Abstract
Purpose: This review aims to determine whether ultrasound-guided platelet-rich plasma injection has any role in improving clinical outcomes in patients with hip osteoarthritis. Methods: A search of the National Institute for Health and Care Excellence database using the Healthcare Databases Advanced Search tool was conducted. The PubMed database was also utilised to search the Medical Literature Analysis and Retrieval System Online, Excerpta Medica database, Cumulative Index of Nursing and Allied Health and Allied and Complimentary Medicine databases. The Preferred Reporting Items for Systematic Review and Meta-Analysis methodology guidance was employed and a quality assessment was performed using the Jadad score. Results: Three randomised clinical trials met the inclusion criteria and were included for analysis. All three trials were of good quality based on the Jadad score. A total of 115 patients out of 254 received platelet-rich plasma injections under ultrasound guidance. The platelet-rich plasma recipient group included 61 males and 54 females with an age range from 53 to 71 years. Outcome scores show an improvement of symptoms and function maintained up to 12 months following platelet-rich plasma injection. Conclusions: Literature to date concludes that intra-articular platelet-rich plasma injections of the hip, performed under ultrasound guidance to treat hip osteoarthritis, are well tolerated and potentially efficacious in delivering long-term and clinically significant pain reduction and functional improvement in patients with hip osteoarthritis. Larger future trials including a placebo group are required to further evaluate these promising results. Level of evidence: Level I, a systematic review of level I studies.
Keywords: platelet-rich plasma, hip joint, osteoarthritis, ultrasound guidance injection
Introduction
Osteoarthritis (OA) is a syndrome of joint pain and dysfunction that is caused by joint degeneration(1). OA affects more people than any other joint disease, and has widespread economic and social consequences. The problem is likely intensified by current demographic trends, including the pandemic of obesity and higher recreational activity levels of our elderly population(2). Currently, there is no known cure for OA. Therefore, treatment is aimed at decreasing pain, maintaining or improving joint mobility, and limiting functional impairment(3). Inflammation has been postulated as a key driver for pain in OA(4), but a recent meta-analysis concluded that disease-modifying antirheumatic drugs (DMARDs) do not provide pain relief beyond placebo in OA(5).
Lately, platelet-rich plasma (PRP) has become increasingly popular among the orthopaedic community as a minimally invasive technique for enhancing tissue healing in different conditions including rotator cuff pathology(6), greater trochanteric pain syndrome(6), knee osteoarthritis(7), lateral epicondylitis(8), osteochondral lesions of the talus(9) and other orthopaedic conditions. It has been postulated that PRP promotes soft tissue healing by delivering a higher than normal concentration of platelets and, therefore, an increased concentration of platelet derived growth factors to the diseased area(10). This has been shown in various studies(11–14).
The use of PRP in treating hip OA has become more prevalent in recent years. The purpose of this review is to summarise the existing knowledge on the role of PRP in hip OA.
Methods
A search of the National Institute for Health and Care Excellence (NICE) database has been completed using the Healthcare Databases Advanced Search (HDAS) tool. In addition, we utilised the PubMed database to search the Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (EMBASE), Cumulative Index of Nursing and Allied Health (CINAHL) and Allied and Complimentary Medicine (AMED) databases. The search was conducted from their year of inception to June 2018 with the keywords “hip joint” and “ultrasound guided injection”.
Broad search keywords were used rather than specific terms to ensure no articles were missed. There was no language limit and all relevant published articles and abstracts were included. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) methodology guidance was employed(15).
Abstracts from the search were reviewed for relevant articles by two authors. All references listed in the relevant articles were also reviewed for any other papers not found in the initial search. Studies were included if they were randomised control trials (RCT), and reported clinical and functional outcomes of patients treated with PRP for hip OA. Due to a small number of studies, we did not set a minimum follow-up period. Case reports, reviews, conference abstracts, case series, studies on animals and technical notes were excluded.
Once relevant RCTs were identified, data was extracted using a standardised form for each of the following: author, year of publication, study design, sample size, demographics, diagnostic test, injection technique, outcome measures and follow-up frequency. Due to the heterogeneity of the included data, a meta-analysis could not be conducted and, therefore, all data was reported descriptively.
Quality assessment
This review utilised the Jadad quality evaluation scale(16) to rank the quality of the included RCTs. Studies ranked 0–2 are of low quality, and studies ranked 3–5 are of high-quality. All studies included in this review ranked 3–5.
