Abstract
Background:
Changing pneumococcal disease epidemiology due to childhood vaccination has prompted re-examination of US adult pneumococcal vaccination policies, as have considerations of greater pneumococcal disease incidence and higher prevalence of conditions that increase risk in underserved minority populations. Prior analyses suggest routine pneumococcal vaccination at age 50 could be considered, which could disproportionately benefit underserved populations.
Methods:
A Markov cohort model estimated the cost-effectiveness of US pneumococcal vaccination policies in hypothetical 50-year-old underserved minority and general population cohorts. Strategies included receiving one or both available pneumococcal vaccines based on age- or chronic condition-specific criteria. US databases and medical literature data calibrated pneumococcal illness incidence, vaccine serotype distributions, age- and race-specific chronic condition distributions, and costs. Black population data were used as a proxy for underserved minorities. We took a US healthcare perspective, discounting at 3%/year. One-way and probabilistic sensitivity analyses were performed and scenarios modeling differing vaccine assumptions were examined.
Results:
In both black and general population 50-year-olds, giving both pneumococcal vaccines to all 50-year-olds prevented the most disease, but cost >$250,000 per quality adjusted life year (QALY) gained. Current CDC recommendations (both vaccines for the immunocompromised, polysaccharide vaccine for other high-risk conditions) were economically favorable in either population when analyses assumed polysaccharide vaccine was ineffective against nonbacteremic pneumococcal pneumonia (NBP). If polysaccharide vaccine is effective against NBP or if less complex age-based vaccination recommendations result in increased vaccine uptake, giving polysaccharide vaccine to all 50-year-olds cost <$100,000/QALY; this effect was more pronounced in black cohorts. Results were robust in 1-way and probabilistic sensitivity analyses.
Conclusions:
Despite changes in pneumococcal epidemiology, current CDC recommendations were favored in underserved minority and general population cohorts. Polysaccharide vaccine for all 50-year-olds could be considered under some vaccine uptake and effectiveness assumptions, particularly if mitigating racial health disparities in pneumococcal disease is a priority.
Keywords: adult pneumococcal vaccination, cost-effectiveness analysis, decision analysis, underserved minorities
Introduction
Recommendations for newly introduced vaccines are frequently limited to population subgroups having a high risk of vaccine-preventable disease or an increased risk of severe illness or complications due to chronic medical conditions. Subsequently, recommendations may be expanded to become age-based for easier determination of vaccine eligibility and consequent increases in vaccine coverage. An example in the US is influenza vaccine, which was initially recommended for seniors and other high-risk adults but gradually expanded to other age, risk and occupational groups to include all persons ≥6 months of age.1
Unlike the entirely age-based US influenza vaccination recommendations, current US adult pneumococcal vaccine recommendations are based on both age and risk. The 2 vaccines available in the US, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13), are recommended for all persons aged ≥65 years and for adults aged 18–64 years with immunocompromising conditions, while PPSV23 alone is recommended for 18–64-year-olds with other conditions placing them at high pneumococcal illness risk.2
Routine childhood PCV13 use in the US has changed pneumococcal disease epidemiology,3–5 with adults benefiting from indirect (herd immunity) effects of childhood immunization, resulting in decreased adult pneumococcal disease risk and decreased likelihood of disease caused by PCV13 serotypes. These effects could eliminate the need for adult PCV13 use, warranting periodic evaluation of pneumococcal vaccination policy and complicating consideration of routine pneumococcal vaccination at age 50 years, which previous research suggests may be favorable.6–8 Other factors to be considered are: 1) greater pneumococcal disease risk in underserved minority adults and in persons with high-risk conditions and 2) high PCV13 cost. Due to greater pneumococcal disease risk, higher likelihood of undiagnosed high-risk chronic medical conditions, and lower likelihood of pneumococcal vaccination in underserved minority adults, the underserved could be disproportionately favored by general population recommendations for routine age-based adult pneumococcal vaccination compared to the population as a whole. In this study, we use Markov cohort decision analysis modeling to compare the cost-effectiveness of several possible general population vaccination strategies for adults aged 50–64 years, with specific examination of those strategies’ effects in underserved minorities. We do not model race-based vaccination strategies, which are not tenable or actionable in the US.9 Instead, we are interested in whether general population routine pneumococcal vaccination at age 50 years might be still be favorable in the underserved and in the general adult population given decreases in disease incidence and vaccine serotype frequency due to childhood pneumococcal vaccination.3–5 As such, our analysis is focused on: 1) whether national general population pneumococcal vaccination policies can either disproportionately benefit or harm underserved subpopulations within the general population compared to the general population as a whole, and 2) estimating the cost-effectiveness of pneumococcal vaccination strategies in each population to better inform US general population vaccination policy recommendations.
Methods
A Markov model estimated the public health impact and cost-effectiveness of pneumococcal vaccination policies in hypothetical 50–64-year-old US black and general population cohorts meant to mirror US census data, with black population data used as a proxy for all underserved minority populations. This choice was made because: 1) black populations make up a substantial proportion of the US underserved (i.e., lowest socioeconomic status groups)10 minority population; and 2) available US bacterial disease surveillance data do not adequately capture underserved minority status, particularly for Hispanic ethnicity.11 A Markov model was chosen to facilitate tracking of health status and disease incidence, health effects, and costs over cohorts’ lifetimes. Mutually exclusive vaccination strategies examined in the model included: 1) no vaccination, 2) present CDC recommendations for this age group (both PCV13 and PPSV23 for persons with immunocompromising conditions and PPSV23 for those with other high-risk conditions), 3) PPSV23 alone for individuals with immunocompromising or high-risk conditions, 4) both PCV13 and PPSV23 for immunocompromising or high-risk conditions, 5) PPSV23 alone for everyone at age 50, or 6) both PCV13 and PPSV23 for everyone at age 50, implemented with vaccination probabilities and costs as observed in the US health care system. Similar analyses were performed in the US non-black population, as shown in the supplementary material.
