Abstract
Background
Diverse aberrancy in genetic background, protein profiling, and biological pathways have emerged as important factors hindering discovery of effective treatment of osteosarcoma. In a previous study, we used a proteomic approach to identify some osteosarcoma-related proteins by analysis of protein profiling in individual patients through primary cell culture. Endoplasmic reticulum protein 29 (ERp29) emerged as a protein of interest for further study since accumulating evidence suggests it has broad functions in tumorigenesis of different types of cancer. Importantly, until now no report on examination of the expression patterns of ERp29 in osteosarcoma has been published.
Methods
In this study, an expression of ERp29 was examined in patient-derived osteosarcoma cells (7 cases) and normal bone graft-derived osteoblasts (7 cases) using western blotting. Expression profile of ERp29 in 94 osteosarcoma cases was investigated using immunohistochemically stained on formalin-fixed paraffin-embedded biopsied tissue. An association with clinicopathologic parameters and the patient survival was evaluated. The doubling time of five osteosarcoma cells lines expressing different levels of ERp29 was determined by a cell number along the exponential phase of the growth curve.
Results
The results substantiate the outcome from the proteomic study in which ERp29 expression was significantly higher in primary osteosarcoma cells compared to osteoblastic cells. Immunohistochemical analysis found that expression of ERp29 was low in 79% of the cases (immunoreactive score (IRS) <6). A significant correlation was observed between expression of ERp29 and patient survival. Lower expression of ERp29 (IRS<6) was statistically significantly associated with shorter overall survival of the patients (P = 0.041). In addition, we found that osteosarcoma cells with low ERp29 expression had a higher growth rate compared with high-ERp29-expressing cells.
Conclusions
These findings suggest a tumor suppressive role of ERp29 in osteosarcoma. In addition, ERp29 might potentially be applied as a prognostic indicator in patients with osteosarcoma.
Keywords: Bone neoplasms, Biomarkers, Heat-shock proteins, Prognosis, Immunohistochemistry
1. Introduction
Osteosarcoma is a cancer of the bone that typically occurs in children and adolescents during their growth spurt. Several risk factors, including tall stature, high birth weight, pubertal hormones, and germline genetic variants have been reported to be associated with the etiology of osteosarcoma [1]. Overall five year survival of patients with non-metastatic disease is approximately 60–70%; however, the outcome is worse in patients with metastasis where the survival rate is significantly decreased to 30% [2]. The overall survival of osteosarcoma patients has not improved for decades, mainly due to inter- and intra-heterogeneity in osteosarcoma that complicate discovery of more effective therapeutic options.
The genetic background of osteosarcoma is extremely chaotic and complex due to the diversity of somatic mutations which include high structural variation and chromosomal aneuploidy [3,4]. More study of the impact of such genetic alterations on biological mechanisms is definitely required to drive therapeutic initiatives. Proteomics is key in this aspect, since protein is considered a direct mediator connecting the genotypes and the phenotypes of the disease [5]. Our previous study identified novel targets for the treatment of osteosarcoma through investigation of proteomic profiling of patient-derived osteosarcoma cells [6]. We found that endoplasmic reticulum resident protein 29 (ERp29) was one of the up-regulated proteins in primary osteosarcoma cells compared to osteoblastic cells.
ERp29 is characterized as a luminal endoplasmic reticulum (ER) protein lacking some redox enzyme properties, therefore it is not a member of the protein disulfide isomerases (PDIs) but is classified as a PDI-like protein [7,8]. The structure of ERp29 consists of N-terminal and C-terminal domains connected by a flexible loop. The N-terminal domain contributes to the dimerization of ERp29, whereas the C-terminal domain facilitates substrate binding and secretion [9,10]. The biological function of ERp29 in protein folding and secretion in cells has been well established. ERp29 was found to assemble with key secretory proteins of the thyroid epithelial cells including thyroid pro-hormone, thyroglobulin (Tg), as well as Bip and GRP94 which are major ER molecular chaperones [11]. The same study indicated the critical role of ERp29 in protein folding and secretion of Tg in thyroid cells. Another study demonstrated a significant function of ERp29 in retention of immature collagen-I in the ER under ascorbate-deficient conditions which prevents secretion of unwanted forms [12].
A number of epithelial cancer studies have demonstrated various roles of ERp29, mainly in ER stress responses, mesenchymal-epithelial transition (MET), cancer cell growth, cell integrity regulation, tumor progression, metastasis, and chemo-sensitivity [13,14]. Non-expression of ERp29 has been significantly associated with shorter overall survival of patients with gallbladder adenocarcinoma as well as other poor clinical outcomes including tumor differentiation, tumor mass growth, lymph node metastasis, and cell invasion [15]. Similar findings have been observed in pancreatic ductal adenocarcinomas (PDAC) [16]. It has been determined that low expression of ERp29 is related to poor prognosis in patients with PDAC. However, until now no studies of the expression and molecular functions of ERp29 in osteosarcoma have been published.
In this study, potential roles of ERp29 in osteosarcoma were investigated through an examination of expression levels of the protein in patient-derived osteosarcoma cells as well as in biopsy tissue specimens for which complete clinical data was available.