Results
Search results
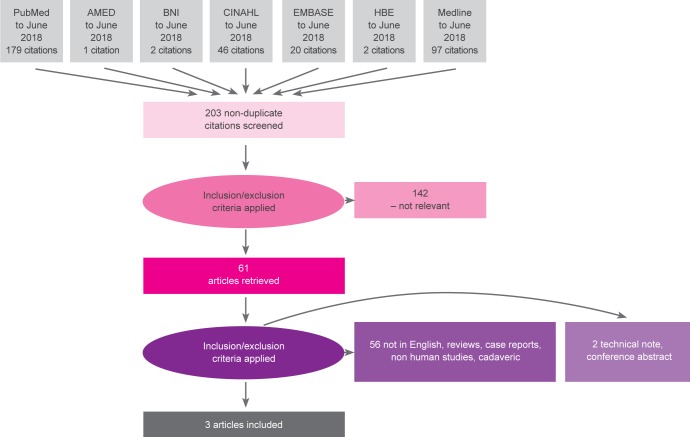
The search threads used resulted in 347 articles (Fig. 1).
Fig. 1.
Study selection process
Summary of studies
Three RCTs(17–19) met the inclusion criteria (Tab. 1). These studies included 254 patients, of whom 115 patients were randomised for PRP and 31 patients received both PRP and the control. The control in all three trials was hyaluronic acid (HA). All injections were administered under ultrasound (US) guidance. The PRP group included 61 males and 54 females with an age range between 53 and 71 years. Patients had different degrees of osteoarthritis based on Kellgren and Lawrence (KL) classification and durations of symptoms were variably reported (Tab. 2).
Tab. 1.
Studies included
| Study | Level of evidence | Jadad | No. of hips | Male / Female | Mean age | Duration of symptoms |
|---|---|---|---|---|---|---|
| Di Sante et al. 2016(17) | Level I | 3 | 43 (22 HA + 21 PRP) | Ia 11/10 | 71 | not reported |
| Dallari et al. 2016(18) | Level I | 5 | 111 (44 PRP + 36 HA + 31 Both) | 20/24 | 50–59 | >4 months |
| Battaglia et al. 2013(19) | Level I | 4 | 100 (50 HA + 50 PRP) | 30/20 | 53 | 6–24 months |
Tab. 2.
Description of studies
| Study | OA grade | Mean FUP | Control | Outcome measures | Preparation | Complications |
|---|---|---|---|---|---|---|
| Di Sante et al. 2016(17) | KL: II i III | 16 weeks | HA | VAS, WOMAC | 8 mls blood, 4 mls PRP 3 weekly injections | None |
| Dallari et al. 2016(18) | KL: I–IV | 12 months | HA | VAS, WOMAC, HHS | 150 mls blood, 4 × 5 mls PRP 3 weekly injections | None |
| Battaglia et al. 2013(19) | KL: II, III i IV | 12 months | HA | VAS, HHS | 150 mls blood, 4 × 5 mls PRP 3 weekly injections | 1 superficial haematoma |
Outcome measures
The visual analogue scale (VAS) was used in all three trials to assess pain at baseline and throughout the follow-up periods. Reported results are summarised in Fig. 2.
Fig. 2.
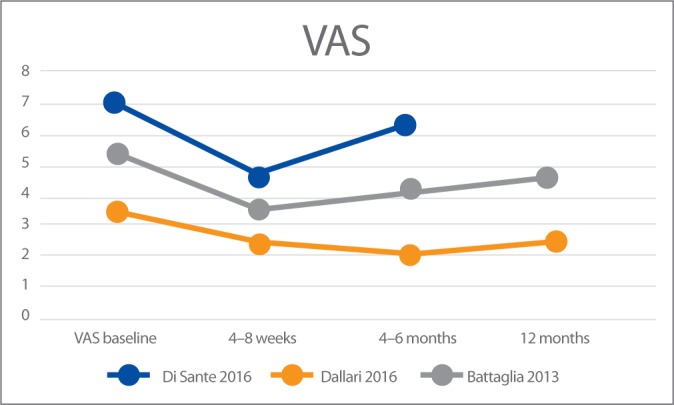
VAS results in the three studies
In addition, Di Sante et al.(17) and Dallari et al.(18) used the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Reported results are summarised in Fig. 3.
Fig. 3.
