Abstract
Neoangiogenesis plays an important role in cutaneous lymphoma pathogenesis. Cutaneous T-cell lymphoma (CTCL) is characterized by the presence of malignant T-cell clones in the skin. Vascular microenvironment of lymphomas accelerates neoangiogenesis through several factors released by tumoral cells: VEGF family, bFGF and PIGF. Tumor stroma (fibroblasts, inflammatory and immune cells) also plays a crucial role, by providing additional angiogenic factors. The angiogenic process through the VEGF-VEGFR axis can promote survival, proliferation and metastasis via autocrine mechanisms in cutaneous lymphomas. Microvascular density (MVD) measures the neo-vascularization of cutaneous lymphoma, generated by the response of tumor cells, proangiogenic stromal cells, and benign T/B lymphocytes within the tumor inflammatory infiltrate. Pro-angiogenic proteins have been found to indicate the evolution and prognosis in patients with CTCL. In conclusion, anti-angiogenic therapeutic protocols can target tumor vasculature or malignant tumor cells directly or through a large number of combinations with other drugs. The integration of proteomics into clinical practice based on high-throughput technologies leads to the development of personalized medicine, adapting the specific biomarkers to the application of cancer-type specific individual drug targets.
Keywords: cutaneous T-cell lymphoma, vascular endothelial growth factors, proteomics, biomarker, antiangiogenic therapy
1. Overview on cutaneous T-cell lymphoma
Molecular biology research has contributed a great deal to advances in medical research, most notably in hematology and oncology. Molecular mechanisms governing hematopoietic differentiation and proliferation, as well as mutations involved in hematopoietic malignancies, are now better understood (1–3).
The established guidelines for the diagnostic and clinical management of hematologic malignancies, including lymphomas, were published in 2008, under WHO coordination, and were revised in 2017 (4). T-cell lymphomas are classified into several categories, based on WHO recommendations, accessible in recent reviews (5–8). CTCLs are characterized by the recruitment of malignant T-cell clones into the skin. Mycosis fungoides (MF) represent the most common type of CTCL and account for ~50% of all primary cutaneous lymphomas, followed by Sézary syndrome. In 2018, the EORTC cutaneous lymphoma task force proposed a uniformity in classification and prognostic of these two entities using flow cytometry (9).
An important player in the pathogeny of most tumors is the vascular niche (10), responsible for increased output of growth factors, such as VEGF (vascular endothelial growth factor) and FGFb, that promote uncontrolled cell growth. An association between angiogenesis and prognostics of MF has been well established (11–13) and repeated attempts have been made to target it therapeutically (14,15). This review is focusing on molecular mechanisms and druggable molecular targets, as well as biomarker progress made in the diagnostics and prognostics of CTCLs.
2. Molecular mechanisms that drive initiation and progression of cutaneous T-cell lymphoma
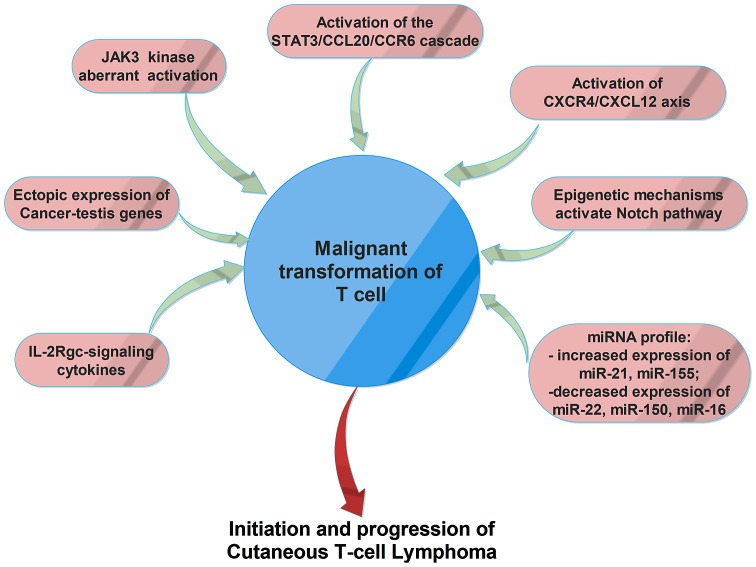
Several dysregulated gene/proteins and signaling pathways have been associated with CTCLs (Fig. 1), but the exact mechanism of initiation and progression of this disorder is not yet known. Recent studies suggested that cancer testis (CT) genes are ectopically expressed in CTCLs and play an important role in carcinogenesis. These genes can sustain cell survival by inhibition of apoptosis, can promote chemo- and radio-therapy resistance and contribute to oncogenesis by targeting p53 and p21 tumor suppressor genes. Moreover, these genes can sustain aneuploidy and genomic instability by producing aberrant chromosomal translocations (16,17).
Figure 1.
The main molecular mechanisms involved in malignant transformation of T-cells in cutaneous T-cell lymphomas.
Another molecular mechanism involved in malignant transformation of CTCLs is represented by the aberrant activation of JAK3 kinase and its key down-stream effectors, STAT3 and STAT5. Therefore, IL-2, IL-4, IL-7, IL-15, and IL-21 cytokines are involved in early pathogenesis, whereas constitutive, interleukin-independent activation of the JAK3/STAT3 pathway seems to be associated with progressive and advanced disease. Aberrant activation of the JAK3/STAT signaling pathway and interleukin-independent proliferation of malignant T-cells are also related to a decreased expression and/or deregulation of function of the negative regulators SOCS-3 and SHP1. Aberrant activation of JAK3/STAT signaling pathway increases the expression of IL-5, IL-10, IL-17A, IL-17F and angiogenic factors, and sustains the resistance to treatment with histone deacetylase (HDAC) inhibitors in malignant T-cells (18).
IL-2Rgc-signaling cytokines, including IL-2, IL-4, IL-7, IL-15, and IL-21, have been associated with the pathogenesis of CTCLs (19). Recent studies suggested that IL-21 could be considered a potent antitumor agent, which increases the cytotoxicity of both natural killer (NK) and CD8+ T-cells. Circulating IL-21 levels in patients with tumor MF were significantly lower than those of healthy controls and plaque MF and the IL-21 expression level decreased during tumoral-stage (20). Another study demonstrated that, in lesional skin, malignant and reactive T-cells produce IL9, a process regulated by STAT3/5. IL9-depleted mice showed a reduction of tumor growth, higher frequencies of regulatory T-cells, and activated CD4 and CD8 T lymphocytes (21). Upregulation of a chemokine receptor CCR6 and its ligand CCL20 was found in advanced CTCL cells; automatic activation of the STAT3/CCL20/CCR6 signaling seems to have an important role in CTCL lymphomagenesis and could be responsible for spreading malignant T-cells to sentinel lymph nodes, the bloodstream, and internal organs (22).
