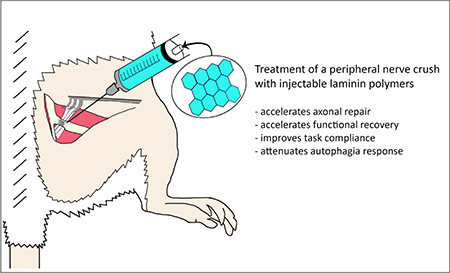
Abstract
Promoting axon growth after peripheral nerve injury may support recovery. Soluble laminin polymers formed at pH 4 (aLam) accelerate axon growth from adult dorsal root ganglion neurons in vitro. We used an adult rat model of a peripheral (peroneal) nerve crush to investigate whether an injection of aLam enhances axon growth and functional recovery in vivo. Rats that received an injection of aLam into the crush at 2 days post-injury show significant improvements in hind limb motor function at 2 and 5 weeks after injury compared with control rats that received phosphate-buffered saline. Functional improvement was not associated with changes in sensitivity to thermal or mechanical stimuli. Treatment with aLam decreased the occurrence of autophagia and abolished non-compliance with treadmill walking. Rats treated with aLam showed increased axon presence in the crush site at 2 weeks post-injury and larger axon diameter at 10 weeks post-injury compared with controls. Treatment with aLam did not affect Schwann cell presence or axon myelination. Our results demonstrated that aLam accelerates axon growth and maturity in a crushed peroneal nerve associated with expedited hind limb motor function recovery. Our data support the therapeutic potential of injectable aLam polymers for treatment of peripheral nerve crush injuries.
Keywords: Extracellular matrix, Biomaterial, Laminin, Autophagia, Axon regeneration, Nerve repair, Peroneal functional index
Graphical Abstract
Introduction
A crush is the prevalent mechanism of peripheral nerve injury in humans and typically causes axon damage and loss of function [1], contributing to widespread individual, familial, and societal consequences, as wages are lost and rehabilitation is extended until nerves can repair and function is recovered. Endogenous recovery after a peripheral nerve crush (PNC) injury is limited [2], which has motivated the search for reparative therapies.
Recovery after PNC injury depends on growth of the damaged axons. In a nerve crush, Schwann cells dedifferentiate and secrete laminin, which promotes axon growth [3]. Chen and Strickland showed that laminin is crucial for successful repair after a sciatic nerve crush [3]. Previously, it was shown that laminin polymers formed at pH 4 (acidic laminin (aLam) or polylaminin [4]) strongly promote axon growth of cultured neurons from adult rat dorsal root ganglia (DRG, [5]), embryonic rat cortex [6], and postnatal day 1 rat retina [7]. Importantly, laminin polymers formed at pH 4 maintain their structure [4] and effect on axon growth [4, 5] when re-suspended at pH 7. These findings supported the concept that aLam can interact with multiple neuron subtypes under physiological conditions and thus may serve as a therapeutic to promote axon growth in an injured peripheral nerve.
In the present study, we used an adult rat model of PNC to investigate the effects of aLam treatment on anatomical and functional repair. After PNC, the epineurium remains intact [8] which facilitates injecting the soluble aLam polymers. We assessed the effects of sub-acute aLam treatment on axon growth, Schwann cell presence, axon myelination, and motor and sensory function performance. In addition, we evaluated the effects of aLam treatment of the crushed peroneal nerve on autophagia (i.e., self-mutilation) and compliance with a treadmill walking test, which both represent potential impediments to recovery and are often associated with peripheral nerve injury.
Materials and Methods
Animals
Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh, Pittsburgh, PA. The animal facility was accredited by the Assessment and Accreditation of Laboratory Animal Care (AAALAC). We used female adult Sprague Dawley rats (n=24; 225–250g; Charles River Laboratory, Wilmington, MA, USA). Rats were housed in pairs under a 12 h light/dark cycle with ad libitum access to food and water.
