Abstract
Confocal laser scanning microscopy (CLSM) is a modern imaging technique that enables the in vivo or ex vivo characterization of skin lesions located in the epidermis and superficial dermis with a high quasi-microscopic resolution. Currently, it is considered to be the most promising imaging tool for the evaluation of superficial skin tumors. The in vivo mode adds the advantage of noninvasive, dynamic, in real-time assessment of the tumor associated vasculature and inflammation. It offers the possibility to repeatedly examine the same skin area without causing any damage and to monitor disease progression and treatment outcome. Furthermore, this novel technology allows the evaluation of the entire lesion and can be used to guide biopsies and to define tumor margins before surgical excision or other invasive therapies. CLSM diagnostic features may differentiate between the various histologic subtypes of skin tumors and therefore helps in choosing the best therapeutic approach. In this study, we present the CLSM characteristic features of the most common melanocytic and non-melanocytic skin tumors, as well as future possible CLSM applications in the study of experimental skin tumorigenesis on animal models.
Keywords: in vivo, real-time, confocal imaging, quasi-microscopic resolution, skin tumors
1. Introduction
Within recent years, dermatologic imaging technology focused on the development of optical, noninvasive tools to improve diagnostic accuracy and to overcome the disadvantages of histopathological examination. Of all these promising in vivo tools, only confocal laser scanning microscopy (CLSM) allows the visualization of cutaneous structures with a resolution that is very close to that of light microscopy, thus performing a skin ‘optical biopsy’ (1). It enables the noninvasive, virtual sectioning of the skin at different depths, with grey-scale images obtained in horizontal planes (en face), parallel to the skin surface and it does not require tissue processing or coloring (2,3). As it allows repeated imaging of the same skin area in real-time, at different time intervals, it is an excellent method for monitoring disease progression and treatment efficacy and studying skin's dynamic behaviour (1,4–10).
Based on the source of image contrast, CLSM can be performed in either fluorescence or reflectance mode. Fluorescence confocal microscopy (FCM) requires the administration of a fluorescent agent to generate contrast (11) and has been used predominantly in experimental studies with promising results in lesional and nonlesional skin (12,13). Reflectance confocal microscopy (RCM) relies on differences in the refractive indices of cellular structures (14) and has been extensively applied in the noninvasive assessment of melanocytic (15–18) and non-melanocytic skin tumors (16,19), with features demonstrating a good correlation with dermoscopic and histologic findings. Furthermore, this novel imaging technique proved to be useful for the diagnosis of various inflammatory skin diseases (20), conditions with dermatologic manifestations (21), as well as for the study of dynamic processes like wound healing (22,23), in real-time assessment of blood flow in response to various topical stimuli (10,24) or leucocyte migration (5,6).
Currently, in vivo RCM is considered to be the most promising noninvasive imaging technique for the quasi-microscopic morphological and dynamic characterization of superficial skin tumors. Moreover, it helps to define tumor margins before surgical excision or other invasive treatment modalities (19). Moreover, ex vivo settings may guide Mohs micrographic surgery (25,26). Recently, novel multilaser devices, combining CLSM in reflectance mode with fluorescence techniques were developed, providing useful additional information when compared with the use of each variant of confocal microscope alone (13).
In this study, we present the RCM characteristic features of the most common melanocytic and non-melanocytic skin tumors (Table I), as well as future possible CLSM applications in the study of experimental skin tumorigenesis on animal models.
Table I.
Summary of the diagnostic reflectance confocal microscopy features for common skin cancers.
| Type of skin cancer | Reflectance confocal microscopy features | Author, year (Refs.) |
|---|---|---|
| Basal cell carcinoma | Bright tumor islands/dark silhouettes | Caruntu et al, 2014 (33) |
| Peritumoral clefting | Ghita et al, 2016 (31) | |
| Streaming of the epidermis | González and Tannous, 2002 (36) | |
| Prominent and tortuous blood vessels | Longo et al, 2014 (32) | |
| Inflammatory cells ± bright dendritic structures | Nori et al, 2004 (35) | |
| Spoke wheel-like structures | Peppelman et al, 2013 (34) | |
| Segura et al, 2007 (41) | ||
| Stephens et al, 2013 (37) | ||
| Ulrich et al, 2010 (38) | ||
| Squamous cell carcinoma | Hyperkeratois | Aghassi et al, 2000 (55) |
| Disarranged honeycomb pattern with enlarged, pleomorphic nuclei in the spinous-granular layers | Branzan et al, 2006 (58) | |
| Peppelman et al, 2014 (56) | ||
| Round, nucleated bright cells in the suprabasal epidermis | Que et al, 2015 (39) | |
| Elongated dermal papillae with looping, round vessels | Rishpon et al, 2009 (57) | |
| Melanoma | Pagetoid spread of roundish or dendritic cells in the epidermis | Carrera et al, 2012 (80) |
| Pleomorphic cells and atypical nests at the dermo-epidermal junction | Guida et al, 2015 (16) | |
| Pellacani et al, 2007 (15) | ||
| Non-edged papillae and atypical nucleated cells in the papillary dermis | Pellacani et al, 2014 (17) | |
| Ulrich and Lange-Asschenfeldt, 2013 (79) | ||
| Poorly defined or absent keratinocytes cell borders | ||
| Mycosis fungoides | Weakly refractile cells (lymphocytes), Vesicle-like spaces (Pautrier collections) within the epidermis | Agero et al, 2007 (90) |
| Fabbrocini et al, 2017 (85) | ||
| Hypo-refractile papillary rings and dilated capillaries | Koller et al, 2009 (89) | |
| Lange-Asschenfeldt et al, 2012 (88) | ||
| Li et al, 2013 (87) | ||
| Mancebo et al, 2016 (86) | ||
| Primary cutaneous folliculocentric lymphoma | Round-shaped, highly-refractive tumor masses | Unpublished study |
| Bright cells of various sizes and numerous bright small cells (lymphocytes) at the periphery of tumor masses |
2. Application of reflectance confocal microscopy for skin cancer diagnosis
Basal cell carcinoma (BCC) is the most common of all cancers in light-skinned individuals (27) and its incidence is still rising with ~10% each year worldwide (28). Very often, a skin biopsy is needed to confirm the diagnosis, despite its associated invasiveness and costs. Early diagnosis and treatment are of paramount importance because it is locally destructive and can lead to disfigurement (29,30). RCM diagnostic features for various clinical types of BCC have been described (31–36), demonstrating a good correlation with certain dermoscopic and histopathologic findings (37,39). BCC consists of aggregates of basaloid cells at the dermo-epidermal junction or papillary dermis that appear in RCM images either as ‘bright tumor islands’, cord-like structures surrounded by cleft-like dark spaces, either as ‘dark silhouettes’, hyporeflective dark areas outlined by bright stromal tissue (36,38–40). These aggregates of basaloid cells often have nuclei that are oriented along the same axis, displaying a ‘peripheral palisading’ at the periphery of tumor islands (35,36). In the above stratum spinosum, the elongated nuclei of keratinocytes that are polarized along the same axis form the typical ‘streaming of the epidermis’ (35). Additionally, prominent and tortuous blood vessels with intense leukocyte traffic are present in the dermis and numerous inflammatory cells with various shape and sizes (lymphocytes, melanophages) surround the tumor nests (36). In pigmented BCC, bright dendritic structures that correspond to dendritic melanocytes can be identified inside aggregates of basaloid cells (41) (Fig. 1).
