Abstract
Background
Idiopathic cervical dystonia (CD) is a chronic movement disorder characterized by impressive clinical symptoms and the lack of clear pathological findings in clinical diagnostics and imaging. At present, the injection of botulinum toxin (BNT) in dystonic muscles is an effective therapy to control motor symptoms and pain in CD.
Objectives
We hypothesized that, although it is locally injected to dystonic muscles, BNT application leads to changes in brain and network activity towards normal brain function.
Methods
Using 3 T functional MR imaging along with advanced analysis techniques (functional connectivity, Granger causality, and regional homogeneity), we aimed to characterize brain activity in CD (17 CD patients vs. 17 controls) and to uncover the effects of BNT treatment (at 6 months).
Results
In CD, we observed an increased information flow within the basal ganglia, the thalamus, and the sensorimotor cortex. In parallel, some of these structures became less responsive to regulating inputs. Furthermore, our results suggested an altered somatosensory integration. Following BNT administration, we noted a shift towards normal brain function in the CD patients, especially within the motor cortex, the somatosensory cortex, and the basal ganglia.
Conclusion
The changes in brain function and network activity in CD can be interpreted as related to the underlying cause, the effort to compensate or a mixture of both. Although BNT is applied in the last stage of the cortico-neuromuscular pathway, brain patterns are shifted towards those of healthy controls.
Abbreviation list: BG, Basal ganglia; BNT, Botulinum toxin; CD, Cervical dystonia; EPI, Echo-planar imaging; FC, Functional connectivity; fMRI, Functional magnetic resonance imaging; FWE, Family wise error; GC, Granger causality; GA, Granger autonomy; GLM, General linear model; KCC, Kendall's coefficient of concordance; ReHo, Regional homogeneity; rs-fMRI, Resting-state fMRI; ROI, Region of interest; S1, Primary somatosensory cortex; S2, Secondary somatosensory cortex; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale
Keywords: Cervical dystonia, Sensorimotor integration, Basal ganglia, Thalamus, Botulinum toxin (BNT)
Highlights
-
•
we characterized brain activity in CD and the effects of BNT using 3T fMR imaging and network analysis techniques
-
•
following treatment with botulinum toxin (BNT), abnormal brain activity patterns in primary dystonia are attenuated
-
•
critical key regions for both the pathophysiology and BNT-induced improvement in cervical dystonia are the BG
1. Introduction
Dystonia is a common movement disorder characterized by involuntary muscle contractions that lead to abnormal repetitive movements or postures (Albanese et al., 2013). A large variety of clinical syndromes can be roughly divided into primary and secondary forms based on their etiology. Primary dystonia occurs in the absence of an associated disease. Secondary dystonia is defined by a known acquired etiology, such as brain injury or medication. Dystonia is also characterized based on clinical features, including a wide spectrum of segmental dystonia forms. The prevalence of primary dystonia is approximately 16.42 per 100,000 people (Steeves et al., 2012). Cervical dystonia (CD), also called spasmodic torticollis, is the most frequent type of primary dystonia and has prevalence of 4.98 / 100,000 people in Europe (Phukan et al., 2011; Steeves et al., 2012). The clinical characteristics of dystonia are often described based on 5 factors: age at onset, body distribution, temporal pattern, coexistence of other movement disorders, and other neurological manifestations (Albanese et al., 2013). CD, together with blepharospasms and writer's cramp, are classified as focal dystonias. Although CD is perceived as a motor disorder, neurophysiological observations have identified sensory dysfunctions, i.e., dysfunction in spatial discrimination (Molloy et al., 2003). The geste antagoniste (sensory ticks) is often observed in patients with CD (84%) and indicates an active contribution of sensory afferent inputs to the manifestation of focal dystonia (Martino et al., 2010). Significant pain is also commonly associated with CD (LeDoux, 2012).
The clinical symptomatology of CD is well defined, but its pathophysiology remains unknown. Because various genes are involved and patients exhibit variable sensitivity to pharmaceuticals, heterogeneous pathogenic effects (Carbon and Eidelberg, 2009; Eidelberg et al., 1998) are indicated.
Traditionally, dystonia was regarded as a disease of the basal ganglia (BG) (Breakefield et al., 2008; M. Hallett, 2006). Clinical associations between dystonic features and other movement disorders have also suggested the involvement of the BG (Louis et al., 1999; Tolosa and Compta, 2006). In the standard model of the functional connections between basal ganglia nuclei, the thalamus, and the cortex, a direct pathway mainly facilitates activity in the motor cortex and the indirect pathway inhibits movements (Albin et al., 1989; DeLong, 1990; Gerfen et al., 1990). The center-surround model of basal ganglia function extends this model and consequently allows its application to hypo- and hyperkinetic movement disorders by introducing the concept of a parallel enhancement of the desired movements and inhibition of unwanted movements (Mink, 1996). Although this model is incomplete, it is suitable for approximating the pathophysiology of CD (Gale et al., 2008). Fig. 1 provides a schematic overview of the known connections and loops relevant to basal ganglia motor function. Furthermore, neuropsychological studies have reported that sensory systems are involved in CD by demonstrating abnormal sensory processing (Molloy et al., 2003; Tinazzi et al., 2009; Bara-Jimenez et al., 2000).
Fig. 1.
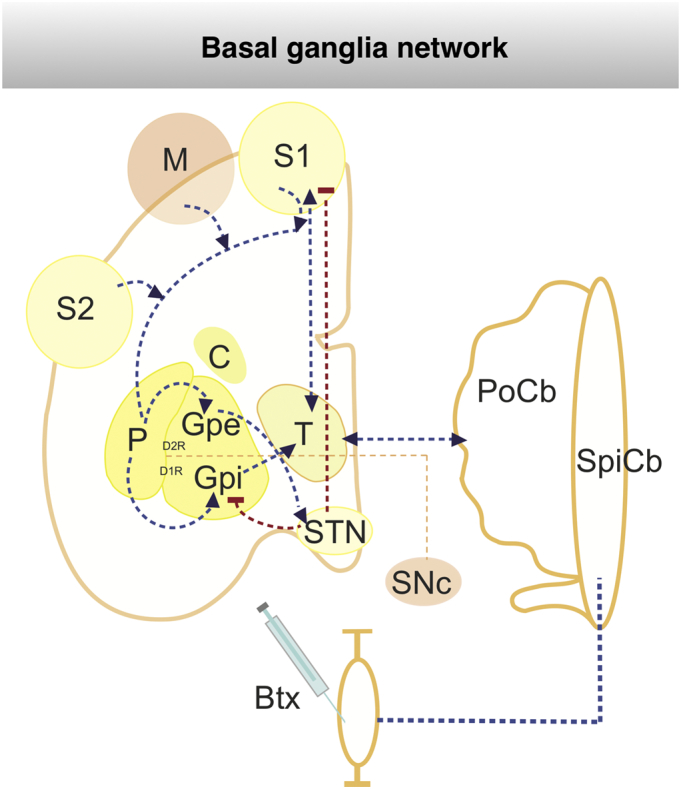
Schematic representation of botox treatment (BNT) in basalganglia network.
The well characterized basal-ganglia loops, thalamo-cortical and cerebellar-thalamic loops are illustrated. In particular the main routes of the striato-fugal connections are displayed: the direct pathway with D2R-expressing striatal MSNs innervating the GPe and the indirect pathway with D1R-expressing striatal MSNs projecting to the GPi and STN. Abbreviations: Btx - Botoliumtoxin, C - caudate ncl, D1R – dopamin receptor 1, D2R – dopamine receptor 2, GPi - internal pallidum, Gpe - external pallidum, M - primary cortex, MSN - medium spiny neurons, STN - subthalamic ncl, PoCb - pontocerebellum, P - putamen, S1/2 - primary and secondary somatosensory cortex, SNC - substantia nigra pars compacta, SpCB - spinocerebellum, T - thalamus, dashed red lines: mainly inhibition connection, dashed blue lines: activating connection, dashed orange lines: dopaminergic innervation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A growing number of imaging studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have aimed to explore the underlying cerebral changes in patients with CD. The authors have shown widespread changes in brain activity and malfunctions in the brainstem, cortical and subcortical structures (Breakefield et al., 2008; Carbon and Eidelberg, 2009; Neychev et al., 2011). Several lines of electrophysical evidence support the hypothesis that a loss of inhibition occurs in several parts of the motor and sensory systems (Tamura et al., 2008; Antelmi et al., 2017; Meunier et al., 2001; Hallett, 2006). Recently, a fMRI study revealed a loss of inhibition and resulting brain overactivation in multiple brain areas (i.e., the cingulate cortex and primary and secondary somatosensory cortices) following kinesthetic sensory stimulation in patients with CD (Obermann et al., 2010). Currently, CD is regarded as a cerebral network disease (Delnooz et al., 2013; Jinnah and Hess, 2006; Jinnah et al., 2017; Poston and Eidelberg, 2012) with a brain-wide loss of inhibition and increased neuroplasticity (Hallett, 2011).
Currently, botulinum neurotoxin (BNT) has been shown to be an effective treatment for the motor and non-motor symptoms of CD (Tsui et al., 1986; Jankovic, 2004; Contarino et al., 2017). A recent Cochrane review analyzed data from 8 RCTs (double-blind, parallel, randomized, placebo-controlled trials) that investigated the application of BNT in patients with CD (Castelão et al., 2017). The BNT treatment reduced the CD severity, disability, and pain; compared to the placebo, its application is well tolerated, and typical adverse events include dysphagia and diffuse weakness. In principle, therapy with BNT exerts inhibitory actions on muscle contraction by blocking acetylcholine-mediated transmission at the neuromuscular junction (Hughes and Whaler, 1962), leading to relaxation of the affected muscle. Based on considerable evidence, BNT may also influence the central nervous system at the spinal and cerebral levels (Gracies, 2004; Hallett, 2018). Consequently, the treatment of dystonic muscles with botulinum toxin (BNT) may cause changes at multiple levels of the sensorimotor system, from transmission at the neuromuscular junction to the cerebral cortex (Abbruzzese and Berardelli, 2003; Kanovský et al., 1998; Walsh and Hutchinson, 2007).
