Abstract
Members of the SLAM family of receptors promote the progression of invariant natural killer T cells through the thymic maturation process and facilitate proper host responses to exogenous lipid antigen through the recruitment and activity of SAP–Fyn and SHP-1, respectively.
Invariant natural killer T cells (iNKT cells) are a small population of cells that recognize lipid antigens presented by CD1d, a non-polymorphic major histocompatibility complex class I–like antigen-presenting molecule, and are characterized by a semi-invariant T cell antigen receptor (TCR). They are highly autoreactive, early responders that require careful selection before they move from the thymus to the tissues. Consistent with that, they undergo a thymic maturation process distinct from that of conventional T cells that involves CD1d-expressing CD4+CD8+ double-positive (DP) thymocytes that present a yet-to-be-defined CD1d-restricted self lipid to another DP thymocyte. This type of homotypic selection process between CD1d-expressing thymocytes is believed to allow cooperative engagement with co-receptors that function in a homophilic fashion to regulate the selection of this highly autoreactive population. Those co-receptors include members of the SLAM family of receptors (SFRs); these associate with the cytoplasmic adaptor SAP, which serves as a molecular bridge to the Src kinase Fyn or phosphatases of the SHP family1,2. However, the role of SFRs in iNKT cell development is poorly understood, as they possess both an inhibitory immunoreceptor tyrosine-based switch motif (ITSM) and an activating ITSM in their cytoplasmic tail that docks with SAP or SHP. In this issue of Nature Immunology, Lu et al. utilize a variety of clever genetic approaches to provide important new insights into the complex ways in which SFRs influence the selection and maturation of iNKT cells in the thymus4.
SFRs are a set of six homophilic (i.e., self ligand) receptors, SLAMF1 (CD150), SLAMF3 (Ly-9 or CD229), SLAMF4 (2B4 or CD244), SLAMF5 (CD84), SLAMF6 (Ly108 in mice; NTB-A in human) and SLAMF7 (CRACC, CD319 or CS1), that are expressed exclusively on hematopoietic cells5,6. These receptors possess an extracellular segment with two or four immunoglobulin-like domains, a single transmembrane domain and an ITSM-bearing cytoplasmic tail that regulates the activation of CD4+ T cells and CD8+ T cells or NK cell–mediated cytotoxicity7. In addition, SFRs promote the development of iNKT cells, which normally proceeds through a four-stage maturation process (stages 0–3)7,8. Deletion of any single member of the SLAM family has a modest influence on iNKT cell development, while deletion of all SFRs leads to a cumulative and severe reduction in the abundance of iNKT cells, but not that of conventional T cells, in both the thymus and peripheral organs. Further, unlike SAP deficiency, which results in mice with defects in all stages of iNKT cell maturation3, deletion of SFRs has no effect on the initial positive selection of DP precursors or the transition to stage 0 but leads to a progressive reduction in the number of iNKT cells in stages 1, 2 and 3, during which iNKT cells differentiate and expand.
Given that the ITSMs of SFRs can mediate both activating signals and inhibitory signals7,9, the role of SFRs in iNKT cell development has remained unknown. To address this, Lu et al. investigate the effect of SFR deficiency on TCR signaling4 through the use of a Nur77 (nuclear hormone receptor) reporter mouse10. Consistent with previous reports, they find that the TCR signaling of iNKT cells in wild-type mice progressively wanes from stage 0, following positive selection, to stage 3 of the maturation process, before extrathymic export to peripheral tissues (Fig. 1a). In contrast, iNKT cells from SFR-deficient mice maintain elevated expression of Nur77, particularly in iNKT cells at stages 1–3, although TCR signaling in iNKT cells at stage 0 or conventional T cells from SFR-deficient mice is similar to that in such cells from wild-type mice. Analysis of bone marrow chimeras confirms that the increased TCR signal strength in SFR-deficient iNKT cells is a genuine consequence of SFR activity and is not merely a compensatory host mechanism developed to mitigate the effects of the decreased number of iNKT cells or increased number of conventional T cells in SFR-deficient mice. Although Lu et al. observe increased proliferation of SFR-deficient iNKT cells, they also detect a stronger association between the excessive TCR signal strength and greater death of SFR-deficient iNKT cells, which exhibit elevated activity of caspase-3 and caspase-7, together with diminished expression of the anti-apoptotic protein Bcl-2, relative to that of wild-type iNKT cells4. Collectively, this demonstrates that following initial positive selection of DP thymocytes, SFRs allow iNKT cells to survive the thymic maturation process by restricting TCR signal strength and, consequently, activation and activation-induced cell death.