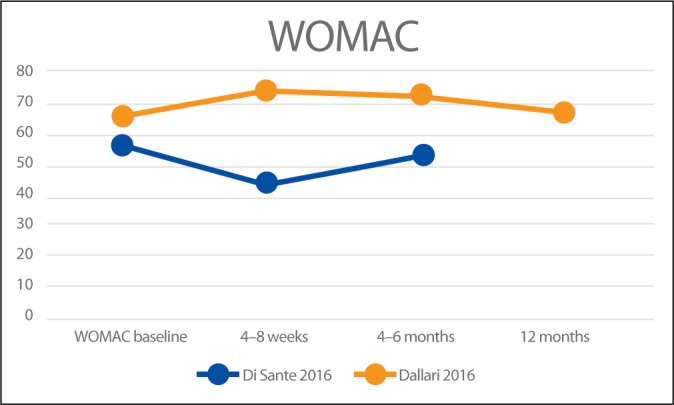
Results of WOMAC scale
Battaglia et al.(19) and Dallari et al.(18) also used the Harris Hip Score (HHS) to report the clinical outcomes of the PRP injections. Reported results are summarised in Fig. 4.
Fig. 4.
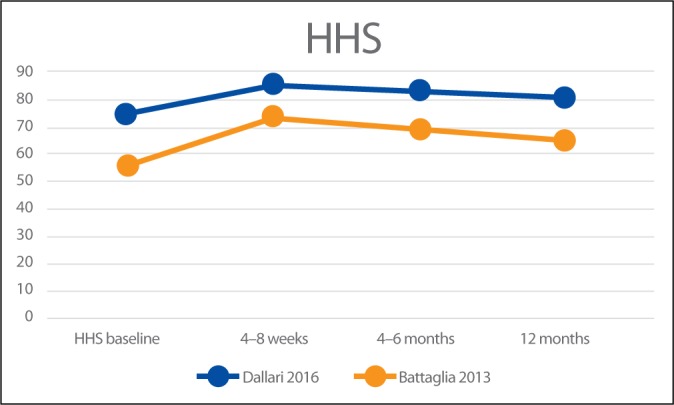
Results of HHS
PRP preparation
Di Sante et al. used the Regen Kit. An 8-ml venous blood sample was obtained for each hip treatment. A complete peripheral blood count was also collected at the time of initial blood donation. The samples were centrifuged twice at 3100 rpm for 9 minutes in order to produce 4 ml of PRP. A random PRP unit was sent to the laboratory for platelet count and microbiology tests. The total number of platelets/ml in the PRP without leukocytes represented a mean increase of around 100–150% compared with whole blood values.
Dallari et al. obtained 150 mL of venous blood for patients with unilateral hip OA and 300 mL for those with bilateral disease. 2 centrifugations were performed at 1480 rpm for 6 minutes and 3400 rpm for 15 minutes. The PRP produced was then divided into 5 ml portions (4 for unilateral disease and 7 for bilateral disease) and stored. 13 patients (at least 25%) from the PRP group were randomly selected and had their samples analysed for proinflammatory and anti-inflammatory markers.
Battaglia et al. obtained 150 mL of venous blood from each patient and performed 2 centrifugations, 1800 rpm for 15 minutes and 3500 rpm for 10 minutes, in order to prepare 4 units, each containing 5 mL of PRP. One unit was sent to the hospital laboratory to perform a platelet count and microbiology tests, and the other three units were stored. The average increase in platelet number per microlitre in the PRP was 600% compared to the whole blood value.
Injection technique
The technique of ultrasound injection was broadly similar in all three studies. All studies performed the injections using an anterior approach, with the patient supine and hip in internal rotation under aseptic precautions. A low-frequency ultrasound probe was used in all studies and aligned to the femoral neck axis. Dallari et al. used a 22-gauge spinal needle targeting the anterior capsular recess at the base of the femoral neck. Di Sante et al. and Battaglia et al. used a 20-gauge spinal needle targeting the junction of the femoral head neck. Synovial fluid aspiration was performed by Dallari et al. and Di Sante et al. Distention of the joint capsule was registered by Di Sante et al. Patients were discharged after the injection in all three studies.