The development of blood and lymphatic vessels was associated with the progression of CTCL. Therefore, malignant T-cells produce angiogenic factors, such as podoplanin (PDPN), lymphatic vessel hyaluronan receptor-1 (LYVE-1), VEGF-C, VEGF-R3, and lymphotoxin-α (LTα), molecules involved in neoangiogenesis and neolymphoangiogenesis by promoting endothelial cell development and tube formation, mainly by stimulation of IL-6 expression (23).
The activation of CXCR4/CXCL12 axis was also associated with MF development. Moreover, administration of anti-CXCL12 and CXCR4 agents, including the anti-CXCR4 drug AMD3100 (plerixafor), the CXCL12 analog CTCE-9908, the anti-CXCL12 aptamer Nox-A12 seems to have promising effects in preclinical and clinical studies as an adjuvant antitumor therapy (24).
Epigenetic mechanisms that activate Notch signaling pathway has also been found associated with CTCL. Notch signaling is involved in cell differentiation, proliferation and stemness. Genome-wide DNA methylation analysis in MF showed a significant methylation and downregulation of the Notch-related microRNAs, especially for miR-200c and miR-124 (25).
Several studies suggested that patients with CTCL present a distinct miRNA expression profile. For example, increased expression of miR-21 and miR-155 is able to promote resistance to apoptosis and malignant proliferation and is associated with poor prognosis and aggressive behavior (19,26). In Sézary syndrome, a downregulation of the miR-22 tumor suppressor was observed, also associated with the activation of JAK3/STAT3 signaling (18). Another study demonstrated that miR-150 was downregulated only in advanced MF patients, but its expression can be restored using pan-HDACI treatment. Same results were obtained in the case of miR-16, whose expression is decreased in early stages of MF, but can also be restored by administration of vorinostat, another compound that inhibits HDACs. These results suggest that dysregulation of miRNAs by HDACs has an important role in the pathogenesis of CTCL, in both early and advanced stages, since these miRNAs are able to inhibit the expression of many oncogenes (27).
Using whole-exome sequencing, da Silva Almeida et al reported a distinctive pattern of somatic copy number alterations in tumor samples from patients with Sézary syndrome and other CTCLs (28). The analyses identified highly prevalent chromosomal deletions involving the TP53, RB1, PTEN, DNMT3A and CDKN1B tumor suppressors. Somatic mutations were found in key genes involved in epigenetic regulation [TET2, CREBBP, KMT2D (MLL2), KMT2C (MLL3), BRD9, SMARCA4 and CHD3]. Signaling pathways are also affected by mutations in MAPK1, BRAF, CARD11 and PRKG1 that result in increased MAPK, NF-κB and NFAT activity upon T-cell receptor stimulation.
3. Tumor niche of cutaneous T-cell lymphoma - emphasis on vascular microenvironment
A niche is a microenvironment, both physical and functional. The physical niche is delineated by several cellular players, centered on the main resident; in this case, tumor cells. In addition to the central resident of the niche, other cellular partners contribute to create a physical or a functional scaffold, the latter mainly via soluble factors. Presently, Pubmed search using the terms ‘cutaneous T-cell lymphoma vascular niche’ yielded no results, although microvascularization has been previously studied in relationship with this class of tumor (12,29). Neovascularization of lymphoma can be quantified by microvessel density (MVD), which is the result of cooperation between tumor cells, proangiogenic stromal cells and infiltrating benign T lymphocytes and myeloid cells. It is used as an early marker in MF (29), but quantification reports vary among different studies due to the heterogeneity of lymphoma stroma, the range of cell surface markers used for staining and differences in scoring methodology (30). In general, MVD scores trend highest in aggressive subtypes including Burkitt's lymphoma and PTCL, compared with intermediate in DLBCL and lower in indolent follicular lymphoma (FL).
Creation of a new vascular niche in CTLCs can be argued by the emerging role of stromal cell derived factor 1 (SDF1, CXCL12) and its receptor C-X-C motif chemokine receptor 4 (CXCR4) axis in progression of MF. Signaling via this axis is known as the main regulating factor for homing of hematopoietic stem cells to the bone marrow niche (31). In MF, neoplastic T-cells express CXCR4, especially in the pretumor stage, to interact with increased levels of CXCL12, playing a critical role in MF progression (32). This axis seems to play an important role in both early and advanced stages of the disease (24).
Endothelial activation and synthesis of growth factors can be driven by another couple ligand/receptor; angiopoietin-1 (Ang1)/Tie2. In bone marrow, activation of this axis is associated with hematopoietic stem cell quiescence (33). Also, Ang1 and its co-family member Ang2 have been associated with angiogenesis. The recent study of Kawaguchi et al showed that circulating levels of Ang2 (not Ang1), might play a role in Sézary syndrome disease activity. In addition, expression of Ang2+ cells in lesional skin of CTCL was higher than in normal skin (34).
The main factor responsible for the angiogenic switch is the family of VEGF. Members of the VEGF family: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor (PlGF), through interactions with their receptors, regulate vascular angiogenesis and lymphangiogenesis (13). These factors create positive feedback loops between endothelium and tumor cells, as well as autocrine feedback, as it has been demonstrated that acute lymphocytic leukemia and aggressive subtypes of lymphoma; peripheral T-cell lymphoma (PTCL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL) and primary effusion lymphoma express both VEGF and VEGFRs (35).
Regarding lymphoid vessels, there are also reports of increased formation of lymphatic vasculature and secondary lymphoid structures in CTLC. In addition to the classic factors, Lauenborg et al studied LTα involvement in the progression and invasion of tumor T-cells. They showed that CTCL cells express LTα in situ, which acts as an autocrine factor and is driven by aberrantly activated JAK3/STAT5 pathway. LTα and LTα-induced expression of IL-6, and together with VEGF, promoted tumor angiogenesis (23). Activation of the same signaling pathway has also been correlated with IL-17A and/or IL-17F secretion that modulate oncogenic angiogenesis (36).