Age- and race-specific population health status data from National Health Interview Survey (NHIS) and National Center for Health Statistics (NCHS) datasets were used to segment general population and black population cohorts of 50-year-olds into the following health status subgroups: average risk, average risk smokers, individuals with immunocompromising conditions and those with other non-immunocompromising pneumococcal high-risk conditions. Per CDC definitions, non-immunocompromising, high-risk conditions for pneumococcal disease included chronic heart disease (including congestive heart failure and cardiomyopathies); chronic lung disease (including chronic obstructive lung disease, emphysema, and asthma); chronic liver disease (including cirrhosis); alcoholism; diabetes mellitus; and smoking. We considered smokers without immunocompromising or other high-risk conditions as average risk smokers, while smokers with high-risk or immunocompromising conditions were considered as part of the high-risk or immunocompromising condition health status subgroup as appropriate. Persons with both immunocompromising conditions and other high-risk conditions were included in the immunocompromising condition group, in keeping with CDC vaccination recommendations for this group. As cohorts aged, the relative likelihood of health status changed, based on age and race, using the data sources above.
For any strategy where vaccination was recommended, vaccination coverage (probability of vaccination) was 23% for ages 50–64 years, based on observed pneumococcal vaccine uptake in high-risk adults aged <65 years.12 Vaccination occurred in the first year that it was indicated. In addition, patients with changes in health status that made them eligible for vaccination at ages <65 years were vaccinated during the year of that transition at the same 23% probability. In sensitivity analyses, vaccine uptake was varied over plausible ranges and the possibility of increased uptake with age-based vaccination, due to less complex eligibility determination, was also considered. Once model cohorts reached age 65 years, regardless of initial vaccination strategy, all patients could receive both pneumococcal vaccines, as currently recommended, at a probability of 63.6%, the current US pneumococcal vaccination rate in persons aged 65 years and older.
Vaccine effectiveness and effectiveness duration estimates for both PCV13 and PPSV23 against invasive pneumococcal disease (IPD) came from Delphi expert panel estimates. PCV13 effectiveness against non-bacteremic pneumococcal pneumonia (NBP) was based on trial observations from the Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA)13 for the first 5 years after vaccination. We assumed similar effectiveness in 50–64-year-olds to that seen in the ≥65-year-old trial population, due to sparse clinical trial data in younger populations. Trial data beyond 5 years are not available, thus subsequent waning beyond 5 years was based on Delphi expert panel estimates,6 using CAPITA trial data as the starting point.
CDC Active Bacterial Core Surveillance (ABCs) data and medical literature data were used to calculate IPD incidence and vaccine serotype-specific illness likelihood. NBP incidence is an area of uncertainty, and was estimated based on treatment setting (inpatient or outpatient). For inpatient NBP, we assumed three cases for every one case of bacteremic pneumonia, based on the estimated proportion of inpatient pneumococcal pneumonia that is bacteremic.14 Bacteremic pneumonia rates came from CDC ABCs data, as a component of IPD rates. Outpatient NBP cases in the general population were estimated15 as a percentage (30%) of all-cause outpatient pneumonia with a further reduction (35.1%)16 to reflect further decreases in IPD among individuals aged 19–64 years. To obtain the outpatient NBP rate in the black population, we applied the ratio of IPD rates between the general and black populations to the estimated outpatient NBP rate in the general population. The base case scenario assumed that PPSV23 was not effective against NBP; however, we relaxed this assumption in an alternative scenario where PPSV23 had a 50% relative effectiveness against NBP compared to its effectiveness against IPD (i.e., one half of the effectiveness values in Supplemental Table 3b). Given substantial uncertainty regarding outpatient pneumococcal pneumonia rates, a separate sensitivity analysis was performed where outpatient NBP was excluded from consideration.
In addition to the assumptions regarding vaccine effectiveness, vaccination strategy uptake, and NBP frequency mentioned above, we made several other modeling assumptions. We assumed that NBP serotype distributions were the same as those of IPD, and that serotype distributions did not differ based on chronic health state. In addition, based on little recent change in US pneumococcal IPD rates and serotype distributions, we assumed no further indirect effect-based changes in those pneumococcal disease rate or serotype distributions in our cohorts as they aged.
Pneumococcal disease costs were obtained from the US National Inpatient Sample (NIS), which approximates a 20% sample of US hospitals.17 NIS data contain total charges for each hospital stay, representing the amount that hospitals bill for services regardless of payer. Hospital-specific cost-to-charge ratios based on all-payer inpatient costs are then applied to charge data to allow researchers to determine costs either by (1) weighted average for hospitals in peer groups defined by hospital characteristics and state, or (2) hospital, if provided. In our analysis, total charges were adjusted by the cost-to-charge ratios to obtain costs. Vaccine and vaccine administration costs were obtained from CDC and Medicare national databases.18,19 Costs are presented as 2014 US dollars, with prior costs inflated using the US Consumer Price Index. Utilities and illness disutilities were drawn from the medical literature.7,15,20,21 We took a US healthcare perspective, discounting costs and effectiveness at 3% per year, the US standard.22
For each strategy analyzed, we used model-calculated illness and mortality risks for IPD and NBP and January 2018 US census data23 to calculate public health outcomes for 50-year-old single-age black and general population cohorts over their remaining lifetimes. NBP disease outcomes included both inpatient and outpatient NBP. To further assess the public health impact of each modeled strategy, we calculated the number of individuals needed to vaccinate in order to prevent one case of pneumococcal disease in each strategy. We assumed that only hospitalized NBP could result in mortality.