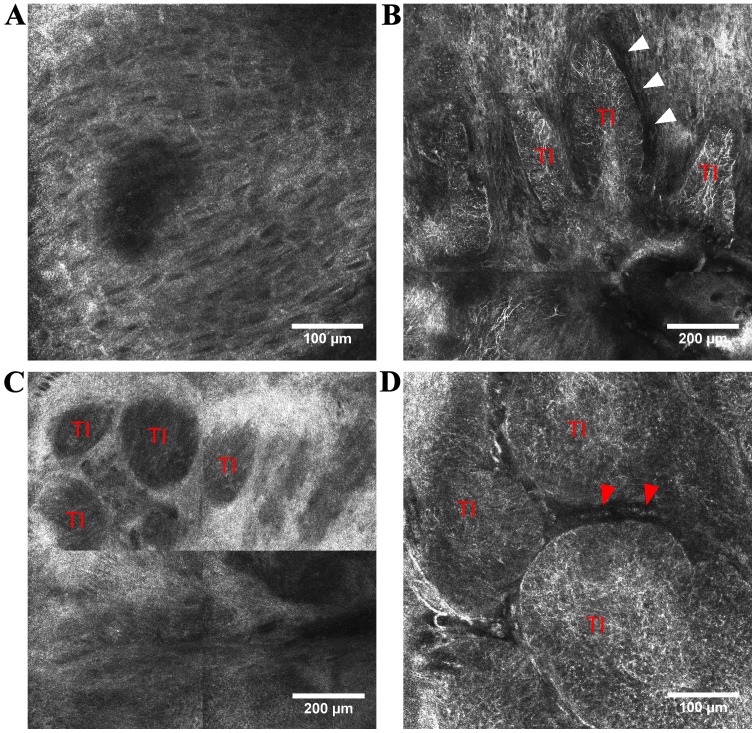
Figure 1.
RCM features of BCC. (A) RCM image (500×500 µm) showing polarization of keratinocytes along the same axis forming epidermal ‘streaming’; (B) RCM mosaic (1×1 mm) in the tumoral area of a pigmented BCC displaying elongated TI infiltrated by bright dendritic cells, peripheral palisading and peritumoral dark spaces (white arrowheads), also known as ‘clefting’; (C) RCM mosaic (1×1 mm) showing ‘dark silhouettes’ representing TI, in the tumor region of a nodular BCC; (D) RCM image (500×500 µm) of BCC showing TI infiltrated by dendritic cells and blood vessels (red arrowheads) surrounding the neoplastic dermal aggregates. TIs, tumor islands.
A retrospective, multicenter study evaluated the sensitivity and specificity of five RCM criteria for BCC, including architectural alteration and cellular pleomorphism of the overlying epidermis, areas of refractile tumor cells with elongated, monomorphic nuclei, nuclear polarization, increased dermal vasculature and prominent inflammatory infiltrate (35). Identification of two or more of these five criteria in a sample showed a sensitivity of 100% for BCC diagnosis, whereas four or more of these had a specificity of 95.7% and a sensitivity of 82.9% (35). Of these criteria, elongated, monomorphic nuclei proved to be the most sensitive (100%) and nuclear polarization the most sensitive (91.6%) and specific (97.1%) (35).
Furthermore, this novel technology may be a diagnostic guide in defining the margins of the lesion before surgical excision (42) or laser ablation (29). During Mohs micrographic surgery, FCM proved to be far superior than RCM for tumor margin assessment in BCC (36). Moreover, it offers the advantage of monitoring noninvasive treatment in superficial-type BCC, thus avoiding the discomfort associated with skin biopsy (43,44).
Squamous cell carcinoma (SCC) is the 2nd most frequent non-melanoma skin cancer after BCC and appears dominantly in sun exposed areas. Besides UV exposure, various risk factors, including immunosuppression, viral infections, exposure to chemical agents, neuro-endocrine factors or chronic inflammation, have been proposed to be involved in SCC pathogenesis (45–51). It has various clinical presentations including in situ lesions (Bowen's disease), invasive superficial lesions or highly infiltrative lesions (52). Actinic keratosis (AK) is the most common precancerous skin lesion with a risk of progression to a full-thickness SCC estimated at 5–10% (53), but some authors consider it as an early form of SCC as it appears (54). Under RCM evaluation, SCC and hypertrophic AK often present a pronounced hyperkeratosis that limits the depth of penetration considerably (55) and provides whitewashed images because of the strong back-reflectance at the keratin-rich surface of the tumor (39). A more pronounced disarranged honeycomb pattern in the spinous-granular layers and the presence of neoplastic aggregates in the dermis can distinguish SCC from AK (56). Moreover, nuclei are enlarged and pleomorphic (55) and roundish, nucleated bright cells with a pagetoid arrangement are often observed in the suprabasal epidermis. When the thickness of the lesion allows the dermo-epidermal junction imaging, dermal papillae may appear elongated with looping, round vessels inside them (39,57) (Fig. 2). However, in case of infiltrative lesions, the level of invasion is usually inaccessible in CLSM (58). Even with ex vivo CLSM during Mohs surgery, the detection of residual SCC is rarely possible also because of the non-reflecting features of keratinization (58,59).
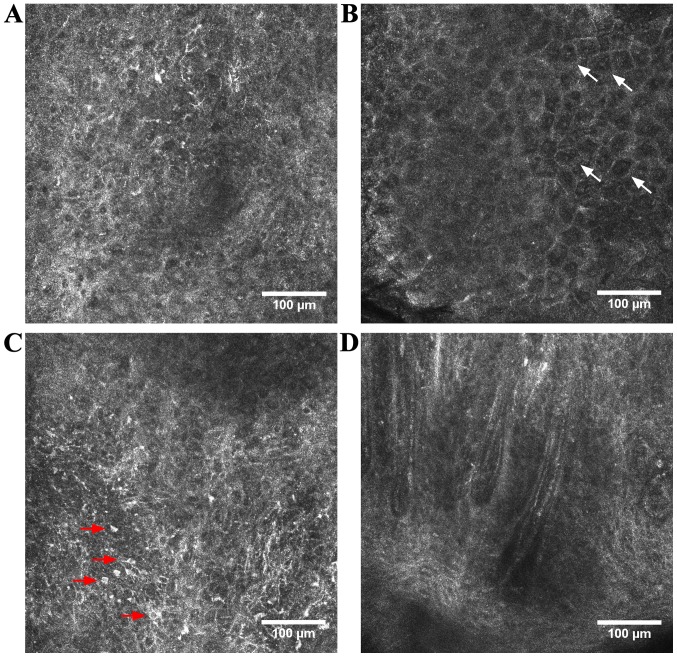
Figure 2.
