Abstract
Chronic low back pain (cLBP) is associated with widespread functional and structural changes in the brain. This study aims to investigate the resting state functional connectivity (rsFC) changes of visual networks in cLBP patients and the feasibility of distinguishing cLBP patients from healthy controls using machine learning methods. cLBP (n = 90) and control individuals (n = 74) were enrolled and underwent resting-state BOLD fMRI scans. Primary, dorsal, and ventral visual networks derived from independent component analysis were used as regions of interest to compare resting state functional connectivity changes between the cLBP patients and healthy controls. We then applied a support vector machine classifier to distinguish the cLBP patients and control individuals. These results were further verified in a new cohort of subjects. We found that the functional connectivity between the primary visual network and the somatosensory/motor areas were significantly enhanced in cLBP patients. The rsFC between the primary visual network and S1 was negatively associated with duration of cLBP. In addition, we found that the rsFC of the visual network could achieve a classification accuracy of 79.3% in distinguishing cLBP patients from HCs, and these results were further validated in an independent cohort of subjects (accuracy = 66.7%). Our results demonstrate significant changes in the rsFC of the visual networks in cLBP patients. We speculate these alterations may represent an adaptation/self-adjustment mechanism and cross-model interaction between the visual, somatosensory, motor, attention, and salient networks in response to cLBP. Elucidating the role of the visual networks in cLBP may shed light on the pathophysiology and development of the disorder.
Keywords: Chronic low back pain, Vision system, fMRI, Cross-modal perception, Attention, Resting state functional connectivity
Highlights
-
•
We investigated rsFC changes of visual networks in cLBP patients.
-
•
rsFC of the primary visual network with S1 and M1 increased in cLBP patients.
-
•
rsFC of the visual networks can differentiate cLBP patients from controls (with 79.3% accuracy).
-
•
Classification results can be validated in an independent cohort (with 66.7% accuracy).
1. Introduction
Chronic low back pain (cLBP) is a serious social and economic problem (Hoy et al., 2010; Smith et al., 2013) that significantly and negatively impacts the lives and work abilities of the individuals who suffer from it (Oberlinner et al., 2015; Yang et al., 2016). Although the pathophysiological mechanisms underlying chronic low back pain remain unclear, studies suggest that compared with healthy controls, cLBP patients show structural and functional changes of the sensory-motor system (Kong et al., 2013; Wasan et al., 2011), attention network (Letzen and Robinson, 2017; Mao et al., 2014), default mode network (Baliki et al., 2008; Goossens et al., 2018; Letzen and Robinson, 2017; Longo et al., 2009; Vachon-Presseau et al., 2016), reward system (Baliki et al., 2008; Hashmi et al., 2013), and pain modulation networks, such as the descending pain modulatory system (Yu et al., 2014). These findings demonstrate that cLBP is associated with widespread brain network changes due to persistent back pain.
The literature suggests that pain is an extremely complex experience that demands the recruitment of many central nervous system components (Peyron et al., 2000). Brain imaging studies have found that brain regions including the insula, anterior cingulate cortex, secondary somatosensory cortex, primary somatosensory cortex, prefrontal cortex, and thalamus are repeatedly activated during experimental pain stimulation (Iannetti and Mouraux, 2010; Kong et al., 2010; Melzack, 2001), and some researchers thus refer to these regions as the “pain matrix” (Melzack, 2005).
Further studies suggest that the so-called “pain matrix” may not represent a neural mechanism uniquely involved in nociception, but rather may act as a defense system that informs the body of potentially damaging events and is involved in “detecting, orienting attention towards, and reacting to the occurrence of salient sensory events” (Legrain et al., 2011; Mouraux et al., 2011). More recently, investigators have suggested that pain perception may represent a continuum of aversive behavior dictated by emotional learning and the physical proximity of the perceived source of danger. Brain regions in the limbic system may play an important role in pain, particularly the chronic pain experience (Baliki and Apkarian, 2015).
Interestingly, studies have found that some brain regions that are not part of the typical “pain matrix,” limbic system, and salient network may also be involved in the central processing of pain signals. For instance, the pain “signature” for experimental pain developed by Wager and colleagues includes brain regions belonging to the visual system, such as the occipital lobe and fusiform gyrus (Wager et al., 2013). Studies from both our group (Kong et al., 2010) and others (Matsuo et al., 2017) found that heat pain stimulation can produce significant fMRI signal decreases at occipital cortices.
In addition to experimental pain, studies have also discovered the involvement of brain regions associated with the visual system in the pathophysiology of chronic pain. For instance, in a previous study that employed vector machine analysis, investigators found that brain gray matter density in the visual cortex and temporal lobe was also useful in the discrimination of cLBP patients from healthy controls (Ung et al., 2014). In addition, studies have found brain functional changes in vision-related brain regions in knee osteoarthritis (Pujol et al., 2017), postherpetic neuralgia (Cao et al., 2018), migraine patients (Liu et al., 2015), and fibromyalgia (Pujol et al., 2014).
Although studies have repeatedly demonstrated the involvement of vision-related brain regions in both experimental and endogenous pain, their role in pain processing remains unclear. Studies suggest that vision is an important part of the selective attention process (Fox et al., 2005a, 2005b; Kawashima et al., 1995). In addition, vision may participate in the integration of brain function in chronic pain through the cross-modal integration of the central nervous system (Haggard et al., 2013; Kong et al., 2009; Pomper et al., 2013), i.e., interactions between two or more different sensory modalities in which one sensory modality is shown to influence the perception, behavioral responses, or neural processing of a stimulus presented in another sensory modality (Bavelier and Neville, 2002; Cavanna and Trimble, 2006; Spence, 2011).