Preparation of aLam polymers
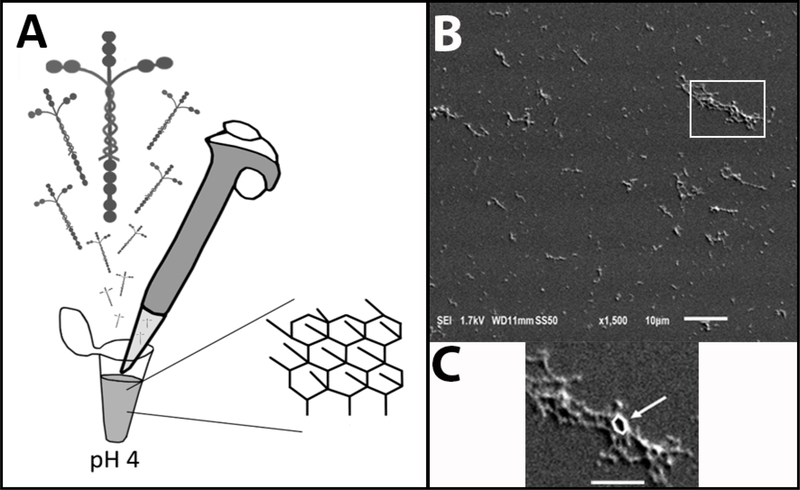
The aLam polymers were made by diluting laminin-111 (L2020 1 mg/ml; Sigma Aldrich, St. Louis, MO, USA) in filtered and autoclaved 20 mM sodium acetate buffer (pH 4) in a sterile hood to a final working concentration of 100 μg/ml (Fig. 1A). It was shown that in this buffer, laminin forms polymers with a regular hexagonal configuration (Fig. 1A) by linking the short arms of the heterotrimer [4]. The polymerization state of the polymer was confirmed with dynamic light scattering [9] and scanning electron microscopy (Fig. 1B, C). Previously, we [5] and others [4] have demonstrated that once polymers are formed at low pH they retain their structure [4] and growth-promoting ability [5] when introduced into a neutral pH environment.
Fig. 1.
aLam preparation. A) Schematic of preparation of aLam polymers from laminin-111 in pH 4 buffer and resulting hexagonal structure. B) Scanning electron microscope image showing example of aLam polymer fragments. Scale bar = 10 μm. C) Enlargement of fragment shown in B with hexagon outlined in white (arrow). Scale bar = 5 μm.
Surgical procedures
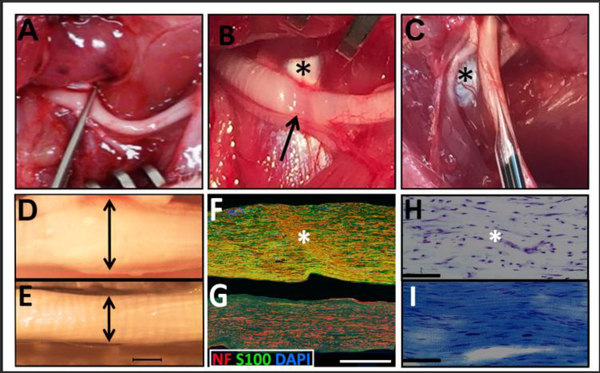
Rats were anaesthetized using an intraperitoneal injection of ketamine (60 mg/kg; Butler Schein, Dublin, OH, USA) and Dexdomitor (dexmetadomidine, 0.5 mg/kg; Pfizer, New York, NY, USA) [10]. The right hind limb was shaved and cleaned with 70% ethanol followed by a Betadine® scrub. The right thigh muscles were exposed and gently separated to reveal the peroneal nerve, which was then crushed for 30 sec using a fully closed fine forceps (#5, Fine Science Tools, Foster City, CA, USA) [11] at the level where the nerve passed over the tendon (Fig. 2A). The crushes were performed by the same experimenter for consistency. The tendon was used as an anatomical landmark to standardize the site of the crush and to identify its location at later time points in the experiment (Fig. 2B). The presence of a translucent band confirmed the crush (Fig. 2B). Two days post-injury (dpi), 3 μl of aLam or phosphate-buffered saline (PBS, 0.01 M) was gently injected into the epicenter of the crush using a 10 μl Hamilton syringe fitted with a pulled glass needle (Fig. 2C). Each experimental group comprised 12 animals that survived for 2 (n = 5) or 10 (n = 7) weeks post-injury (wpi). A sterile suture loop was used to stabilize the crush site during the injection procedure. The experimenter performing the injections was blinded to the treatment groups. After injections, the muscles were repositioned, sutured, and the overlaying skin closed with wound clips.