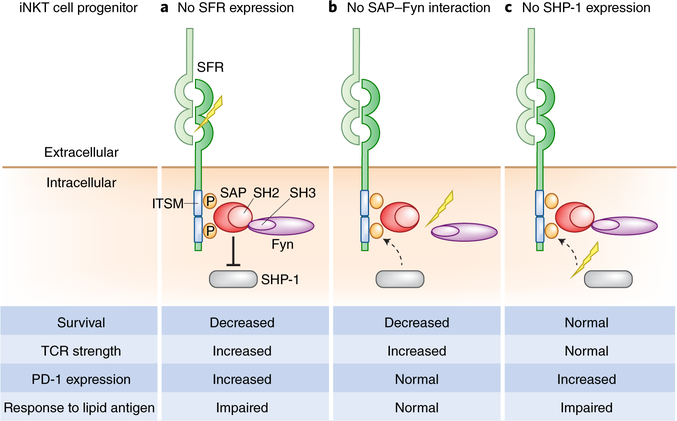
Fig. 1 |. SFR-mediated interactions between thymic progenitor cells mediate proper expansion and maturation of iNKT cells.
a, Homophilic interactions between SFRs promote the proper development and expansion of iNKT cells in the thymus through alternative interactions with SAP–Fyn and SHP-1. Cumulative deficiency in SFRs leads to progressive loss of iNKT cells in the thymus and peripheral tissues through the disruption of direct, ITSM-mediated, cytosolic associations with SAP–Fyn and/ or SHP-1, in part due to increased basal TCR signaling and decreased survival of iNKT cell progenitors after positive selection. In response, iNKT cells compensate for SFR deficiency by upregulating inhibitory molecules, such as PD-1, that prevent further depletion of thymic iNKT cell progenitors but are associated with impaired cytokine response to exogenous lipid presented by CD1d. b, Abolishing SFR-SAP interactions leads to a severe deficiency in iNKT cells that is partially mediated through the activity of Fyn. Specific disruption of SAP–Fyn interactions, in the presence of SHP-1, results in iNKT cell deficiencies associated with increased TCR signaling and decreased cell survival, with no compensatory upregulation of PD-1, and a normal systemic host response to exogenous lipid antigen. c, In contrast to b, in the absence of SHP-1, iNKT cells undergo normal thymic expansion and differentiation but experience upregulation of PD-1, which is associated with impaired responses to exogenous lipid antigens.
The SFR-deficient iNKT cells also exhibit attenuated inflammatory responses after exposure to α-galactosyl ceramide (α-GC), a high-affinity CD1d-restricted stimulus, relative to the response of wild-type cells, as shown by their reduced expression of Nur77 and diminished intracellular cytokine production in response to this agonist. This suggests that in the absence of SFRs, iNKT cells exhibit a cell-intrinsic inability to respond to exogenous stimuli, consistent with an ‘exhausted’ or anergic T cell response secondary to hyper-activation, as a consequence of SFR deficiency. Indeed, consistent with that, Lu et al. observe specific upregulation of other inhibitory immunoreceptors, such as PD-1, LAG-3 and CD160, on SFR-deficient iNKT cells, relative to the abundance of these immunoreceptors on wild-type cells, which serves to limit the response to exogenous α-GC exposure4. Interestingly, mice with dual deletion of SFRs and PD-1 exhibit depletion of iNKT cells that is even more severe than the depletion that results from deletion of SFRs or PD-1 alone, which suggests that upregulation of PD-1 is an active compensatory process to further limit the detrimental effect of SFR deficiency on iNKT cells. Overall, this highlights the important need for a receptor ‘brake’ that allows an iNKT cell to survive the positive selection process.