Recently, both resting state fMRI and machine learning have been widely used to study chronic pain. Resting state fMRI allows us to investigate the pathophysiological mechanisms of chronic pain at a network level (Fransson, 2005), while machine learning gives us the capability to identify brain signatures of patients and make accurate classifications (Arbabshirani et al., 2017).
Previous studies have identified three vision-related networks: (1) the primary visual network, which is specialized for processing information about static and moving objects, (2) the dorsal stream, which is associated with spatial awareness and guidance of actions, and (3) the ventral stream, which is associated with object recognition (James et al., 2003; Nassi and Callaway, 2009). Thus, in this study, we systemically investigated the resting state functional connectivity of the visual networks (the primary, dorsal, and ventral visual networks) in cLBP patients as compared to healthy controls. In addition, we applied machine learning methods to discriminate cLBP patients from healthy controls and tested the accuracy of this method in an independent cohort of cLBP subjects. We hypothesized that cLBP patients would have altered visual network rsFC, which in turn would be associated with the duration of chronic back pain.
2. Materials and methods
2.1. Participants
We used two cohorts of cLBP patients and controls for this study. The first cohort was a large group of cLBP patients and age- and gender-matched healthy controls, which included 90 cLBP patients between 20 and 50 years of age and 70 healthy controls matched by age and gender. Cohort two, which was used for validation of machine learning results obtained from cohort one, included 18 cLBP patients and 19 healthy controls matched by age and gender. The demographic data and pain-related parameters for cLBP and HC are presented in Table 1. The study was approved by the Institutional Review Board at Massachusetts General Hospital, and all subjects signed informed consent forms.
Table 1.
Demographic and clinical characteristics of study participants (mean ± SD).
| Characteristic | Dataset 1 |
Dataset 2 |
||
|---|---|---|---|---|
| cLBP | Controls | cLBP | Controls | |
| Age | 34.46 ± 8.97 | 32.44 ± 8.38 | 36.11 ± 9.85 | 37.16 ± 9.07 |
| Gender (male/female) | 38/52 | 31/43 | 7/11 | 7/12 |
| Duration (years) | 6.94 ± 6.21 | NA | 5.27 ± 3.66 | NA |
| BDI | 6.12 ± 6.00 | NA | 6.50 ± 7.19 | NA |
| Pain Bothersomeness | 5.06 ± 1.88 | NA | NA | NA |
All eligible participants were required to meet the following inclusion/exclusion criteria. Inclusion criteria: 1) 20–50 years old, 2) Presence of nonspecific cLBP for a duration of at least 6 months, with the condition established by a clinical evaluation, including the use of X-ray/MRI reports, when available (Werneke and Hart, 2004), 3) Pain intensity averaging at least 4 on the 0–10 visual analog scale (VAS) in the week preceding the screening. Exclusion Criteria: 1) Specific causes of back pain (e.g., cancer, fractures, spinal stenosis, infections), 2) Complicated back problems (e.g., prior back surgery, medicolegal issues), 3) Major systemic diseases or history of head injury or coma, 4) Presence of any contraindications to MRI scanning. For example: cardiac pacemaker, metal implants, claustrophobia, pregnancy, cannot lie still in fMRI scanner, 5) History of substance abuse or dependence.
Pain Bothersomeness Scale (0–10, 0 indicates "not at all bothersome", 10 indicates "extremely bothersome") was used to measure the average low back pain severity in the preceding week. The Beck Depression Inventory-II (BDI-II) was self-administered by cLBP patients to assess depression symptoms (Beck et al., 1996).
2.2. MRI data acquisition
The fMRI brain imaging data from the first cohort was acquired with a 3 T Siemens whole-body scanner using a 32-channel radio-frequency head coil at the Martinos Center for Biomedical Imaging. T2*-weighted functional images encompassing the whole brain were acquired with the gradient-echo EPI sequence (echo time: 30 ms, repetition time: 3000 ms, flip angle: 90°, slice thickness: 2.6 mm, 44 slices, voxel size: 2.62 × 2.62 × 3.12 mm3, field of view: 220 × 220 mm2, matrix: 84 × 84 mm2, slice orientation: axial, order of slice accession: interleaved). During the 6-min resting state fMRI scan, subjects were instructed to keep their eyes open and blink normally. High-resolution brain structural images were also acquired with a T1-weighted three-dimensional multi-echo magnetization-prepared rapid gradient-echo (MPRAGE) sequence (repetition time: 2530 ms, echo time: 1.69 ms, slice thickness 1 mm, flip angle: 7°, 176 sagittal slices covering the whole brain, voxel size: 1 × 1 × 1 mm3, field of view: 256 × 256 mm2, matrix: 256 × 256 mm2, inversion time: 1100 ms).
The second cohort of participants were scanned using parameters similar to those of a previous experiment (Kong et al., 2013) with a different MRI scanner. RS-fMRI parameters were: gradient-echo EPI sequence, echo time: 30 ms, repetition time: 3000 ms, flip angle: 85°, slice thickness: 3 mm, 47 slices, voxel size: 3 × 3 × 3 mm3, field of view: 240 × 240 mm2, matrix: 64 × 64 mm2, slice orientation: axial, order of slice accession: interleaved. Structural imaging parameters were: MPRAGE sequence, repetition time: 2200 ms, echo time: 1.54 ms, slice thickness 1.2 mm, flip angle: 7°, and 144 sagittal slices covering the whole brain, voxel size: 1.2 × 1.2 × 1.2 mm3, field of view: 230 × 230 mm2, inversion time: 1100 ms.
2.3. Resting-state fMRI preprocessing
Functional data was preprocessed using SPM12 (Statistical Parametric Mapping. Wellcome Department of Cognitive Neurology, London, UK; implemented by MATLAB R2015b, Math Works, Inc., Natick, MA, USA). The preprocessing, including removing the first 5-time points, realignment, slice timing, outlier detection, functional normalization, structural segmentation and normalization, were used in normalizing images to the standard Montreal Neurological Institute (MNI) template. Images were also smoothed using a 4 mm full-width at half-maximum (FWHM) Gaussian kernel, filtered with a frequency window of 0.008–0.09 Hz. Subsequently, the brain was segmented into gray matter, white matter, and cerebrospinal fluid (CSF) for the removal of temporal confounding factors (white matter and CSF).