Malignant stroma (fibroblasts, inflammatory and infiltrated immune cells, such as monocytes and dendritic cells), provides additional angiogenic and pro-proliferative cues for tumor cells. In MF, tumor-associated macrophages were activated by stroma-produced periostin, to create a tumor niche (37). Stromal cells produce thymic stromal lymphopoietin and IL-16 that trigger T-cell recruitment to the skin (38). The stroma of MF can be highlighted by the presence of (LT)β and CCL21 (39).
Cells from tumor stroma, such as macrophages, can be targeted for destruction either by treatments aimed primarily at tumor cells, such as interferons (40), or directly, using cell-targeted treatments (in this case, macrophage-targeted) (41).
Molecular pathways associated with tumor cells and modified stroma are further targeted in biomarker research and for therapeutic purposes, as discussed further.
4. Proteomic biomarkers for diagnostic and prognostic of CTLC
An article by Humphrey et al (42) argues the utility of serum ribonuclease as a biomarker for leukemia diagnosis. In this paper, the authors refer to an even earlier work from the same decade (43). Since then, as the term entered in current research language, most notably in protein research, different definitions have been suggested, trying to harmonize terms and concepts.
The current widely accepted definition of a ‘biomarker’ was first provided by Biomarkers Definition Working Group in 2001: ‘A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or biological responses to a therapeutic intervention’ (44,45). This definition was modified in 2016 by the FDA-NIH Biomarker Working Group to ‘characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions’ (46).
The nature of a biomarker may vary from molecular, to a histological, imagistic or physiologic characteristic. Following advances in molecular biology technologies, molecular biomarkers can now be classified into four main types: i) Genomic biomarkers, based on the analysis of specific DNA sequences; ii) transcriptomic biomarkers, based on the analysis of RNA expression profiles; iii) proteomic biomarkers, based on the analysis of peptide/protein profiles in a wide range of biological samples from cell cultures and tissue biopsies to cerebrospinal fluid and tears; and iv) metabolomic biomarkers, based on the analysis of final- or by-products of different metabolic pathways.
Various studies emphasize the relevance of proteomics as a useful tool for biomarker identification, through minimally invasive procedures, allowing a precise approach by providing the proteomic signature of the disease. High-throughput proteomic technologies e.g., ultrasensitive microarray, are nowadays focused on discovery of specific biomarkers, expanding our knowledge regarding the mechanisms responsible for disease development, enabling early diagnostic, prognostic, monitoring and even tailored therapy of the disease (8,47–54).
Given the indolent nature of CTCLs, with majority of cases evolving over many years with a very slow progression, identification of circulating biomarkers for early diagnosis, differentiation and prognosis would be of great benefit for patients suffering of CTLC (55). Serum markers are helpful tools in determining the tumor burden in cutaneous lymphoma and thus might prove useful for disease monitoring during treatment. There is also a prognostic value that they may have for predicting the clinical course.
Circulating endothelial progenitor cells (EPC) and VEGF levels appear to correlate with tumor volumes. The phenotype CD133+CD34+VEGFR-2+ EPC present in peripheral blood and lymph nodes in patients with non-Hodgkin lymphoma; the blood levels are increased in younger patients and those with aggressive lymphomas (56). It has been shown that circulating levels of the soluble interleukin-2 receptor (sIL-2R) of α-chain and lactate dehydrogenase (LDH) are significantly correlated with lymph node size, however the correlation with cutaneous clinical severe symptoms are significant only with regard to sIL-2R in erythrodermic patients. Furthermore, it has been established that tissue-based lymphoma cells express low levels of sIL-2R, whilst large-cell transformation in CTCL contributes to lowering high levels of sIL-2R in several patients. Besides sIL-2R, other two molecules-neopterin and β2-microglobulin seem to express significant high levels in serum samples from patients suffering from Sézary syndrome. Of these considered biomarkers, only sIL-2R appears to be the most sensitive; nevertheless, in terms of prognosis, only neopterin revealed a substantial significance in nonleukemic CTCL patients (57).
Circulating markers like neopterin, β2-microglobulin, sIL-2R, IL-6 and −4 have been described to be elevated in various malignancies. Studies show that increased cytoplasmic IL-4 is the sole predictor of advanced CTCL disease. Concerning the outcome of the disease (progression versus non-progression), only neopterin showed a significant prognostic value in CTCL patients (58).
The histological and molecular characteristics play a key-role in establishing the prognosis of CTLC. Various protein biomarkers useful in CTLC diagnosis have been established, comprising CD2, CD3, CD4, CD5, CD7, CD8, CD14, CD16/56, CD19, CD25, CD45, CD45RA, CD45R0. Moshkovskii et al analyzed the differential cytokine expressions of IL-1Ra, IL-4, G-CSF, IP-10 in serum samples of MF in the attempt to estimate the probability of an accurate diagnosis based on serum protein profiling using mass spectrometry SELDI-TOF. In their study, the authors concluded that IP-10 may be considered a candidate biomarker for the differentiation between MF and other skin conditions (59,60).
Moreover, molecules engaged in signaling pathways, regulation of cellular proliferation, and apoptosis such as Jun, Myc, c-myb, p53, STATs, Bcl-2, Fas/CD95 and SOCS-3, or involved in immunopathology such as expression of inhibitory MHC receptors (ILT2/CD85j), NK receptors (p140/KIR3DL2) and dendritic cell defects (CD40 abnormal expression) could be of significant relevance concerning CTLC prognosis (61). There are few studies on the characterization of DCs in cancer, with implication of their expression of CD40. It is well recognized that CD40 is a co-stimulatory molecule, belonging to the tumor necrosis factor superfamily, essential in DCs activation, and suggested that CD40 expression in DCs is impaired in cancer, particularly in metastatic disease.
It has been highlighted that aberrant activation of the JAK/STAT signaling pathway was encountered in almost all T-cell lymphomas, leading to activation of pathways downstream of the T-cell receptor (TCR), co-stimulatory proteins, and/or cytokine receptors (62,63). Considering the high prevalence of CTCL, it is necessary to determine specific biomarkers in order to discriminate between more or less aggressive forms CTLC.