Although key regions that are potentially associated with CD pathophysiology have been identified, further studies are needed to understand how their deviation from normal physiological brain function and communication should be classified. We had two main objectives in the present study. We aimed to do the following: (1) identify which brain regions are involved in CD and how changes in these regions lead to abnormal brain network function, and eventually (2) evaluate the effect of a BNT treatment. Here, we hypothesized that the treatment of patients with CD with BNT would also affect patterns of brain activity and partially restore normal brain function by decreasing the output of the motor cortex towards the affected muscles. Thus, changes in brain function and network activity in patients with CD are potentially related to the underlying cause, to compensatory efforts or, most likely, to a combination of the two; one might argue that BNT treatment possibly helps to unmask causes from effects on patients with CD. Brain function was assessed using 3.0 T functional magnetic resonance imaging (fMRI). We applied functional and effective connectivity analyses to quantify changes in brain and network activity in patients with CD and the effect of the BNT treatment. We employed a regional homogeneity analysis to measure the degree of synchronicity of brain activity in specific structures (Zang et al., 2004).
2. Methods
2.1. Subjects and treatment with BNT
The study was approved by the local ethics committee, and all subjects gave their written informed consent according to the Declaration of Helsinki. The study is registered within the German Clinical Trials Register (DRKS0003696).
We studied 17 patients with a clinically confirmed diagnosis of primary CD (9 females, age 61.3 ± 8.3 years) (initially we investigated 19 patients, 2 were excluded due to severe head motion artifacts in the MR scans). All patients had pure neck involvement (10 left- and 7 right-sided torticollis deviation, disease duration 11.6 ± 5.15 years) and were naive to treatment with BNT at the time of the first fMRI. The severity of CD was assessed with the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS). After 6 months of treatment of the dystonic neck muscles with BNT-A (in total 3 applications), a second fMRI examination followed by a clinical evaluation took place. Supplemental Fig. S4 provides a schematic overview of the timelines of BNT application and MRI acquisition.
Healthy age- and sex-matched controls (N = 17, 8 females, age 63.5 ± 10.5 years) were given a routine examination by an expert neurologist, which revealed the absence of any present clinical manifestations of neurologic or psychiatric diseases. The demographic and clinical data are shown in Table 1, Table 2.
Table 1.
Study participants: controls and patients with CD.
| controls |
Patients with CD |
||||||
|---|---|---|---|---|---|---|---|
| Age (yrs)/gender | Handedness | MMSE | Age (yrs)/gender | Handedness | MMSE | Disease duration (yrs) | |
| 1 | 34/M | R | 30 | 54/F | L | 30 | 3 |
| 2 | 55/M | R | 30 | 48/M | L | 30 | 18 |
| 3 | 56/M | R | 30 | 47/M | R | 30 | 13 |
| 4 | 56/M | R | 30 | 68/F | R | 29 | 21 |
| 5 | 58/F | R | 30 | 65/M | R | 30 | 9 |
| 6 | 61/F | L | 30 | 68/F | R | 29 | 6 |
| 7 | 61/M | R | 30 | 67/F | R | 28 | 11 |
| 8 | 63/F | R | 30 | 67/F | R | 30 | 16 |
| 9 | 63/M | L | 30 | 66/F | R | 30 | 7 |
| 10 | 68/F | R | 30 | 60/M | R | 30 | 15 |
| 11 | 70/F | R | 30 | 70/F | R | 28 | 17 |
| 12 | 70/F | R | 30 | 62/M | R | 30 | 12 |
| 13 | 71/F | R | 30 | 45/M | R | 30 | 8 |
| 14 | 71/M | R | 28 | 70/M | R | 30 | 9 |
| 15 | 73/M | R | 29 | 55/F | R | 29 | 4 |
| 16 | 74/F | R | 30 | 66/M | R | 30 | 13 |
| 17 | 75/M | R | 30 | 64/F | R | 30 | 16 |
| Mean ± std | 63.5 ± 10.5, 8 F/9 M | 15 R/2 L | 61.3 ± 8.3,9 F/8 M | 15 R/2 L | 11.6 ± 5.15 | ||
Seventeen patients with cervical dystonia (CD, mean age ± SD = 61.3 ± 10.5 years; nine females) and 17 age-matched controls (mean age ± SD = 63.5 ± 10.5 years; 8 female) participated in this study. MMSE: Mini-Mental Statu Examination.
Table 2.
Patients with CD - disease duration and clinical degree of severity before and after BNT application.
| Age (yrs)/gender | Disease duration (yrs) | TWSTRS (naive) | TWSTRS (BNT) | Last treatment/duration | |
|---|---|---|---|---|---|
| 1 | 45/M | 8 | 27 | 16 | 100 Xeomin/6 months |
| 2 | 47/M | 13 | 21 | 15 | 100 Xeomin/6 months |
| 3 | 48/M | 18 | 17 | 9 | 105 Botox/6 months |
| 4 | 54/F | 3 | 23 | 16 | 150 Xeomin/6 months |
| 5 | 55/F | 4 | 22 | 7 | 100 Xeomin/6 months |
| 6 | 60/M | 15 | 20 | 14 | 300 Dysport/6 months |
| 7 | 62/M | 12 | 20 | 10 | 80 Xeomin/6 months |
| 8 | 64/F | 16 | 18 | 13 | 150 Xeomin/6 months |
| 9 | 65/M | 9 | 22 | 7 | 150 Xeomin/6 months |
| 10 | 66/F | 7 | 23 | 16 | 100 Botox/6 months |
| 11 | 66/M | 13 | 22 | 15 | 120 Xeomin/6 months |
| 12 | 67/F | 11 | 17 | 10 | 80 Botox/6 months |
| 13 | 67/F | 16 | 21 | 13 | 100 Xeomin/6 months |
| 14 | 68/F | 21 | 28 | 20 | 105 Xeomin/6 months |
| 15 | 68/F | 6 | 39 | 27 | 100 Botox/6 months |
| 16 | 70/F | 17 | 26 | 18 | 300 Dysport/6 months |
| 17 | 70/M | 9 | 28 | 11 | 150 Xeomin/6 months |
| Mean ± std | 61.3 ± 8.3, 9F/8 M | 11.6 ± 5.15 | 23 ± 5.3 | 14 ± 5.0 |
All patients with CD underwent treatment with BNT (6 months, 3 total applications), and clinical severity was measured using the TWSTRS (Toronto Western Spasmodic Torticollis Rating Scale). The TWSTRS score decreased from mean of 23 to a mean of 14 after the administration of BNT. The nonparametric signed-rank test (Wilcoxon) revealed a significant group difference at p ≤ 0.001 (Z = −3.633).
2.1.1. Functional magnetic resonance imaging recordings
All experiments were performed on a 3 T MR scanner (Trio, Siemens, Erlangen, Germany) to obtain echo-planar T2*-weighted image volumes (EPI) and transaxial T1-weighted structural images. The EPI images (voxel size = 3 mm × 3 mm × 3 mm; repetition time = 2.52 s; TE = 35 ms; 40 transaxial slices; 740 images in total) covered the entire cerebrum and cerebellum. Functional data were acquired in two EPI sessions with 203 (resting-state fMRI) and 403 (event design with stimulation of the right hand) images. The high-resolution T1-weighted structural images had a voxel size of 1 mm × 1 mm × 1 mm to allow precise anatomical localization.
2.1.2. Resting-state fMRI (rs-fMRI)
For the recording of the rs-fMRI, participants were instructed to lie still and keep their eyes closed for the whole recording (203 images, approx. 8.5 min).
2.1.3. Event design fMRI
While lying in the fMR scanner, subjects were stimulated by an air-driven pneumatic device (air-puff). Stimuli were applied to the index finger of the right and left hand in a pseudorandomized order (403 images, approximately 17 min, stimuli duration 2 s, interstimulus interval ranged between 20 and 30 s, 20 stimuli to the left and 20 to the right hand separately). A similar fMRI paradigm was employed in our previous studies (Brodoehl et al., 2013; Brodoehl et al., 2015a; Klingner et al., 2016).
2.2. Data analysis
Data analysis was performed using MATLAB 2015b (Mathworks, Natick, MA) and SPM12 software (Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm). Standard preprocessing included rejection of the first 3 images, realignment (Kiebel et al., 1997) and normalization to the Montreal Neurological Institute (MNI) standard brain (Evans et al., n.d.), and smoothing with a 6-mm full-width-at-half-maximum Gaussian kernel. For temporal filtering and denoising of rs-fMRI data, we employed the methods provided in the REST toolbox for MATLAB (Song et al., 2011).
2.3. Motion artifacts
Involuntary head movements are a key feature of patients with CD. Therefore, the mounting apparatus in the MR scanner included a firm foam padding around the head and restraining straps across the chin. We calculated the maximum motion using the first three parameters of the rigid body correction to quantify the head motion in the MRI data: maximum movement = in mm). A detailed description of the measurement of head motion and its influence on rs-fMRI data is provided in the study by Van Dijk et al. (2012). In recent studies, relative movements of > 0.1 mm are considered micro movements and > 0.55 mm as gross movements (Satterthwaite et al., 2012; Van et al. 2012). In our study, we counted the micro movements, and subjects with interscan movements > 0.4 mm were excluded (we excluded 2 patients with CD prior to data analysis according to this criterion, and these subjects are not included in the present study). Supplementary Figs. 1 and 2 show the results of the head motion analysis.