Di Sante et al. administered 3 consecutive injections of PRP (3 ml, 1 week apart) and 1 injection of HA (30 mg/2 ml). Battaglia et al. administered 3 consecutive injections of PRP (5 ml, 2 weeks apart) and 1 injection of HA (30 mg/2 ml). Dallari et al. administered 3 consecutive injections of PRP (5 ml, 1 week apart) and 1 injection of HA (30 mg/2 ml).
Summary of results
Di Sante et al.(17) compared the efficacy of US-guided injection of PRP versus hyaluronic acid (HA) for hip OA. Patients were evaluated at baseline, 4 weeks and 16 weeks using VAS and WOMAC pain subscale. Compared to baseline, VAS scores in the PRP group significantly decreased at 4 weeks but not at 16 weeks. No differences in WOMAC pain scale were discernible compared to baseline at 4 weeks or 16 weeks in the PRP group. In the HA group a significant decrease of both VAS and WOMAC values was registered only at the 16 week follow-up. The authors concluded that intra-articular PRP had an immediate effect on pain that was not maintained at longer-term follow-up when, on the contrary, the effects of intra-articular HA were evident.
Battaglia et al.(19) compared the clinical efficacy of PRP versus HA at 12 months of follow-up, and evaluated the influence of the type of infiltration, patient age, sex, body mass index, and degree of OA on temporal clinical evolution. Patients were evaluated at baseline and after 1, 3, 6, and 12 months using HHS and VAS. An overall improvement was detected in both groups at 1- and 3-month follow-up. Despite a slightly progressive worsening between 6- and 12-month follow-up, the final HHS and VAS values remained lower compared to baseline (p <.0005), with no significant differences between PRP and HA. They concluded that intra-articular injections of PRP are efficacious in terms of functional improvement and pain reduction but are not superior to HA in patients with symptomatic hip OA at 12-month follow-up.
Dallari et al.(18) compared the therapeutic efficacy of PRP, HA and the combination of both in hip OA. HHS, WOMAC and VAS were used at 2, 6, and 12 months after treatment to measure the clinical outcomes. In addition, they evaluated the concentration of growth factors in PRP and their correlation to clinical outcomes. At all follow-ups, the PRP group had the lowest VAS scores. The WOMAC score of the PRP group was significantly better at 2 and 6-month follow-up but not at 12-month follow-up. The study demonstrates significant improvements in reducing pain and ameliorating the quality of life and functional recovery in the PRP group. A significant “moderate” correlation was also found between interleukin-10 and variations of the VAS score in this study (r = 0.392; p = .040). The authors concluded that PRP injections offer a significant clinical improvement in patients with hip OA without relevant side effects. The benefit was significantly more stable up to 12 months as compared with the other tested treatments. The addition of PRP + HA did not lead to a significant reduction in pain.
One patient in Battaglia’s series developed a superficial haematoma, however no other significant complications were reported in all series (Tab. 1).
Discussion
The hip joint is the second most affected joint with OA after the knee, with a high prevalence in patients older than 50 years(20). OA of the hip can be very devastating, and the significance of the disease is manifested in the persistent pain and functional impairment which undermine the patient’s quality of life. Currently, cartilage loss is not considered to be the main factor triggering OA. More accurately, the triggering factor is suggested to be a malfunction in the affected joint, including all the tissues that maintain the articular homeostasis. Subchondral bone is identified as the starting place for pathologic changes, and cartilage is the victim of this process. Subchondral bone pathology inevitably leads to the onset of OA if not properly treated(21).
To date, non-operative treatments remain very limited, and none were proven to stop or slow the progressive nature of the disease. Therefore, hip arthroplasty usually comes as the only definitive solution for patients with hip OA. Since PRP-based therapies have broken into clinical practice, they have been increasingly used as a non-operative option to treat earlier stages of joint degeneration to provide symptomatic relief and delay surgical intervention(22). The high amount of growth factors in PRP is thought to have an inductive and protective effect on chondrocytes as well as anti-inflammatory action, eventually restoring joint homeostasis.
The evidence from this review suggests that ultrasound-guided hip PRP injections may be efficacious in delivering long-term and clinically significant pain reduction in patients with hip OA, and may also lead to an improvement in function. The treatment effect appears to be of rapid onset, with better outcomes reported in the first 8 weeks post-injection. The magnitude of pain reduction and functional improvement decreases thereafter, although two trials report clinically significant differences in both pain and function at 12 months post-injection. Although these trials did not report details of the clinicians administering the injection, the procedure itself seemed well tolerated by patients, and only one significant minor complication was reported.