In this regard, future studies are needed to establish the suitable biomarkers for an accurate early diagnostic of cutaneous lymphoma. The integration of proteomics into clinical practice needs a high-throughput laboratory infrastructure, leading to the development of personalized medicine, adapting the specific biomarkers to application of cancer-type specific drug targets to individuals.
5. Molecular targets in vascular niche factors
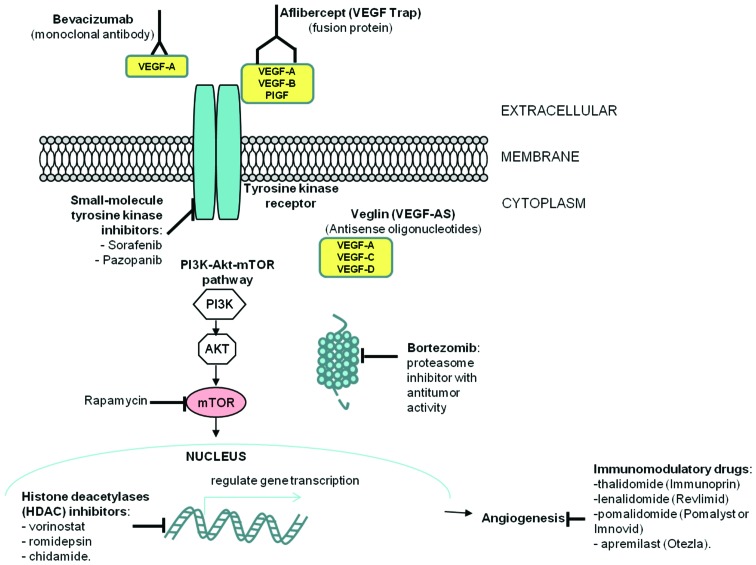
The growing list of antiangiogenic therapies available (summarized in Fig. 2) includes:
Figure 2.
Targeted antiangiogenic therapy strategies in cutaneous T-cell lymphomas.
i) Direct anti-VEGF (bevacizumab, VEGF-Trap, VEGF-antisense); due to the importance of the VEGF axis for the angiogenic process, research efforts focused on direct targeting of VEGF. The prototypic therapeutic agent is the humanized monoclonal antibody bevacizumab, which in combination with chemotherapy improves progression free-survival for patients with metastatic colorectal, renal, breast and advanced non-small cell lung cancer (64–66). Other strategies directly targeting VEGF include the use of antisense oligonucleotides against VEGF, reported to cause a partial response in one CTCL patient (14) and aflibercept (VEGF Trap), a fusion protein consisting of the extracellular domains of VEGFR receptors 1 and 2 fused to the Fc portion of human IgG. Aflibercept was reported to be well tolerated and have antitumoral activity in patients with advanced solid tumors in a phase I clinical trial (67).
ii) Immunomodulatory drugs with antiangiogenic properties; this group includes a number of anticancer agents, among which the prototype drug thalidomide (Immunoprin) (68), and its more recently developed analogues: Lenalidomide (Revlimid), pomalidomide (Pomalyst or Imnovid) and apremilast (Otezla). A phase III clinical study of lenalidomide maintenance after debulking therapy in patients with advanced CTCL revealed that lenalidomide maintenance increased progression free survival. However, these results were not statistically significant due to the reduced number of patients enrolled in the study (69,70).
iii) Metronomic chemotherapy refers to the administration of relatively low doses of medications at close regular intervals without prolonged drug-free break periods rather than the conventional ‘maximum tolerated dose’ (71). This approach preferentially damages endothelial cells in tumor blood vessels presumably due to a simultaneous blockade of VEGF-A blunting a key survival signal for endothelial cells, thus selectively amplifying the endothelial cell targeting effects of chemotherapy, leading to improved subsequent killing of cancer cells (72).
iv) Other novel antiangiogenic strategies, which include: a) Mammalian target of rapamycin (mTOR) inhibitors; mTOR inhibitors have shown antiangiogenic activity by inhibiting VEGF production through HIF-1α (73,74) and mTOR has emerged a promising target for cancer therapy in both solid and hematological tumors. Primary cells from patients with CTCL or Sézary syndrome were reported to be sensitive to rapamycin (75) and the inhibition of mTOR was shown to induce apoptosis in CTCL cells (76); b) HDAC inhibitors; the first clinical data to support the use of HDAC inhibitors in CTCL came from a 2001 phase-1 trial that looked at the effect of romidepsin on a variety of cancers. The 3 patients with CTCL enrolled had partial remission, while a PTCL patient had complete remission (77). Currently, HDAC inhibitors approved for the treatment of CTCL include vorinostat; c) Proteasome inhibitors; ubiquitin-proteasome pathway inhibition in tumor cells impedes tumor growth by inducing cell cycle arrest, apoptosis and inhibiting tumor metastasis and angiogenesis. Bortezomib is a proteasome inhibitor with antitumor activity (78,79) that has several downstream effects, including activation of p53, inhibition of NF-κB and accumulation of pro-apoptotic proteins (80). Bortezomib was approved in 2003 by the FDA for the treatment of multiple myeloma and for relapsed or refractory mantle cell lymphoma (81,82) and several reports and clinical trials reveal that it can also be used for the treatment of solid tumors, alone or in combination (83–85). Bortezomib has shown promising results in patients with relapsed or refractory CTCL (86–88).
6. Conclusion
Neoangiogenesis plays potentially important pathogenic roles in CTLC initiation and prognostic, by stimulating homing of tumor cells and generation of pro-proliferative soluble cues. The main signaling pathway involved in autocrine stimulation of proliferation and survival of lymphoma tumor cells is VEGF-VEGF receptor axis, although other axes, described in homing studies (CXCR4/CXCL12) or signaling pathways (JAK3/STAT5) associated with hematopoietic development were recently associated with clinical and fundamental studies of CTLC. Proteomic studies are useful to highlight more members of these signaling pathways that are modified in the lesions or serum of patients and that may be used in prognostic or therapy follow-up. These studies can also highlight novel molecular therapy targets, possibly some with higher specificity for characterized subsets of patients, in the framework of personalized medicine.
Acknowledgements
We would like to thank Ms. Irina Radu, certified translator in Medicine and Pharmacy (certificate credentials: series E no. 0048), for professional linguistic assistance.