2.4. Defining regions of interest (ROI)
As shown in Fig. 1, pathophysiology and recent literature suggest the involvement of the somatosensory and motor cortices, the basal ganglia, and the thalamus in the manifestation of CD. Accordingly, we defined the following ROIs: primary motor cortex (M1), primary and secondary somatosensory cortices (S1/S2), thalamus, subthalamic nucleus, putamen, pallidum, and caudate nucleus. The selected brain regions and representative MNI coordinates are shown in Table 3.
Table 3.
Definition of regions of interest (ROIs).
| Size in voxels |
XYZ (MNI coordinates) |
||
|---|---|---|---|
| Left | Right | ||
| M1 (primary motor cortex) | 324 | −40 −29 59 | 36 25 56 |
| S1 (primary somatosensory cortex) | 486 | −52 −29 56 | 44 –29 56 |
| S2 (secondary somatosensory cortex) | 162 | −41 20 13 | 47 –23 14 |
| Putamen | 485 | −24 4 4 | 26 3 4 |
| Pallidum | 162 | −20 −6 −1 | 15 –4 −1 |
| Caudate ncl. | 385 | −16 4 14 | 13 5 14 |
| Thalamus | 324 | −16 −23 −4 | 14 −21 −4 |
| Subthalamic ncl. | 38 | −3 −20 −12 | 8 −18 −12 |
| Occipital cortex | 500 | −24 −94 8 | 32 −91 8 |
We included the primary and secondary somatosensory cortex (S1 and S2) and the primary motor cortex (M1) along with the BG and the thalamus. The table provides the final size of each ROI in voxels within the coregistered and normalized structural T1 image. MNI coordinates are given for the left and right hemispheres of each ROI.
We applied two different strategies for the definition of the regions of interest (ROIs) for the following analysis. First, we manually defined the BG and the thalamus in each individual coregistered T1 image and stored the ROIs in NIFI-file format. Then, the ROIs were normalized using the transformation matrix of the normalized T1 image, and a mean ROI was created by averaging all individually mapped ROI images (thresholded above 0.5). For the definition of the somatosensory and the motor cortex, we performed a general linear model (GLM) analysis of the event design fMRI images, as implemented in SPM software. Second-level results were thresholded and corrected for multiple comparisons at P ≤ 0.01 FWE. Significantly activated regions within the left and right hemispheres were assigned according to the SPM toolbox Anatomy (Eickhoff et al., 2005). Supplementary Fig. 3 shows the brain regions that were activated by the stimulation of the left hand.
The occipital cortex was included for the regional homogeneity analysis and was composed of 500 randomly chosen voxels of V1-3 from the anatomy toolbox. We chose the occipital cortex as the area for validation because, to date, there is no valid evidence that the occipital region is critical for CD pathophysiology.
2.5. Connectivity and regional homogeneity (ReHo) analysis
Several sources of variance were removed from the data using linear regression: (1) six parameters obtained by rigid body correction of head motion, (2) signal from a ventricular region of interest, (3) signal from a region centered in the white matter, and (4) mean global signal of the whole brain. All signal intensity time courses were bandpass filtered (0.01 < f < 0.1 Hz) to reduce the effects of low-frequency drift and high-frequency noise.
Within each region, each voxels' time series were extracted. For the functional and effective connectivity analysis, average time courses for each ROI were estimated by averaging the time series of all voxels within an ROI.
2.5.1. Functional connectivity
Changes in the functional connectivity between ROI-specific time series were measured by calculating Pearson correlation coefficients. R-values were transformed to z-values by the Fisher r-z transformation. Findings were considered significant at p ≤ 0.05 (2-sample t-test for independent or dependent samples, corrected for multiple comparisons using a Bonferroni/FWE-correction).
2.5.2. Effective connectivity
Conditional Granger causality analysis is an approach used to explore the dynamic causal relationships between time series (Granger, 1969) and has been used in several fMRI studies (Goebel et al., 2003; Roebroeck et al., 2005; Valdes-Sosa et al., 2011). In this study, Conditional Granger causality was performed using the toolbox implemented by Seth (Seth, 2010). Findings were considered significant at p ≤ 0.01 (2-sample t-test for independent or rather dependent samples, corrected for multiple comparisons using a Bonferroni/FWE-correction). A more detailed description of functional and effective connectivity analyses is given in our previous work (Brodoehl et al., 2015b)
2.5.3. Regional homogeneity (ReHo)
To evaluate the synchronicity within a brain region, we applied a voxel-based measure (Kendall's coefficient of concordance, KCC) to quantify the similarity of a voxel and its 26 surrounding neighbors in a time series. KCC values can range from 0 to 1, where higher values indicate a greater similarity between the activation pattern of a specific voxel and its surrounding neighbors (Zang et al., 2004). For each brain region, KCC values were averaged and compared between groups of healthy and CD subjects. Findings were considered significant at p ≤ 0.01 (2-sample t-test for independent or dependent samples, corrected for multiple comparisons using a Bonferroni correction).
3. Results
First, we examined changes in the local brain and network activity in patients with CD prior to treatment with BNT in comparison to brain function in healthy controls.
3.1. Changes of local brain and network activity in cervical dystonia
3.1.1. Functional connectivity
To detect changes in the sensorimotor and basal ganglia network, we performed functional connectivity analysis between members of this network as well as substructures of the basal ganglia and the thalamus. For a more detailed description of these seed regions see Table 3.
Functional connectivity can quantify the connections between these brain regions based on time-coursed correlations, because normal brain activity is realized by a group of synchronized neurons (Varela et al., 2001).
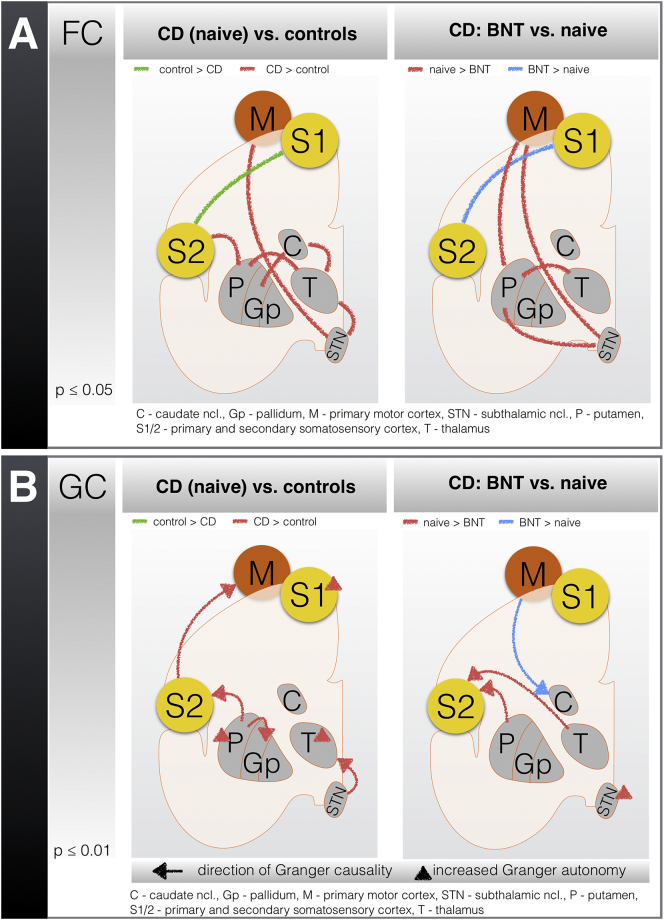
Compared to CD, in controls (Fig. 2, left / green; Supplementary Table 1), we found increased communication within the primary (S1) and secondary (S2) somatosensory cortex. In contrast, the CD group (Fig. 2, left / red; Supplementary Table 1) yielded increased information flow within the basal ganglia and between the basal ganglia and the thalamus, as well as between the somatosensory cortex and the motor cortex. The thalamus was highly connected to the putamen, the caudate body, and the subthalamic nucleus.
Fig. 2.
Results from the functional (A) and effective (B) connectivity analyses of patients with cervical dystonia (CD) and the effects of treatment with botulinum toxin (BNT).
Schematic representation of (A) functional connectivity (FC) and (B) Granger causality (GC) in resting-state fMRI from (left panels) the controls compared with the patients with CD (naive to treatment with BNT) and (right panels) the patients with CD who were treated without (naive) and with BNT (6 months). The results for the comparisons between patients with CD and controls (independent samples) and between patients with CD before and after BNT treatment (dependent samples) were corrected for multiple comparisons using a Bonferroni/FWE correction. In the figure, the lines indicate increased connectivity between specific brain regions; in GC, the direction of the arrow indicates the direction of the effective connectivity. A / left panel: in controls, FC between S1/S2 is increased; in patients with CD, increased connectivity within the BG, the thalamus, the motor cortex, and S2 is observed. A / right panel: the connectivity between S1/S2 is increased in patients with CD following BNT treatment; before the BNT application, the FC between the putamen and the thalamus, the STN, and the motor cortex is increased. B / left panel: in patients with CD, increased coupling of the putamen towards the pallidum and S2, S2 towards the motor cortex, and STN towards the thalamus is observed; increased autonomy in the pallidum and the thalamus is also detected. B / right panel: increased coupling of the motor cortex towards the caudate nucleus is observed in patients with CD following the BNT application; before the BNT application, increased GC exists between the putamen and the thalamus towards S2 and increased autonomy of the STN.