The limitations of this review include a small number of high-quality studies performed, and a relatively small number of participants. Small trials are known to potentially overestimate treatment effect sizes or report a significant effect when none is present. Furthermore, participants in these trials presented with variable degrees of OA. The authors reported outcomes at different follow-up intervals, and to enable comparison, it was necessary to use ranges (4–8 weeks and 4–6 months). The heterogeneity in methodology between trials, coupled with the limited reporting of trial statistics, makes it difficult to accurately compare results. This highlights the importance of the development and use of core outcomes for clinical trials in this area. All included RCTs compared PRP with HA, and there were no trials comparing PRP with placebo, limiting our ability to draw a firm conclusion.
Conclusion
Hip intra-articular PRP injections, when performed under ultrasound guidance, appear to be well tolerated and may be efficacious in delivering long-term and clinically significant pain reduction in patients with hip OA, and may also lead to an improvement in function, though the quality of evidence is relatively poor. Future trials should be sufficiently large and include a placebo group. Standardised outcomes, such as those recommended by the Osteoarthritis Research Society International (OARSI), should be used, and the results should be presented in a manner which will facilitate their inclusion in future meta-analyses.
Footnotes
Funding
None.
Conflict of interest
The authors declare they have no conflicts of interest.
References
- 1.Buckwalter JA, Martin JA: Osteoarthritis. Adv Drug Deliv Rev 2006; 58: 150–167. [DOI] [PubMed] [Google Scholar]
- 2.Cochran L, Banerjee P: Baby boomers and seniors: Understanding their leisure values enhances programs. Activ Adapt Aging 2010; 34: 196–215. [Google Scholar]
- 3.Bhatia D, Bejarano T, Novo M: Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci 2013; 5: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM. et al. : Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016; 12: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson MSM, Sarmanova A, Doherty M, Zhang W: Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: A meta-analysis of randomized controlled trials. Rheumatology 2018; 57: 1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali M, Oderuth E, Atchia I, Malviya A: The use of platelet-rich plasma in the treatment of greater trochanteric pain syndrome: A systematic literature review. J Hip Preserv Surg 2018; 5: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD: Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy 2016; 32: 495–505. [DOI] [PubMed] [Google Scholar]
- 8.Mishra AK, Skrepnik NV, Edwards SG, Jones GL, Sampson S, Vermillion DA. et al. : Efficacy of platelet-rich plasma for chronic tennis elbow: A double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med 2014; 42: 463–471. [DOI] [PubMed] [Google Scholar]
- 9.Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M: Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med 2012; 40: 534–541. [DOI] [PubMed] [Google Scholar]
- 10.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA: Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009; 37: 2259–2272. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Kwon OH, Kim TK, Cho YK, Choi KY, Chung HY. et al. : Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg 2013; 40: 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakudo N, Morimoto N, Kushida S, Ogawa T, Kusumoto K: Plateletrich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol 2014; 47: 83–89. [DOI] [PubMed] [Google Scholar]
- 13.Kajikawa Y, Morihara T, Sakamoto H, Matsuda K, Oshima Y, Yoshida A. et al. : Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol 2008; 215: 837–845. [DOI] [PubMed] [Google Scholar]
- 14.Yin W, Qi X, Zhang Y, Sheng J, Xu Z, Tao S. et al. : Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J Transl Med 2016; 14: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Altman DG; PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. : Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 17.Di Sante L, Villani C, Santilli V, Valeo M, Bologna E, Imparato L. et al. : Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason 2016; 18: 463–468. [DOI] [PubMed] [Google Scholar]
- 18.Dallari D, Stagni C, Rani N, Sabbioni G, Pelotti P, Torricelli P. et al. : Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: A randomized controlled study. Am J Sports Med 2016; 44: 664–671. [DOI] [PubMed] [Google Scholar]
- 19.Battaglia M, Guaraldi F, Vannini F, Rossi G, Timoncini A, Buda R. et al. : Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics 2013; 36: e1501–e1508. [DOI] [PubMed] [Google Scholar]
- 20.Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E: The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011; 19: 1270–1285. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C. et al. : Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res Ther 2013; 15: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mlynarek RA, Kuhn AW, Bedi A: Platelet-rich plasma (PRP) in orthopedic sports medicine. Am J Orthop (Belle Mead NJ) 2016; 45: 290–326. [PubMed] [Google Scholar]