RCM features of SCC. (A) RCM image (500×500 µm) of an atypical honeycomb pattern in the stratum spinosum; (B) RCM image (500×500 µm) of dyskeratotic cells, also known as ‘targetoid cells’ (white arrows); (C) RCM image (500×500 µm) at epidermal level showing roundish, nucleated, bright cells with a pagetoid arrangement (red arrows); (D) RCM image (500×500 µm) showing looped vessels in the tumoral region of an SCC.
When it comes to RCM evaluation of SCC localized on the lips, distinctive features were described (60). Moreover, RCM evaluation has the potential to distinguish between features of normal mucosa, dysplasia and lip SCC in real-time and therefore may be useful for the preoperative assessment of tumor resection margins (61).
Cutaneous melanoma is one of the most aggressive human malignancies, associated with high mortality rates, despite latest advances in therapy (62–65). An important genetic background and several environmental factors are key players in melanoma development and progression (66–70). Two crucial points have to be taken into account in this form of aggressive skin cancer. One is particularly in high-risk patients, where melanomas may be complicated to distinguish from nevi (71) and the fact that numerous biopsy specimens for screening are associated with patient morbidity. Therefore, if a dermatologist is confronted with a lesion obeying the ABCDE rule of melanoma (72) or if the atypical lesion is solitary/is the ‘ugly duckling’ (73) there are no particular issues for a dermatologist. Conversely, in patients with numerous and clinically atypical nevi, visually identifying the lesion with the greatest atypical features that may represent a new or developing melanoma is almost impossible. Removing high numbers of nevi in such patients for finding one melanoma can be a screening method, but although there are extended publications on how many nevi should be removed in high-risk patients to identify one melanoma (74–77), there are still issues such as removing too few nevi can be associated with overlooked melanomas and/or significant medical system costs (78).
In particular, for this aggressive type of skin cancer RCM allows a noninvasive in vivo imaging at cellular-level from superficial melanomas to dermis invading melanomas. This important new tool has an emerging diagnostic role in the characterization of melanomas as a noninvasive in vivo histomorphological analysis and as an added device in following the clinical management of skin cancer patients (79).
In the case of melanoma, the melanocytic lesions have in the upper parts of the tumor pagetoid roundish or dendritic cells in the superficial epidermis, atypical nests at the dermo-epidermal junction, non-edged papillae and atypical nucleated cells in the papillary dermis. The benefit of RCM in vivo examination in real-time is important also in particular cases of melanoma like lentigo maligna melanoma and amelanotic melanoma. Moreover, this technology can add information on management of subclinical margins, recurrences, or monitoring noninvasive treatment of tumors (80).
Studies that focused on the application of this technology in melanoma diagnosis have shown that melanocyte-derived tumor cells can be demarcated from non-melanocytic ones. Thus, our experience has shown that, while benign nevi have monomorphic cells, round to oval in shape, with bright appearance, in melanomas cells are bright, polymorphic and irregular, roundish or with branching dendrites (Fig. 3). In benign nevi, junctional and dermal nevus cell nests can be found, while in melanomas there is a disarray of the melanocytic cell architecture. Keratinocyte cell borders can be detected readily but are poorly defined or absent in melanomas. The horizontal optical sections in RCM offer a better visualization of malignant melanocyte morphology than classical hematoxylin and eosin stained histologic sections (15,81). In addition, based on their cell morphology in RCM, four types of melanomas have been identified, namely dendritic cell melanomas, melanomas with roundish melanocytes, melanomas with predominant dermal nesting proliferation and combined type melanomas, each with different tumor and patient characteristics (15).
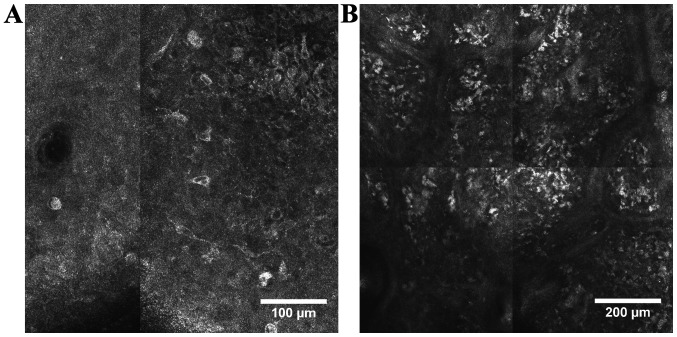
Figure 3.
RCM features of cutaneous melanoma. (A) RCM image (500×500 µm) displaying big, roundish and dendritic, bright, pagetoid cells infiltrating the epidermis; (B) RCM mosaic (1×1 mm) showing large heterogeneous nests of melanocytes with the presence of numerous atypical cells in the tumoral region of a cutaneous melanoma.
More elaborated studies seeking to evaluate specificity and sensitivity of this technology have reported that there is a good differentiation between benign versus malignant tumors. Thus, depending on the observers, the sensitivity ranged from 90.42 to 97.62% and the specificity from 96.67 to 100%. These values generated good performance of the investigation: sensitivity, 94.65%; specificity, 96.67%; positive predictive value 97.50%; and negative predictive value 92.99% (82).
Gathering important information from large studies, this quasi-histological in vivo evaluation has no restrictions for age, sex, ethnicity and has a good association with clinical, dermoscopic and histopathologic findings. Therefore, diagnostic accuracy, sensitivity and specificity of the technique were a good backbone to implement it in the diagnosis of melanoma (16). This new technology brings, besides non-invasiveness characteristics, new mapping possibilities of difficult melanomas like lentigo maligna of the face (78).
Cutaneous lymphomas are a heterogeneous group of lymphoproliferative disorders involving the skin that are characterized by clonal proliferation of mature T-lymphocytes (>60% of all cases), B-lymphocytes or NK cells (83,84). Histopathological examination combined with immunohistochemistry of the skin biopsy specimen is the mainstay of the diagnosis, although sequential biopsies are often needed, especially in case of early stage lesions.
RCM has already been reported to be useful for the in vivo diagnosis (85–91) and therapeutic follow-up of cutaneous T-cell lymphomas (92), with the majority of studies referring to its most common type, mycosis fungoides (85–90) and one to lymphomatoid papulosis (91).
Mycosis fungoides, early patch lesions in particular, can imitate a wide variety of erythematosquamous skin diseases and its clinical and histopathological diagnosis is often a challenge. Most commonly reported RCM features of mycosis fungoides correlate with histopathologic findings and include weakly refractile cells (lymphocytes) and vesicle-like spaces (Pautrier collections) within the epidermis, hypo-refractile papillary rings and dilated capillaries with thick walls at the dermo-epidermal junction (90). Detection of Pautrier collections with RCM is associated with improved histopathologic diagnosis and presence of TCR gene clonality (86).