3.1.2. Identifying changed network dynamics with predictive causality network analysis–Granger causality
To further identify the driving force behind the above mentioned changes in the information flow within the somatosensory and basal ganglia network, we applied Granger causality analysis. While Granger causality can reveal the direction of the information transfer within a time series, a rising Granger autonomy is a measure to uncover increasing independence of a time series from other sources (Seth, 2010).
Compared to the CD patients, there was no significant increased directed information flow within the sensorimotor network in the control group (Fig. 2, left/green; Supplementary Table 1). Although in CD, effective connectivity as measured by Granger causality was increased in all subunits of the sensorimotor network (Fig. 2, left/red; Supplementary Table 1). We discovered most changes within the putamen which directly influenced the information flow within the pallidum and S2. Additionally, the putamen was stripped of other influencing and controlling sources as indicated by the increase in Granger autonomy. Increases in the Granger autonomy were also found within S1 and the thalamus. Furthermore, the effective connectivity was increased from S2 towards the primary motor cortex and from the subthalamic nucleus towards the thalamus.
In summary, in the CD patients, the putamen, the thalamus, and S1 became less modifiable and more independent from other brain regions. In particular, the putamen increasingly influenced information processing within the whole sensorimotor network.
3.1.3. Regional homogeneity (ReHo)
A method to evaluate the synchronicity within brain regions is regional homogeneity (ReHo)(Zang et al., 2004). By calculating Kendall's coefficient of concordance (KCC) of each voxel and its nearest neighbors within a specific brain region, an index of signal similarity is achieved. As stated by Jiang et al. (2015), the ReHo across large brain areas might differ largely. We therefore used the ROIs as described in Table 3 and limited the voxel count to a maximum of 500 voxels (Jiang et al., 2015).
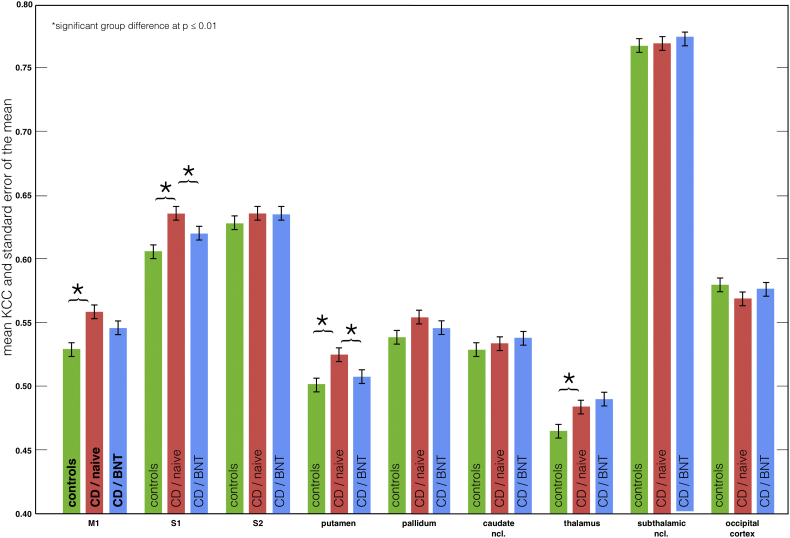
The average KCC was significantly increased in the CD patients within the primary motor cortex, S1, the putamen and the thalamus (independent sample t-tests at p ≤ 0.01) (Fig. 3, green vs. red; Supplemental Table 3). No significant increase in KCC was found in the healthy controls.
Fig. 3.
Regional homogeneity analysis in cervical dystonia (CD) and effects of treatment with botulinum toxin (BNT).
Kendall's coefficient of concordance (KCC) and standard error of the mean of the defined ROIs in the primary motor cortex (M1), the primary and secondary somatosensory cortex (S1 / S2), the BG, and the thalamus in the controls and the CD patients without (naive) and with BNT (6 month) treatment. In CD (naive) KCC values were significantly increased (p ≤ 0.01, 2-samples t-test: red vs. green) in M1, S1, putamen, and thalamus (compared to healthy controls). With BNT treatment KCC values in CD were again significantly decreased (p ≤ 0.01, paired t-test: blue vs. red) in S1 and putamen. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Effect of BNT treatment on the altered brain and network activity in CD
All patients with CD were treated three times with BNT-A at months 0, 3, and 6. Two weeks after the last BNT injection, the second fMRI examination was performed (please refer to Fig. S4 for more details). According to a decrease in the TWSTRS score, all CD patients experienced an improvement of dystonic symptoms at the time point of the second fMRI (day 0 mean TWSTRS score was 23 ± 5.3; day 180 mean TWSTRS score was 14 ± 5.0) (clinical details in Table 2).
Based on the aforementioned changes in brain activity, we compared the functional and effective connectivity, as well as the ReHo, in the CD patients before and after BNT treatment (2-sample t-test for dependent samples). No correlations were found between connectivity changes and the effect of clinical treatment (TWSTRS scores).
After correction for multiple comparisons, there were no significant connectivity changes in the group comparison at both time points (control vs. CD (naive to treatment) and control vs. CD (following BNT treatment)).
3.2.1. Functional connectivity (FC)
FC analysis revealed two main effects of BNT treatment in CD. Following BNT treatment, the communication between S1 and S2 increased (Fig. 2, right/blue; Supplementary Table 2). Furthermore, connectivity within the basal ganglia and between the basal ganglia, the thalamus and the sensorimotor cortex decreased (Fig. 2, right/red; Supplementary Table 2).
Comparing the changes on both sides of Fig. 1, multiple matches can be found. This indicates a partial convergence of connectivity patterns between the controls and the BNT-treated CD patients.
3.2.2. Identifying changed network dynamics with predictive causality network analysis – Granger causality
After BNT treatment, several changes in the effective connectivity between the sensorimotor network and the basal ganglia were observed (Fig. 2, right; Supplementary Table 2). First, the influence of the thalamus and the putamen on the somatosensory cortex was reduced. Second, the input to the striatum via the motor cortex increased. Finally, the autonomy of the subthalamic nucleus decreased.
3.2.3. Regional homogeneity (ReHo)
There were 4 brain regions with increased KCC values in CD: the primary motor cortex, S1, the putamen and the thalamus. Treatment with BNT reduced KCC values in the putamen and S1 (Fig. 3; Supplementary Table 3). However, there were no significant changes in the thalamus and the motor cortex.
For all group comparisons (FC, GC, and ReHo), we performed a two-way ANOVA with disease duration and severity (TWSTRS) as independent factors; no significant interactions were identified.
4. Discussion
In the current study, we identified two striking distinctive features in CD. First, there was increased connectivity within the basal ganglia and between the basal ganglia, the thalamus and the sensorimotor cortex. The most connections were derived from the putamen and the thalamus. Some of these key regions, namely, the putamen, the thalamus and the somatosensory cortex, became less responsive to other potential regulating inputs. Second, our data suggested an impaired integration of higher somatosensory functions in CD.
Our data support that CD is a functional network disease (Breakefield et al., 2008). A deficient integration between the basal ganglia (i.e., putamen) and somatosensory cortex seemed to play a pivotal role in sustaining abnormal brain activity in CD.
With clinical improvement after BNT treatment, some of these changes in brain activity declined. Connectivity originating from the BG and especially from the putamen decreased, and communication within the somatosensory cortex improved. In the following subsections, these key findings will be discussed in more detail.
4.1. Basal ganglia function
In the basal ganglia, different parallel information pathways exist. These pathways include several common input (i.e., the striatum) and output (i.e., the globus pallidus internus (GPi) and thalamus) structures, as well as exclusive structures such as the subthalamic nucleus (STN) and the globus pallidus externus (GPE) (Albin et al., 1989; DeLong, 1990). Moreover, there are both excitatory (mainly glutamatergic) and inhibitory (mainly GABAergic) connections (Kreitzer and Malenka, 2008; Nelson and Kreitzer, 2014; Utter and Basso, 2008). Dopaminergic input from the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA), for instance, can result in both inhibition and excitation of striatal projection neurons (so-called medium spiny neurons within the striatum) (Gerfen et al., 1990; Schultz, 2002, Schultz, 2007). Accordingly, high and low dopamine levels in the striatum can cause dystonic symptoms (Breakefield et al., 2008; Wagner et al., 1996). Another main glutamatergic excitatory input to the BG via the striatum comes from cortical regions (Kemp and Powell, 1970, Kemp and Powell, 1971) and the thalamus (Smith et al., 1998; Smith et al., 2009). Depending on the inhibitory (GABAergic) input from the GPi, the overall output of the thalamus towards the cortex (especially the supplementary motor cortex) can facilitate movements (as part of the so-called direct pathway) or inhibit movements (part of the indirect pathway) (Gerfen et al., 1990; Utter and Basso, 2008). In regard to the increased motor activity that is pathognomonic for CD, the observed increase in connectivity of the basal ganglia in our study is most likely associated with an increased involvement of the direct striatal pathway. Our findings are supported by increased striatal D1 receptors in dystonia, which might be an underlying cause of the increased excitatory striatal output (Simonyan et al., 2017). The increased connectivity between the motor cortex and the STN, as observed in our study, might reflect a hyperdirect cortical-subcortical input to activate the indirect striatal pathway and achieve a compensatory thalamic inhibition (Jahanshahi et al., 2015; Obermann et al., 2008).