2.4. Motion-related analyses
The artifact detection toolbox (http://www.nitrc.org/projects/artifact_detect/) was applied to detect motion during the resting state fMRI scan. Time points in subjects' images were marked as outliers if the global signal exceeded three standard deviations from the mean or if the scan-to-scan motion exceeded 0.5 mm.
We compared the group level head motion between cLBP patients and HCs. To express instantaneous head motion as a scalar quantity, we use the framewise displacement (FD) as:
where ∆dix = d(i−1)x − dix and similarly for other rigid body parameters [dixdiydizαiβiγi].
To explore the potential effects of head motion in discriminating cLBP patients from HCs, we compared mean FD between these two groups and did not observe a significant difference (cLBP: 0.10 ± 0.05; HC: 0.09 ± 0.05; p = .22, two sample t-test).
2.5. Seed based functional connectivity analysis
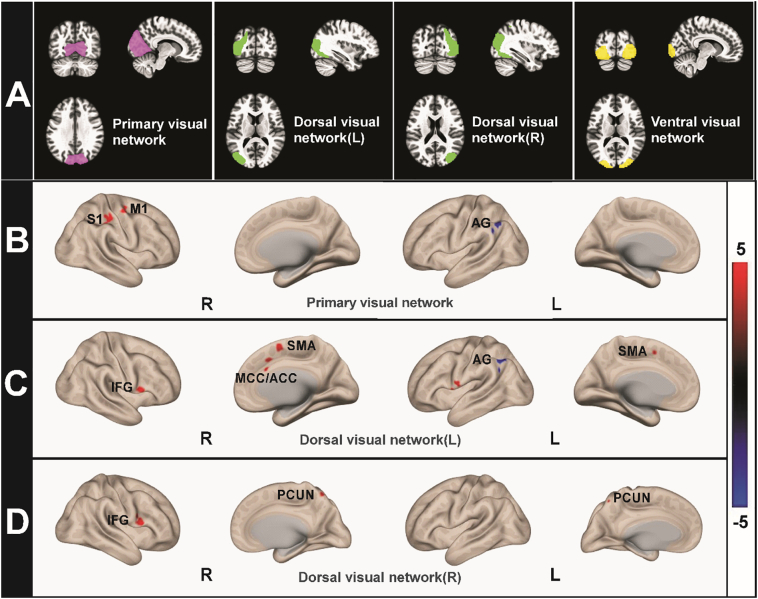
Resting-state functional connectivity analysis was conducted using the CONN toolbox v17.f(http://www.nitrc.org/projects/conn; Whitfield-Gabrieli and Nieto-Castanon, 2012). The visual network seeds used were the primary visual network (center coordinates: 2, −79, 12), dorsal visual network (L, center coordinates: −37, −79, 10; R, center coordinates: 38, −72, 13), and ventral visual network (central coordinates: 0, −93, 4) (Fig. 1a). All coordinates of seeds were provided by CONN toolbox and were originally derived from ICA analyses based on the Human Connectome Project (HCP) dataset of 497 subjects. Using publicly available ROIs benefits the external validations of our findings from other groups and minimizes the potential bias when manually selecting independent components from ICA analysis with our own dataset.
Fig. 1.
Seed Locations and Regions Showing Between-Group Differences in Mean rsFC. (A) The visual networks ROI is divided into four compartments, such as primary visual network (pink), left dorsal visual network (green), right dorsal visual network (green), ventral visual network (yellow). (B, C and D) cLBP patients showed increased rsFC (red) and decreased rsFC (blue) compared with HCs when using different visual networks as the ROI (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Functional connectivity measures were computed between a seed region of interest (ROI) and every other voxel in the brain; nuisance covariates (white matter, cerebro-spinal fluid, and six motion parameters) were also included in the data analysis. In the calculations, we first extracted the residual BOLD time course from a given seed and then estimated its first-level correlation maps by computing Pearson's correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were transformed into Fisher's Z-scores to increase normality and allow for improved second-level General Linear Model analyses. Visual network seed-to-voxel functional connectivity was estimated for each subject. Between-group analyses were performed to compare rsFC changes using two sample t-tests with age and gender included in the model as covariates. Thresholds of voxel-wise p < .001 uncorrected and cluster-level p < .05 family-wise error corrected were applied.
2.6. Classify LBP and HCs
All discriminative FCs obtained in the seed-based functional connectivity analysis were used for distinguishing cLBP patients from HCs. Machine learning models were trained using a support vector machine (SVM) classifier and based on LIBSVM (Chang and Lin, 2011). To quantify performance, classification accuracy, sensitivity, and specificity were calculated. The sensitivity and specificity represent the proportion of patients and HCs correctly classified. To further assess the performance of the classifier and evaluate the significance of classification accuracy, we ran permutation testing. In each testing, we randomly permuted the class labels of the data prior to training. 5-fold CV was then performed on the permuted dataset and the procedure was repeated 10,000 times. If the classifier trained on real class labels had an accuracy exceeding the 95% confidence interval generated from the accuracies of the classifiers trained on randomly relabeled class labels, this classifier was considered to be well-performing (Gu et al., 2018; Noirhomme et al., 2014).
To evaluate the generalizability of the classifier, we applied the trained classifier to an independent cohort of cLBP patients and HCs scanned in a different MRI machine but processed with the same pipeline. We extracted the identified FCs and used the classifier to discriminate cLBP patients from HCs.