The rest of the RCM findings are non-specific and reflect the heterogeneous clinical and histopathologic presentation of the lesions (86). However, in vivo RCM seems to be reliable in guiding skin biopsy collection, therefore reducing the number of unsuccessful histopathological examinations for mycosis fungoides lesions (85,87).
In contrast to cutaneous T-cell lymphomas, to date no RCM features have been described for the diagnosis of B-cell lymphomas. Our research team recently described the in vivo RCM features observed in primary cutaneous folliculocentric lymphoma lesions (unpublished results). These correlate with histopathology and include round-shaped, highly-refractive tumor masses in the dermis, bright cells of various sizes dispersed throughout the dermis and aggregates of bright small cells (lymphocytes) at the periphery of tumor masses (Fig. 4).
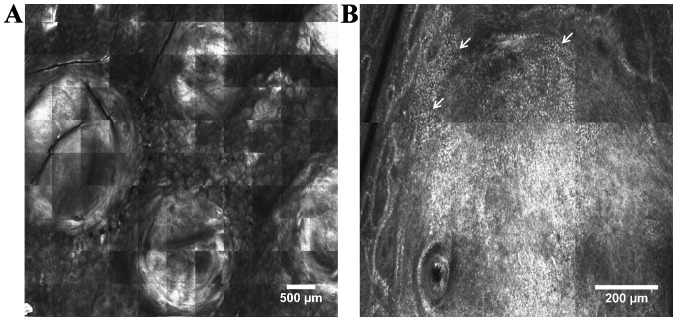
Figure 4.
RCM features of cutaneous lymphoma. (A) RCM mosaic (5.5×5.5 mm) showing well-demarcated, round-shaped, highly-refractive tumor masses; (B) RCM mosaic (1×1 mm) displaying aggregates of bright small cells (white arrows) at the periphery of a tumor mass.
3. Application of confocal scanning laser microscopy for skin oncology research
Skin carcinogenesis is a complex, multifactorial process and the topic of intensive research given the continuously increasing incidence of skin cancer. In addition to the recognized genetic and environmental factors (93), prolonged exposure to pro-inflammatory cytokines and chemokines within chronic inflammation is experimentally sustained to favor initiation and progression of skin cancer (94).
Mouse models of chemically induced skin carcinogenesis are one of the most available and cost-effective models to analyze early alterations and pathways involved in skin tumorigenesis (95). The two-stage skin carcinogenesis model has been used to study mechanisms of epithelial cancers (96) and it refers to the two-step topical administration of chemicals to mouse skin for the initiation and promotion phases of skin tumorigenesis. This delimitation of phases allows the observation of premalignant lesions (96) and it offers more reliable results when testing the effects of environmental factors and drugs on skin tumors (95).
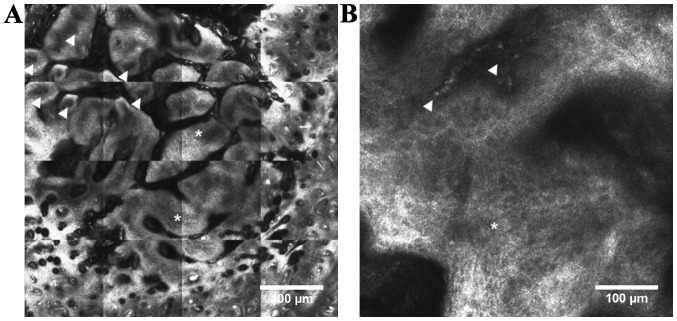
In vivo CLSM is a new imaging technology, not yet fully explored for investigating murine skin structures within experimental tumorigenesis. Reflectance mode CLSM was reported to allow real-time observation of abnormal tissue architecture, atypical structures, as well as the blood flow and vasculature that accompanies skin tumors (95) (Fig. 5). Dendritic immune cells are difficult to differentiate from melanocytes under RCM as they have similar morphologic features (97), but activated Langerhans cells seem to have a more superficial epidermal localization (41).
Figure 5.
Chemically induced carcinogenesis in CD1-Foxn1nu mouse. (A) RCM mosaic (2×2 mm) showing a tumor with multi-lobular structure with altered keratinocyte architecture (white asterisks) and numerous blood vessels (white arrowheads); (B) detailed RCM image (500×500 µm) showing atypical cells (white asterisk) and an enlarged, tortuous blood vessel (white arrowheads).
Fluorescence mode CLSM studies have been done on transgenic mice using green fluorescent protein marker to visualize cellular details of the skin (11). As it allows sequential noninvasive examination of the same skin area, CLSM technology seems to be ideal for monitoring tumor progression (98) and therapeutic effects of anticancer agents in mouse models of skin carcinogenesis (95). Recently, a dual mode in vivo reflectance and fluorescence CLSM has been developed and holds significant promise for imaging tumor progression in murine skin (98). This system combines the fluorescence contrast of targeted tumor cells with the acquired reflectance contrast of examined cells and tissues and place them within a histologically meaningful framework (98). In addition to the in situ visualization of tumor cell proliferation and vascular structures, it has been shown that combined reflectance/fluorescence in vivo CLSM has the ability to image dendritic immune cell trafficking to inoculated tumors and to monitor tumor induced immune response in the skin (98).
4. Limitations and future perspectives
Despite the great advantages CLSM adds to dermatological practice, it also has some limitations, the most recognized being the restricted depth of penetration to 200–300 µm, that allows imaging only of the epidermis and upper dermis (3,4). Therefore, the deeper part of the dermis and the hypodermis cannot be visualized using the currently commercially available confocal microscopes. Examination of deeper skin structures could be achieved using higher laser power, but at the expense of damaging the skin area under evaluation (52). There are attempts to develop new devices that improve light collection from the examined plane in order to increase depth of penetration (99). Moreover, examination of skin lesions by means of CLSM is more time consuming than clinical evaluation or dermoscopy and it needs training and experience for the interpretation of CLSM images. Recent technological breakthroughs have led to the development of new, smaller and more practical hand-held devices that offer faster image acquisition and allow the examination of lesions located in less accessible body areas (52). Unlike vertical sections obtained in conventional histology, CLSM enables virtual sectioning of the skin at different depths, in horizontal planes (en face), parallel to the skin surface (3). For a better correlation with histology sections, current efforts are aimed at developing devices that could also perform optical sections of vertical planes and then compile 3-D reconstructions of the lesions (100). In addition, CLSM does not require tissue processing or coloring and images are obtained in greyscale, similar to X-rays or ultrasonography (2,3). To improve contrast of epidermal and dermal structures and toward color-enhanced in vivo CSLM imaging, fluorescent dyes like indocyanine-green are being tested (101).