Glossary
Abbreviations
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- bFGF
basic fibroblast growth factor
- PIGF
placenta growth factor
- STAT
signal transducer and activator of transcription
- JAK
Janus kinase
- CCL20
chemokine (C-C motif) ligand 20
- CCR6
C-C motif chemokine receptor 6
- PDPN
podoplanin
- LYVE-1
lymphatic vessel hyaluronan receptor-1
- LTα
lymphotoxin-α
- CXCR4
C-X-C chemokine receptor type 4
- CXCL12
C-X-C motif chemokine ligand 12
- TP53
tumor protein p53
- RB1
retinoblastoma protein 1
- PTEN
phosphatase and tensin homolog
- DNMT3A
DNA methyltransferase 3α
- CDKN1B
cyclin-dependent kinase inhibitor 1B
- TET2
tet methylcytosine dioxygenase 2
- CREBBP
CREB binding protein
- CREB
cAMP-response element binding protein
- KMT2D (MLL2)
histone-lysine N-methyltransferase 2D
- KMT2C (MLL3)
lysine N-methyltransferase 2C
- BRD9
bromodomain-containing protein 9
- SMARCA4
transcription activator BRG1
- CHD3
chromodomain helicase DNA binding protein 3
- MAPK1
mitogen-activated protein kinase 1
- BRAF
v-raf murine sarcoma viral oncogene homolog B1
- CARD11
caspase recruitment domain-containing protein 11
- PRKG1
cGMP-dependent protein kinase 1
- NF-κB
nuclear factor-κB
- NFAT
nuclear factor of activated T-cells
- SDF1
stromal cell-derived factor 1
- CXCL12
C-X-C motif chemokine ligand 12
- Ang1
angiopoietin 1
- Tie2
angiopoietin receptor
- LDH
lactate dehydrogenase
- G-CSF
granulocyte colony-stimulating factor
- IP-10
interferon γ-induced protein 10
- Jun
AP-1 transcription factor
- Bcl-2
B-cell lymphoma 2
- SOCS-3
suppressor of cytokine signaling-3
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CT, IDP, AME, AAGG, EC, SM, LA, LN and RA contributed to the gathering of the data, writing the manuscript and revising it critically for an important intellectual content. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Patterson-Fortin J, Moliterno AR. Molecular pathogenesis of myeloproliferative neoplasms: Influence of age and gender. Curr Hematol Malig Rep. 2017;12:424–431. doi: 10.1007/s11899-017-0411-0. [DOI] [PubMed] [Google Scholar]
- 2.Kwan W, North TE. Netting novel regulators of hematopoiesis and hematologic malignancies in zebrafish. Curr Top Dev Biol. 2017;124:125–160. doi: 10.1016/bs.ctdb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Deininger MW, Tyner JW, Solary E. Turning the tide in myelodysplastic/myeloproliferative neoplasms. Nat Rev Cancer. 2017;17:425–440. doi: 10.1038/nrc.2017.40. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow SH, Harris NL, Campo E, Pileri SA, Stein H, Jaffe ES, Thiele J. 4th. Vol. 2. IARC press; Lyon: 2017. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 5.Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms. Expert Rev Hematol. 2017;10:239–249. doi: 10.1080/17474086.2017.1281122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matutes E. The 2017 WHO update on mature T- and natural killer (NK) cell neoplasms. Int J Lab Hematol. 2018;40:97–103. doi: 10.1111/ijlh.12817. [DOI] [PubMed] [Google Scholar]
- 8.Lupu M, Caruntu A, Caruntu C, Papagheorghe LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki M, et al. Neuroendocrine factors: The missing link in non melanoma skin cancer (Review) Oncol Rep. 2017;38:1327–1340. doi: 10.3892/or.2017.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarisbrick JJ, Hodak E, Bagot M, Stranzenbach R, Stadler R, Ortiz-Romero PL, Papadavid E, Evison F, Knobler R, Quaglino P, et al. Blood classification and blood response criteria in mycosis fungoides and Sézary syndrome using flow cytometry: Recommendations from the EORTC cutaneous lymphoma task force. Eur J Cancer. 2018;93:47–56. doi: 10.1016/j.ejca.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 10.Shahrabi S, Rezaeeyan H, Ahmadzadeh A, Shahjahani M, Saki N. Bone marrow blood vessels: Normal and neoplastic niche. Oncol Rev. 2016;10:306. doi: 10.4081/oncol.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vacca A, Moretti S, Ribatti D, Pellegrino A, Pimpinelli N, Bianchi B, Bonifazi E, Ria R, Serio G, Dammacco F. Progression of mycosis fungoides is associated with changes in angiogenesis and expression of the matrix metalloproteinases 2 and 9. Eur J Cancer. 1997;33:1685–1692. doi: 10.1016/S0959-8049(97)00186-X. [DOI] [PubMed] [Google Scholar]
- 12.Mazur G, Woźniak Z, Wróbel T, Maj J, Kuliczkowski K. Increased angiogenesis in cutaneous T-cell lymphomas. Pathol Oncol Res. 2004;10:34–36. doi: 10.1007/BF02893406. [DOI] [PubMed] [Google Scholar]
- 13.Miyagaki T, Sugaya M, Oka T, Takahashi N, Kawaguchi M, Suga H, Fujita H, Yoshizaki A, Asano Y, Sato S. Placental growth factor and vascular endothelial growth factor together regulate tumour progression via increased vasculature in cutaneous T-cell lymphoma. Acta Derm Venereol. 2017;97:586–592. doi: 10.2340/00015555-2623. [DOI] [PubMed] [Google Scholar]
- 14.Levine AM, Tulpule A, Quinn DI, Gorospe G, III, Smith DL, Hornor L, Boswell WD, Espina BM, Groshen SG, Masood R, et al. Phase I study of antisense oligonucleotide against vascular endothelial growth factor: Decrease in plasma vascular endothelial growth factor with potential clinical efficacy. J Clin Oncol. 2006;24:1712–1719. doi: 10.1200/JCO.2005.03.4801. [DOI] [PubMed] [Google Scholar]