2. Materials and methods
2.1. Patient characteristics
The osteosarcoma cohort in this study included 94 patients who had been diagnosed and treated at Maharaj Nakorn Chiang Mai Hospital, Thailand, between 2000 and 2015. The osteosarcomas in all patients had been histopathology confirmed by a bone and soft tissue pathologist (JS). All patients were followed up for survival analysis until 30 June 2016. The patients’ clinicopathological parameters are shown in Table 1. This research protocol has been approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University.
Table 1.
Characteristics of osteosarcoma patients in study cohort and association with ERp29 expression.
| Factor | All patients | Expression of ERp29(IRS score; Mean ± SD) | P-value |
|---|---|---|---|
| Age at diagnosis, years [mean=18.56 ± 12.02, median=15 (range 5–73)] | |||
| ≤15 | 49 | 4.78 ± 1.88 | 0.041 |
| >15 | 45 | 3.97 ± 2.46 | |
| Gender | |||
| Male | 54 | 4.42 ± 2.25 | 0.982 |
| Female | 40 | 4.41 ± 2.24 | |
| Enneking stage | |||
| IIB | 48 | 4.60 ± 2.32 | 0.337 |
| III | 32 | 4.12 ± 1.98 | |
| Site | |||
| Extremities | 78 | 4.60 ± 2.11 | 0.001 |
| Axial | 8 | 2.06 ± 1.54 | |
| Tumor size, cm [mean=9.06 ± 4.01, median=7.9 (range 2.4–21.4)] | |||
| <8 cm | 40 | 4.21 ± 2.01 | 0.333 |
| ≥8 cm | 37 | 4.70 ± 2.38 | |
| Metastasis at initial diagnosis or at follow-up | |||
| No | 29 | 4.67 ± 2.03 | 0.421 |
| Yes | 51 | 4.26 ± 2.28 | |
| Chemoresistance | |||
| Good responders (Tumor necrosis > 90%) | 7 | 4.92 ± 1.84 | 0.393 |
| Poor responders (Tumor necrosis ≥ 90%) | 35 | 4.19 ± 2.08 | |
P-values were calculated with Mann-Whitney U test, P-value < 0.05 shown in bold.
2.2. Patient-derived osteosarcoma and osteoblastic cells
Patient-derived osteosarcoma and osteoblastic cells were extracted, cultured, and characterized following previously reported protocol [6]. Primary osteosarcoma cells were obtained from patients at the time of biopsy from chemo-naïve osteosarcoma tissues (7 cases). Primary osteoblastic cells were obtained from bone grafts from healthy donors (7 cases) who had been diagnosed with non-cancer orthopedic conditions and who had required autologous bone grafts for substitution procedures. All primary cells were extracted from clinical samples using collagenase type I-trypsin (Gibco, Boston, MA, USA) digestion and cultured in freshly prepared Dulbecco's modified Eagle's medium (Gibco, Boston, MA, USA) supplemented with 10% fetal bovine serum at 37 °C in a humidified 5% CO2 incubator. All osteosarcoma primary cells were characterized for osteogenicity; cancer markers including expression levels of MMP-9 and collagen type X were determined by real-time RT-PCR following previously described protocol [6].
2.3. Immunoblotting analysis
Protein extraction was done using RIPA buffer containing 50 mmol/L Tris–HCl (pH 8.0), 150 mmol/L NaCl, 1% (vol/vol) Triton X-100, 0.5% (wt/vol) sodium deoxycholate, 0.1% SDS, 1 mmol/L EDTA, 5 mmol/L sodium fluoride, and 1% protease inhibitor cocktail (Sigma-Aldrich, Darmstadt, Germany). Concentration of protein was determined using Bradford assay. Extracted proteins (10 μg) were separated in 10% sodium dodecyl sulfate using polyacrylamide gel electrophoresis (SDS–PAGE) then transferred to nitrocellulose membranes. Blots were probed with antibody against ERp29 protein (dilution 1:3000; ab176573; Abcam, Cambridge, UK). The ERp29-immunoblots was re-probed with anti-actin antibody (dilution 1:3000; ab8227; Abcam, Cambridge, UK) for loading control evaluation. Protein band intensity was determined using an ECL-Advance Western Blotting Detection kit (GE Healthcare, Chicago, IL, USA). Chemiluminescent signals were captured using Gel documentation system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
2.4. Immunohistochemistry and scoring
Formalin-fixed paraffin-embedded (FFPE) biopsy tissues (N = 94) were obtained from archival paraffin blocks from the Department of Pathology, Faculty of Medicine, Chiang Mai University. FFPE tissues were immunostained using the Ventana automated staining system (Ventana Medical Systems, Tucson, AZ, USA). An Ultraview Universal DAB Detection Kit (Ventana Medical Systems, Tucson, AZ, USA), an indirect biotin-free system, was used to detect primary antibodies. The immune-staining was performed using standard protocol. Antigen retrieval was performed by heating the FFPE tissue in citrate buffer (pH 6) and subsequently incubating it with anti-ERp29 at 1:100 dilution (ab176573; Abcam, Cambridge, UK). The positive controls for ERp29 staining were human kidney, liver, testis, pancreatic tissues. Staining of ERp29 in osteosarcoma tissue was evaluated by PP and JS without prior knowledge of clinical data using a semi-quantitative immunoreactive scoring (IRS) system [17]. The percentage of immunoreactive cells was estimated and scored as follows: negative = 0, positive staining <10% = 1, positive staining ≥10% and <33% = 2, positive staining ≥33% and <66% = 3, positive staining ≥66% = 4. Intensity of staining was scored on a scale of 0 to 3: no color reaction = 0, mild reaction = 1, moderate reaction = 2, and intense reaction = 3. Immunoreactive score (IRS) was derived by multiplying immunoreactive cell scores and intensity of staining scores to compute an immunoreactive score ranging from 0 to 12.