Cost and effectiveness outputs from the model generated the per person cost and effectiveness, in quality adjusted life years (QALY), over their lifetime, for each strategy. Strategies were then ordered by cost, and incremental cost and effectiveness calculated through row-by-row comparisons and expressed as cost per QALY gained. Dominated strategies (more expensive and less effective than other modeled strategies, or having greater incremental cost-effectiveness ratios than more effective strategies) were excluded from the analysis.22 Cost-effectiveness analysis results were reported using the commonly cited US acceptability benchmark of $100,000/QALY gained.24
One-way and probabilistic sensitivity analyses were performed to test the robustness of model results. In the probabilistic sensitivity analyses, parameters were assigned distributions in keeping with SMDM/ISPOR guidelines:25 gamma distributions for costs and beta distributions for probabilities and utilities, fitted to approximate ranges listed in Table 1. Parameter distributions were simultaneously sampled 3000 times, with results summarized in cost-effectiveness acceptability curves.
Table 1.
Parameter Values Examined in Model
| Parameter | Value | Range | Source |
|---|---|---|---|
| Probabilities | |||
| Receiving vaccination | 23% | 17.9% – 27.7% | 12 |
| PCV13 serotype coverage | |||
| Black Population | 18.6% | 12.7% – 25.2% | CDC ABCs |
| General Population | 24.5% | 21.1% – 28.5% | CDC ABCs |
| PPSV23 serotype coverage | |||
| Black Population | 57.5% | 47.8% – 69.6% | CDC ABCs |
| General Population | 66.9% | 57.7% – 78.1% | CDC ABCs |
| Vaccine Effectiveness | |||
| PCV against IPD | Supplemental Table 3a | ||
| PCV against NBP | Supplemental Table 3a | ||
| PPSV against IPD | Supplemental Table 3b | ||
| IPD yearly risk per 100,000 | |||
| Black Population | 28.0 | 25.0 – 31.4 | CDC ABCs |
| General Population | 16.3 | 15.1 – 17.5 | CDC ABCs |
| Non-bacteremic pneumonia yearly risk per 100,000 | |||
| Inpatient | |||
| Black Population | 78.3 | 26.3 – 135.6 | Estimate |
| General Population | 45.9 | 20.0 – 102.9 | Estimate |
| Outpatient | |||
| Black Population | 200.9 | 30.4 – 309.4 | Estimate |
| General Population | 117.9 | 23.2 – 235.8 | Estimate |
| IPD case fatality | |||
| Black Population | 8% | 5.7% – 10.5% | CDC ABCs |
| General Population | 10% | 9.1% – 11.8% | CDC ABCs |
| Disability post IPD | |||
| Black Population | 6% | 4.5% – 8.8% | CDC ABCs |
| General Population | 6% | 4.7% – 6.7% | CDC ABCs |
| Costs | |||
| Vaccine | |||
| PPSV23 | $94.51 | $48.78 – $152.45 | 18 |
| PCV13 | $180.05 | $103 – $277 | 18 |
| Administration | $25.84 | $21.33 – $31.18 | 19 |
| Vaccine side effects (per occurrence) | $0.76 | $0 – $2 | Estimate |
| IPD-discharged alive | $26,001 | $13,000 – $39.001 | NIS |
| IPD-death | $49,066 | $24,533 – $73,599 | NIS |
| Pneumonia- discharged alive | $19,984 | $9,992 – $29,976 | NIS |
| Outpatient pneumonia | $131.23 | $65.62 – $196.85 | NIS |
| Pneumonia-death | $46,647 | $23,323 – $69,970 | NIS |
| Initial IPD symptom treatment | $5 | $0 – $10 | Estimate |
| Disability (per yr) | $14,239 | $7,947 – $21,763 | 41 |
| Utility weights | |||
| Disability | 0.4 | 0.21 – 0.59 | Estimate21 |
| Hospitalization | 0.2 | 0.11 – 0.30 | 7,20 |
| Vaccine side effects | 0.9 | 0.77 – 0.97 | Estimate21 |
| Well | 0.83 | 0.78–0.88 | 7 |
| Disutility- outpatient NBP | 0.004 | 0.002 – 0.006 | 15 |
| Illness durations (days) | |||
| IPD | 34 | 17–51 | 7,20 |
| Hospitalized pneumonia | 34 | 17–51 | 7,20 |
| Vaccine side effects | 3 | 1–8 | 42 |
Role of the funding source: This study was funded by the US National Institute for Allergy and Infectious Diseases. The funding source had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Results
From a public health perspective, giving both PPSV23 and PCV13 to all 50-year-olds resulted in the fewest IPD and NBP cases and deaths for both the general and black populations and in scenarios when PPSV23 was or was not effective against NBP (Table 2). The next fewest number of IPD and NBP cases and deaths resulted from giving PPSV23 alone to all 50-year-olds in all but one scenario, i.e., IPD deaths among the general population when PPSV23 was ineffective against NBP. In this scenario, it was more advantageous to give both vaccines to individuals with high-risk and immunocompromising conditions. For context, in cohorts of 50-year-olds over their remaining lifetime, the strategies with the greatest public health impact, compared to no vaccination when PPSV was assumed to be ineffective against NBP, reduced pneumococcal disease cases by 652 (number needed to vaccinate [NNV] to prevent 1 case = 194) and deaths by 23 among a cohort of 549,197 blacks. In the same analysis examining the general population cohort (n = 4,034,327), pneumonia cases were reduced by 3,543 (NNV = 262) and deaths by 126 compared to no vaccination. Morbidity and mortality reductions when PPSV was effective against NBP were 1,108 cases (NNV 114) and 32 deaths in the black population and 5,981 cases (NNV = 156) and 189 deaths in the general population.