- 15.Zain J, O'Connor OA. Targeting histone deacetylases in the treatment of B- and T-cell malignancies. Invest New Drugs. 2010;28:S58–S78. doi: 10.1007/s10637-010-9591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litvinov IV, Netchiporouk E, Cordeiro B, Zargham H, Pehr K, Gilbert M, Zhou Y, Moreau L, Woetmann A, Ødum N, et al. Ectopic expression of embryonic stem cell and other developmental genes in cutaneous T-cell lymphoma. OncoImmunology. 2014;3:e970025. doi: 10.4161/21624011.2014.970025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanase C, Albulescu R, Codrici E, Calenic B, Popescu ID, Mihai S, Necula L, Cruceru ML, Hinescu ME. Decreased expression of APAF-1 and increased expression of cathepsin B in invasive pituitary adenoma. OncoTargets Ther. 2014;8:81–90. doi: 10.2147/OTT.S70886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sibbesen NA, Kopp KL, Litvinov IV, Jønson L, Willerslev-Olsen A, Fredholm S, Petersen DL, Nastasi C, Krejsgaard T, Lindahl LM, et al. Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget. 2015;6:20555–20569. doi: 10.18632/oncotarget.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagherani N, Smoller BR. An overview of cutaneous T cell lymphomas. F1000 Res. 2016;5:5. doi: 10.12688/f1000research.8829.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabasawa M, Sugaya M, Oka T, Takahashi N, Kawaguchi M, Suga H, Miyagaki T, Takahashi T, Shibata S, Fujita H, et al. Decreased interleukin-21 expression in skin and blood in advanced mycosis fungoides. J Dermatol. 2016;43:819–822. doi: 10.1111/1346-8138.13278. [DOI] [PubMed] [Google Scholar]
- 21.Vieyra-Garcia PA, Wei T, Naym DG, Fredholm S, Fink-Puches R, Cerroni L, Odum N, O'Malley JT, Gniadecki R, Wolf P. STAT3/5-dependent IL9 overexpression contributes to neoplastic cell survival in mycosis fungoides. Clin Cancer Res. 2016;22:3328–3339. doi: 10.1158/1078-0432.CCR-15-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda S, Kitadate A, Ito M, Abe F, Nara M, Watanabe A, Takahashi N, Miyagaki T, Sugaya M, Tagawa H. Disruption of CCL20-CCR6 interaction inhibits metastasis of advanced cutaneous T-cell lymphoma. Oncotarget. 2016;7:13563–13574. doi: 10.18632/oncotarget.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauenborg B, Christensen L, Ralfkiaer U, Kopp KL, Jønson L, Dabelsteen S, Bonefeld CM, Geisler C, Gjerdrum LM, Zhang Q, et al. Malignant T-cells express lymphotoxin α and drive endothelial activation in cutaneous T-cell lymphoma. Oncotarget. 2015;6:15235–15249. doi: 10.18632/oncotarget.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maj J, Jankowska-Konsur AM, Hałoń A, Woźniak Z, Plomer-Niezgoda E, Reich A. Expression of CXCR4 and CXCL12 and their correlations to the cell proliferation and angiogenesis in mycosis fungoides. Postepy Dermatol Alergol. 2015;32:437–442. doi: 10.5114/pdia.2015.48034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallardo F, Sandoval J, Díaz-Lagares A, Garcia R, D'Altri T, González J, Alegre V, Servitje O, Crujeiras AB, Stefánsson ÓA, et al. Notch1 pathway activation results from the epigenetic abrogation of notch-related microRNAs in mycosis fungoides. J Invest Dermatol. 2015;135:3144–3152. doi: 10.1038/jid.2015.328. [DOI] [PubMed] [Google Scholar]
- 26.Lindahl LM, Fredholm S, Joseph C, Nielsen BS, Jønson L, Willerslev-Olsen A, Gluud M, Blümel E, Petersen DL, Sibbesen N, et al. STAT5 induces miR-21 expression in cutaneous T cell lymphoma. Oncotarget. 2016;7:45730–45744. doi: 10.18632/oncotarget.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe F, Kitadate A, Ikeda S, Yamashita J, Nakanishi H, Takahashi N, Asaka C, Teshima K, Miyagaki T, Sugaya M, et al. Histone deacetylase inhibitors inhibit metastasis by restoring a tumor suppressive microRNA-150 in advanced cutaneous T-cell lymphoma. Oncotarget. 2017;8:7572–7585. doi: 10.18632/oncotarget.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Silva Almeida AC, Abate F, Khiabanian H, Martinez-Escala E, Guitart J, Tensen CP, Vermeer MH, Rabadan R, Ferrando A, Palomero T. The mutational landscape of cutaneous T-cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465–1470. doi: 10.1038/ng.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosseila M, Sayed Sayed K, El-Din Sayed SS, Abd El Monaem A. Evaluation of angiogenesis in early mycosis fungoides patients: Dermoscopic and immunohistochemical study. Dermatology. 2015;231:82–86. doi: 10.1159/000382124. [DOI] [PubMed] [Google Scholar]
- 30.Gratzinger D, Zhao S, Tibshirani RJ, Hsi ED, Hans CP, Pohlman B, Bast M, Avigdor A, Schiby G, Nagler A, et al. Prognostic significance of VEGF, VEGF receptors, and microvessel density in diffuse large B cell lymphoma treated with anthracycline-based chemotherapy. Lab Invest. 2008;88:38–47. doi: 10.1038/labinvest.3700697. [DOI] [PubMed] [Google Scholar]
- 31.Mendt M, Cardier JE. Stromal-derived factor-1 and its receptor, CXCR4, are constitutively expressed by mouse liver sinusoidal endothelial cells: Implications for the regulation of hematopoietic cell migration to the liver during extramedullary hematopoiesis. Stem Cells Dev. 2012;21:2142–2151. doi: 10.1089/scd.2011.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daggett RN, Kurata M, Abe S, Onishi I, Miura K, Sawada Y, Tanizawa T, Kitagawa M. Expression dynamics of CXCL12 and CXCR4 during the progression of mycosis fungoides. Br J Dermatol. 2014;171:722–731. doi: 10.1111/bjd.13054. [DOI] [PubMed] [Google Scholar]
- 33.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi M, Sugaya M, Suga H, Miyagaki T, Ohmatsu H, Fujita H, Asano Y, Tada Y, Kadono T, Sato S. Serum levels of angiopoietin-2, but not angiopoietin-1, are elevated in patients with erythrodermic cutaneous T-cell lymphoma. Acta Derm Venereol. 2014;94:9–13. doi: 10.2340/00015555-1633. [DOI] [PubMed] [Google Scholar]