3. Results
3.1. Demographic and clinical scales
90 cLBP patients and 74 HC subjects from cohort one were included in data analysis. There were no significant differences in age (t (162) = 1.472 p = .14) and gender (χ2(1, N = 164) = 0.002 p = .97) between the two groups. The average pain bothersomeness scores measured the week prior was 5.06 ± 1.88 for cLBP patients, and the average duration of cLBP was 6.94 ± 6.21 years. The Beck Depression Inventory-II (BDI-II) in patients showed very low mean values; the average depression score was 6.12 ± 6.00, less than mild depression (score ≥ 14) as defined by the BDI-II manual.
3.2. Functional connectivity analysis results
Whole brain rsFC analysis showed that when using the primary visual network as a seed, cLBP patients showed significant rsFC increases at the right postcentral (S1) and precentral gyri (M1) and rsFC decreases at the left angular gyrus/lateral occipital cortex.
Using the left dorsal visual network as a seed, we found significantly increased connectivity in multiple regions, including the right middle cingulate cortex/anterior cingulate cortex (MCC/ACC), bilateral supplementary motor area (SMA), left precentral gyrus/temporal pole (PreCG/TP), and right inferior frontal gyrus (IFG), while the left angular gyrus (BA40)/left lateral occipital cortex showed decreased connectivity. When using the right dorsal visual network as a seed, the right inferior frontal gyrus and bilateral precuneus exhibited increased connectivity. No significant cluster decreases were found. When the ventral visual network was used as a seed, no significant brain regions were detected for both the HC > cLBP and cLBP > HC contrasts. Results of the functional connectivity contrast map and coordinates are shown in Fig. 1 and listed in Table 2.
Table 2.
Regions with significantly changed resting-state functional connectivity between the primary visual network, bilateral dorsal visual network, and other cortical brain regions, controlling for age and gender as a covariate (voxel-wise, p < .001, uncorrected; cluster-wise, p < .05, FWE corrected).
| Regions of interest | Contrast | Brain region | k | MNI |
Z value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Primary visual network | LBP > HC | S1 | 128 | 46 | −30 | 50 | 5.30 |
| M1 | 55 | 24 | −12 | 54 | 3.89 | ||
| HC > LBP | Left AG/LOC | 180 | −48 | −58 | 42 | 4.79 | |
| Dorsal visual network (L) | LBP > HC | Right MCC/ACC | 53 | 6 | 20 | 30 | 4.29 |
| Bilateral SMA | 128 | 6 | 4 | 60 | 4.40 | ||
| Right IFG | 83 | 54 | 10 | 12 | 5.23 | ||
| Left PreCG / TP | 91 | −60 | 8 | −2 | 4.48 | ||
| HC > LBP | Left AG/LOC | 171 | −48 | −58 | 46 | 4.65 | |
| Dorsal visual network (R) | LBP > HC | Right IGF | 109 | 54 | 10 | 12 | 5.11 |
| Bilateral PCUN | 51 | −2 | −62 | 60 | 3.84 | ||
| HC > LBP | NA | ||||||
| Ventral visual network | NA | ||||||
Brain area abbreviation: L left, R right, S1 primary somatosensory cortex, M1 primary motor gyrus, AG angular gyrus, LOC lateral occipital cortex, MCC mid-cingulate cortex, ACC anterior cingulate cortex, SMA, supplementary motor area, IFG inferior frontal gyrus, PreCG precentral gyrus, TP temporal pole, PCUN precuneous.
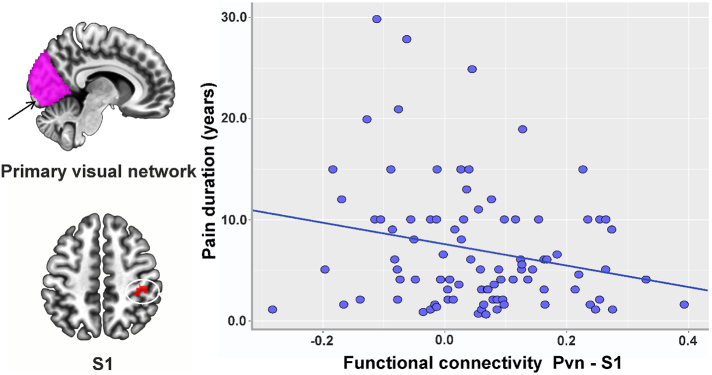
Due to the important role of the S1 in chronic low back pain processing (Kong et al., 2013), we extracted the z value of the functional connectivity between the primary visual network seed and S1. We performed a correlation analysis between the rsFC of the primary visual system with S1 and pain duration and average bothersomeness scores in the past week, adjusted for age, gender, and head motion. We found a significant association between cLBP duration and the rsFC of the primary visual network with S1 (r = −0.24, p = .05 after FDR p-value correction, Fig. 2).
Fig. 2.
rsFC changes were related to behavioral data in the cLBP groups. We extracted the z value of the functional connectivity between the primary visual network seed and S1 and performed a correlation analysis between the rsFC and behavioral data, adjusted for age, gender, and head motion. Pain duration was negatively associated with functional connectivity between the primary visual seed and S1 in the cLBP subjects. Brain area abbreviation: Pvn primary visual network.
3.3. Classification results
The classification accuracy for discriminating cLBP patients from HCs was 79.3% (p < .001; sensitivity: 83.3%, specificity: 74.3%) in the first cohort of subjects. The classifier achieved an accuracy of 66.7% (p = .008; sensitivity: 72.2%, specificity: 61.1%) in cohort 2, the independent dataset.
4. Discussion
In this study, we compared the resting state functional connectivity of the visual networks between cLBP patients and healthy controls. We found that the functional connectivity of the primary visual network with the somatosensory/motor areas was significantly enhanced in cLBP patients. Primary visual network-S1 rsFC was negatively associated with duration of low back pain. In addition, using machine learning methods, we found that the rsFC of the visual network could achieve a classification accuracy of 79.3% in discriminating cLBP patients from HCs. These results were further validated in an independent cohort of subjects and achieved an accuracy of 66.7%. Our results suggest that the visual network may be involved in the central pathophysiology of chronic low back pain.