5. Conclusions
CLSM is a modern imaging technique that enables the noninvasive characterization of skin lesions located in the epidermis and superficial dermis with a high resolution. Currently, it is considered to be the most promising imaging technique for the quasi-microscopic morphological and dynamic characterization of superficial skin tumors. The in vivo mode adds the advantage of noninvasive, dynamic, in real-time assessment of the tumor associated vasculature and inflammation. It allows sequential noninvasive examination of the same skin area without causing any damage and to monitor disease progression and treatment outcome. Furthermore, CLSM technology seems to be ideal for monitoring tumor progression and therapeutic effects of anticancer agents in mouse models of experimental skin carcinogenesis.
Acknowledgements
Not applicable.
Funding
This study was partially supported by a grant of the Romanian Ministry of Research and Innovation (CCCDI-UEFISCDI; project no. 61PCCDI⁄2018 PN-III-P1-1.2-PCCDI-2017-0341) within PNCDI–III.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MAI, CC, ML, DL, MT, SGR, AB, CC, MN, SAZ and DB were responsible for acquisition of references, analysis and systematization of data, and contributed to writing the manuscript and revising it critically for important intellectual content. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Diaconeasa A, Boda D, Neagu M, Constantin C, Căruntu C, Vlădău L, Guţu D. The role of confocal microscopy in the dermato-oncology practice. J Med Life. 2011;4:63–74. [PMC free article] [PubMed] [Google Scholar]
- 2.Aghassi D, González E, Anderson RR, Rajadhyaksha M, González S. Elucidating the pulsed-dye laser treatment of sebaceous hyperplasia in vivo with real-time confocal scanning laser microscopy. J Am Acad Dermatol. 2000;43:49–53. doi: 10.1067/mjd.2000.105566. [DOI] [PubMed] [Google Scholar]
- 3.González S, Swindells K, Rajadhyaksha M, Torres A. Changing paradigms in dermatology: Confocal microscopy in clinical and surgical dermatology. Clin Dermatol. 2003;21:359–369. doi: 10.1016/j.clindermatol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Rajadhyaksha M, González S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: Advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 5.González S, Sackstein R, Anderson RR, Rajadhyaksha M. Real-time evidence of in vivo leukocyte trafficking in human skin by reflectance confocal microscopy. J Invest Dermatol. 2001;117:384–386. doi: 10.1046/j.0022-202x.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 6.Peppelman M, Wolberink EA, Gerritsen MJ, van de Kerkhof PC, van Erp PE. Application of leukotriene B4 and reflectance confocal microscopy as a noninvasive in vivo model to study the dynamics of skin inflammation. Skin Res Technol. 2015;21:232–240. doi: 10.1111/srt.12181. [DOI] [PubMed] [Google Scholar]
- 7.Batani A, Brănișteanu DE, Ilie MA, Boda D, Ianosi S, Ianosi G, Caruntu C. Assessment of dermal papillary and microvascular parameters in psoriasis vulgaris using in vivo reflectance confocal microscopy. Exp Ther Med. 2018;15:1241–1246. doi: 10.3892/etm.2017.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Căruntu C, Boda D, Căruntu A, Rotaru M, Baderca F, Zurac S. In vivo imaging techniques for psoriatic lesions. Rom J Morphol Embryol. 2014;55(Suppl 3):1191–1196. [PubMed] [Google Scholar]
- 9.Ghiţă MA, Căruntu C, Rosca AE, Căruntu A, Moraru L, Constantin C, Neagu M, Boda D. Real-time investigation of skin blood flow changes induced by topical capsaicin. Acta Dermatovenerol Croat. 2017;25:223–227. [PubMed] [Google Scholar]
- 10.Căruntu C, Boda D. Evaluation through in vivo reflectance confocal microscopy of the cutaneous neurogenic inflammatory reaction induced by capsaicin in human subjects. J Biomed Opt. 2012;17:085003. doi: 10.1117/1.JBO.17.8.085003. [DOI] [PubMed] [Google Scholar]
- 11.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/S0014-5793(97)00313-X. [DOI] [PubMed] [Google Scholar]
- 12.Meyer LE, Otberg N, Sterry W, Lademann J. In vivo confocal scanning laser microscopy: Comparison of the reflectance and fluorescence mode by imaging human skin. J Biomed Opt. 2006;11:044012. doi: 10.1117/1.2337294. [DOI] [PubMed] [Google Scholar]
- 13.Skvara H, Plut U, Schmid JA, Jonak C. Combining in vivo reflectance with fluorescence confocal microscopy provides additive information on skin morphology. Dermatol Pract Concept. 2012;2:3–12. doi: 10.5826/dpc.0201a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. J Invest Dermatol. 1995;104:946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 15.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759–2765. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 16.Guida S, Longo C, Casari A, Ciardo S, Manfredini M, Reggiani C, Pellacani G, Farnetani F. Update on the use of confocal microscopy in melanoma and non-melanoma skin cancer. G Ital Dermatol Venereol. 2015;150:547–563. [PubMed] [Google Scholar]
- 17.Pellacani G, De Pace B, Reggiani C, Cesinaro AM, Argenziano G, Zalaudek I, Soyer HP, Longo C. Distinct melanoma types based on reflectance confocal microscopy. Exp Dermatol. 2014;23:414–418. doi: 10.1111/exd.12417. [DOI] [PubMed] [Google Scholar]
- 18.Serban ED, Farnetani F, Pellacani G, Constantin MM. Role of in vivo reflectance confocal microscopy in the analysis of melanocytic lesions. Acta Dermatovenerol Croat. 2018;26:64–67. [PubMed] [Google Scholar]
- 19.González S, Sánchez V, González-Rodríguez A, Parrado C, Ullrich M. Confocal microscopy patterns in nonmelanoma skin cancer and clinical applications. Actas Dermosifiliogr. 2014;105:446–458. doi: 10.1016/j.ad.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Białek-Galas K, Wielowieyska-Szybińska D, Dyduch G, Wojas-Pelc A. The use of reflectance confocal microscopy in selected inflammatory skin diseases. Pol J Pathol. 2015;66:103–108. doi: 10.5114/pjp.2015.53001. [DOI] [PubMed] [Google Scholar]
- 21.Constantin MM, Bucur S, Serban DE, Caruntu C, Orzan OA, Constantin T. Dermoscopic and laser confocal features of an exogenous ochronosis case. G Ital Dermatol Venereol. 2018 Jun 29; doi: 10.23736/S0392-0488.18.06024-8. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Lange-Asschenfeldt S, Bob A, Terhorst D, Ulrich M, Fluhr J, Mendez G, Roewert-Huber HJ, Stockfleth E, Lange-Asschenfeldt B. Applicability of confocal laser scanning microscopy for evaluation and monitoring of cutaneous wound healing. J Biomed Opt. 2012;17:076016. doi: 10.1117/1.JBO.17.7.076016. [DOI] [PubMed] [Google Scholar]