- 35.Alshenawy HA. Prognostic significance of vascular endothelial growth factor, basic fibroblastic growth factor, and microvessel density and their relation to cell proliferation in B-cell non-Hodgkin's lymphoma. Ann Diagn Pathol. 2010;14:321–327. doi: 10.1016/j.anndiagpath.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Lauenborg B, Litvinov IV, Zhou Y, Willerslev-Olsen A, Bonefeld CM, Nastasi C, Fredholm S, Lindahl LM, Sasseville D, Geisler C, et al. Malignant T-cells activate endothelial cells via IL-17 F. Blood Cancer J. 2017;7:e586. doi: 10.1038/bcj.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Asano M, Watabe A, Aiba S. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp Dermatol. 2016;25:107–112. doi: 10.1111/exd.12873. [DOI] [PubMed] [Google Scholar]
- 38.Tuzova M, Richmond J, Wolpowitz D, Curiel-Lewandrowski C, Chaney K, Kupper T, Cruikshank W. CCR4+ T-cell recruitment to the skin in mycosis fungoides: Potential contributions by thymic stromal lymphopoietin and interleukin-16. Leuk Lymphoma. 2015;56:440–449. doi: 10.3109/10428194.2014.919634. [DOI] [PubMed] [Google Scholar]
- 39.Hashikawa K, Yasumoto S, Nakashima K, Arakawa F, Kiyasu J, Kimura Y, Saruta H, Nakama T, Yasuda K, Tashiro K, et al. Microarray analysis of gene expression by microdissected epidermis and dermis in mycosis fungoides and adult T-cell leukemia/lymphoma. Int J Oncol. 2014;45:1200–1208. doi: 10.3892/ijo.2014.2524. [DOI] [PubMed] [Google Scholar]
- 40.Furudate S, Fujimura T, Kakizaki A, Hidaka T, Asano M, Aiba S. Tumor-associated M2 macrophages in mycosis fungoides acquire immunomodulatory function by interferon alpha and interferon gamma. J Dermatol Sci. 2016;83:182–189. doi: 10.1016/j.jdermsci.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Fujimura T, Kambayashi Y, Fujisawa Y, Hidaka T, Aiba S. Tumor-associated macrophages: Therapeutic targets for skin cancer. Front Oncol. 2018;8:3. doi: 10.3389/fonc.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humphrey RL, Karpetsky TP, Neuwelt EA, Levy CC. Levels of serum ribonuclease as an indicator of renal insufficiency in patients with leukemia. Cancer Res. 1977;37:2015–2022. [PubMed] [Google Scholar]
- 43.Serban M, Cucu C, Mihăilescu E, Micu D. Value of ribonuclease and guanase activity for the diagnosis of leukemias. Rev Roum Med Intern. 1974;11:319–324. [PubMed] [Google Scholar]
- 44.Biomarkers Definitions Working G, Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 45.Pistol-Tanase C, Raducan E, Dima SO, Albulescu L, Alina I, Marius P, Cruceru LM, Codorean E, Neagu TM, Popescu I. Assessment of soluble angiogenic markers in pancreatic cancer. Biomarkers Med. 2008;2:447–455. doi: 10.2217/17520363.2.5.447. [DOI] [PubMed] [Google Scholar]
- 46.FDA-NIH Biomarker Working Group . Silver Spring; MA, USA: 2016. BEST (Biomarkers, EndpointS, and other Tools) Resource (Internet) [PubMed] [Google Scholar]
- 47.Caruntu C, Boda D, Dumitrascu G, Constantin C, Neagu M. Proteomics focusing on immune markers in psoriatic arthritis. Biomarkers Med. 2015;9:513–528. doi: 10.2217/bmm.14.76. [DOI] [PubMed] [Google Scholar]
- 48.Neagu M, Caruntu C, Constantin C, Boda D, Zurac S, Spandidos DA, Tsatsakis AM. Chemically induced skin carcinogenesis: Updates in experimental models. (Review) Oncol Rep. 2016;35:2516–2528. doi: 10.3892/or.2016.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihai S, Codrici E, Popescu ID, Enciu AM, Rusu E, Zilisteanu D, Albulescu R, Anton G, Tanase C. Proteomic biomarkers panel: New insights in chronic kidney disease. Dis Markers. 2016;2016:3185232. doi: 10.1155/2016/3185232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matei C, Tampa M, Caruntu C, Ion RM, Georgescu SR, Dumitrascu GR, Constantin C, Neagu M. Protein microarray for complex apoptosis monitoring of dysplastic oral keratinocytes in experimental photodynamic therapy. Biol Res. 2014;47:33. doi: 10.1186/0717-6287-47-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanase CP, Albulescu R, Neagu M. Application of 3D hydrogel microarrays in molecular diagnostics: Advantages and limitations. Expert Rev Mol Diagn. 2011;11:461–464. doi: 10.1586/erm.11.30. [DOI] [PubMed] [Google Scholar]
- 52.Caruntu C. Catecholamines increase in vitro proliferation of murine B16F10 melanoma cells. Acta Endocrinol (Bucur) 2014;10:545–558. doi: 10.4183/aeb.2014.545. [DOI] [Google Scholar]
- 53.Boda D. Cellomics as integrative omics for cancer. Curr Proteomics. 2013;10:237–245. doi: 10.2174/1570164611310030006. [DOI] [Google Scholar]
- 54.Zurac S, Neagu M, Constantin C, Cioplea M, Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et al. Variations in the expression of TIMP1, TIMP2 and TIMP3 in cutaneous melanoma with regression and their possible function as prognostic predictors. Oncol Lett. 2016;11:3354–3360. doi: 10.3892/ol.2016.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ion A, Popa IM, Papagheorghe LM, Lisievici C, Lupu M, Voiculescu V, Caruntu C, Boda D. Proteomic approaches to biomarker discovery in cutaneous T-cell lymphoma. Dis Markers. 2016;2016:9602472. doi: 10.1155/2016/9602472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Igreja C, Courinha M, Cachaço AS, Pereira T, Cabeçadas J, Da Silva MG, Dias S. Characterization and clinical relevance of circulating and biopsy-derived endothelial progenitor cells in lymphoma patients. Haematologica. 2007;92:469–477. doi: 10.3324/haematol.10723. [DOI] [PubMed] [Google Scholar]
- 57.Schadendorf D, Matharoo-Ball B, Rees R, Ugurel S, Utikal J. Prognostic biomarkers of cutaneous malignancies - serological, immunohistochemical and proteomic approaches. Curr Cancer Ther Rev. 2008;4:96–104. doi: 10.2174/157339408784310061. [DOI] [Google Scholar]
- 58.Hassel JC, Meier R, Joller-Jemelka H, Burg G, Dummer R. Serological immunomarkers in cutaneous T-cell lymphoma. Dermatology. 2004;209:296–300. doi: 10.1159/000080852. [DOI] [PubMed] [Google Scholar]