Table 2.
Public Health Results in a 50-year-old single-year cohort over their remaining lifetimes
| Assuming PPSV23 is NOT effective vs. non-bacteremic pneumonia | IPD cases | IPD deaths | NBP cases | NBP deaths |
| Black Population (cohort size = 549,197) | ||||
| No vaccination | 7,617 | 904 | 67,258 | 1,097 |
| High-risk, immunocompromised get PPSV23 | 7,523 | 894 | 67,198 | 1,097 |
| Present recs, (high-risk get PPSV23, immunocompromised get PCV13/PPSV23) | 7,512 | 893 | 67,169 | 1,095 |
| High-risk, immunocompromised get PCV13/PPSV23 | 7,512 | 893 | 66,985 | 1,092 |
| All get PPSV23 | 7,484 | 890 | 67,199 | 1,097 |
| All get PCV13/PPSV23 | 7,469 | 889 | 66,755 | 1,089 |
| General Population (cohort size = 4,034,327) | ||||
| No vaccination | 32,635 | 3,792 | 420,634 | 8,593 |
| High-risk, immunocompromised get PPSV23 | 32,163 | 3,745 | 420,401 | 8,600 |
| Present recs, (high-risk get PPSV23, immunocompromised get PCV13/PPSV23) | 32,110 | 3,739 | 420,260 | 8,590 |
| High-risk, immunocompromised get PCV13/PPSV23 | 32,104 | 3,739 | 419,306 | 8,558 |
| All get PPSV23 | 31,975 | 3,726 | 420,406 | 8,600 |
| All get PCV13/PPSV23 | 31,897 | 3,718 | 417,829 | 8,540 |
| Assuming PPSV23 IS effective vs. non-bacteremic pneumonia | IPD cases | IPD deaths | NBP cases | NBP deaths |
| Black Population (cohort size = 549,197) | ||||
| No vaccination | 7,618 | 904 | 67,109 | 1,087 |
| High-risk, immunocompromised get PPSV23 | 7,524 | 894 | 66,664 | 1,078 |
| Present recs, (high-risk get PPSV23, | 7,512 | 893 | 66,636 | 1,076 |
| immunocompromised get PCV13/PPSV23) | ||||
| High-risk, immunocompromised get PCV13/PPSV23 | 7,512 | 893 | 66,589 | 1,075 |
| All get PPSV23 | 7,485 | 890 | 66,333 | 1,073 |
| All get PCV13/PPSV23 | 7,469 | 889 | 66,149 | 1,070 |
| General Population (cohort size = 4,034,327) | ||||
| No vaccination | 32,636 | 3,792 | 419,602 | 8,506 |
| High-risk, immunocompromised get PPSV23 | 32,165 | 3,745 | 417,512 | 8,443 |
| Present recs, (high-risk get PPSV23, | 32,112 | 3,740 | 417,371 | 8,433 |
| immunocompromised get PCV13/PPSV23) High-risk, immunocompromised get PCV13/PPSV23 | 32,105 | 3,739 | 41 7,095 | 8,425 |
| All get PPSV23 | 31,977 | 3,726 | 415,534 | 8,418 |
| All get PCV13/PPSV23 | 31,898 | 3,718 | 414,360 | 8,390 |
Reductions in illness were then viewed in the context of cost. In a cost-effectiveness analysis of the 50-year-old black cohort when PPSV23 was assumed to be ineffective against NBP (Table 3, top), continuing present CDC recommendations (both vaccines for the immunocompromised, PPSV23 for ages <65 with other high-risk conditions) cost $37,346 per quality adjusted life year (QALY) gained compared to no vaccination. In the black population analysis, using PPSV23 in all 50-year olds cost $123,087/QALY and using both vaccines in all 50-year olds cost $284,654/QALY gained. Both strategies cost more than the US acceptability benchmark of $100,000/QALY gained.24 Using PPSV23 alone or both PPSV23 and PCV13 in high-risk groups (with no vaccination for non-high-risk persons) were dominated (i.e., they were either more expensive and less effective, or had higher cost-effectiveness ratios than more effective strategies). Further details on this analysis, on the analyses below, and on the analysis on the non-black populations are shown in the supplemental material (Supplemental Table 1).
Table 3.