2.5. Osteosarcoma cell growth analysis
Osteosarcoma cell lines including 143B (CRL‐8303), MG-63 (CRL‐1427), and Saos-2 (HTB‐85) cells were purchased from ATCC (Manassas, VA, USA). U2OS (CLS‐300364) and MNNG/HOS (CLS 300289) were from Cell Lines Service (GmbH, Eppelheim, Germany). All cell lines were cultured and maintained at 37̊C in humidified 5% CO2 incubator following manufacture's instruction. All osteosarcoma cells were seeded at a density of 20,000 cells per well in a 24 well plate. To determine number of osteosarcoma cells, the cells were trypsinized and counted in hemocytometer counting chamber every 24 h for 7 days using trypan blue exclusion assay. The doubling time could be determined by a cell number along the exponential phase of the growth curve. The doubling time of each cell lines was calculated using the following formula Doubling time = duration of culture (h)× ln(2)/ln(c2/c1), where c is the number of cells at each time of collection and ln is a neperian logarithm (Roth V. 2006 Doubling Time Computing, Available from: http://www.doubling-time.com/compute.php)
2.6. Statistical analysis
Statistical analyses were carried out using STATA version 11. Survival curves were estimated by the Kaplan–Meier method together with the log-rank test to evaluate association between ERp29 expression and overall survival of osteosarcoma patients. Cox regression of proportional hazards was applied to probe for significance at the 95% confidence interval (CI). The significance of correlation between staining patterns of ERp29 and clinicopathological data was determined using Student's t-test for parametric data and Mann-Whitney U test for nonparametric data. P values < 0.05 were considered to be statistically significant.
3. Results
3.1. Expression of ERp29 in primary osteosarcoma and osteoblastic cells
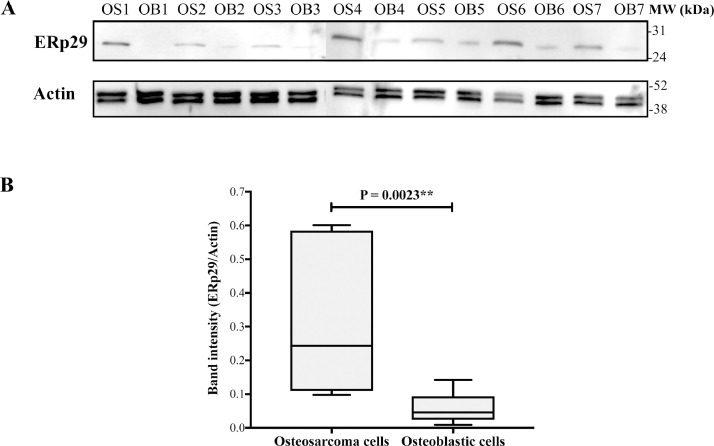
To initially explore the importance of ERp29 in osteosarcoma, expression levels of ERp29 were observed in primary osteoblastic and osteosarcoma cells using western blotting analysis. All primary cells collected from healthy donors and osteosarcoma patients were cultured under optimized conditions and characterized to confirm their osteogenic and oncogenic properties [17]. Results showed that ERp29 was statistically significantly up-regulated in osteosarcoma cells compared to osteoblastic cells (Fig. 1).
Fig. 1.
Expression of ERp29 in primary osteoblastic and osteosarcoma cells. (A) Western blots of ERp29 and actin of osteoblastic (OB) and osteosarcoma (OS) cells. (B) Box plot of ERp29 band intensities of individual samples relative to actin.
3.2. Expression of ERp29 in osteosarcoma tissues in the study cohort
Expression of ERp29 protein was investigated in the osteosarcoma cohort. Immunohistochemically staining of ERp29 was performed using paraffin blocks from 94 osteosarcoma cases with complete clinicopathologic data. In this cohort, patients were followed for 166 months. The median of overall survival after primary diagnosis was 18 months. One-year and 5-year survival rates were 67% and 28%, respectively.