Fig. 2.
Surgical procedures and histological characterization of the injured peroneal nerve. A) Exposure and crush of peroneal nerve using fine forceps; B) Translucent band resulting from crush (arrow). Note that the tendon (asterisk) was used as anatomical landmark; C) Injection into injury site at 2 days post-injury (dpi) with pulled glass capillary needle. The asterisk denotes the tendon; D) Swollen, crushed nerve at 2 dpi; E) Normal uninjured nerve; Scale bar = 500 μm for D/E; F) Disorganized axonal, NF-hpositive profiles (anti-RT-97; red), Schwann cell profiles, (anti-S-100; green) and nuclear staining (DAPI; blue) were present within the crush epicenter (asterisk) at 2 dpi G) Normal uninjured nerve with organized continuous NF-h-positive profiles (anti-RT-97; red), Schwann cell profiles, (anti-S-100; green) and nuclear staining (DAPI; blue); Scale bar = 500 μm for F/G; H) Luxol fast blue and Cresyl Violet staining reveal diminished presence of myelin and disrupted cytoarchitecture within the epicenter (asterisk) and surrounding tissue in 2 dpi crushed nerve; Scale bar = 250 μm I) Luxol fast blue and cresyl violet staining showing presence of myelin and organized cytoarchitecture in a healthy, uninjured nerve. Scale bar = 250 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Post-surgical procedures
After surgery, rats received Antisedan (1.5 mg/kg, SC; Pfizer) to reverse the effects of the anesthesia. Starting immediately after surgery and once a day for the ensuing five days, rats were treated with gentamicin (6 mg/kg, IM; VWR, Radnor, PA, USA), Rimadyl (5 mg/kg, SC; Pfizer), and lactated Ringer’s solution (5 ml, SC). Note that this time period includes two days after initial crush plus three days following the injection of aLam or PBS into the nerve crush. Rats were monitored at least once daily throughout the course of the experiment. Throughout the experiment, autoclaved paper huts environmentally enriched the rat cages. At the first signs of autophagia (i.e., blood under or near the toenails or lacerations/ulcers on the right hind paw), each self-mutilated paw was treated with a combination of topical lidocaine and Zymox® with 1% hydrocortisone combined with a bitter spray to discourage further self-mutilation.
Hind limb motor function assessment
Hind limb motor function was analyzed by examiners blinded to the experimental groups using the DigiGait™ Image Analysis System (Mouse Specifics, Framingham, MA, USA), which is a transparent, speed-controlled treadmill with a high-speed camera capturing dynamic foot prints from below. Before the start of the experiment, for acclimation, rats were kept in the DigiGait™ chamber with the treadmill off for about 8 min under dimmed lights. The following 4 days, rats were kept in the DigiGait™ chamber for a maximum of 8 min/day with the treadmill running at 3 cm/sec on day 1 and then at gradually increasing speeds until 20 cm/sec on days 2–4. When the rats were comfortably and consistently walking at 20 cm/sec, baseline measurements were taken. Our target speed was 20 cm/sec because rats with PNC are typically able to walk at that speed. Rats that were not able or refused to walk at the target speed at baseline, prior to injury, were excluded from further participation in the experiment. Measurements were taken when the rat made at least 4 consecutive steps without hopping by each of the hind paws. The results were used to calculate the peroneal functional index (PFI) at 1 day prior to injury (baseline) and 2, 5, and 10 wpi.
Peroneal functional index (PFI)
The PFI is a sub-index of the sciatic functional index (SFI) that focuses on significant differences in toe spread, the main peroneal contributing factor, and ranges from 0 (intact, naïve nerve) to −100 (completely transected nerve). The PFI is defined as:
In which, E=experimental, N=normal and TS=toe spread [12, 13]. DigiGait™ analysis software was used to calculate the PFI from high-speed images of rats walking at target speed.