Cost-effectiveness Analysis Results*
| Assuming PPSV23 is NOT effective vs. non-bacteremic pneumonia | Incremental Cost- Effectiveness |
| Black Population | |
| Present recommendations (high-risk get PPSV23, immunocompromised get PCV13/PPSV23) | $37,346 |
| All get PPSV23 | $123,087 |
| All get PCV13/PPSV23 | $284,654 |
| General Population | |
| Present recommendations | $42,068 |
| High-risk, immunocompromised get PCV13/PPSV23 | $220,251 |
| All get PCV13/PPSV23 | $258,573 |
| Assuming PPSV23 IS effective vs. non-bacteremic pneumonia | |
| Black Population | |
| High-risk, immunocompromised get PPSV23 | $14,786 |
| Present recommendations | $15,357 |
| All get PPSV23 | $43,957 |
| All get PCV13/PPSV23 | $752,245 |
| General Population | |
| High-risk, immunocompromised get PPSV23 | $14,556 |
| Present recommendations | $24,497 |
| All get PPSV23 | $81,002 |
| All get PCV13/PPSV23 | $522,774 |
Dominated strategies not shown
In the general population analysis, present recommendations cost about $42,000/QALY gained, while using both vaccines in high-risk groups or in all 50 year olds cost >$200,000/QALY gained; strategies giving PPSV23 to all 50 year olds or only PPSV23 (without PCV13) to high risk groups were dominated.
Conversely, when PPSV23 was assumed to be effective in preventing NBP (Table 3, bottom), giving PPSV to all 50 year olds was the most effective strategy that cost less than $100,000/QALY gained in both black and general populations, costing about $44,000/QALY in the black population analysis and about $81,000/QALY in the general population. Giving both vaccines to all 50 year olds cost >$500,000/QALY gained in all population groups under this assumption.
Sensitivity Analyses
Because of considerable uncertainty regarding inpatient and outpatient NBP rates, we varied those rates jointly and examined those variations’ effects on results (Supplemental Table 2). When PPSV23 was assumed to be ineffective against NBP, present recommendations remained the favored strategy, at a $100,000/QALY threshold, regardless of population or variation in pneumonia rates, while giving PPSV23 to all 50 year olds in the black population analysis varied from $105,000-$147,000/QALY gained. When PPSV23 was assumed to prevent NBP, giving all 50 year olds PPSV23 remained the favored strategy at a $100,000/QALY threshold with one exception: in the general population at low NBP rates, this strategy cost about $120,000/QALY gained. PCV13/PPSV23 for all at age 50 remained unfavorable regardless of NBP rate in all population and PPSV23 effectiveness scenarios, with the lowest cost of approximately $160,000/QALY gained when high range NBP rates were modeled.
Age-based recommendations for pneumococcal vaccination (rather than health status-based recommendations) could be easier to implement and consequently may lead to greater vaccine uptake. Therefore, we examined absolute vaccine uptake improvements of 5% and 10% in strategies where vaccines were recommended for all 50-year-olds (Supplemental Table 3). In these analyses, giving both vaccines to all 50 year olds remained unfavorable regardless of population examined, assumed uptake increase, or PPSV effectiveness scenario, costing ≥$258,000/QALY gained. When PPSV23 was assumed to be effective against NBP, giving PPSV23 to all 50 year olds became more favorable with increased uptake. When PPSV was assumed to be not effective against NBP, giving PPSV23 to all 50 year olds cost about $80,000/QALY in the black population and about $144,000/QALY in the general population if uptake increased 10 percentage points when age-based strategies were used.
Finally, we examined a scenario where outpatient NBP was not considered in the model, due to even greater uncertainty regarding its true frequency. This analysis did not substantially change model results favoring the present recommendations strategy, increasing the cost-effectiveness ratio of present recommendations by about $8,000/QALY gained in the black population.
Probabilistic Sensitivity Analyses
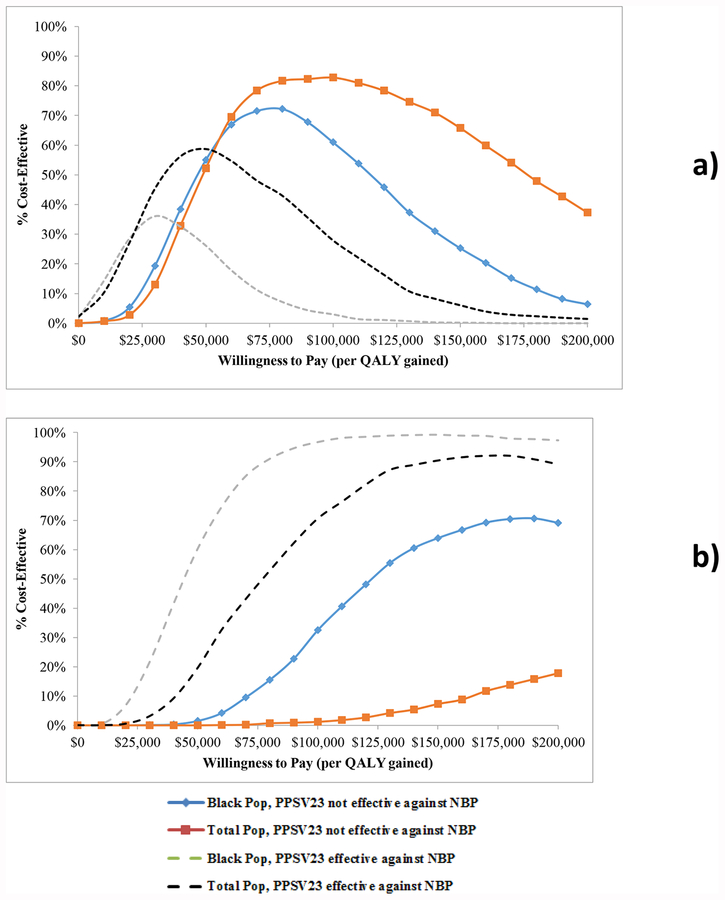
Figure 1 depicts probabilistic sensitivity analyses, varying all parameters over distributions, for two vaccination strategies; present recommendations and giving PPSV23 alone to everyone at age 50, in all population- and PPSV23 effectiveness scenarios. At a $50,000/QALY willingness-to-pay threshold, present recommendations (top panel) were favored more than half the time in all but one scenario (black population with PPSV23 effective against NBP). At a $100,000/QALY threshold, present recommendations were most favorable (82.8% cost-effective) in the general population when PPSV23 is assumed not effective against NBP. Giving PPSV23 alone to everyone at age 50 (bottom panel) was more favorable in the black population analysis, at all willingness-to-pay thresholds, compared to the favorability of this strategy in the general population cohort under either PPSV effectiveness assumption. A probabilistic sensitivity analysis in the non-black population had similar results to that performed in the general population (Supplemental Figure 1).