- 59.Moshkovskii SA, Sokolova EE, Brattseva EV, Karpova MA, Pyatnitskiy MA, Kubanova AA, Archakov AI. Proteome and cytokine serum profiling to diagnose a mycosis fungoides. Proteomics Clin Appl. 2011;5:432–439. doi: 10.1002/prca.201000165. [DOI] [PubMed] [Google Scholar]
- 60.Popescu I, Raducan E, Dinischiotu A, Tanase C. Applications of SELDI-TOF technology in cancer biomarkers discovery. Rom Biotechnol Lett. 2010;15:5654–5667. [Google Scholar]
- 61.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085–1102. doi: 10.1002/ajh.24876. [DOI] [PubMed] [Google Scholar]
- 62.Van Arnam JS, Lim MS, Elenitoba-Johnson KSJ. Novel insights into the pathogenesis of T-cell lymphomas. Blood. 2018;131:2320–2330. doi: 10.1182/blood-2017-11-764357. [DOI] [PubMed] [Google Scholar]
- 63.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, Totoki Y, Chiba K, Sato-Otsubo A, Nagae G, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 64.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 65.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 66.Miller KD. E2100: A phase III trial of paclitaxel versus paclitaxel/bevacizumab for metastatic breast cancer. Clin Breast Cancer. 2003;3:421–422. doi: 10.3816/CBC.2003.n.007. [DOI] [PubMed] [Google Scholar]
- 67.Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L, Buzenet G, Koehler E, Sosman JA, Schwartz LH, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bagot M, Hasan B, Whittaker S, Beylot-Barry M, Knobler R, Shah E, Marreaud S, Morris S, Dalle S, Servitje O, et al. A phase III study of lenalidomide maintenance after debulking therapy in patients with advanced cutaneous T-cell lymphoma; EORTC 21081 ( NCT01098656): Results and lessons learned for future trial designs. Eur J Dermatol. 2017;27:286–294. doi: 10.1684/ejd.2017.3008. [DOI] [PubMed] [Google Scholar]
- 70.Neagu M, Constantin C, Zurac S. Immune parameters in the prognosis and therapy monitoring of cutaneous melanoma patients: Experience, role, and limitations. BioMed Res Int. 2013;2013:107940. doi: 10.1155/2013/107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 72.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 73.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 74.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 75.Kremer M, Sliva K, Klemke CD, Schnierle BS. Cutaneous T-cell lymphoma cells are sensitive to rapamycin. Exp Dermatol. 2010;19:800–805. doi: 10.1111/j.1600-0625.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 76.Marzec M, Liu X, Wysocka M, Rook AH, Odum N, Wasik MA. Simultaneous inhibition of mTOR-containing complex 1 (mTORC1) and MNK induces apoptosis of cutaneous T-cell lymphoma (CTCL) cells. PLoS One. 2011;6:e24849. doi: 10.1371/journal.pone.0024849. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Piekarz RL, Robey R, Sandor V, Bakke S, Wilson WH, Dahmoush L, Kingma DM, Turner ML, Altemus R, Bates SE. Inhibitor of histone deacetylation, depsipeptide, in the treatment of peripheral and cutaneous T-cell lymphoma: A case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.V98.9.2865. [DOI] [PubMed] [Google Scholar]
- 78.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 79.Shah JJ, Orlowski RZ. Proteasome inhibitors in the treatment of multiple myeloma. Leukemia. 2009;23:1964–1979. doi: 10.1038/leu.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jain S, Zain J, O'Connor O. Novel therapeutic agents for cutaneous T-cell lymphoma. J Hematol Oncol. 2012;5:24. doi: 10.1186/1756-8722-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buac D, Shen M, Schmitt S, Kona FR, Deshmukh R, Zhang Z, Neslund-Dudas C, Mitra B, Dou QP. From bortezomib to other inhibitors of the proteasome and beyond. Curr Pharm Des. 2013;19:4025–4038. doi: 10.2174/1381612811319220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 83.Kozuch PS, Rocha-Lima CM, Dragovich T, Hochster H, O'Neil BH, Atiq OT, Pipas JM, Ryan DP, Lenz HJ. Bortezomib with or without irinotecan in relapsed or refractory colorectal cancer: Results from a randomized phase II study. J Clin Oncol. 2008;26:2320–2326. doi: 10.1200/JCO.2007.14.0152. [DOI] [PubMed] [Google Scholar]
- 84.Morris MJ, Kelly WK, Slovin S, Ryan C, Eicher C, Heller G, Scher HI. A phase II trial of bortezomib and prednisone for castration resistant metastatic prostate cancer. J Urol. 2007;178:2378–2383. doi: 10.1016/j.juro.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 85.Schmid P, Kühnhardt D, Kiewe P, Lehenbauer-Dehm S, Schippinger W, Greil R, Lange W, Preiss J, Niederle N, Brossart P, et al. A phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol. 2008;19:871–876. doi: 10.1093/annonc/mdm569. [DOI] [PubMed] [Google Scholar]
- 86.Heider U, Rademacher J, Lamottke B, Mieth M, Moebs M, von Metzler I, Assaf C, Sezer O. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in cutaneous T-cell lymphoma. Eur J Haematol. 2009;82:440–449. doi: 10.1111/j.1600-0609.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 87.Kim SJ, Yoon DH, Kang HJ, Kim JS, Park SK, Kim HJ, Lee J, Ryoo BY, Ko YH, Huh J, et al. Consortium for improving survival of lymphoma (CISL) investigators: Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: A multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48:3223–3231. doi: 10.1016/j.ejca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Zinzani PL, Musuraca G, Tani M, Stefoni V, Marchi E, Fina M, Pellegrini C, Alinari L, Derenzini E, de Vivo A, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:4293–4297. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.