Hind limb sensory function assessment
In an additional cohort of rats, we analyzed sensory function two weeks after treatment of the crushed nerve with aLam (200 μg/ml). Mechanical allodynia was analyzed using an electronic von Frey anesthesiometer (IITC Life Science, Inc, Woodland Hills, CA) [14, 15]. Rats were acclimated in clear plastic holding boxes on a raised grated platform until their exploratory behavior ceased. Examiners blinded to the experimental groups placed the filament directly below the middle of the plantar surface of each hind paw and increased the force gradually until paw withdrawal. The force at the time of paw withdrawal was recorded. Five measurements were taken with 5 min intervals; the middle three values were averaged and expressed relative to baseline measure.
Thermal hyperalgesia was assessed using Hargreaves’ method (Harvard Apparatus, Holliston, MA, USA) [16] by examiners blinded to the experimental groups. Before testing, rats were acclimated in the clear plastic holding cells on a raised glass platform until their exploratory behavior ceased. The examiners aimed a high-intensity light beam at the center of the plantar surface of each hind paw with the light resting intensity set to 5%, active intensity set to 25%, and a cut-off at 20 sec. The time (sec) to withdrawal of the paw was recorded five times with 5 min intervals; the middle three values were averaged and expressed relative to baseline measure.
Autophagia assessment
The presence of blood under or near the toenails and/or lacerations on the right hind paw determined autophagia. Autophagia occurred after PNC either before or after injection. Examiners blinded to the experimental groups recorded the presence or absence of autophagia. Rats were discouraged from self-mutilation by treatment with topical lidocaine, Zymox® with 1% hydrocortisone, and a bitter spray. The response to this treatment was not different between groups.
Histological procedures
At the designated times, rats were deeply anaesthetized and transcardially perfused with 300 ml PBS (0.01 M, pH 7.4) followed by 400 ml 4% paraformaldehyde in 0.01 M phosphate buffer (PFA, pH 7.4). Next, a 5 mm-long segment of the right peroneal nerve centered on the crush, a 3 mm-long segment just distal thereof, and the lumbar enlargement of the spinal cord (~15 mm in total length) were removed. The 5 mm-long nerve segment and the lumbar enlargement were post-fixed in 4% PFA, transferred to 30% sucrose in 0.01 M PBS for 24 h at 4 °C, and then embedded and cut in 15 (nerve) or 20 (spinal cord) μm-thick longitudinal sections on a cryostat at −24°C..
The 3 mm-long distal segment was used for semi-thin sections. The segment was transferred to 2.5% glutaraldehyde in 0.01 M PBS and post-fixed overnight at 4 °C, rinsed in 0.01 M PBS, kept in OsO4 overnight, and then washed and dried using increasing concentrations of ethanol. The tissue blocks were treated with propylene oxide (PO), PO:Epon® (3:1, 1:1, and 1:3),100% Epon®, and then embedded in Epon® resin using silicone molds and kept for 24 h at 37 °C followed by 48 h at 65 °C. These plastic blocks were then cut in semi-thin (400 nm) cross sections and mounted on glass slides.
Retrograde tracing of lumbar spinal motor neurons
The presence of lumbar spinal motor neurons with an axon projecting beyond the crush site was investigated at 10 wpi using retrograde tracing with CTB [17–18]. Details on the tracing method, and on the immunocytochemistry to detect labeled neurons, are described in Supplementary data.
Immunohistochemistry and staining
Cryostat sections were exposed to 5% normal goat serum in 0.01 M PBS with 0.03% Triton X-100 at room temperature for 1 h prior to the primary antibody incubation. Nerve sections were incubated for 1 h at room temperature followed by overnight incubation at 4 °C with rabbit polyclonal antibodies against high molecular weight neurofilament (NF; 1:250, Millipore AB1991) to stain axons, mouse monoclonal antibodies against S100 (1:200, Millipore MAB079–1) combined with rabbit polyclonal antibodies against P75 (1:100, Millipore 07–476) to stain Schwann cells. After the primary incubation, nerve tissue sections were washed three times for 5 min with 0.01 M PBS (pH 7.4) and then incubated with goat anti-rabbit or goat anti-mouse antibodies coupled to AlexaFluor 488 or AlexaFluor 555 (1:500; Molecular Probes, Eugene, OR, USA) for 1 h at room temperature. Sections were then washed 3 times for 5 min with 0.01 M PBS (pH 7.4), counterstained with DAPI (4’,6-Diamidino-2-Phenylindole, Dihydrochloride; ThemoFisher), rinsed twice more, and covered with glass slips with anti-fade fluorescent mounting medium (Dako, Carpenteria, CA, USA). For detection of myelinated axons, plastic sections were stained with toluidine blue, covered with glass slips with VectaMount mounting medium (Vector Laboratories, Burlingame, CA, USA).