In this study, we investigated the rsFC of three visual networks (Milner and Goodale, 2006) in patients with chronic pain. The primary visual network is mainly located at visual area 1 (V1). The dorsal visual network area is adjacent to the parietal lobe in the dorsal stream, which stretches from V1 into the parietal lobe. The ventral visual network area is adjacent to the temporal area in the ventral stream, which runs downward from V1 into the anterior inferotemporal lobule (Kravitz et al., 2013; Siegel et al., 2008). Based on the previous studies, the primary visual network is specialized for processing information about static and moving objects, the dorsal stream is associated with spatial awareness and guidance of actions, and the ventral stream is associated with object recognition (James et al., 2003; Nassi and Callaway, 2009).
We found increased rsFC between the primary visual cortex and primary somatosensory cortex in cLBP patients compared to healthy controls. This result is partly consistent with a previous study in which Pujol and colleagues found that altered functional connectivity extended beyond the somatosensory domain and implicated visual and auditory sensory modalities in fibromyalgia patients (Pujol et al., 2014). We speculate that this increase may be due to an adaptive neural remodeling in the brain following repeated pain stimulation (Seminowicz et al., 2011) through a cross-modal mechanism (Filbrich et al., 2017; Senkowski et al., 2014). Specifically, pain sensation is generally accompanied with other sensory inputs, including vision (Pomper et al., 2013), audition (Hauck et al., 2013) and olfaction (Perkins et al., 2016), which may consequently interfere with each other. The visual system is involved in the detection of potentially meaningful stimuli for the body. These visual stimuli can enhance the saliency of painful input, leading to increased pain experience. For instance, watching a needle pricking another person's hand enhances pain perception and anticipatory pupil dilation responses in the viewer (Hofle et al., 2012).
In addition, visual distraction can increase pain tolerance by diverting one's attention away from pain, thereby reducing its perceived strength (Gu and Han, 2007; Harvie et al., 2015; Maltzman, 1988). Viewing pleasant pictures (Godinho et al., 2008) or viewing one's own body has also been shown to reduce pain perception (Longo et al., 2009). This pain-relieving strategy has been applied in clinics during a variety of potentially painful operative procedures (Carwile et al., 2014; Xiaolian et al., 2015).
The dorsal visual pathway perceives salient visual features (Olague et al., 2014; Zheng et al., 2013) and can enhance visual attention, which may be mediated by the prefrontal and parietal cortices (Corbetta, 1998; Kastner and Ungerleider, 2000). The intrinsically defined dorsal attention system extends from V1 beyond the intraparietal sulcus and frontal eye field and includes the midline SMA/pre-SMA region (Fox et al., 2006; Vossel et al., 2014). Attention counts as a basic cognitive capacity in integrating information in the multisensory mind (Bekrater-Bodmann et al., 2014). Noxious stimuli can capture our attention through the salience network, including the anterior cingulate cortex (Buffington et al., 2005; Li et al., 2009) and the orbital frontoinsular cortices (Seeley et al., 2007). We found that the dorsal visual network was significantly connected with the MCC/ACC, a region that implements a domain-general process that is integral to negative affect, pain, and cognitive control (Shackman et al., 2011). We speculate that the visuospatial attention from the dorsal stream and signal of pain perception integrated into the MCC/ACC may enhance avoidance learning for events associated with negative outcomes, which may represent an adaptive pain coping strategy.
We found increased reciprocal connections between the primary visual cortex and motor cortex (M1) (Rizzolatti and Luppino, 2001). This finding may reflect the preparation of a defense response. Because of the increased impact of avoidance behavior, low back pain patients need to pay attention to position adjustment with the aid of their visual system (Mok et al., 2004; Salavati et al., 2016). We also detected an increased interregional synchronization between the dorsal visual system, precuneus, and IFG. Recent literature suggests that the precuneus is involved in directing attention in space when making or preparing movements (Cavanna and Trimble, 2006; Margulies et al., 2009). The right inferior frontal gyrus (rIFG) activates when stopping incipient action (Bartoli et al., 2018). Taken together, the increased connectivity provides further support that the visual network is involved in avoidance behavior and position adjustment in cLBP patients.
We did not find significant functional connectivity differences in the ventral visual network, which is primarily responsible for the processing of memory and learning of visual information. In contrast to the dorsal pathway's involvement in somatosensation, spatial attention, and visually-guided action, the ventral pathway is focused on object vision, such as shape, color, and texture (Kravitz et al., 2013). This finding further endorses our hypothesis that visual system changes in cLBP patients may reflect an automatic self-adaptation process through attention and movement adjustment.
To further demonstrate the role of the visual system in the pathophysiology of cLBP, we also applied multivariate pattern analysis (MVPA) to distinguish cLBP patients from healthy controls based on visual system functional connectivity changes. MVPA is a sensitive machine learning approach (e.g., general linear model (Haxby, 2012)) that has been used to explore neurophysiology in migraine (Chong et al., 2017), chronic low back pain (Ung et al., 2014), and fibromyalgia (Lopez-Sola et al., 2017). We found that functional connectivity changes among the visual, sensory, motor, salience, and attention networks can distinguish cLBP patients from healthy controls with an accuracy rate of 79.3%. Most importantly, these changes were further validated in an independent cohort of subjects. Our results are consistent with a previous report in which the authors found that the visual system can be used to distinguish cLBP patients from healthy controls (Ung et al., 2014). These findings provide further support for the involvement of the visual system in the pathophysiology of cLBP.