- 23.Altintas AA, Altintas MA, Ipaktchi K, Guggenheim M, Theodorou P, Amini P, Spilker G. Assessment of microcirculatory influence on cellular morphology in human burn wound healing using reflectance-mode-confocal microscopy. Wound Repair Regen. 2009;17:498–504. doi: 10.1111/j.1524-475X.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 24.Altintas AA, Guggenheim M, Oezcelik A, Gehl B, Aust MC, Altintas MA. Local burn versus local cold induced acute effects on in vivo microcirculation and histomorphology of the human skin. Microsc Res Tech. 2011;74:963–969. doi: 10.1002/jemt.20982. [DOI] [PubMed] [Google Scholar]
- 25.Longo C, Ragazzi M, Rajadhyaksha M, Nehal K, Bennassar A, Pellacani G, Malvehy Guilera J. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol Clin. 2016;34:497–504. doi: 10.1016/j.det.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennàssar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170:360–365. doi: 10.1111/bjd.12671. [DOI] [PubMed] [Google Scholar]
- 27.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/S0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 28.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen CS, Sierra H, Cordova M, Rajadhyaksha M. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: A promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994–998. doi: 10.1001/jamadermatol.2013.10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lupu M, Caruntu C, Ghita MA, Voiculescu V, Voiculescu S, Rosca AE, Caruntu A, Moraru L, Popa IM, Calenic B, et al. Gene expression and proteome analysis as sources of biomarkers in basal cell carcinoma. Dis Markers. 2016;2016:9831237. doi: 10.1155/2016/9831237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghita MA, Caruntu C, Rosca AE, Kaleshi H, Caruntu A, Moraru L, Docea AO, Zurac S, Boda D, Neagu M, et al. Reflectance confocal microscopy and dermoscopy for in vivo, non-invasive skin imaging of superficial basal cell carcinoma. Oncol Lett. 2016;11:3019–3024. doi: 10.3892/ol.2016.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longo C, Lallas A, Kyrgidis A, Rabinovitz H, Moscarella E, Ciardo S, Zalaudek I, Oliviero M, Losi A, Gonzalez S, et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J Am Acad Dermatol. 2014;71:716–724. doi: 10.1016/j.jaad.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 33.Căruntu C, Boda D, Guţu DE, Căruntu A. In vivo reflectance confocal microscopy of basal cell carcinoma with cystic degeneration. Rom J Morphol Embryol. 2014;55:1437–1441. [PubMed] [Google Scholar]
- 34.Peppelman M, Wolberink EA, Blokx WA, van de Kerkhof PC, van Erp PE, Gerritsen MJ. In vivo diagnosis of basal cell carcinoma subtype by reflectance confocal microscopy. Dermatology. 2013;227:255–262. doi: 10.1159/000354762. [DOI] [PubMed] [Google Scholar]
- 35.Nori S, Rius-Díaz F, Cuevas J, Goldgeier M, Jaen P, Torres A, González S. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: A multicenter study. J Am Acad Dermatol. 2004;51:923–930. doi: 10.1016/j.jaad.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 36.González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869–874. doi: 10.1067/mjd.2002.124690. [DOI] [PubMed] [Google Scholar]
- 37.Stephens A, Fraga-Braghiroli N, Oliviero M, Rabinovitz H, Scope A. Spoke wheel-like structures in superficial basal cell carcinoma: A correlation between dermoscopy, histopathology, and reflective confocal microscopy. J Am Acad Dermatol. 2013;69:e219–e221. doi: 10.1016/j.jaad.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 38.Ulrich M, Roewert-Huber J, González S, Rius-Diaz F, Stockfleth E, Kanitakis J. Peritumoral clefting in basal cell carcinoma: Correlation of in vivo reflectance confocal microscopy and routine histology. J Cutan Pathol. 2011;38:190–195. doi: 10.1111/j.1600-0560.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- 39.Que SK, Fraga-Braghiroli N, Grant-Kels JM, Rabinovitz HS, Oliviero M, Scope A. Through the looking glass: Basics and principles of reflectance confocal microscopy. J Am Acad Dermatol. 2015;73:276–284. doi: 10.1016/j.jaad.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 40.Lupu M, Caruntu C, Solomon I, Popa A, Lisievici C, Draghici C, Papagheorghe L, Voiculescu VM, Giurcaneanu C. The use of in vivo reflectance confocal microscopy and dermoscopy in the preoperative determination of basal cell carcinoma histopathological subtypes. DermatoVenerol. 2017;62:7–13. [Google Scholar]
- 41.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Dendritic cells in pigmented basal cell carcinoma: A relevant finding by reflectance-mode confocal microscopy. Arch Dermatol. 2007;143:883–886. doi: 10.1001/archderm.143.7.883. [DOI] [PubMed] [Google Scholar]
- 42.Pan ZY, Lin JR, Cheng TT, Wu JQ, Wu WY. In vivo reflectance confocal microscopy of basal cell carcinoma: Feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945–1950. doi: 10.1111/j.1524-4725.2012.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webber SA, Wurm EMT, Douglas NC, Lambie D, Longo C, Pellacani G, Soyer HP. Effectiveness and limitations of reflectance confocal microscopy in detecting persistence of basal cell carcinomas: A preliminary study. Australas J Dermatol. 2011;52:179–185. doi: 10.1111/j.1440-0960.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 44.Ahlgrimm-Siess V, Horn M, Koller S, Ludwig R, Gerger A, Hofmann-Wellenhof R. Monitoring efficacy of cryotherapy for superficial basal cell carcinomas with in vivo reflectance confocal microscopy: A preliminary study. J Dermatol Sci. 2009;53:60–64. doi: 10.1016/j.jdermsci.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Voiculescu V, Calenic B, Ghita M, Lupu M, Caruntu A, Moraru L, Voiculescu S, Ion A, Greabu M, Ishkitiev N, et al. From normal skin to squamous cell carcinoma: A quest for novel biomarkers. Dis Markers. 2016;2016:4517492. doi: 10.1155/2016/4517492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boda D, Neagu M, Constantin C, Voinescu RN, Caruntu C, Zurac S, Spandidos DA, Drakoulis N, Tsoukalas D, Tsatsakis AM. HPV strain distribution in patients with genital warts in a female population sample. Oncol Lett. 2016;12:1779–1782. doi: 10.3892/ol.2016.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE, Voiculescu V, et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review) Int J Oncol. 2018;52:637–655. doi: 10.3892/ijo.2018.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Georgescu SR, Sârbu MI, Matei C, Ilie MA, Caruntu C, Constantin C, Neagu M, Tampa M. Capsaicin: Friend or foe in skin cancer and other related malignancies? Nutrients. 