Quantitative analyses
Images were taken from the stained cryostat and semi-thin sections by an experimenter blinded to experimental groups using a Zeiss Plan Apochromat (20x air, NA 0.8) equipped with an ORCA digital CCD camera (C7780–20) or Olympus VS120 virtual slide microscope (Olympus, Tokyo, Japan) (S100 cell count data) using an Olympus UPLSAPO Super Apochromat objective (20x air, NA 0.75) equipped with an ORCA-Flash4.0 camera (Hamamatsu Photonics, Japan). For longitudinal sections, one series was stained and images 3 mm in height, centered on the injury site were taken of the four middle tissue sections for analysis. For counting and intensity measurements in semi-thin and cross-sections, two sections completely spanning the nerve were imaged and averaged per animal. For G Ratio measurement, ImageJ G Ratio plugin was used to evaluate 10 randomly chosen myelinated axons from each semithin image. ImageJ software [19] was used to quantify the number, fluorescence intensity, and diameter of NF-positive axons, the number and G Ratio [20] of myelinated axons, and the number (S100/DAPI co-labeled) and fluorescence intensity of S100 positive Schwann cells. The number of CTB-labeled motor neurons was counted using StereoInvestigator software (MBF Bioscience, VT, USA) (see Supplementary data). For all image quantifications, the experimenter was blinded to the identity of the group.
Statistical Analysis
SPSS statistical software package (SPSS version 21, IBM) was used for statistical analysis. Student T-test was used to determine the significance of differences between group averages when determined at a single time point. Repeated measures mixed ANOVA test was used to determine the significance of differences in behavioral tests at successive time points. Compliance and autophagia data was evaluated using likelihood ratio Chi Square. Significance was set at p < 0.05.
Results
PNC caused axon damage and a disorganized cytoarchitecture
The crush of the peroneal nerve (Fig. 2A) was recognizable as a translucent band (Fig. 2B). Two days later, the day of treatment, the nerve at the crush site was swollen (Fig. 2D) compared with an uninjured nerve (Fig. 2E). Within the crush epicenter, few NF-positive profiles were present, while many disorganized NF-positive profiles were found just proximal and distal to the epicenter (Fig. 2F). This was in contrast to the uninjured nerve, which contained many continuous NF-positive profiles (Fig. 2G). Schwann cells in and just proximal and distal to the crush were highly variable and disorganized with areas of diminished and very intense S100 expression (Fig. 2F). Myelin appeared less in the crush (Fig. 2H) compared with an uninjured nerve (Fig. 2I).
Treatment with aLam improved functional recovery after PNC
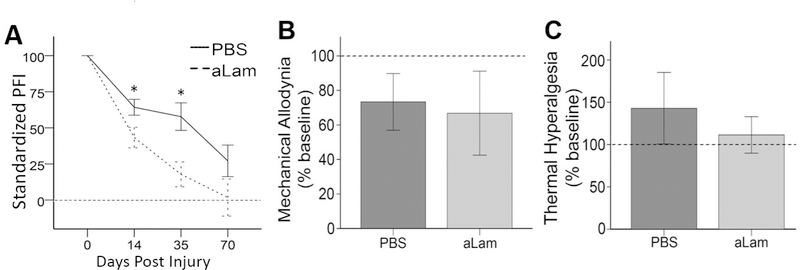
Hind limb function was assessed using the PFI, which was calculated from images acquired by the DigiGait™. Values were expressed relative to the individual baseline measurements of uninjured rats. After PNC, the average PFI (absolute value) of compliant, PBS treated rats was 78.7 at 2, 65.8 at 5, and 27.2 at 10 wpi (Fig. 3A). The average PFI values of aLam treated rats were 43.3 at 2, 17.8 at 5, and 1.7 at 10 wpi. These data show that treatment with aLam significantly improved recovery of hind limb motor function by 45% (p = 0.04) at 2 wpi and 72.9% (p = 0.03) at 5 wpi compared with controls (Fig. 3A). At 10 wpi, aLam-treated rats had lower, but not statistically different, PFI scores in comparison to control rats. Our data indicated that the rate of hind limb motor recovery after PNC in rats treated with aLam was more than 2 × faster than rats treated with PBS.