There are several limitations to this study. Although we found that the rsFC between the visual network and the sensory area is increased in patients with back pain, we cannot determine the nature of functional connectivity changes (e.g., increase vs. decrease in neuronal activity) in these regions. In addition, we do not know whether the visual network connectivity changes observed in cLBP patients can extend to other chronic pain disorders. Abnormalities of activity and connectivity in the visual system have been reported in other chronic pain disorders such as knee OA (Pujol et al., 2017) and migraine (Liu et al., 2015). Future studies directly comparing visual network rsFC changes across different chronic pain disorders are needed. Finally, we only measured the BDI for cLBP patients, as most of the healthy controls did not have a BDI score. Thus, we were not able to include the BDI score or other measures of emotional distress in data analysis. However, because the average BDI score in our study is quite low (with an average score of 6.12 ± 6.0), less than mild depression (score ≥ 14) as defined by the BDI manual, we believe it is unlikely that depressive symptoms may have confounded the group comparison. Future studies are needed to replicate our findings.
In summary, we found significant rsFC alteration of the visual networks in cLBP patients, and these visual networks allowed us to distinguish cLBP from healthy controls. Our findings suggest that functional connectivity alteration in chronic low back pain patients extends beyond the somatosensory, limbic, and salient domains and into visual sensory modalities. We speculate that these changes may represent an adaptation mechanism of the visual, somatosensory, motor, attention, salient and default mode networks to persistent low back pain. Elucidating the role of the visual networks in cLBP may shed light on the pathophysiology and development of the disorder.
Disclosure of potential conflicts of interest
J.K. has a disclosure to report (holding equity in a startup company (MNT) and pending patents to develop new neuromodulation tools) but declares no conflict of interest. All other authors declare no conflict of interest.
Research involving human participants and/or animals
The study was approved by the Institutional Review Board at Massachusetts General Hospital.
Informed consent
All participants signed consent forms.
Funding
Jian Kong is supported by P01AT006663, R01AT008563, R21AT008707, and R33AT009310 from USA NIH/NCCIH.
References
- Arbabshirani M.R., Plis S., Sui J., Calhoun V.D. Single subject prediction of brain disorders in neuroimaging: promises and pitfalls. Neuroimage. 2017;145:137–165. doi: 10.1016/j.neuroimage.2016.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Apkarian A.V. Nociception, pain, negative moods, and behavior selection. Neuron. 2015;87:474–491. doi: 10.1016/j.neuron.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli E., Aron A.R., Tandon N. Topography and timing of activity in right inferior frontal cortex and anterior insula for stopping movement. Hum. Brain Mapp. 2018;39:189–203. doi: 10.1002/hbm.23835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D., Neville H.J. Cross-modal plasticity: where and how? Nat. Rev. Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of Beck depression inventories-IA and –II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bekrater-Bodmann R., Foell J., Diers M., Kamping S., Rance M., Kirsch P., Trojan J., Fuchs X., Bach F., Cakmak H.K., Maass H., Flor H. The importance of synchrony and temporal order of visual and tactile input for illusory limb ownership experiences – an FMRI study applying virtual reality. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington A.L., Hanlon C.A., McKeown M.J. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain. 2005;113:172–184. doi: 10.1016/j.pain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Cao S., Qin B., Zhang Y., Yuan J., Fu B., Xie P., Song G., Li Y., Yu T. Herpes zoster chronification to postherpetic neuralgia induces brain activity and grey matter volume change. Am. J. Transl. Res. 2018;10:184–199. [PMC free article] [PubMed] [Google Scholar]
- Carwile J.L., Feldman S., Johnson N.R. Use of a simple visual distraction to reduce pain and anxiety in patients undergoing colposcopy. J. Low Genital. Tract Dis. 2014;18:317–321. doi: 10.1097/LGT.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chang C.-C., Lin C.-J. LIBSVM: a library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011;2:27. [Google Scholar]
- Chong C.D., Gaw N., Fu Y., Li J., Wu T., Schwedt T.J. Migraine classification using magnetic resonance imaging resting-state functional connectivity data. Cephalalgia. 2017;37:828–844. doi: 10.1177/0333102416652091. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc. Natl. Acad. Sci. U. S. A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbrich L., Alamia A., Blandiaux S., Burns S., Legrain V. Shaping visual space perception through bodily sensations: testing the impact of nociceptive stimuli on visual perception in peripersonal space with temporal order judgments. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P.T., Laird A.R., Lancaster J.L. Coordinate-based voxel-wise meta-analysis: dividends of spatial normalization. Report of a virtual workshop. Hum. Brain Mapp. 2005;25:1–5. doi: 10.1002/hbm.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho F., Frot M., Perchet C., Magnin M., Garcia-Larrea L. Pain influences hedonic assessment of visual inputs. Eur. J. Neurosci. 2008;27:2219–2228. doi: 10.1111/j.1460-9568.2008.06196.x. [DOI] [PubMed] [Google Scholar]
- Goossens N., Rummens S., Janssens L., Caeyenberghs K., Brumagne S. Association between sensorimotor impairments and functional brain changes in patients with low Back pain: a critical review. Am. J. Phys. Med. Rehabil. 2018;97:200–211. doi: 10.1097/PHM.0000000000000859. [DOI] [PubMed] [Google Scholar]