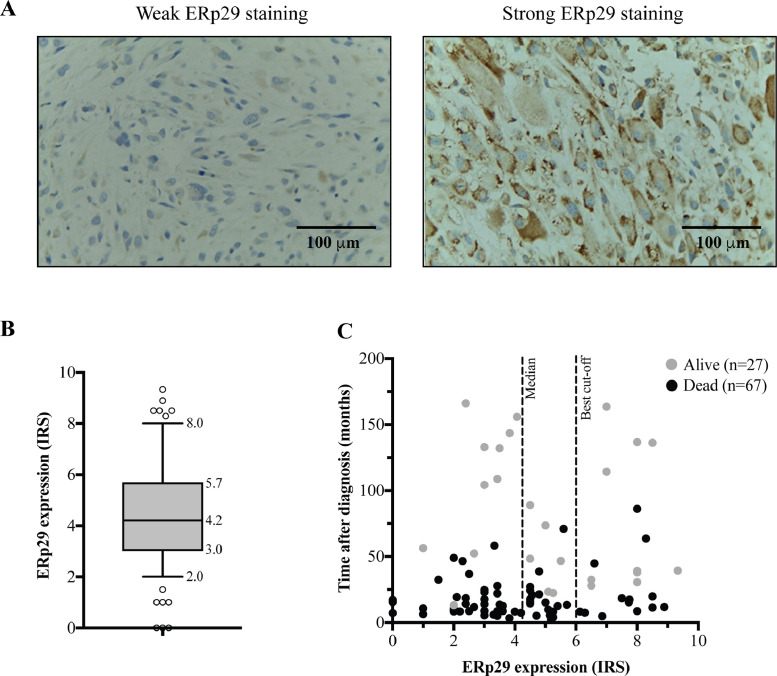
ERp29 protein was expressed in the nuclear and cytoplasmic compartments of OS cells (Fig. 2(A)). Negative staining was found in 3 cases. The median immunoreactive scores (IRS) in all patients was 4.2 (IRS range 0–9.33) as shown in Fig. 2(B). Survival data of individual patients is shown in Fig. 2(C). The IRS cut-off point for ERp29 expression levels was defined by ROC curves. Overall, 79% of the cases were weakly stained (IRS < 6) and 21% were strongly stained (IRS ≥ 6).
Fig. 2.
Immunohistochemical staining of ERp29 in osteosarcoma tissues (X400). (A) Weak ERp29 staining (IRS < 6) and strong ERp29 staining (IRS ≥ 6). (B) The boxplot shows median and distribution of ERp29 expression levels in osteosarcoma cases. (C) Survival scatter plot of individual patient data.
3.3. Association between ERp29 expression and clinicopathological factors
To determine the correlation of ERp29 expression and clinicopathological factors, the average of the immunoreactive scores were directly compared for each group of patients (Table 1). Expression levels of ERp29 were significantly lower in adults (age > 15 years) (P = 0.041) and in the axial location group (P = 0.001).
3.4. Correlation of ERp29 expression and survival rates of osteosarcoma
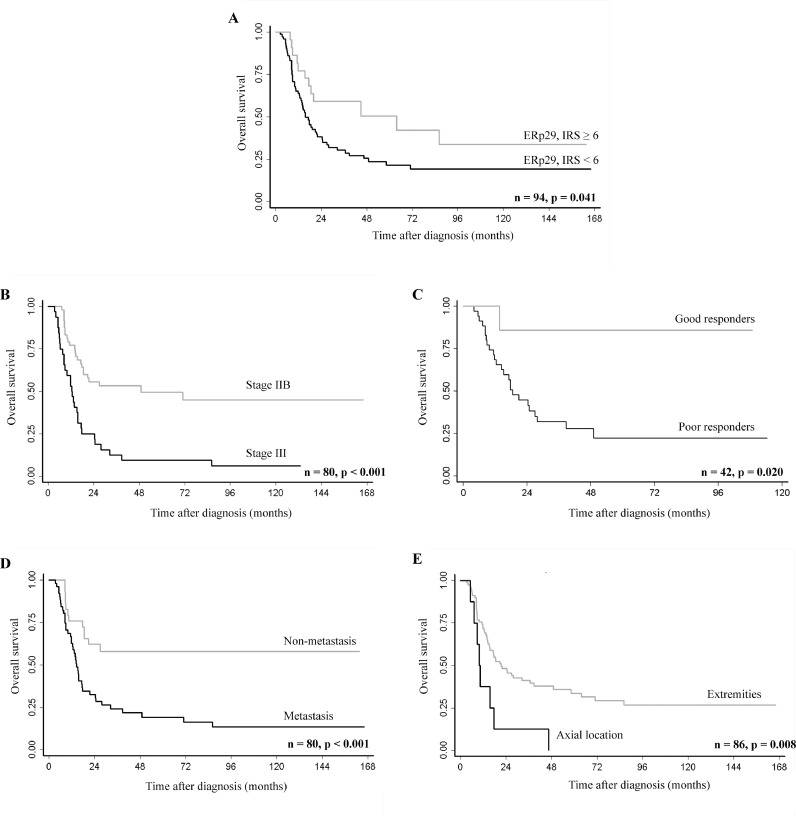
Results of univariate survival analysis showed that low levels of ERp29 expression (IRS < 6) significantly predicted shorter overall survival compared to high ERp29 expression levels (IRS ≥ 6) (P = 0.044) (Fig. 3). Five-year survival rates were 22% in patients with low levels of ERp29 (IRS < 6). Patients with higher levels of ERp29 expression (IRS ≥ 6) had better prognosis, with a 5-year survival of 51%. Other significant prognostic indicators observed in this study were advanced stage (P < 0.001), axial location (P = 0.011), poorly responded to chemotherapy (P = 0.044), and the presence of metastasis (either at initial diagnosis or at follow-up) (P = 0.002) (Fig. 3 and Table 2). Neither multivariate association analysis nor multivariate survival analysis detected any significant correlation between ERp29 expression and the studied parameters (data not shown).