2017;9:1365. doi: 10.3390/nu9121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupu M, Caruntu A, Caruntu C, Papagheorghe LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki M, et al. Neuroendocrine factors: The missing link in non-melanoma skin cancer (Review) Oncol Rep. 2017;38:1327–1340. doi: 10.3892/or.2017.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solomon I, Voiculescu VM, Caruntu C, Lupu M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C, et al. Neuroendocrine factors and head and neck squamous cell carcinoma: An affair to remember. Dis Markers. 2018;2018:9787831. doi: 10.1155/2018/9787831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tampa M, Caruntu C, Mitran M, Mitran C, Sarbu I, Rusu LC, Matei C, Constantin C, Neagu M, Georgescu SR. Markers of oral lichen planus malignant transformation. Dis Markers. 2018;2018:1959506. doi: 10.1155/2018/1959506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.González S, Ahlgrimm-Siess V. Reflectance confocal microscopy in dermatology: Fundamentals and clinical applications. Aula Médica. 2012:p111. [Google Scholar]
- 53.Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895–904. doi: 10.1111/j.1365-4632.2007.03166.x. [DOI] [PubMed] [Google Scholar]
- 54.Wolff K, Johnson RA. 6th. McGraw-Hill; New York, NY: 2009. Fitzpatrick's Color Atlas and Synopsis of Clinical Dermatology; pp. 267–270. [Google Scholar]
- 55.Aghassi D, Anderson RR, González S. Confocal laser microscopic imaging of actinic keratoses in vivo: A preliminary report. J Am Acad Dermatol. 2000;43:42–48. doi: 10.1067/mjd.2000.105565. [DOI] [PubMed] [Google Scholar]
- 56.Peppelman M, Nguyen KP, Hoogedoorn L, van Erp PE, Gerritsen MJ. Reflectance confocal microscopy: Non-invasive distinction between actinic keratosis and squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2015;29:1302–1309. doi: 10.1111/jdv.12806. [DOI] [PubMed] [Google Scholar]
- 57.Rishpon A, Kim N, Scope A, Porges L, Oliviero MC, Braun RP, Marghoob AA, Fox CA, Rabinovitz HS. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766–772. doi: 10.1001/archdermatol.2009.134. [DOI] [PubMed] [Google Scholar]
- 58.Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology. Lasers Med Sci. 2007;22:73–82. doi: 10.1007/s10103-006-0416-8. [DOI] [PubMed] [Google Scholar]
- 59.Chung VQ, Dwyer PJ, Nehal KS, Rajadhyaksha M, Menaker GM, Charles C, Jiang SB. Use of ex vivo confocal scanning laser microscopy during Mohs surgery for nonmelanoma skin cancers. Dermatol Surg. 2004;30:1470–1478. doi: 10.1097/00042728-200412010-00009. [DOI] [PubMed] [Google Scholar]
- 60.Lupu M, Caruntu A, Caruntu C, Boda D, Moraru L, Voiculescu V, Bastian A. Non-invasive imaging of actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin Oncol. 2018;8:640–646. doi: 10.3892/mco.2018.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark AL, Gillenwater AM, Collier TG, Alizadeh-Naderi R, El-Naggar AK, Richards-Kortum RR. Confocal microscopy for real-time detection of oral cavity neoplasia. Clin Cancer Res. 2003;9:4714–4721. [PubMed] [Google Scholar]
- 62.Zurac S, Neagu M, Constantin C, Cioplea M, Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et al. Variations in the expression of TIMP1, TIMP2 and TIMP3 in cutaneous melanoma with regression and their possible function as prognostic predictors. Oncol Lett. 2016;11:3354–3360. doi: 10.3892/ol.2016.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neagu M, Constantin C, Zurac S. Immune parameters in the prognosis and therapy monitoring of cutaneous melanoma patients: Experience, role, and limitations. BioMed Res Int. 2013;2013:107940. doi: 10.1155/2013/107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neagu M, Constantin C, Manda G, Margaritescu I. Biomarkers of metastatic melanoma. Biomarkers Med. 2009;3:71–89. doi: 10.2217/17520363.3.1.71. [DOI] [PubMed] [Google Scholar]
- 65.Neagu M, Constantin C, Tanase C. Immune-related biomarkers for diagnosis/prognosis and therapy monitoring of cutaneous melanoma. Expert Rev Mol Diagn. 2010;10:897–919. doi: 10.1586/erm.10.81. [DOI] [PubMed] [Google Scholar]
- 66.Surcel M, Constantin C, Caruntu C, Zurac S, Neagu M. Inflammatory cytokine pattern is sex-dependent in mouse cutaneous melanoma experimental model. J Immunol Res. 2017;2017:9212134. doi: 10.1155/2017/9212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caruntu C, Mirica A, Rosca A, Mirica R, Caruntu A, Tampa M, Matei C, Constantin C, Neagu M, Badarau A, et al. The role of estrogens and estrogen receptors in melanoma development and progression. Acta Endo Buc. 2016;12:234–241. doi: 10.4183/aeb.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caruntu C, Boda D, Constantin C, Caruntu A, Neagu M. Catecholamines increase in vitro proliferation of murine B16F10 melanoma cells. Acta Endo Buc. 2014;10:545–558. doi: 10.4183/aeb.2014.545. [DOI] [Google Scholar]
- 69.Neagu M, Constantin C, Dumitrascu GR, Lupu AR, Caruntu C, Boda D, Zurac S. Inflammation markers in cutaneous melanoma - edgy biomarkers for prognosis. Discoveries (Craiova) 2015;3:e38. doi: 10.15190/d.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ene CD, Anghel AE, Neagu M, Nicolae I. 25-OH vitamin D and interleukin-8: Emerging biomarkers in cutaneous melanoma development and progression. Mediators Inflamm. 2015;2015:904876. doi: 10.1155/2015/904876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solovastru LG, Vâta D, Statescu L, Constantin MM, Andrese E. Skin cancer between myth and reality, yet ethically constrained. Rev Rom Bioet. 2014;12:47–52. [Google Scholar]
- 72.Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE - an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141:1032–1034. doi: 10.1001/archderm.141.8.1032. [DOI] [PubMed] [Google Scholar]
- 73.Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: Identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134:103–104. doi: 10.1001/archderm.134.1.103-a. [DOI] [PubMed] [Google Scholar]
- 74.Hansen C, Wilkinson D, Hansen M, Argenziano G. How good are skin cancer clinics at melanoma detection? Number needed to treat variability across a national clinic group in Australia. J Am Acad Dermatol. 2009;61:599–604. doi: 10.1016/j.jaad.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Sidhu S, Bodger O, Williams N, Roberts DL. The number of benign moles excised for each malignant melanoma: The number needed to treat. Clin Exp Dermatol. 2012;37:6–9. doi: 10.1111/j.1365-2230.2011.04148.x. [DOI] [PubMed] [Google Scholar]