- Gu X., Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Gu Y., Miao S., Han J., Liang Z., Ouyang G., Yang J., Li X. Identifying ADHD children using hemodynamic responses during a working memory task measured by functional near-infrared spectroscopy. J. Neural Eng. 2018;15 doi: 10.1088/1741-2552/aa9ee9. [DOI] [PubMed] [Google Scholar]
- Haggard P., Iannetti G.D., Longo M.R. Spatial sensory organization and body representation in pain perception. Curr. Biol. 2013;23:R164–R176. doi: 10.1016/j.cub.2013.01.047. [DOI] [PubMed] [Google Scholar]
- Harvie D.S., Broecker M., Smith R.T., Meulders A., Madden V.J., Moseley G.L. Bogus visual feedback alters onset of movement-evoked pain in people with neck pain. Psychol. Sci. 2015;26:385–392. doi: 10.1177/0956797614563339. [DOI] [PubMed] [Google Scholar]
- Hashmi J.A., Baliki M.N., Huang L., Baria A.T., Torbey S., Hermann K.M., Schnitzer T.J., Apkarian A.V. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck M., Metzner S., Rohlffs F., Lorenz J., Engel A.K. The influence of music and music therapy on pain-induced neuronal oscillations measured by magnetencephalography. Pain. 2013;154:539–547. doi: 10.1016/j.pain.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Haxby J.V. Multivariate pattern analysis of fMRI: the early beginnings. Neuroimage. 2012;62:852–855. doi: 10.1016/j.neuroimage.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofle M., Hauck M., Engel A.K., Senkowski D. Viewing a needle pricking a hand that you perceive as yours enhances unpleasantness of pain. Pain. 2012;153:1074–1081. doi: 10.1016/j.pain.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Hoy D., March L., Brooks P., Woolf A., Blyth F., Vos T., Buchbinder R. Measuring the global burden of low back pain. Best Pract. Res. Clin. Rheumatol. 2010;24:155–165. doi: 10.1016/j.berh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Iannetti G.D., Mouraux A. From the neuromatrix to the pain matrix (and back) Exp. Brain Res. 2010;205:1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- James T.W., Culham J., Humphrey G.K., Milner A.D., Goodale M.A. Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain. 2003;126:2463–2475. doi: 10.1093/brain/awg248. [DOI] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L.G. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kawashima R., O'Sullivan B.T., Roland P.E. Positron-emission tomography studies of cross-modality inhibition in selective attentional tasks: closing the "mind's eye". Proc. Natl. Acad. Sci. U. S. A. 1995;92:5969–5972. doi: 10.1073/pnas.92.13.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Kaptchuk T.J., Webb J.M., Kong J.T., Sasaki Y., Polich G.R., Vangel M.G., Kwong K., Rosen B., Gollub R.L. Functional neuroanatomical investigation of vision-related acupuncture point specificity – a multisession fMRI study. Hum. Brain Mapp. 2009;30:38–46. doi: 10.1002/hbm.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Loggia M.L., Zyloney C., Tu P., Laviolette P., Gollub R.L. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148:257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Spaeth R.B., Wey H.Y., Cheetham A., Cook A.H., Jensen K., Tan Y., Liu H., Wang D., Loggia M.L., Napadow V., Smoller J.W., Wasan A.D., Gollub R.L. S1 is associated with chronic low back pain: a functional and structural MRI study. Mol. Pain. 2013;9:43. doi: 10.1186/1744-8069-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz D.J., Saleem K.S., Baker C.I., Ungerleider L.G., Mishkin M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn. Sci. 2013;17:26–49. doi: 10.1016/j.tics.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V., Iannetti G.D., Plaghki L., Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog. Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Letzen J.E., Robinson M.E. Negative mood influences default mode network functional connectivity in patients with chronic low back pain: implications for functional neuroimaging biomarkers. Pain. 2017;158:48–57. doi: 10.1097/j.pain.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wu J., Han H., Chui D. Complex Medical Engineering. CME; ICME International Conference on: 2009. The contribution of IPL (BA7/39) and ACC (BA24/33) in visual spatial voluntary attention. [Google Scholar]
- Liu J., Zhao L., Lei F., Zhang Y., Yuan K., Gong Q., Liang F., Tian J. Disrupted resting-state functional connectivity and its changing trend in migraine suffers. Hum. Brain Mapp. 2015;36:1892–1907. doi: 10.1002/hbm.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M.R., Betti V., Aglioti S.M., Haggard P. Visually induced analgesia: seeing the body reduces pain. J. Neurosci. 2009;29:12125–12130. doi: 10.1523/JNEUROSCI.3072-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sola M., Woo C.W., Pujol J., Deus J., Harrison B.J., Monfort J., Wager T.D. Towards a neurophysiological signature for fibromyalgia. Pain. 2017;158:34–47. doi: 10.1097/j.pain.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman S. Visual stimuli in distraction strategies for increasing pain tolerance. The confounding of affect with other stimulus characteristics. Pavlov. J. Biol. Sci. 1988;23:67–74. [PubMed] [Google Scholar]
- Mao C.P., Zhang Q.L., Bao F.X., Liao X., Yang X.L., Zhang M. Decreased activation of cingulo-frontal-parietal cognitive/attention network during an attention-demanding task in patients with chronic low back pain. Neuroradiology. 2014;56:903–912. doi: 10.1007/s00234-014-1391-6. [DOI] [PubMed] [Google Scholar]
- Margulies D.S., Vincent J.L., Kelly C., Lohmann G., Uddin L.Q., Biswal B.B., Villringer A., Castellanos F.X., Milham M.P., Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y., Kurata J., Sekiguchi M., Yoshida K., Nikaido T., Konno S.I. Attenuation of cortical activity triggering descending pain inhibition in chronic low back pain patients: a functional magnetic resonance imaging study. J. Anesth. 2017;31:523–530. doi: 10.1007/s00540-017-2343-1. [DOI] [PubMed] [Google Scholar]
- Melzack R. Pain and the neuromatrix in the brain. J. Dent. Educ. 2001;65:1378–1382. [PubMed] [Google Scholar]
- Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi raj lecture: presented at the third world congress of world Institute of Pain, Barcelona 2004. Pain Practice. 2005;5:85–94. doi: 10.1111/j.1533-2500.2005.05203.x. [DOI] [PubMed] [Google Scholar]