Fig. 3.
Motor and sensory function after peroneal crush. A) Peroneal functional index (PFI) standardized to baseline measurements for animals injected with aLam (dashed line) or PBS control (solid line) showing significant improvements at 14 and 35 dpi (repeated measures ANOVA, ⋆p < 0.05) with aLam treatment; B) Mechanical allodynia (standardized to baseline values) was not adversely affected at 14 days post-injury by aLam treatment (light gray bar) compared with PBS treatment (dark gray bar); C) Thermal hyperalgesia (standardized to baseline values) was not adversely affected at 14 days post-injury by aLam treatment (light gray bar) compared with PBS treatment (dark gray bar). A–C) Dashed horizontal lines represent baseline values from uninjured animals; error bars represent SEM.
Treatment with aLam did not exacerbate sensory impairment after PNC
Interventions to increase axon growth in the injured peripheral nerve can exacerbate sensory impairment [21]. We assessed the effects of aLam treatment on sensory function by measuring mechanical allodynia and thermal hyperalgesia. Both aLam treated and control rats were more sensitive to mechanical stimuli and less sensitive to thermal stimuli after injury. There was no significant difference between experimental groups in the magnitude of mechanical allodynia (Fig. 3B) or thermal hyperalgesia (Fig. 3C). The data suggest that aLam treatment of the crushed peripheral nerve does not have adverse effects on sensory function.
Treatment with aLam increased compliance for treadmill walking after PNC
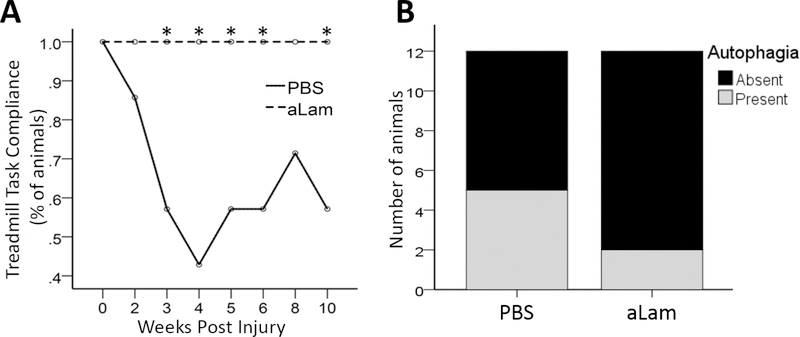
Assessment of motor function in models of peripheral nerve injury may be hampered by non-compliance of the injured animal. We examined compliance for treadmill walking in the DigiGait™ during our experiment. We found that 100% of rats treated with aLam walked on the treadmill running at the target speed during the study (Fig. 4A). In contrast, several of the control rats were unable and/or refused to walk on the treadmill running at the target speed at each of the examinations (Fig. 4A). The difference between groups was statistically significant (p < 0.05). These results indicated that aLam treatment significantly improved the compliance of rats to walk on a treadmill after PNC.
Fig. 4.
Compliance for treadmill walking and autophagia. A) Rats with aLam treatment showed 100% compliance for treadmill walking which was significantly better than rats with PBS treatment (likelihood ratio Chi Square, ⋆p < 0.05); B) Rats with aLam treatment displayed decreased incidence of autophagia (14% vs. 43%). The incidence of autophagia was equal across groups prior to injection.