Figure 1. Probabilistic sensitivity analysis.
Panel a) depicts the likelihood that the current US adult pneumococcal recommendations are favored (y-axis), compared to other vaccination strategies, over a range of willingness-to-pay (or acceptability) thresholds (x-axis) for population and pneumococcal polysaccharide vaccine (PPSV23) effectiveness scenarios. Panel b) depicts the likelihood that giving all 50-year-olds PPSV23 is favored. QALY = quality adjusted life year, NBP = nonbacteremic pneumococcal pneumonia.
Discussion
This analysis found that, for 50–64-year-olds in either the black population or the general population, current general population recommendations for pneumococcal vaccination (both vaccines for persons with immunocompromising conditions and PPSV23 alone for those with other high-risk conditions) were economically favorable under base case assumptions. In analyses where age-based strategies could increase vaccine uptake compared to uptake under current recommendations, we found that general population recommendations for giving PPSV23 to all 50 year olds could be economically favorable in the black population. For example, if uptake increased 5 percentage points (from 23% to 28%) due to giving PPSV23 to all 50-year-olds in the general population, this strategy cost about $92,000/QALY gained in the black population analysis.
These findings could be particularly pertinent if addressing racial disparities in vaccination protection is a priority in policy deliberations, given the US black population’s higher prevalence of risk factors, higher likelihood of undiagnosed chronic illness, and greater risk of pneumococcal disease.26–28 Please note that we did not model race- or underserved-specific vaccination policies, as such policies are not currently tenable or actionable, based on CDC reluctance to make race-based vaccination recommendations. Instead, we modeled the potential effects of general population policies on that population segment, along with effects on the population as a whole. This analysis found that vaccinating all 50–64 year olds could have greater impact and be more economically favorable in the underserved, due to their greater chronic disease burden and pneumococcal disease risk. In addition, current adult pneumococcal vaccination rates are suboptimal (23% for high-risk adults aged 18–64 years) and racial disparities exist. For example, as of 2015, 50% percent of black American adults over age 65 years ever received a pneumococcal vaccine compared with 66% percent of white American adults of the same age.29 One reason for the overall low rates may be the complexity of current chronic disease-based pneumococcal vaccination recommendations.30,31 Making those recommendations age-based, and less complex to implement, could lead to greater uptake.
We also found that recommending routine PPSV23 for all 50–64-year-olds is favored when the vaccine is assumed to be effective against NBP. While evidence of PPSV23 effectiveness in preventing IPD is clear and widely supported, its effectiveness in preventing nonbacteremic pneumonia is less clear and subject to contentious debate.32–34 Our analysis examined results in either PPSV23 effectiveness scenario, and, not unexpectedly, made a stronger case for using PPSV23 in all 50-year-olds if PPSV23 effectiveness vs. NBP was assumed.
In 2012, similar analyses suggested that PCV13 use in all 50-year-olds could be economically reasonable, depending on assumptions made regarding pneumococcal disease incidence and serotype frequency.6 Now, using updated age- and race-specific data on pneumococcal illness rates and serotype frequency, our analysis found routine PCV13 use in strategies where both pneumococcal vaccines are given to all 50 year olds to be economically unfavorable regardless of the population studied. This change in findings is likely due to fuller indirect effects from childhood PCV13 vaccination, which began in 2010 in the US and has reached 82% uptake in children for all 4 recommended doses.35 Thus, successful PCV13 programs in children have substantially decreased pneumococcal disease from PCV13 serotypes in adults through herd immunity effects, likely decreasing the value of routine PCV13 use in adults <65 years old. ABCs data show that IPD rates among adults ≥65 years stabilized from 2014–17, including both types within PCV13 (plus type 6c) and non-vaccine types.36 This stabilization started just before the introduction of the ACIP recommendation for age-based adult vaccination in those ≥65 years. In the US, replacement disease has not been seen whereas it has been seen in other countries.37 Thus, increased PCV13 use in US adults aged 50–64 years may not pose a risk for increasing serotype replacement. However, if herd immunity from childhood PCV13 vaccination were eventually to eliminate disease caused by PCV13 types among adults, then vaccinating adults aged 50–64 years would be unnecessary. CDC currently recommends both pneumococcal vaccines for all adults aged 65 years or older, but is revisiting that decision, as planned, due to changes in epidemiology.38
Previous research suggested that routine PPSV use in all 50-year-olds could be economically reasonable.7,8 Our results for routine PPSV23 use, though affected by the same herd immunity effects on pneumococcal disease and serotype epidemiology, outline circumstances where PPSV23 for all 50 year olds could be considered. Routine PCV13 use at age 50 years was considered in the US and Belgium that found, similar to our analysis, that this strategy would not be economically favorable under reasonable herd immunity projections.15,39
The future herd immunity effects of childhood pneumococcal vaccination are uncertain, a limitation of our analysis. Pneumococcal vaccine serotype epidemiology appears to have stabilized in the US,40 but it is unclear if this will remain the case. Further decreases in PCV13 serotype frequency will make adult use of that vaccine less valuable, while increases in those serotypes will have the opposite effect. Another limitation is uncertainly regarding the incidence and serotype epidemiology of inpatient and outpatient nonbacteremic pneumococcal pneumonia. For incidence rates, we used previously described approximation techniques for base case values, then varied those values widely in sensitivity analyses. Serotype frequency for inpatient and outpatient NBP, were assumed to be similar to IPD serotype distributions but were similarly varied widely in sensitivity analyses. Once again, the value of pneumococcal vaccination strategies in adults aged 50–64 year could have been misestimated if these approximations were inaccurate.