Fig. 3.
Kaplan–Meier curves showing overall survival according to (A) ERp29 expression levels, (B) Enneking stage, (C) chemo-responsiveness (%tumor necrosis), (D) metastatic status, and (E) location of the tumor. P-values were obtained from the log-rank test.
Table 2.
Cox regression analysis of factors affecting overall survival.
| Factor | Patients | Events (Death) | HR(95% CI) | P-value |
|---|---|---|---|---|
| Age at diagnosis, years | ||||
| ≤15 | 49 | 32 | 1.00 | – |
| >15 | 45 | 35 | 1.23 (0.76–1.99) | 0.404 |
| Gender | ||||
| Male | 54 | 43 | 1.00 | – |
| Female | 40 | 24 | 0.73 (0.44–1.20) | 0.209 |
| Enneking stage | ||||
| IIB | 48 | 24 | 1.00 | – |
| III | 31 | 29 | 3.09 (1.79–5.34) | <0.001 |
| Site | ||||
| Extremities | 78 | 52 | 1.00 | – |
| Axial | 8 | 8 | 2.67 (1.25–5.70) | 0.011 |
| Tumor size | ||||
| <8 cm | 40 | 24 | 1.00 | – |
| ≥8 cm | 37 | 28 | 1.69 (0.98–2.93) | 0.060 |
| Metastasis at initial diagnosis or at follow-up | ||||
| No | 30 | 13 | 1.00 | – |
| Yes | 51 | 42 | 2.68 (1.43–5.00) | 0.002 |
| Chemoresistance | ||||
| Good responders | 7 | 1 | 1.00 | – |
| Poor responders | 35 | 25 | 7.50 (1.01–55.43) | 0.048 |
| ERp29 expression | ||||
| Low (Immunoreactive score < 6) | 72 | 55 | 1.00 | – |
| High (Immunoreactive score ≥ 6) | 22 | 12 | 0.52 (0.28–0.98) | 0.044 |
P-values were obtained with Cox regression of proportional hazards, P-value < 0.05 shown in bold.
3.5. Association of ERp29 expression and osteosarcoma cell growth
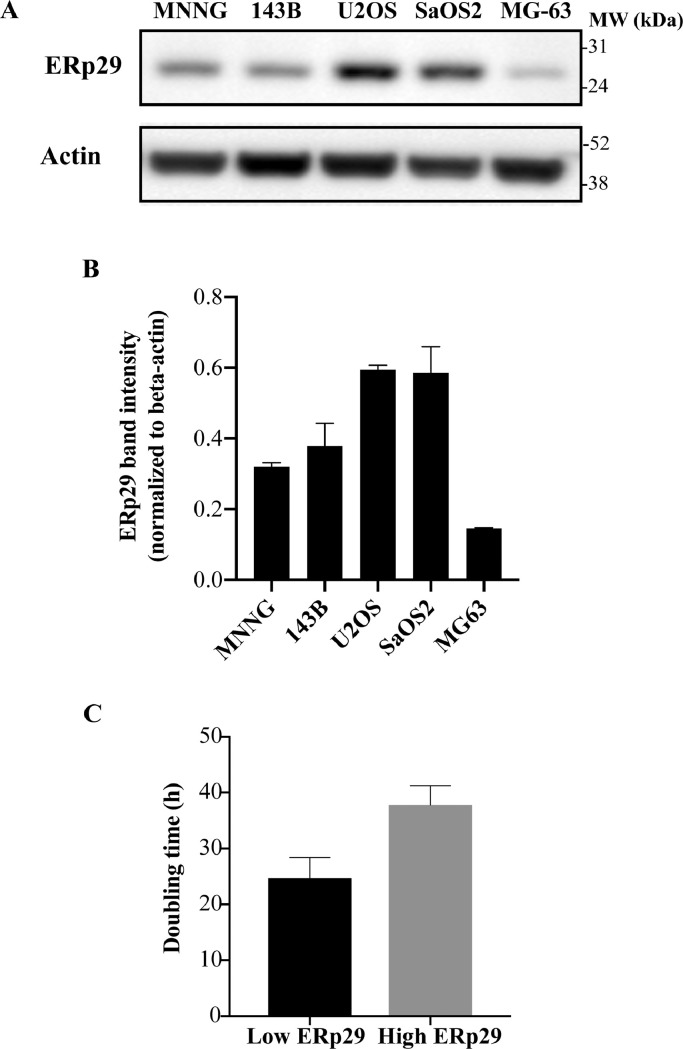
To examine functions of ERp29 as a tumor suppressor, we investigated whether expression level of ERp29 associate with an aggressiveness of osteosarcoma cell lines. Expression of ERp29 was then investigated in 5 osteosarcoma cell lines using western blotting. The result demonstrated that MNNG/HOS,143B, and MG-63 cell lines expressed low level of ERp29, while U2OS and Saos-2 cells expressed higher level of ERP29 (Fig. 4(A) and (B)). The high-ERp29-expressing osteosarcoma cells had a tendency toward higher doubling time compared with low-ERp29-expressing osteosarcoma group (Fig. 4(C)).
Fig. 4.