4.2. Loss of inhibition
We provide further evidence that the putamen, as the main input structure of the basal ganglia, was increasingly connected to cortical areas, the thalamus and to other basal ganglia structures in CD (Kreitzer and Malenka, 2008; Lanciego et al., 2012). We assume that this increased connectivity was associated with the flawed inhibitory control and increase in excitatory influences on the basal ganglia and cortical level (Blood et al., 2004; Peller et al., 2006). While there is increased information flow from the putamen towards the main output structure (thalamus), our analysis showed that both regions became less responsive to regulating influences in CD. In particular, the putaminal input from the cortex and the substantia nigra is less likely to be integrated. For the thalamus, regulating corticothalamic connections as well as the influence of the indirect striatal-thalamic pathway are diminished (Kreitzer and Malenka, 2008). For CD, different manifestations of loss of inhibition, ranging from short to long intracortical inhibitory dysfunctions, have been described (M. Hallett, 2006; Schicatano et al., 1997; Sohn and Hallett, 2004). The loss of inhibition affected motor and sensory functions as well as brain stem reflexes (Breakefield et al., 2008; Mink, 1996; Sohn and Hallett, 2004). Our study extended this knowledge and indicated that by diminishing the regulatory and inhibitory basal ganglia output to the motor cortex, the cortical activation of desired and undesired movements became blurred in CD (Mink, 1996; Sohn and Hallett, 2004). This was also supported by our findings of increased synchronicity within the sensorimotor cortex, suggesting that local inhibition was disrupted.
Analysis of the regional homogeneity of brain activity has been previously used to analyze brain function (Li et al., 2017; Ni et al., 2017). While higher degrees of synchronicity can be associated with task-specific brain activity and better performance in healthy controls (Haag et al., 2015; Zang et al., 2004), variations in cortical ReHo can also be discussed as a deficit in intracortical inhibition (Li et al., 2017). An indisputable argument for the latter is that intracortical inhibition is also energy consuming and therefore linked to fluctuations of the BOLD signal (Kelly and McCulloch, 1983).
4.3. Effects of BNT
BNT has been shown to improve muscle function and pain in dystonia without any sign of muscular weakness (Cohen et al., 1989; Tsui et al., 1986). However, its effect is not restricted to the block of the release of acetylcholine (Hughes and Whaler, 1962). BNT can also affect both alpha and gamma motor neurons, selectively weaken highly active muscles, and affect muscle spindles, thereby indirectly reducing the excitability of motoneurons (Filippi et al., 1993; Trompetto et al., 2006). BNT exhibits, to some degree, a retrograde transport into the central nervous system, at least to the level of Renshaw cells (Restani et al., 2011). Although transcranial magnetic stimulation suggested that BNT may affect the brain directly (Byrnes et al., 1998), it is most likely that the central effects of BNT are explained by cortical plasticity following an altered cortical representation of the affected muscles and limbs (Byrnes et al., 1998; Gilio et al., 2000; M. Hallett, 2001; Hallett, 2018).
As our study shows, a main effect of BNT treatment was the reduction of connectivity between the putamen, the sensorimotor cortex, the thalamus and the STN. The synchronous activation of the putamen, thalamus and motor cortex in CD was most likely an indication of the hyperactive direct striatal-thalamic-cortical pathway that is regulated by the neuroplasticity-inducing effects of BNT treatment (Albin et al., 1989; DeLong, 1990; Mink, 1996). By weakening dystonic muscles, BNT created a sensory-motor discrepancy and influenced the motor output of the brain. A similar principle has been debated regarding facial palsy (Klingner et al., 2014). Most likely, the brain could not differentiate whether the motor output or the sensory input was the cause of the prediction error. Essentially high-order processing of somatosensory information within S2 is necessary for the detection of the mismatch between perceived and expected movements (Mutschler et al., 2009; Servos et al., 2001; Simões and Hari, 1999). Analogous to facial palsy, the altered sensory-motor feedback in the CD brain after BNT treatment was associated with a decreased connectivity within the sensorimotor network (Klingner et al., 2013; Klingner et al., 2014). This mechanism potentially affects the overactive excitatory connections that underlie the pathomechanisms of CD to a larger extent than the moderate inhibitory connections. An observation that conceivably supports this assumption was the altered function and increased responsiveness of the STN following BNT application (Fig. 3). As part of the indirect striatothalamic pathway (Albin et al., 1989; DeLong, 1990), this led to enhanced cortical inhibition as measured by a decrease in local brain synchronization (Fig. 3). Conveniently for this interpretation, the CD patients with BNT treatment expressed lower levels of basal ganglia activation in a finger tapping task (Nevrlý et al., 2018; Opavský et al., 2011).
4.4. Sensorimotor integration
A disturbed sensorimotor integration has been a common object of discussion in dystonia (Hallett, 2006). Although CD is primarily a movement disorder, there are many subclinical sensory dysfunctions and sensory tricks, such as the geste antagoniste, that can relieve dystonic postures (Hallett, 1995; Leis et al., 1992; Naumann et al., 2000) and inflict involved as well as noninvolved limbs or body parts (Bara-Jimenez et al., 2000; Molloy et al., 2003). Changes in task-induced activity within the somatosensory cortex have been reported to be less prominent or even absent in CD (Contarino et al., 2007; Dolberg et al., 2011; Meunier et al., 2001; Tamura et al., 2009). Our data showed that communication within the somatosensory cortex was altered in CD. Compared to the healthy controls, the CD patients had a significantly reduced information transfer between S1 and S2, while S1 became less responsive to signals derived from the sensorimotor network, the thalamus and the basal ganglia. Furthermore, the directed information flow from S2 towards the motor cortex was increased. Notably, treatment with BNT improved the communication between S1 and S2. After BNT treatment, the thalamic-cortical influences upon S2 were decreased. In CD, deficient somatosensory processing may lead to distorted and aberrant sensorimotor integration, which then triggers motor function abnormalities such as coactivation of agonistic and antagonistic muscles (Abbruzzese and Berardelli, 2003). In some forms of focal dystonia, there is a clear association between high sensory stimulation and the repetition of a specific motor task; in blepharospasm, for instance, the sensation of a dry eye leads to escalating eye blinking (Quartarone et al., 2006). However, it is difficult to differentiate whether improper sensorimotor integration is a trigger for dystonia or a consequence. We hypothesize that CD affects the levels of somatosensory processing to a differential degree. In particular, higher levels of processing with S2 are compromised. These assumptions are supported by previous electrophysiological and neuroimaging studies that demonstrated most pronounced deficits in the spatial and temporal processing of somatosensory stimuli (Antelmi et al., 2017; Kimmich et al., 2014; Molloy et al., 2003). An MEG study in focal hand dystonia likewise demonstrated a decreased functional connectivity within the somatosensory cortex (Cheng et al., 2016; Opavský et al., 2011).
By reducing the effective movement through BNT application, the load of sensorimotor integration is globally reduced. Consequently, there remain less distorted and aberrant signals leading to inappropriate motor program activation and basal ganglia input which again reduces potential prediction errors (Friston, 2010; Lee et al., 2013). The effects of BNT on the sensorimotor network and a partial normalization of brain function have been reported previously (Delnooz et al., 2013; Nevrlý et al., 2018; Opavský et al., 2011; Opavský et al., 2012); here, we want to emphasize again that the effects of BNT are an indirect cause of cortical plasticity most likely induced by alterations in sensorimotor integration (Klingner et al., 2019). Therefore, partially diverging effects are to be expected, and to fully cover and systemize these effects, much larger groups of patients and subtypes of dystonia need to be observed in the future.
4.5. Multiple causes for CD manifestation (concept of a second hit)
Findings in monogenetic dystonia syndromes (>14 genes have been identified to date) have suggested that there is often more than one additional environmental factor necessary to develop dystonia symptoms (Breakefield et al., 2008). These extrinsic factors in the form of a “second hit” can include injury, viral infections, and emotional or physical stress (Edwards et al., 2003; Saint Hilaire et al., 1991) These observations, however, make it plausible that there are also multiple intrinsic alterations that lead to the onset of dystonia. Accordingly, the combination of GABA inhibitory deficits, especially within the basal ganglia, accompanied by impaired sensorimotor integration, might be essential for the breakthrough of dystonic symptoms.
4.6. Study limitations
We acknowledge several limitations of the study that temper our conclusions and should be addressed in future research. Although there is some evidence for the involvement of other brain regions in CD (Delnooz et al., 2013; Dresel et al., 2014; Jinnah et al., 2017), we restricted the number of analyzed brain regions to a set relevant to our working hypotheses. As illustrated in Fig. 1, we were mainly interested in brain regions that are known to be involved in the pathophysiology of CD: the motor cortex, basal ganglia, thalamus loop and the somatosensory integration. Due to technical reasons, we did not differentiate between GPi and GPe. Although differential extraction and time series analysis of GPi and GPe is feasible (Manes et al., 2018), in our preprocessing process <20 voxels for each GPi survived; therefore we extracted the globus pallidus as a whole.
In general, a clinically prominent lateralization of the involved neck muscles is observed in patients with CD. Debate exists regarding the presence of a corresponding lateralization of abnormal brain function. Higher sensory functions have consistently been reported to be impaired bilaterally (Molloy et al., 2003); similar impairments in brainstem reflexes have been reported in patients with CD (Nakashima et al., 1990). Recent MEP and SEP studies only found abnormal surround inhibition in the hemisphere contralateral to the affected body part; median nerve stimulation, for instance, revealed a lateralization of the precentral P22/N30 components to the contralateral hemisphere, when the splenius capitis muscle was involved, and to the ipsilateral hemisphere, when the sternocleidomastoid muscle was involved (Kanovský et al., 2003; Kanovský et al., 1997)(McDougall et al., 2015). Asymmetric BG output has also been described in patients with CD (Moll et al., 2014). In the present study, we did not differentiate the left and right hemispheres. All ROIs were selected from the right and left sides of the brain in a balanced manner because we intended to study global changes in brain function in patients with CD.