Treatment with aLam decreased the occurrence of autophagia after PNC
Autophagia may occur after an injury to a peripheral nerve. We examined the occurrence of autophagia throughout the experiment and observed no difference between the treated and control groups during the first two days after PNC; 29% of the rats in each group exhibited signs of autophagia (Fig. 4B). After injection, 14% of the rats treated with aLam showed signs of autophagia compared with 43% of the rats injected with PBS (Fig. 4B). These findings indicated that aLam treatment decreased the incidence of autophagia after PNC.
Treatment with aLam promoted axon growth and maturation in the crushed nerve
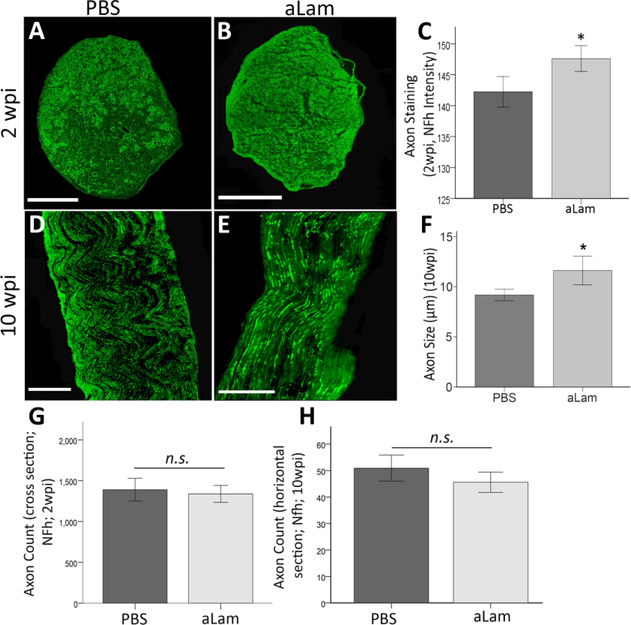
Axon growth across an injured peripheral nerve is crucial for recovery of function. We analyzed the presence of the axon cytoskeletal protein, high molecular weight neuro-filament (NF) in the crushed peroneal nerve (Fig. 5A-H) and found a significant increase (p = 0.04) in staining intensity [20] in axons in rats that were treated with aLam compared with controls at 2 wpi (Fig. 5A-C). There was no difference in axon count at either time point (Fig. 5G/H). However, at the 10 wpi time point, NF-h labeled axons in the crush had a significantly larger diameter (p = 0.01) in aLam-treated rats compared with controls (Fig. 5D-F). Our data suggested that aLam enhanced axon growth and maturation in the crushed peripheral nerve.
Fig. 5.
Axonal response to aLam. aLam treatment increased axon presence and maturation after injury. A/B) Cross section of injured nerve at 2 wpi treated with PBS (A) or aLam (B) and stained with antibodies recognizing NF-h (α-RT97, green); C) Bar graph illustrating significantly higher axon (NF-h) fluorescent staining intensity (integrated density) on tissue cross sections from the aLam treated group at 2 wpi (Student T-test, ⋆p = 0.04); D/E) Longitudinal section of injured nerve at 10 wpi treated with PBS (D) or aLam (E) and stained with antibodies recognizing NF-h (α-RT97, green); F) Bar graph illustrating significantly increased axon diameter (lm; NF-h particle diameter) with aLam treatment at 10 wpi (Student t-test, *p = 0.01). G/H Bar graphs illustrating similar numbers (counts) of NF-h+ axon at 2 and 10 wpi. Error bars represent SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Treatment with aLam increased myelin thickness without affecting Schwann cell and myelinated axon number in the crushed peroneal nerve
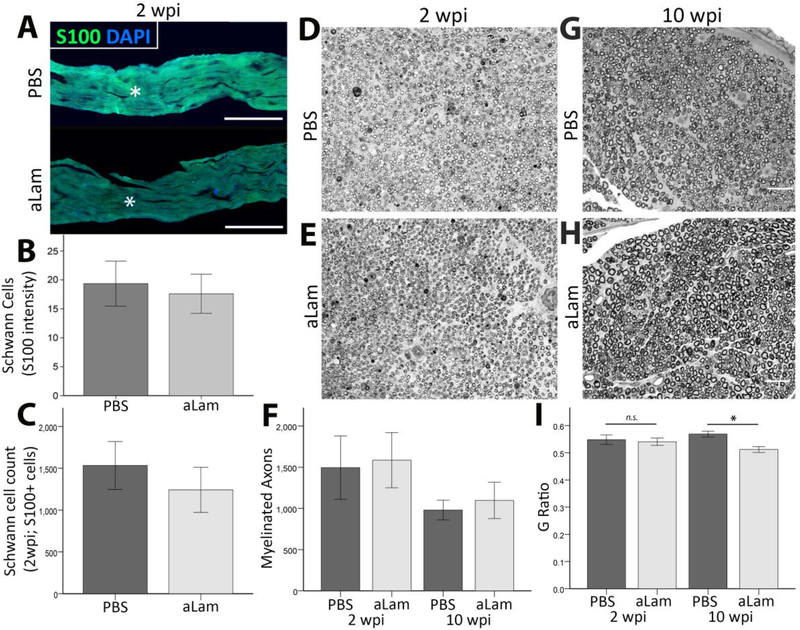