Deletion of any single SFR has a minimal, if any, effect on iNKT cells9. However, iterative deletion of SFRs leads to cumulative depletion of iNKT cells. Consistent with that, Lu et al. show that iNKT cells express multiple SFRs, although the expression of any given SFR varies depending on the development state of the iNKT cell4. For example, SLAMF6 has its maximal expression at stage 0, and its expression diminishes during the maturation process2. It is therefore interesting that retroviral delivery of human or mouse SLAMF6 to SFR-deficient iNKT cells is associated with restoration of proper iNKT cell development and the downregulation of inhibitory receptors such as PD-1. On the other hand, the authors observe only partial restoration of iNKT cells after re-expression of SLAMF1 in SFR-deficient mice. As SLAMF1 follows a developmental expression profile distinct from that of SLAMF6 in wild-type iNKT cells, these studies suggest that SFRs have varying capacities to influence iNKT cell maturation and may not be completely functionally redundant molecules.
As noted above, an important feature of SFRs is the presence of a cytoplasmic ITSM capable of promoting TCR activity via SAP– Fyn but that can also exert an inhibitory effect by binding SHPs such as SHP-13. When the authors eliminate the ITSM of SLAMF6 and thus disrupt its cytoplasmic interactions with SAP or Src-homology phosphatases, SLAMF6 fails to restore iNKT cell development. Furthermore, when the authors use a genetic model in which SAP binds SFRs normally but has an impaired capacity to recruit and activate Fyn11, this results in partial iNKT cell defects in association with increased TCR signal strength (Fig. 1b). However, there is no compensatory upregulation of inhibitory molecules, and the mice expressing the SAP mutant unable to bind Fyn respond to exogenous lipid antigen (α-GC) similarly to wild-type mice. In contrast, loss of SHP-1 itself has no effect on the expansion and maturation of thymic iNKT cells but leads to increased expression of PD-1 and reduced expression of Bcl-2 by iNKT cells, in association with impaired responses to α-GC (Fig. 1c).
Thus, the work of Lu et al. suggests a model in which SFRs contribute to iNKT cell maturation and subsequent responses to exogenous stimuli at multiple levels4 (Fig. 1). Most importantly, they show that SFRs limit the activation of iNKT cells and that this effect is at its maximum when SAP, Fyn and SHP interactions are present together, as shown with SLAMF6. It is interesting that Fyn, a kinase, and SHP-1, a phosphatase, have partial, overlapping effects, with the former controlling mainly TCR signal strength and the latter controlling survival (Bcl-2); this suggests distinct and non-redundant, but cumulative, effects of SFR signaling during the various phases of T cell activation, despite their opposing consequences on the state of tyrosine phosphorylation. In the physiological context, the data of Lu et al. also indirectly raise the possibility that excessively potent agonistic lipid antigens may engage the cooperation of not only SFRs but also other inhibitory immunoreceptors such as PD-1, LAG-3 and CD160, as shown here, and potentially others4. As SAP deficiency leads to a much more profound iNKT cell defect that occurs earlier during thymic development, relative to the defect found in SFR deficiency, it can be further surmised that there are other yet-to-be-defined SAP-dependent immune molecules important for iNKT cell development waiting to be discovered. In the end, these studies highlight the complex layers of regulation that are required, and that SFRs provide, to prevent the inappropriate selection of this extremely autoreactive population of lymphocytes that can contribute to autoimmunity throughout the body.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Griewank K et al. Immunity 27, 751–762 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S et al. J. Exp. Med 214, 475–489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detre C, Keszei M, Romero X, Tsokos GC & Terhorst C Semin. Immunopathol 32, 157–171 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y et al. Nat. Immunol 10.1038/s41590-019-0334-0 (2019). [DOI] [PubMed]
- 5.Engel P, Eck MJ & Terhorst C Nat. Rev. Immunol 3, 813–821 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Veillette A Immunol. Rev 214, 22–34 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Detre Cynthia et al. Semin. Immunopathol 32, 157–171 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H & Hogquist KA Front. Immunol 9, 1450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannons JL, Tangye SG & Schwartzberg PL Annu. Rev. Immunol 29, 665–705 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Moran AE et al. J. Exp. Med 208, 1279–1289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunez-Cruz S et al. J. Immunol 181, 2311–2320 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]