A further issue of debate is the method of fMRI data acquisition itself. Image sampling below 1 Hz, as is common in fMRI, is not able to model fast signal fluctuations that are observed within the somatosensory network and the basal ganglia (Cheng et al., 2016). In addition to the “rate model” to explain basal ganglia function, there have been plenty of reports that the temporal patterns of activity in the basal ganglia circuits is of importance in normal motor function as well as in dystonia (M. DeLong and Wichmann, 2010; Gale et al., 2008; Nelson and Kreitzer, 2014). To further address this matter, combined fMRI and MEG studies must be performed. Further limitations include the known alterations of autonomic functions caused by the application of BNT (Tintner et al., 2005). Although the potential impacts on blood pressure, the heart, and the respiratory rate might affect the fMRI signal (Iacovella and Hasson, 2011), the overall known effect of BNT A application in CT appears to be minor (Nebe et al., 1996). However, we must not completely neglect the influence of BNT on autonomic functions; thus, we did not administer BNT to our control group. Additionally, an analysis of head motion in our fMRI data revealed significant differences between controls and patients with CD (Supplemental Figs. 1 and 2). Signal changes in MR data caused by head motion can affect measures of a connectivity analysis (i.e., decreased coupling of distributed networks and increased coupling in local networks) (Van Dijk et al., 2012; Satterthwaite et al., 2012). As shown in a recent study by Parkes et al. (2018), the type of motion correction used in our preprocessing pipeline, including the regression of all 6 head motion parameters (provided by the rigid body correction), the average time series signal from the white matter and the cerebral spinal fluid (CSF) and the global signal, provides adequate control of motion artifacts; however, no currently available method provides perfect motion compensation.
Another aspect that should be discussed is the time intervals at which BNT is applied and the timing of the pre- and post fMRI scans. The time intervals of ≥ 12 weeks are consistent with clinical standards (Castelão et al., 2017; Evidente et al., 2013). In our study, we re-assessed the clinical data obtained two weeks after every BNT application. Based on these validation experiments, we adjusted the dose and localization of the BNT application in the next session. In our general clinical experience, after 2–3 BNT applications, we achieve good and reproductive outcomes. However, since many recent studies that investigated the effect of BNT on brain activity used fewer BNT applications, different outcomes are conceivable.
The underlying pathophysiological mechanisms in primary dystonias clearly extend beyond the pure motor network. The hallmarks of CD are an impaired neurotransmitter system (i.e., GABA-mediated inhibition), changes in basal ganglia connectivity and a malfunction or an imbalance of higher somatosensory integration and sensorimotor pathways. Therefore, to fully uncover its causes, research on a cellular and global network level must be carried out and combined. Understanding how dystonia, which is both a single receptor and a system-wide disease, develops will not only help to improve and monitor treatment but also to deepen our understanding of fundamental neural mechanisms related to motor learning and sensorimotor integration.
5. Conclusion
Alterations in local brain function and connectivity in CD are characterized by increased connectivity within the basal ganglia and the sensorimotor network as well as a loss of responsiveness in key regions, such as the putamen, the thalamus, and the somatosensory cortex. Unarguably, the cause and effect of CD cannot be dissociated from these results alone. However, by monitoring the effect of an effective treatment that only interferes with the direct output of the motor cortex (BNT), we observed a partial normalization of brain activity and connectivity within the BG and the sensorimotor network. Although BNT treatment did not influence the loss of responsiveness within the putamen and the thalamus (which might be associated with the concept of the loss of inhibition in CD) in a detectable manner, it had decreased their overall output. It is arguable that BNT treatment will be an essential tool for dissociating effects from cause in CD in future studies.
Authors' roles
-
1.
Research project: SB / FW / AG / TP, Conception; SB / FW / AG / OW, Organization; SB / FW / AG / TP, Execution.
-
2.
Statistical Analysis: SB / FW / CK, Design; SB / CK / TP, Execution; SB / FW / CK / TP, Review and Critique.
-
3.
Manuscript: SB / FW, Writing of the first draft; SB / FW / CK / AG / TP / OW, Review and Critique.
Financial disclosures of all authors
The authors declare that they have no competing interests or financial relations.
Funding sources
The authors received support from Deutsche Forschungsgemeinschaft (DFG) for 1738 B2; BMBF Bernstein Fokus (FKZ 01GQ0923); BMBF Gerontosys JenAge (FKZ 031 5581B); EU BrainAge (FP 7/HEALTH.2011.2.22-2 GA No. 2798219); and BMBF Irestra (FKZ 16SV7209).
This study was supported by Merz Pharmaceuticals GmbH, Frankfurt, Germany.
Acknowledgments
Not applicable.
Appendix A. Supplementary data
Supplementary material
References
- Abbruzzese G., Berardelli A. Sensorimotor integration in movement disorders. Mov. Disord. 2003;18(3):231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Albanese A., Bhatia K., Bressman S.B., Delong M.R., Fahn S., Fung V.S.C.…Teller J.K. Phenomenology and classification of dystonia: a consensus update. Mov. Disord. 2013;28(7):863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin R.L., Young A.B., Penney J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Antelmi E., Erro R., Rocchi L., Liguori R., Tinazzi M., Di Stasio F.…Bhatia K.P. Neurophysiological correlates of abnormal somatosensory temporal discrimination in dystonia. Mov. Disord. 2017;32(1):141–148. doi: 10.1002/mds.26804. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W., Shelton P., Hallett M. Spatial discrimination is abnormal in focal hand dystonia. Neurology. 2000;55(12):1869–1873. doi: 10.1212/wnl.55.12.1869. [DOI] [PubMed] [Google Scholar]
- Blood A.J., Flaherty A.W., Choi J.-K., Hochberg F.H., Greve D.N., Bonmassar G.…Jenkins B.G. Basal ganglia activity remains elevated after movement in focal hand dystonia. Ann. Neurol. 2004;55(5):744–748. doi: 10.1002/ana.20108. [DOI] [PubMed] [Google Scholar]
- Breakefield X.O., Blood A.J., Li Y., Hallett M., Hanson P.I., Standaert D.G. The pathophysiological basis of dystonias. Nature reviews. Neuroscience. 2008;9(3):222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Brodoehl S., Klingner C., Stieglitz K., Witte O.W. Age-related changes in the somatosensory processing of tactile stimulation--an fMRI study. Behav. Brain Res. 2013;238:259–264. doi: 10.1016/j.bbr.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Brodoehl S., Klingner C., Stieglitz K., Witte O.W. The impact of eye closure on somatosensory perception in the elderly. Behav. Brain Res. 2015;293:89–95. doi: 10.1016/j.bbr.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Brodoehl S., Klingner C.M., Witte O.W. Eye closure enhances dark night perceptions. Sci. Rep. 2015;5:10515. doi: 10.1038/srep10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes M.L., Thickbroom G.W., Wilson S.A., Sacco P., Shipman J.M., Stell R., Mastaglia F.L. The corticomotor representation of upper limb muscles in writer's cramp and changes following botulinum toxin injection. Brain. 1998;121(Pt 5):977–988. doi: 10.1093/brain/121.5.977. [DOI] [PubMed] [Google Scholar]
- Carbon M., Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164(1):220–229. doi: 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelão M., Marques R.E., Duarte G.S., Rodrigues F.B., Ferreira J., Sampaio C.…Costa J. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst. Rev. 2017;12 doi: 10.1002/14651858.CD003633.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.-H., Tseng Y.-J., Chen R.-S., Lin Y.-Y. Reduced functional connectivity of somatosensory network in writer's cramp patients. Brain Behav. 2016;6(3) doi: 10.1002/brb3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L.G., Hallett M., Geller B.D., Hochberg F. Treatment of focal dystonias of the hand with botulinum toxin injections. J. Neurol. Neurosurg. Psychiatry. 1989;52(3):355–363. doi: 10.1136/jnnp.52.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino M.F., Kruisdijk J.J.M., Koster L., Ongerboer de Visser B.W., Speelman J.D., Koelman J.H.T.M. Sensory integration in writer's cramp: comparison with controls and evaluation of botulinum toxin effect. Clin. Neurophysiol. 2007;118(10):2195–2206. doi: 10.1016/j.clinph.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Contarino M.F., Van Den Dool J., Balash Y., Bhatia K., Giladi N., Koelman J.H.…Tijssen M.A.J. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front. Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnooz C.C.S., Pasman J.W., Beckmann C.F., van de Warrenburg B.P.C. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong M., Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin. EEG Neurosci. 2010;41(2):61–67. doi: 10.1177/155005941004100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolberg R., Hinkley L.B.N., Honma S., Zhu Z., Findlay A.M., Byl N.N., Nagarajan S.S. Amplitude and timing of somatosensory cortex activity in task-specific focal hand dystonia. Clin. Neurophysiol. 2011;122(12):2441–2451. doi: 10.1016/j.clinph.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresel C., Li Y., Wilzeck V., Castrop F., Zimmer C., Haslinger B. Multiple changes of functional connectivity between sensorimotor areas in focal hand dystonia. J. Neurol. Neurosurg. Psychiatry. 2014;85(11):1245–1252. doi: 10.1136/jnnp-2013-307127. [DOI] [PubMed] [Google Scholar]