Other limitations of our study include the use of multiple and varied data sources that, when combined in the model, may not accurately reflect reality. As previously described when estimating nonbacteremic pneumococcal pneumonia, we made several assumptions where information was either missing or unavailable, which included setting vaccination effectiveness reported in individuals aged ≥65 years to be similar in 50–64-year-olds. Moreover, we applied the same methodology in both general and black populations when estimating inpatient NBP rates from IPD rates, potentially masking any actual differences or similarities between the populations. As NBP rates were one of the greatest uncertainties in the analysis, we varied both inpatient and outpatient rates widely in sensitivity analysis in both populations. Our findings are also limited by modeling assumptions made regarding pneumococcal serotype distributions in NBP and differing health states, and regarding future pneumococcal illness rates and serotypes.
Conclusion
As the epidemiology of pneumococcal disease continues to evolve, periodic cost-effectiveness evaluation of vaccination policy is essential to maximize its public health impact and protect the most vulnerable populations. Although pneumococcal disease caused by serotypes contained in PCV13 has decreased due to routine childhood PCV13 vaccination, current CDC adult pneumococcal vaccination recommendations for adults aged <65 years were favored in black and general population cohorts in base case analyses, where PPSV23 was considered ineffective against NBP. PPSV23 given to all adults at age 50 may be favorable if mitigating racial health disparities is a priority, particularly if a simpler age-based vaccination indication results in greater vaccine uptake. Giving all 50-year-olds PPSV23 might also be considered if PPSV23 is conclusively shown to be effective against NBP.
Supplementary Material
Funding source:
This work was supported by the National Institutes of Health (grant number R01 AI11657503).
Conflict of interest: Dr. Zimmerman and Dr. Lin have an active research grant from Sanofi Pasteur and past grants from Merck & Co., Inc. and Pfizer Inc. Dr. Nowalk has received or currently receives grant funding from Merck & Co., Inc.,and Pfizer, Inc LLC. Dr. Schaffner is a member of data safety monitoring boards for Merck and Pfizer, and has served as a consultant for Dynavax, Novavax, GSK, Sanofi-Pasteur and Seqirus. Dr. Harrison is on a scientific advisory board for GSK. All other authors have no competing interests to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm. Rep 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 2.Centers for Disease Contol and Prevention (CDC). Adult Conditions Immunization Schedule. National Center for Immunization and Respiratory Diseases. 2016; http://www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html. Accessed 6/15/2016.
- 3.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–1186. [DOI] [PubMed] [Google Scholar]
- 4.Lexau CA, Lynfield R, Danila R, et al. Changing Epidemiology of Invasive Pneumococcal Disease Among Older Adults in the Era of Pediatric Pnemococcal Conjugate Vaccine. J. Am. Med. Assoc 2005;294(16):2043–2051. [DOI] [PubMed] [Google Scholar]
- 5.Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect. Dis 2015;15:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of Adult Vaccination Strategies Using Pneumococcal Conjugate Vaccine Compared With Pneumococcal Polysaccharide Vaccine. J. Am. Med. Assoc 2012;307(8):804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sisk JE, Whang W, Butler JC, Sneller V-P, Whitney CG. Cost-Effectiveness of Vaccination against Invasive Pneumococcal Disease among People 50 through 64 Years of Age: Role of Comorbid Conditions and Race. Ann. Intern. Med 2003;138(12):960–968. [DOI] [PubMed] [Google Scholar]
- 8.Smith KJ, Zimmerman RK, Lin CJ, et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: A cost-effectiveness analysis. Vaccine. 2008;26(11):1420–1431. [DOI] [PubMed] [Google Scholar]
- 9.Hebert PL, Frick KD, Kane RL, McBean AM. The causes of racial and ethnic differences in influenza vaccination rates among elderly Medicare beneficiaries. Health Serv. Res 2005;40(2):517–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorpe RJ Jr., Fesahazion RG, Parker L, et al. Accelerated Health Declines among African Americans in the USA. Journal of urban health : bulletin of the New York Academy of Medicine. 2016;93(5):808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.See I, Wesson P, Gualandi N, et al. Socioeconomic Factors Explain Racial Disparities in Invasive Community-Associated Methicillin-Resistant Staphylococcus aureus Disease Rates. Clin. Infect. Dis 2017;64(5):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of Vaccination Coverage among Adult Populations - United States, 2015. MMWR Surveill. Summ 2017;66(11):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. The New England Journal of Medicine. 2015;372:1114–1125. [DOI] [PubMed] [Google Scholar]
- 14.Said MA, Johnson HL, Nonyane BAS, Deloria-Knoll M, O’Brien KL. Estimating the Burden of Pneumococcal Pneumonia among Adults: A Systematic Review and Meta-Analysis of Diagnostic Techniques. PLoS One. 2013;8(4):e60273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental Cost-Effectiveness of 13-valent Pneumococcal Conjugate Vaccine for Adults Age 50 Years and Older in the United States. J. Gen. Intern. Med 2016;31(8):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilishvili T, Gierke R, Farley M, et al. Direct and Indirect Impact of 13-valent Pneumococcal Conjugate Vaccine (PCV13) on Invasive Pneumococcal Disease (IPD) Among Children and Adults in the U.S. Open Forum Infectious Diseases. 2017;4(Suppl 1):S66–S67. [Google Scholar]
- 17.HCUP Databases: Healthcare Cost and Utilization Project (HCUP). August 2018. Agency for Healthcare Research and Quality. Rockville, MD: https://www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 18.CDC Vaccine Price List. Centers for Disease Control and Prevention. http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/. Accessed May 26, 2018.