Association of expression levels of ERp29 and osteosarcoma cell growth. (A) Representative immunoblotting of ERp29 in 5 osteosarcoma cell lines. (B) Bar graph demonstrating expression levels of ERp29 normalized to actin. (C) Doubling time (h) of osteosarcoma cell lines expressing low-ERp29 (MNNG/HOS, 143B, and MG-63) and high-ERp29 (U2OS and Saos-2).
4. Discussion
From our previous proteomic study of patient-derived osteosarcoma and osteoblastic cells, we found that ERp29 protein was up-regulated significantly in osteosarcoma [6]. In this study, the results from immunoblotting confirmed higher expression of ERp29 in primary osteosarcoma cells compared to osteoblasts (Fig. 1). Even though many previous studies have shown an elevation of ERp29 in various epithelial cancers, e.g., basal cell carcinoma [18], lung cancer [19], and ovarian cancer [20], this study is the first observation of an expression profile of ERp29 in osteosarcoma.
Accumulating evidence indicates that an up-regulation of ERp29 in cancer is primarily involved in ER stress stimulation. This is the case because ERp29 is an ER resident protein and ER chaperone that is, by nature, induced by ER stress. In cancer cells, ER stress response is vigorously triggered by various stimuli as well as by a hostile environment, e.g., oncogenic activation, genomic instability, hypoxia, hyper-proliferation, nutrient deprivation, oxidative stress, and DNA-damage. Under these perturbations, cancer cells restore their proteostasis by activating an adaptive mechanism, the so-called unfolded protein response (UPR). It has been proposed that upon ER stress, an expression of ERp29 facilitates the UPR by acting as a transporter carrying secretory proteins from the ER to Golgi [13,21]. The transcriptional activation of ERp29 in cancer, however, is not fully understood. Transcription of ERp29 is not directly regulated by ER stress due to a lack of an ER-stress response element in the promoter region [8,22]. Various transcription factors have been reported to be involved in up-regulation of ERp29 under ER stress stimulation. For example, a study of the breast cancer cell line MDA-MB-231 revealed a regulation of ERp29 expression through p38 and XBP1 which is also involved in the UPR [23]. Presently, only limited evidence is available regarding the contribution of UPR to osteosarcoma. A study testing osteosarcoma cell lines demonstrated that stimulation of UPR induced cisplatin resistance through activation of the NF-κB pathway [24]. Several studies of canine osteosarcoma cells have demonstrated an inhibition of UPR attenuated tumor cell growth by stabilizing p53 protein [25,26].
Although many studies have indicated important roles of ERp29 as a regulator of various biological effects in epithelial cancer, no reports on ERp29 function in sarcoma have been published. There is much evidence demonstrating still controversial roles of ERp29 as a tumor suppressor or oncogenic inducer which depends mainly on cellular context [13,14]. In this study, the results showed a protective role of ERp29 in an osteosarcoma cohort as indicated by the shorter overall survival of patients with low ERp29 expression (IRS score < 6) (Fig. 3). Furthermore, we found that low-ERp29-expressing osteosarcoma cells could grow faster than the cells with high level of ERp29 (Fig. 4). These results are consistent with studies of various cancers including breast cancer, pancreatic ductal adenocarcinoma (PDAC), and gallbladder cancer [15,16,27]. We also observed lower expression of ERp29 in tumors located in axial bones. Osteosarcoma patients with a tumor mass at these sites had a poorer prognosis than cases where the tumor was in the extremity skeleton (Tables 1 and 2). A study of breast cancer demonstrated that ERp29 plays an important role as a tumor suppressor regulating tumor growth and in cell survival. A study by Bambang et al. reported an effect of an overexpression of ERp29 on cell cycle arrest at G0/G1 phase resulting in attenuated proliferation of fast growing MDA-MB-231 breast cancer cells [27]. Subsequent results indicated a down-regulation of several key cell cyclins including CCND1, CCND2 and CCND3. The in vivo xenograft model of MDA-MB-231 cells concomitantly revealed the important finding that overexpression of ERp29 leads to significantly delayed tumor initiation and reduced tumor growth. Taken together, this evidence indicates it is likely that ERp29 might play a critical role as a tumor suppressor regulating tumor cell growth and cell survival of osteosarcoma.
The present study included a large cohort (94 cases) of osteosarcoma samples, all with complete clinicopathologic data. This is the first report of an association between ERp29 protein and survival rate of osteosarcoma patients. This finding also provides substantial additional evidence supporting the results of our previously reported proteomic study regarding the important role of ERp29 in osteosarcoma. A limitation of the present study is that it covers a long period (2000–2015) during which there were changes in treatment protocols.
5. Conclusions
In summary, this study demonstrated higher expression of ERp29 in patient-derived osteosarcoma cells compared to osteoblastic cells, suggesting that the ER stress response is activated in osteosarcoma cells. The results also uncovered an association between lower expression of ERp29 and shorter overall survival, implying a protective role of ERp29 in osteosarcoma as well as substantiating the use of ERp29 as a prognostic marker. In addition, an alteration of ERp29 expression during tumor progression, has some relation to the aggressiveness of the disease. Further in-depth study of molecular mechanisms of ER stress and biological functions of ERp29 involved in tumorigenesis of osteosarcoma may lead to a discovery of a novel therapeutic target.