- Milner D., Goodale M. Oxford University Press; 2006. The Visual Brain in Action. [Google Scholar]
- Mok N.W., Brauer S.G., Hodges P.W. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine (Phila Pa 1976) 2004;29:E107–E112. doi: 10.1097/01.brs.0000115134.97854.c9. [DOI] [PubMed] [Google Scholar]
- Mouraux A., Diukova A., Lee M.C., Wise R.G., Iannetti G.D. A multisensory investigation of the functional significance of the "pain matrix". Neuroimage. 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Nassi J.J., Callaway E.M. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirhomme Q., Lesenfants D., Gomez F., Soddu A., Schrouff J., Garraux G., Luxen A., Phillips C., Laureys S. Biased binomial assessment of cross-validated estimation of classification accuracies illustrated in diagnosis predictions. Neuroimage Clin. 2014;4:687–694. doi: 10.1016/j.nicl.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlinner C., Yong M., Nasterlack M., Pluto R.P., Lang S. Combined effect of back pain and stress on work ability. Occup. Med. (Lond.) 2015;65:147–153. doi: 10.1093/occmed/kqu190. [DOI] [PubMed] [Google Scholar]
- Olague G., Dozal L., Clemente E., Ocampo A. EVOLVE-A Bridge between Probability, Set Oriented Numerics, and Evolutionary Computation III. Springer; 2014. Optimizing an artificial dorsal stream on purpose for visual attention; pp. 141–166. [Google Scholar]
- Perkins M., de Bruyne M., Giummarra M.J. A pain in the bud? Implications of cross-modal sensitivity for pain experience. Atten. Percept. Psychophys. 2016;78:2348–2356. doi: 10.3758/s13414-016-1217-1. [DOI] [PubMed] [Google Scholar]
- Peyron R., Laurent B., Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol. Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Pomper U., Hofle M., Hauck M., Kathmann N., Engel A.K., Senkowski D. Crossmodal bias of visual input on pain perception and pain-induced beta activity. Neuroimage. 2013;66:469–478. doi: 10.1016/j.neuroimage.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Pujol J., Macia D., Garcia-Fontanals A., Blanco-Hinojo L., Lopez-Sola M., Garcia-Blanco S., Poca-Dias V., Harrison B.J., Contreras-Rodriguez O., Monfort J., Garcia-Fructuoso F., Deus J. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain. 2014;155:1492–1503. doi: 10.1016/j.pain.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Pujol J., Martinez-Vilavella G., Llorente-Onaindia J., Harrison B.J., Lopez-Sola M., Lopez-Ruiz M., Blanco-Hinojo L., Benito P., Deus J., Monfort J. Brain imaging of pain sensitization in patients with knee osteoarthritis. Pain. 2017;158:1831–1838. doi: 10.1097/j.pain.0000000000000985. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Salavati M., Akhbari B., Takamjani I.E., Bagheri H., Ezzati K., Kahlaee A.H. Effect of spinal stabilization exercise on dynamic postural control and visual dependency in subjects with chronic non-specific low back pain. J. Bodyw. Mov. Ther. 2016;20:441–448. doi: 10.1016/j.jbmt.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D.A., Wideman T.H., Naso L., Hatami-Khoroushahi Z., Fallatah S., Ware M.A., Jarzem P., Bushnell M.C., Shir Y., Ouellet J.A., Stone L.S. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D., Hofle M., Engel A.K. Crossmodal shaping of pain: a multisensory approach to nociception. Trends Cogn. Sci. 2014;18:319–327. doi: 10.1016/j.tics.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Oostenveld R., Fries P., Engel A.K. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60:709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Smith M., Davis M.A., Stano M., Whedon J.M. Aging baby boomers and the rising cost of chronic back pain: secular trend analysis of longitudinal medical expenditures panel survey data for years 2000 to 2007. J. Manipulative Physiol. Ther. 2013;36:2–11. doi: 10.1016/j.jmpt.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C. Crossmodal correspondences: a tutorial review. Atten. Percept. Psychophysiol. 2011;73:971–995. doi: 10.3758/s13414-010-0073-7. [DOI] [PubMed] [Google Scholar]
- Ung H., Brown J.E., Johnson K.A., Younger J., Hush J., Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb. Cortex. 2014;24:1037–1044. doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon-Presseau E., Tetreault P., Petre B., Huang L., Berger S.E., Torbey S., Baria A.T., Mansour A.R., Hashmi J.A., Griffith J.W., Comasco E., Schnitzer T.J., Baliki M.N., Apkarian A.V. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139:1958–1970. doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.W., Kross E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan A.D., Loggia M.L., Chen L.Q., Napadow V., Kong J., Gollub R.L. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115:364–374. doi: 10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke M.W., Hart D.L. Categorizing patients with occupational low back pain by use of the Quebec task force classification system versus pain pattern classification procedures: discriminant and predictive validity. Phys. Ther. 2004;84:243–254. [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Xiaolian J., Xiaolin L., Lan Z.H. Effects of visual and audiovisual distraction on pain and anxiety among patients undergoing colonoscopy. Gastroenterol. Nurs. 2015;38:55–61. doi: 10.1097/SGA.0000000000000089. [DOI] [PubMed] [Google Scholar]
- Yang H., Haldeman S., Lu M.L., Baker D. Low Back pain prevalence and related workplace psychosocial risk factors: a study using data from the 2010 National Health Interview Survey. J. Manipulative Physiol. Ther. 2016;39:459–472. doi: 10.1016/j.jmpt.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Gollub R.L., Spaeth R., Napadow V., Wasan A., Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 2014;6:100–108. doi: 10.1016/j.nicl.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Zheng X., Lin Z., Tang W., Zhou C. Awareness Science and Technology and Ubi-Media Computing (iCAST-UMEDIA) 2013. A dorsal pathway guided visual attention model. International Joint Conference on. [Google Scholar]