- Edwards M., Wood N., Bhatia K. Unusual phenotypes in DYT1 dystonia: a report of five cases and a review of the literature. Mov. Disord. 2003;18(6):706–711. doi: 10.1002/mds.10411. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eidelberg D., Moeller J.R., Antonini A., Kazumata K., Nakamura T., Dhawan V.…Fahn S. Functional brain networks in DYT1 dystonia. Ann. Neurol. 1998;44(3):303–312. doi: 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- Evidente V.G.H., Fernandez H.H., LeDoux M.S., Brashear A., Grafe S., Hanschmann A., Comella C.L. A randomized, double-blind study of repeated incobotulinumtoxinA (Xeomin(®)) in cervical dystonia. J. Neural Trans. (Vienna, Austria : 1996) 2013;120(12):1699–1707. doi: 10.1007/s00702-013-1048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi G.M., Errico P., Santarelli R., Bagolini B., Manni E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993;113(3):400–404. doi: 10.3109/00016489309135834. [DOI] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a unified brain theory? Nature reviews. Neuroscience. 2010;11(2):127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Gale J.T., Amirnovin R., Williams Z.M., Flaherty A.W., Eskandar E.N. From symphony to cacophony: pathophysiology of the human basal ganglia in Parkinson disease. Neurosci. Biobehav. Rev. 2008;32(3):378–387. doi: 10.1016/j.neubiorev.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Gerfen C.R., Engber T.M., Mahan L.C., Susel Z., Chase T.N., Monsma F.J., Sibley D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science (New York, N.Y.) 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gilio F., Currà A., Lorenzano C., Modugno N., Manfredi M., Berardelli A. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann. Neurol. 2000;48(1):20–26. [PubMed] [Google Scholar]
- Goebel R., Roebroeck A., Kim D.-S., Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn. Reson. Imaging. 2003;21(10):1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Gracies J.-M. Physiological effects of botulinum toxin in spasticity. Mov. Disord.Society. 2004;19(Suppl. 8):S120–S128. doi: 10.1002/mds.20065. [DOI] [PubMed] [Google Scholar]
- Granger C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3) [Google Scholar]
- Haag L.M., Heba S., Lenz M., Glaubitz B., Höffken O., Kalisch T.…Schmidt-Wilcke T. Resting BOLD fluctuations in the primary somatosensory cortex correlate with tactile acuity. Cortex. 2015;64:20–28. doi: 10.1016/j.cortex.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Is dystonia a sensory disorder? Ann. Neurol. 1995;38(2):139–140. doi: 10.1002/ana.410380203. [DOI] [PubMed] [Google Scholar]
- Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res. Rev. 2001;36(2–3):169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- Hallett M. Pathophysiology of dystonia. Journal of neural transmission. Supplementum. 2006;70:485–488. doi: 10.1007/978-3-211-45295-0_72. [DOI] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol. Dis. 2011;42(2):177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Mechanism of action of botulinum neurotoxin: unexpected consequences. Toxicon. 2018;147:73–76. doi: 10.1016/j.toxicon.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R., Whaler B.C. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Cl. botulinum type A toxin. J. Physiol. 1962;160:221–233. doi: 10.1113/jphysiol.1962.sp006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovella V., Hasson U. The relationship between BOLD signal and autonomic nervous system functions: implications for processing of “physiological noise”. Magn. Reson. Imaging. 2011;29(10):1338–1345. doi: 10.1016/j.mri.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M., Obeso I., Rothwell J.C., Obeso J.A. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Neuroscience. 2015;16(12):719–732. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Treatment of cervical dystonia with botulinum toxin. Mov. Disord. 2004;19(Suppl. 8):S109–S115. doi: 10.1002/mds.20024. [DOI] [PubMed] [Google Scholar]
- Jiang L., Xu T., He Y., Hou X.-H., Wang J., Cao X.-Y.…Zuo X.-N. Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Struct. Funct. 2015;220(5):2485–2507. doi: 10.1007/s00429-014-0795-8. [DOI] [PubMed] [Google Scholar]
- Jinnah H.A., Hess E.J. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67(10):1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- Jinnah H.A., Neychev V., Hess E.J. The anatomical basis for dystonia: the motor network model. Tremor Other Hyperkinetic Mov. (New York, N.Y.) 2017;7:506. doi: 10.7916/D8V69X3S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanovský P., Streitová H., Dufek J., Rektor I. Lateralization of the P22/N30 component of somatosensory evoked potentials of the median nerve in patients with cervical dystonia. Mov. Disord. 1997;12(4):553–560. doi: 10.1002/mds.870120412. [DOI] [PubMed] [Google Scholar]
- Kanovský P., Streitová H., Dufek J., Znojil V., Daniel P., Rektor I. Change in lateralization of the P22/N30 cortical component of median nerve somatosensory evoked potentials in patients with cervical dystonia after successful treatment with botulinum toxin A. Mov. Disord. 1998;13(1):108–117. doi: 10.1002/mds.870130122. [DOI] [PubMed] [Google Scholar]
- Kanovský P., Bares M., Streitová H., Klajblová H., Daniel P., Rektor I. Abnormalities of cortical excitability and cortical inhibition in cervical dystonia evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J. Neurol. 2003;250(1):42–50. doi: 10.1007/s00415-003-0942-2. [DOI] [PubMed] [Google Scholar]
- Kelly P.A., McCulloch J. The effects of the GABAergic agonist muscimol upon the relationship between local cerebral blood flow and glucose utilization. Brain Res. 1983;258(2):338–342. doi: 10.1016/0006-8993(83)91162-9. [DOI] [PubMed] [Google Scholar]
- Kemp J.M., Powell T.P. The cortico-striate projection in the monkey. Brain. 1970;93(3):525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kemp J.M., Powell T.P. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos. Trans. R. Soc. Lond. Series B. 1971;262(845):429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- Kiebel S.J., Ashburner J., Poline J.B., Friston K.J. MRI and PET coregistration--a cross validation of statistical parametric mapping and automated image registration. NeuroImage. 1997;5(4 Pt 1):271–279. doi: 10.1006/nimg.1997.0265. [DOI] [PubMed] [Google Scholar]
- Kimmich O., Molloy A., Whelan R., Williams L., Bradley D., Balsters J.…Hutchinson M. Temporal discrimination, a cervical dystonia endophenotype: penetrance and functional correlates. Mov. Disord. 2014;29(6):804–811. doi: 10.1002/mds.25822. [DOI] [PubMed] [Google Scholar]
- Klingner C.M., Hasler C., Brodoehl S., Axer H., Witte O.W. Perceptual plasticity is mediated by connectivity changes of the medial thalamic nucleus. Hum. Brain Mapp. 2013;34(9):2343–2352. doi: 10.1002/hbm.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner C.M., Volk G.F., Brodoehl S., Witte O.W., Guntinas-Lichius O. The effects of deefferentation without deafferentation on functional connectivity in patients with facial palsy. NeuroImage Clin. 2014;6:26–31. doi: 10.1016/j.nicl.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner C.M., Brodoehl S., Huonker R., Witte O.W. The processing of somatosensory information shifts from an early parallel into a serial processing mode: a combined fMRI/MEG study. Front. Syst. Neurosci. 2016;10:103. doi: 10.3389/fnsys.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner C.M., Brodoehl S., Witte O.W., Guntinas-Lichius O., Volk G.F. The impact of motor impairment on the processing of sensory information. Behav. Brain Res. 2019;359:701–708. doi: 10.1016/j.bbr.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Kreitzer A.C., Malenka R.C. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego J.L., Luquin N., Obeso J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harbor Perspect. Med. 2012;2(12):a009621. doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux M.S. Dystonia: phenomenology. Parkinsonism Relat. Disord. 2012;18(Suppl. 1):S162–S164. doi: 10.1016/S1353-8020(11)70050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Furuya S., Karst M., Altenmüller E. Alteration in forward model prediction of sensory outcome of motor action in focal hand dystonia. Front. Hum. Neurosci. 2013;7:172. doi: 10.3389/fnhum.2013.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis A.A., Dimitrijevic M.R., Delapasse J.S., Sharkey P.C. Modification of cervical dystonia by selective sensory stimulation. J. Neurol. Sci. 1992;110(1–2):79–89. doi: 10.1016/0022-510x(92)90013-b. [DOI] [PubMed] [Google Scholar]
- Li Z., Prudente C.N., Stilla R., Sathian K., Jinnah H.A., Hu X. Alterations of resting-state fMRI measurements in individuals with cervical dystonia. Hum. Brain Mapp. 2017;38(8):4098–4108. doi: 10.1002/hbm.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E.D., Lee P., Quinn L., Marder K. Dystonia in Huntington's disease: prevalence and clinical characteristics. Mov. Disord. 1999;14(1):95–101. doi: 10.1002/1531-8257(199901)14:1<95::aid-mds1016>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Manes J.L., Tjaden K., Parrish T., Simuni T., Roberts A., Greenlee J.D.…Kurani A.S. Altered resting-state functional connectivity of the putamen and internal globus pallidus is related to speech impairment in Parkinson's disease. Brain Behav. 2018;8(9) doi: 10.1002/brb3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino D., Liuzzi D., Macerollo A., Aniello M.S., Livrea P., Defazio G. The phenomenology of the geste antagoniste in primary blepharospasm and cervical dystonia. Mov. Disord. 2010;25(4):407–412. doi: 10.1002/mds.23011. [DOI] [PubMed] [Google Scholar]
- McDougall L., Kiernan D., Kiss Z.H.T., Suchowersky O., Welsh T.N. Abnormal surround inhibition does not affect asymptomatic limbs in people with cervical dystonia. Neurosci. Lett. 2015;604:7–11. doi: 10.1016/j.neulet.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Meunier S., Garnero L., Ducorps A., Mazières L., Lehéricy S., du Montcel S.T.…Vidailhet M. Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann. Neurol. 2001;50(4):521–527. doi: 10.1002/ana.1234. [DOI] [PubMed] [Google Scholar]