Acknowledgments
This work was supported by the Faculty of medicine, Chiang Mai University, the National Science and Technology Development Agency (NSTDA), the National Research University (NRU) fund, and the Musculoskeletal Science and Translational Research Center. The authors would also like to express their sincere thanks to Dr. G. Lamar Robert, PhD, and Assoc. Prof. Dr. Chongchit Sripun Robert, PhD, for editing the English manuscript, Ms. Nutnicha Sirikaew and Ms. Piyaporn Budprom for their assistance in in vitro experiment.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Parunya Chaiyawat, Email: p_chaiyawat@hotmail.com.
Dumnoensun Pruksakorn, Email: dumnoensun.p@cmu.ac.th.
Prach Pipatwattana, Email: title_holder@msn.com.
Areerak Phanphaisarn, Email: aphanphaisarn@hotmail.com.
Pimpisa Teeyakasem, Email: pimpis.mk@gmail.com.
Jeerawan Klangjorhor, Email: jeerawan.klangjorhor@gmail.com.
Jongkolnee Settakorn, Email: jsettakorn@gmail.com.
References
- 1.Gianferante D.M., Mirabello L., Savage S.A. Germline and somatic genetics of osteosarcoma – connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017;13(8):480–491. doi: 10.1038/nrendo.2017.16. [DOI] [PubMed] [Google Scholar]
- 2.Friebele J.C., Peck J., Pan X., Abdel-Rasoul M., Mayerson J.L. Osteosarcoma: a meta-analysis and review of the literature. Am. J. Orthop. 2015;44(12):547–553. [PubMed] [Google Scholar]
- 3.Kovac M., Blattmann C., Ribi S., Smida J., Mueller N.S., Engert F., Castro-Giner F., Weischenfeldt J., Kovacova M., Krieg A., Andreou D., Tunn P.U., Durr H.R., Rechl H., Schaser K.D., Melcher I., Burdach S., Kulozik A., Specht K., Heinimann K., Fulda S., Bielack S., Jundt G., Tomlinson I., Korbel J.O., Nathrath M., Baumhoer D. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat. Commun. 2015;6:8940. doi: 10.1038/ncomms9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X., Bahrami A., Pappo A., Easton J., Dalton J., Hedlund E., Ellison D., Shurtleff S., Wu G., Wei L., Parker M., Rusch M., Nagahawatte P., Wu J., Mao S., Boggs K., Mulder H., Yergeau D., Lu C., Ding L., Edmonson M., Qu C., Wang J., Li Y., Navid F., Daw N.C., Mardis E.R., Wilson R.K., Downing J.R., Zhang J., Dyer M.A., P. St. Jude Children's Research Hospital-Washington University Pediatric Cancer Genome Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7(1):104–112. doi: 10.1016/j.celrep.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis M.J., Gillette M., Carr S.A., Paulovich A.G., Smith R.D., Rodland K.K., Townsend R.R., Kinsinger C., Mesri M., Rodriguez H., Liebler D.C., C. Clinical Proteomic Tumor Analysis Connecting genomic alterations to cancer biology with proteomics: the NCI clinical proteomic tumor analysis consortium. Cancer Discov. 2013;3(10):1108–1112. doi: 10.1158/2159-8290.CD-13-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pruksakorn D., Teeyakasem P., Klangjorhor J., Chaiyawat P., Settakorn J., Diskul-Na-Ayudthaya P., Chokchaichamnankit D., Pothacharoen P., Srisomsap C. Overexpression of KH-type splicing regulatory protein regulates proliferation, migration, and implantation ability of osteosarcoma. Int. J. Oncol. 2016;49(3):903–912. doi: 10.3892/ijo.2016.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liepinsh E., Baryshev M., Sharipo A., Ingelman-Sundberg M., Otting G., Mkrtchian S. Thioredoxin fold as homodimerization module in the putative chaperone ERp29: NMR structures of the domains and experimental model of the 51kDa dimer. Structure. 2001;9(6):457–471. doi: 10.1016/s0969-2126(01)00607-4. [DOI] [PubMed] [Google Scholar]
- 8.Mkrtchian S., Fang C., Hellman U., Ingelman-Sundberg M. A stress-inducible rat liver endoplasmic reticulum protein, ERp29. Eur. J. Biochem. 1998;251(1–2):304–313. doi: 10.1046/j.1432-1327.1998.2510304.x. [DOI] [PubMed] [Google Scholar]
- 9.Rainey-Barger E.K., Mkrtchian S., Tsai B. Dimerization of ERp29, a PDI-like protein, is essential for its diverse functions. Mol. Biol. Cell. 2007;18(4):1253–1260. doi: 10.1091/mbc.E06-11-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barak N.N., Neumann P., Sevvana M., Schutkowski M., Naumann K., Malesevic M., Reichardt H., Fischer G., Stubbs M.T., Ferrari D.M. Crystal structure and functional analysis of the protein disulfide isomerase-related protein ERp29. J. Mol. Biol. 2009;385(5):1630–1642. doi: 10.1016/j.jmb.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 11.Sargsyan E., Baryshev M., Szekely L., Sharipo A., Mkrtchian S. Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J. Biol. Chem. 2002;277(19):17009–17015. doi: 10.1074/jbc.M200539200. [DOI] [PubMed] [Google Scholar]