- 19.Medicare Physician Fee Schedule. https://www.cms.gov/apps/physician-fee-schedule/.
- 20.Mangen MJ, Huijts SM, Bonten MJ, de Wit GA. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect. Dis 2017;17(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold MR, Franks P, McCoy K, Fryback DG. Toward Consistency in Cost-Utility Analyses: Using National Measures to Create Condition-Specific Values. Med. Care 1998;36(6):778–792. [DOI] [PubMed] [Google Scholar]
- 22.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost effectiveness in health and medicine. Second edition. ed. New York: Oxford University Press. [Google Scholar]
- 23.US Census Bureau. https://www2.census.gov/programs-surveys/popest/datasets/2010-2017/national/asrh. data file:nc-est2017-alldata-r-file17.csv
- 24.Neumann PJ, Cohen JT, Weinstein MC. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. N. Engl. J. Med 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 25.Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model Parameter Estimation and Uncertainty Analysis:A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group–6. Med. Decis. Making 2012;32(5):722–732. [DOI] [PubMed] [Google Scholar]
- 26.Kim EJ, Kim T, Conigliaro J, Liebschutz JM, Paasche-Orlow MK, Hanchate AD. Racial and Ethnic Disparities in Diagnosis of Chronic Medical Conditions in the USA. J. Gen. Intern. Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton DC, Flannery B, Bennett NM, et al. Socioeconomic and Racial/Ethnic Disparities in the Incidence of Bacteremic Pneumonia Among US Adults. Am. J. Public Health 2010;100(10):1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman RK, Lauderdale DS, Tan SM, Wagener DK. Prevalence of high-risk indications for influenza vaccine varies by age, race, and income. Vaccine. 2010;28(39):6470–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. US Department of Health and Human Services;2017. [PubMed] [Google Scholar]
- 30.Hurley LP, Allison MA, Pilishvili T, et al. Primary Care Physicians’ Struggle with Current Adult Pneumococcal Vaccine Recommendations. J. Am. Board Fam. Med 2018;31(1):94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurley LP, Bridges CB, Harpaz R, et al. U.S. physicians’ perspective of adult vaccine delivery. Ann. Intern. Med 2014;160(3):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin. Infect. Dis 2012;55(2):255–258. [DOI] [PubMed] [Google Scholar]
- 33.Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin. Infect. Dis 2012;55(2):265–267. [DOI] [PubMed] [Google Scholar]
- 34.Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin. Infect. Dis 2012;55(2):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination Coverage Among Children Aged 19–35 Months - United States, 2016. MMWR Morb. Mortal. Wkly. Rep 2017;66(43):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilishvili T PCV13 Impact on IPD and serotype distribution for the remaining disease burden. ACIP meeting; October 24–25, 2018; Atlanta, GA. [Google Scholar]
- 37.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MPE, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. The Lancet Infectious Diseases. 2015;15(5):535–543. [DOI] [PubMed] [Google Scholar]
- 38.Lee G ACIP presentation: Pneumococcal Vaccines. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Pneumo-01-Lee-508.pdf.
- 39.Blommaert A, Bilcke J, Willem L, Verhaegen J, Goossens H, Beutels P. The cost-effectiveness of pneumococcal vaccination in healthy adults over 50: An exploration of influential factors for Belgium. Vaccine. 2016;34:2106–2112. [DOI] [PubMed] [Google Scholar]
- 40.Matanock A Invasive Pneumococcal Disease in the U.S.—2008–2016. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/pneumo-04-matanock.pdf.
- 41.Anderson WL, Armour BS, Finkelstein EA, Wiener JM. Estimates of state-level health-care expenditures associated with disability. Public Health Rep. 2010;125(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson LA, Benson P, Sneller V-P, et al. Safety of Revaccination With Pneumococcal Polysaccharide Vaccine. J. Am. Med. Assoc 1999;281(3):243–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.