of Alzheimer's disease. Neuroimage Clin. 2013;2:497–511. doi: 10.1016/j.nicl.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P., Wiste H.J., Weigand S.D., Shaw L.M., Trojanowski J.Q., Weiner M.W., Knopman D.S., Petersen R.C., Jack C.R., Jr. MRI and CSF biomarkers in normal, MCI, and AD subjects. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne V.L., Fodero-Tavoletti M.T., Masters C.L., Rowe C.C. Tau imaging: early progress and future directions. Lancet Neurol. 2014;14:114–124. doi: 10.1016/S1474-4422(14)70252-2. [DOI] [PubMed] [Google Scholar]
- Visser P.J., Verhey F.R.J., Hofman P.A.M., Scheltens P., Jolles J. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2002;72:491–497. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S., van Rossum I., Burns L., Knol D., Scheltens P., Soininen H., Wahlund L.O., Hampel H., Tsolaki M., Minthon L., Handels R., L'Italien G., van der Flier W., Aalten P., Teunissen C., Barkhof F., Blennow K., Wolz R., Rueckert D., Verhey F., Visser P.J. Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol. Aging. 2012;33:2272–2281. doi: 10.1016/j.neurobiolaging.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Vos S.J.B., Gordon B.A., Su Y., Visser P.J., Holtzman D.M., Morris J.C., Fagan A.M., Benzinger T.L.S. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol. Aging. 2016;44:1–8. doi: 10.1016/j.neurobiolaging.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Brewer J., McEvoy L.K., Fennema-Notestine C., Hagler D.J., Jennings R.G., Karow D., Dale A.M., the Alzheimer's Disease Neuroimaging Initiative Combining MR imaging, positron emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. Am. J. Neuroradiol. 2010;31:347–354. doi: 10.3174/ajnr.A1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Dale A.M., McEvoy L.K., Brewer J., Karow D.S., Salmon D.P., Fennema-Notestine C. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol. Aging. 2010;31:1107–1121. doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Xiong C., McDade E.M., Hassenstab J., Aschenbrenner A.J., Fagan A.M., Benzinger T.L.S., Gordon B.A., Morris J.C., Li Y., Bateman R.J., Network T.D.I.A. Simultaneously evaluating the effect of baseline levels and longitudinal changes in disease biomarkers on cognition in dominantly inherited Alzheimer's disease. Alzheimers Dement. 2018;4:669–676. doi: 10.1016/j.trci.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M., Madison C.M., Rabinovici G.D., Oh H., Landau S.M., Jagust W.J. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J. Neurosci. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Dean R.A., Hall S.D., Qi Y., Sethuraman G., Willis B.A., Siemers E.R., Martenyi F., Tauscher J.T., Schwarz A.J. Enriching amnestic mild cognitive impairment populations for clinical trials: optimal combination of biomarkers to predict conversion to dementia. J. Alzheimers Dis. 2012;32:373–385. doi: 10.3233/JAD-2012-120832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material