- 12.DiChiara A.S., Taylor R.J., Wong M.Y., Doan N.D., Rosario A.M., Shoulders M.D. Mapping and exploring the collagen-I proteostasis network. ACS Chem. Biol. 2016;11(5):1408–1421. doi: 10.1021/acschembio.5b01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S., Zhang D. Friend or foe: endoplasmic reticulum protein 29 (ERp29) in epithelial cancer. FEBS Open Bio. 2015;5:91–98. doi: 10.1016/j.fob.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D., Richardson D.R. Endoplasmic reticulum protein 29 (ERp29): an emerging role in cancer. Int. J. Biochem. Cell Biol. 2011;43(1):33–36. doi: 10.1016/j.biocel.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Yuan L.W., Liu D.C., Yang Z.L. Correlation of S1P1 and ERp29 expression to progression, metastasis, and poor prognosis of gallbladder adenocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2013;12(2):189–195. doi: 10.1016/s1499-3872(13)60030-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K., Yao H., Yang Z., Li D., Yang L., Zou Q., Yuan Y., Miao X. Comparison of ILK and ERP29 expressions in benign and malignant pancreatic lesions and their clinicopathological significances in pancreatic ductal adenocarcinomas. Clin. Transl. Oncol. 2016;18(4):352–359. doi: 10.1007/s12094-015-1331-x. [DOI] [PubMed] [Google Scholar]
- 17.Chaiyawat P., Pruksakorn D., Phanphaisarn A., Teeyakasem P., Klangjorhor J., Settakorn J. Expression patterns of class I histone deacetylases in osteosarcoma: a novel prognostic marker with potential therapeutic implications. Mod. Pathol. 2018;31(2):264–274. doi: 10.1038/modpathol.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheretis C., Dietrich F., Chatzistamou I., Politi K., Angelidou E., Kiaris H., Mkrtchian S., Koutselini H. Expression of ERp29, an endoplasmic reticulum secretion factor in basal-cell carcinoma. Am. J. Dermatopathol. 2006;28(5):410–412. doi: 10.1097/01.dad.0000211521.49810.ac. [DOI] [PubMed] [Google Scholar]
- 19.Shnyder S.D., Mangum J.E., Hubbard M.J. Triplex profiling of functionally distinct chaperones (ERp29/PDI/BiP) reveals marked heterogeneity of the endoplasmic reticulum proteome in cancer. J. Proteome Res. 2008;7(8):3364–3372. doi: 10.1021/pr800126n. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson S., Krogh M., Szigyarto C.A., Uhlen M., Schedvins K., Silfversward C., Linder S., Auer G., Alaiya A., James P. Large-scale proteomics analysis of human ovarian cancer for biomarkers. J. Proteome Res. 2007;6(4):1440–1450. doi: 10.1021/pr060593y. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch I., Weiwad M., Prell E., Ferrari D.M. ERp29 deficiency affects sensitivity to apoptosis via impairment of the ATF6-CHOP pathway of stress response. Apoptosis. 2014;19(5):801–815. doi: 10.1007/s10495-013-0961-0. [DOI] [PubMed] [Google Scholar]
- 22.Sargsyan E., Baryshev M., Backlund M., Sharipo A., Mkrtchian S. Genomic organization and promoter characterization of the gene encoding a putative endoplasmic reticulum chaperone, ERp29. Gene. 2002;285(1–2):127–139. doi: 10.1016/s0378-1119(02)00417-1. [DOI] [PubMed] [Google Scholar]
- 23.Bambang I.F., Lu D., Li H., Chiu L.L., Lau Q.C., Koay E., Zhang D. Cytokeratin 19 regulates endoplasmic reticulum stress and inhibits ERp29 expression via p38 MAPK/XBP-1 signaling in breast cancer cells. Exp. Cell Res. 2009;315(11):1964–1974. doi: 10.1016/j.yexcr.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Yan M., Ni J., Song D., Ding M., Huang J. Activation of unfolded protein response protects osteosarcoma cells from cisplatin-induced apoptosis through NF-kappaB pathway. Int. J. Clin. Exp. Pathol. 2015;8(9):10204–10215. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R., Thamm D.H., Misra V. The effect of Zhangfei/CREBZF on cell growth, differentiation, apoptosis, migration, and the unfolded protein response in several canine osteosarcoma cell lines. BMC Vet. Res. 2015;11:22. doi: 10.1186/s12917-015-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergeron T., Zhang R., Elliot K., Rapin N., MacDonald V., Linn K., Simko E., Misra V. The effect of Zhangfei on the unfolded protein response and growth of cells derived from canine and human osteosarcomas. Vet. Comp. Oncol. 2013;11(2):140–150. doi: 10.1111/j.1476-5829.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- 27.Bambang I.F., Xu S., Zhou J., Salto-Tellez M., Sethi S.K., Zhang D. Overexpression of endoplasmic reticulum protein 29 regulates mesenchymal-epithelial transition and suppresses xenograft tumor growth of invasive breast cancer cells. Lab. Investig. 2009;89(11):1229–1242. doi: 10.1038/labinvest.2009.87. [DOI] [PubMed] [Google Scholar]