- Mink J.W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Moll C.K.E., Galindo-Leon E., Sharott A., Gulberti A., Buhmann C., Koeppen J.A.…Engel A.K. Asymmetric pallidal neuronal activity in patients with cervical dystonia. Front. Syst. Neurosci. 2014;8:15. doi: 10.3389/fnsys.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy F.M., Carr T.D., Zeuner K.E., Dambrosia J.M., Hallett M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126(Pt 10):2175–2182. doi: 10.1093/brain/awg219. [DOI] [PubMed] [Google Scholar]
- Mutschler I., Wieckhorst B., Kowalevski S., Derix J., Wentlandt J., Schulze-Bonhage A., Ball T. Functional organization of the human anterior insular cortex. Neurosci. Lett. 2009;457(2):66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Rothwell J.C., Thompson P.D., Day B.L., Berardelli A., Agostino R.…Marsden C.D. The blink reflex in patients with idiopathic torsion dystonia. Arch. Neurol. 1990;47(4):413–416. doi: 10.1001/archneur.1990.00530040055019. [DOI] [PubMed] [Google Scholar]
- Naumann M., Magyar-Lehmann S., Reiners K., Erbguth F., Leenders K.L. Sensory tricks in cervical dystonia: perceptual dysbalance of parietal cortex modulates frontal motor programming. Ann. Neurol. 2000;47(3):322–328. [PubMed] [Google Scholar]
- Nebe A., Schelosky L., Wissel J., Ebersbach G., Scholz U., Poewe W. No effects on heart-rate variability and cardiovascular reflex tests after botulinum toxin treatment of cervical dystonia. Mov. Disord. 1996;11(3):337–339. doi: 10.1002/mds.870110324. [DOI] [PubMed] [Google Scholar]
- Nelson A.B., Kreitzer A.C. Reassessing models of basal ganglia function and dysfunction. Annu. Rev. Neurosci. 2014;37:117–135. doi: 10.1146/annurev-neuro-071013-013916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevrlý M., Hluštík P., Hok P., Otruba P., Tüdös Z., Kaňovský P. Changes in sensorimotor network activation after botulinum toxin type A injections in patients with cervical dystonia: a functional MRI study. Exp. Brain Res. 2018;236(10):2627–2637. doi: 10.1007/s00221-018-5322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neychev V.K., Gross R.E., Lehéricy S., Hess E.J., Jinnah H.A. The functional neuroanatomy of dystonia. Neurobiol. Dis. 2011;42(2):185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M.-F., Huang X.-F., Miao Y.-W., Liang Z.-H. Resting state fMRI observations of baseline brain functional activities and connectivities in primary blepharospasm. Neurosci. Lett. 2017;660:22–28. doi: 10.1016/j.neulet.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Obermann M., Yaldizli O., de Greiff A., Konczak J., Lachenmayer M.L., Tumczak F.…Maschke M. Increased basal-ganglia activation performing a non-dystonia-related task in focal dystonia. Eur. J. Neurol. 2008;15(8):831–838. doi: 10.1111/j.1468-1331.2008.02196.x. [DOI] [PubMed] [Google Scholar]
- Obermann M., Vollrath C., de Greiff A., Gizewski E.R., Diener H.-C., Hallett M., Maschke M. Sensory disinhibition on passive movement in cervical dystonia. Mov. Disord. 2010;25(15):2627–2633. doi: 10.1002/mds.23321. [DOI] [PubMed] [Google Scholar]
- Opavský R., Hluštík P., Otruba P., Kaňovský P. Sensorimotor network in cervical dystonia and the effect of botulinum toxin treatment: a functional MRI study. J. Neurol. Sci. 2011;306(1–2):71–75. doi: 10.1016/j.jns.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Opavský R., Hluštík P., Otruba P., Kaňovský P. Somatosensory cortical activation in cervical dystonia and its modulation with botulinum toxin: an fMRI study. Int. J. Neurosci. 2012;122(1):45–52. doi: 10.3109/00207454.2011.623807. [DOI] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- Peller M., Zeuner K.E., Munchau A., Quartarone A., Weiss M., Knutzen A.…Siebner H.R. The basal ganglia are hyperactive during the discrimination of tactile stimuli in writer's cramp. Brain. 2006;129(Pt 10):2697–2708. doi: 10.1093/brain/awl181. [DOI] [PubMed] [Google Scholar]
- Phukan J., Albanese A., Gasser T., Warner T. Primary dystonia and dystonia-plus syndromes: clinical characteristics, diagnosis, and pathogenesis. Lancet Neurol. 2011;10(12):1074–1085. doi: 10.1016/S1474-4422(11)70232-0. [DOI] [PubMed] [Google Scholar]
- Poston K.L., Eidelberg D. Functional brain networks and abnormal connectivity in the movement disorders. NeuroImage. 2012;62(4):2261–2270. doi: 10.1016/j.neuroimage.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A., Siebner H.R., Rothwell J.C. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 2006;29(4):192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Restani L., Antonucci F., Gianfranceschi L., Rossi C., Rossetto O., Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J. Neurosci. 2011;31(44):15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroeck A., Formisano E., Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. NeuroImage. 2005;25(1):230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Saint Hilaire M.H., Burke R.E., Bressman S.B., Brin M.F., Fahn S. Delayed-onset dystonia due to perinatal or early childhood asphyxia. Neurology. 1991;41(2):216–222. doi: 10.1212/wnl.41.2_part_1.216. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H.…Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicatano E.J., Basso M.A., Evinger C. Animal model explains the origins of the cranial dystonia benign essential blepharospasm. J. Neurophysiol. 1997;77(5):2842–2846. doi: 10.1152/jn.1997.77.5.2842. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30(5):203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Servos P., Lederman S., Wilson D., Gati J. fMRI-derived cortical maps for haptic shape, texture, and hardness. Cogn. Brain Res. 2001;12(2):307–313. doi: 10.1016/s0926-6410(01)00041-6. [DOI] [PubMed] [Google Scholar]
- Seth A.K. A MATLAB toolbox for Granger causal connectivity analysis. J. Neurosci. Methods. 2010;186(2):262–273. doi: 10.1016/j.jneumeth.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Simões C., Hari R. Relationship between responses to contra- and ipsilateral stimuli in the human second somatosensory cortex SII. NeuroImage. 1999;10(4):408–416. doi: 10.1006/nimg.1999.0476. [DOI] [PubMed] [Google Scholar]
- Simonyan K., Cho H., Hamzehei Sichani A., Rubien-Thomas E., Hallett M. The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain. 2017;140(12):3179–3190. doi: 10.1093/brain/awx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y., Bevan M.D., Shink E., Bolam J.P. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–387. doi: 10.1016/s0306-4522(98)00004-9. http://www.ncbi.nlm.nih.gov/pubmed/9881853 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Smith Y., Raju D., Nanda B., Pare J.-F., Galvan A., Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res. Bull. 2009;78(2–3):60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn Y.H., Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann. Neurol. 2004;56(4):595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y., Li S.-F., Zuo X.-N., Zhu C.-Z.…Zang Y.-F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves T.D., Day L., Dykeman J., Jette N., Pringsheim T. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov. Disord. 2012;27(14):1789–1796. doi: 10.1002/mds.25244. [DOI] [PubMed] [Google Scholar]
- Tamura Y., Matsuhashi M., Lin P., Ou B., Vorbach S., Kakigi R., Hallett M. Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov. Disord. 2008;23(4):558–565. doi: 10.1002/mds.21870. [DOI] [PubMed] [Google Scholar]
- Tamura Y., Ueki Y., Lin P., Vorbach S., Mima T., Kakigi R., Hallett M. Disordered plasticity in the primary somatosensory cortex in focal hand dystonia. Brain. 2009;132(Pt 3):749–755. doi: 10.1093/brain/awn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M., Fiorio M., Fiaschi A., Rothwell J.C., Bhatia K.P. Sensory functions in dystonia: insights from behavioral studies. Mov. Disord. 2009;24(10):1427–1436. doi: 10.1002/mds.22490. [DOI] [PubMed] [Google Scholar]
- Tintner R., Gross R., Winzer U.F., Smalky K.A., Jankovic J. Autonomic function after botulinum toxin type A or B: a double-blind, randomized trial. Neurology. 2005;65(5):765–767. doi: 10.1212/01.wnl.0000174433.76707.8c. [DOI] [PubMed] [Google Scholar]
- Tolosa E., Compta Y. Dystonia in Parkinson's disease. J. Neurol. 2006;253(Suppl. 7):VII7–VI13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- Trompetto C., Currà A., Buccolieri A., Suppa A., Abbruzzese G., Berardelli A. Botulinum toxin changes intrafusal feedback in dystonia: a study with the tonic vibration reflex. Mov. Disord. 2006;21(6):777–782. doi: 10.1002/mds.20801. [DOI] [PubMed] [Google Scholar]
- Tsui J.K., Eisen A., Stoessl A.J., Calne S., Calne D.B. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet (London, England) 1986;2(8501):245–247. doi: 10.1016/s0140-6736(86)92070-2. [DOI] [PubMed] [Google Scholar]
- Utter A.A., Basso M.A. The basal ganglia: an overview of circuits and function. Neurosci. Biobehav. Rev. 2008;32(3):333–342. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa P.A., Roebroeck A., Daunizeau J., Friston K. Effective connectivity: influence, causality and biophysical modeling. NeuroImage. 2011;58(2):339–361. doi: 10.1016/j.neuroimage.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F., Lachaux J.P., Rodriguez E., Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature reviews. Neuroscience. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Wagner M.L., Fedak M.N., Sage J.I., Mark M.H. Complications of disease and therapy: a comparison of younger and older patients with Parkinson's disease. Annals Clin. Lab. Sci. 1996;26(5):389–395. http://www.ncbi.nlm.nih.gov/pubmed/8879356 Retrieved from. [PubMed] [Google Scholar]
- Walsh R., Hutchinson M. Molding the sensory cortex: spatial acuity improves after botulinum toxin treatment for cervical dystonia. Mov. Disord. 2007;22(16):2443–2446. doi: 10.1002/mds.21759. [DOI] [PubMed] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]