- 76.Goodson AG, Florell SR, Hyde M, Bowen GM, Grossman D. Comparative analysis of total body and dermatoscopic photographic monitoring of nevi in similar patient populations at risk for cutaneous melanoma. Dermatol Surg. 2010;36:1087–1098. doi: 10.1111/j.1524-4725.2010.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moloney FJ, Guitera P, Coates E, Haass NK, Ho K, Khoury R, O'Connell RL, Raudonikis L, Schmid H, Mann GJ, et al. Detection of primary melanoma in individuals at extreme high risk: A prospective 5-year follow-up study. JAMA Dermatol. 2014;150:819–827. doi: 10.1001/jamadermatol.2014.514. [DOI] [PubMed] [Google Scholar]
- 78.March J, Hand M, Grossman D. Practical application of new technologies for melanoma diagnosis: Part I. Noninvasive approaches. J Am Acad Dermatol. 2015;72:929–942. doi: 10.1016/j.jaad.2015.02.1140. [DOI] [PubMed] [Google Scholar]
- 79.Ulrich M, Lange-Asschenfeldt S. In vivo confocal microscopy in dermatology: From research to clinical application. J Biomed Opt. 2013;18:061212. doi: 10.1117/1.JBO.18.6.061212. [DOI] [PubMed] [Google Scholar]
- 80.Carrera C, Puig S, Malvehy J. In vivo confocal reflectance microscopy in melanoma. Dermatol Ther (Heidelb) 2012;25:410–422. doi: 10.1111/j.1529-8019.2012.01495.x. [DOI] [PubMed] [Google Scholar]
- 81.Longo C, Farnetani F, Ciardo S, Cesinaro AM, Moscarella E, Ponti G, Zalaudek I, Argenziano G, Pellacani G. Is confocal microscopy a valuable tool in diagnosing nodular lesions? A study of 140 cases. Br J Dermatol. 2013;169:58–67. doi: 10.1111/bjd.12259. [DOI] [PubMed] [Google Scholar]
- 82.Gerger A, Koller S, Weger W, Richtig E, Kerl H, Samonigg H, Krippl P, Smolle J. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193–200. doi: 10.1002/cncr.21910. [DOI] [PubMed] [Google Scholar]
- 83.Sokołowska-Wojdyło M, Olek-Hrab K, Ruckemann-Dziurdzińska K. Primary cutaneous lymphomas: Diagnosis and treatment. Postepy Dermatol Alergol. 2015;32:368–383. doi: 10.5114/pdia.2015.54749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ion A, Popa IM, Papagheorghe LML, Lisievici C, Lupu M, Voiculescu V, Caruntu C, Boda D. Proteomic approaches to biomarker discovery in cutaneous T-cell lymphoma. Dis Markers. 2016;2016:9602472. doi: 10.1155/2016/9602472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fabbrocini G, Mazzella C, Cantelli M, Baldo A, Russo D, De Rosa G, Monfrecola G. Reflectance confocal microscopy as new diagnostic tool in folliculotropic mycosis fungoides. Skin Appendage Disord. 2018;4:118–121. doi: 10.1159/000479822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mancebo SE, Cordova M, Myskowski PL, Flores ES, Busam K, Jawed SI, Skripnik A, Rajadhyaksha M, Querfeld C. Reflectance confocal microscopy features of mycosis fungoides and Sézary syndrome: Correlation with histopathologic and T-cell receptor rearrangement studies. J Cutan Pathol. 2016;43:505–515. doi: 10.1111/cup.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W, Dai H, Li Z, Xu AE. Reflectance confocal microscopy for the characterization of mycosis fungoides and correlation with histology: A pilot study. Skin Res Technol. 2013;19:352–355. doi: 10.1111/srt.12049. [DOI] [PubMed] [Google Scholar]
- 88.Lange-Asschenfeldt S, Babilli J, Beyer M, Ríus-Diaz F, González S, Stockfleth E, Ulrich M. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001. doi: 10.1117/1.JBO.17.1.016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koller S, Gerger A, Ahlgrimm-Siess V, Weger W, Smolle J, Hofmann-Wellenhof R. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536–540. doi: 10.1111/j.1600-0625.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- 90.Agero AL, Gill M, Ardigo M, Myskowski P, Halpern AC, González S. In vivo reflectance confocal microscopy of mycosis fungoides: A preliminary study. J Am Acad Dermatol. 2007;57:435–441. doi: 10.1016/j.jaad.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 91.Ardigò M, Donadio C, Vega H, Cota C, Moscarella E, Agozzino M. Concordance between in vivo reflectance confocal microscopy and optical histology of lymphomatoid papulosis. Skin Res Technol. 2013;19:308–313. doi: 10.1111/srt.12046. [DOI] [PubMed] [Google Scholar]
- 92.Ardigò M, El Shabrawi-Caelen L, Tosti A. In vivo reflectance confocal microscopy assessment of the therapeutic follow-up of cutaneous T-cell lymphomas causing scalp alopecia. Dermatol Ther (Heidelb) 2014;27:248–251. doi: 10.1111/dth.12129. [DOI] [PubMed] [Google Scholar]
- 93.de Vries E, Trakatelli M, Kalabalikis D, Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, Sotiriadis D, Ioannides D, Aquilina S, Apap C, et al. EPIDERM Group Known and potential new risk factors for skin cancer in European populations: A multicentre case-control study. Br J Dermatol. 2012;167(Suppl 2):1–13. doi: 10.1111/j.1365-2133.2012.11081.x. [DOI] [PubMed] [Google Scholar]
- 94.Maru GB, Gandhi K, Ramchandani A, Kumar G. The role of inflammation in skin cancer. Adv Exp Med Biol. 2014;816:437–469. doi: 10.1007/978-3-0348-0837-8_17. [DOI] [PubMed] [Google Scholar]
- 95.Neagu M, Caruntu C, Constantin C, Boda D, Zurac S, Spandidos DA, Tsatsakis AM. Chemically induced skin carcinogenesis: Updates in experimental models (Review) Oncol Rep. 2016;35:2516–2528. doi: 10.3892/or.2016.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: Fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hashemi P, Pulitzer MP, Scope A, Kovalyshyn I, Halpern AC, Marghoob AA. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: A challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452–462. doi: 10.1016/j.jaad.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Gonzalez S, Terwey TH, Wolchok J, Li Y, Aranda I, Toledo-Crow R, Halpern AC. Dual mode reflectance and fluorescence confocal laser scanning microscopy for in vivo imaging melanoma progression in murine skin. J Invest Dermatol. 2005;125:798–804. doi: 10.1111/j.0022-202X.2005.23786.x. [DOI] [PubMed] [Google Scholar]
- 99.Izatt JA, Kulkarni MD, Hsing-Wen W, Kobayashi K, Sivak MV. Optical coherence tomography and microscopy in gastrointestinal tissues. IEEE J Sel Top Quantum Electron. 1996;2:1017–1028. doi: 10.1109/2944.577331. [DOI] [Google Scholar]
- 100.Ono I, Sakemoto A, Ogino J, Kamiya T, Yamashita T, Jimbow K. The real-time, three-dimensional analyses of benign and malignant skin tumors by confocal laser scanning microscopy. J Dermatol Sci. 2006;43:135–141. doi: 10.1016/j.jdermsci.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Skvara H, Kittler H, Schmid JA, Plut U, Jonak C. In vivo fluorescence confocal microscopy: Indocyanine green enhances the contrast of epidermal and dermal structures. J Biomed Opt. 2011;16:096010. doi: 10.1117/1.3625255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.