Schwann cells play a role in repair of peripheral nerve injuries, both in regeneration and remyelination [3, 22–26]. We examined S-100-positive Schwann cell (Fig. 6A) and myelinated axon (Fig. 6D/E/G/H) presence in the crushed peroneal nerve in two separate areas, centered on, and adjacent (distal to) the injury site. The presence of Schwann cells (Fig. 6B/C) and myelinated axons (Fig. 6F) were similar between groups at both 2 and 10 wpi. The G Ratio of myelin sheaths was similar between groups at 2 wpi and significantly decreased in the aLam group at 10 wpi. Our findings suggested that aLam did not affect proliferation of Schwann cells or their ability to myelinate axons at 2 weeks, but was associated with increased thickness of myelin sheaths at 10 weeks after injury.
Fig. 6.
Schwann cell response to aLam. Number of Schwann cells (S-100 positive) and myelinated axons (toluidine blue) were similar between groups at both 2 and 10 wpi. G Ratio was similar between groups at 2 wpi but significantly reduced in the aLam group relative to PBS control at 10 wpi, indicative of thicker myelin sheath. A) Injured peroneal nerve showing Schwann cells (S-100, green; DAPI, blue) at and around the injury site; B) Bar graph showing similar S-100 fluorescence intensity (integrated density); C) Bar graphs showing similar S-100 positive cell counts between groups; D/E) Semi-thin cross-section of injured nerve treated with PBS (D) or aLam (E) stained with toluidine blue at 2 wpi; D) Bar graph showing similar counts of myelinated axons between groups at 2 and 10 wpi. G/H) Semi-thin cross-section of injured nerve treated with PBS (G) or aLam (H) stained with toluidine blue at 10 wpi; Scale bars in G/H represent 50 μm for D/E/G/H; I) Bar graph showing significantly reduced G Ratio of myelinated axons in the aLam group relative to PBS control at 10 wpi. Error bars represent SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Motor axon presence was similar between groups at 10 weeks after injury and treatment
At 10 wpi, we did not observe differences in CTB-labeled motor neuron presence in lumbar spinal cord segments between the experimental groups. Details are described in Supplementary data.
Discussion
We investigated the effects of soluble aLam polymers on peripheral nerve repair following PNC using a clinically relevant adult rat model. We found that aLam treatment of the crushed peroneal nerve at 2 dpi resulted in enhanced axon growth and maturation, which was associated with accelerated hind limb motor recovery without exhibiting adverse effects on sensory function. We also found that aLam treatment decreased autophagia and abolished non-compliance for treadmill walking. Our data suggest that aLam treatment has no ill effects within the parameters of our study. These promising results, combined with the knowledge that laminin is approved by the FDA, may open avenues for the development of aLam-based strategies to accelerate recovery after peripheral nerve damage in humans.
Treatment of a crushed peroneal nerve with aLam led to a significant increase in neurofilament fluorescent staining intensity, which indicates more neurofilament, and potentially a higher amount or more stable axons in the crush at 2 wpi compared with controls. In addition, the nerves treated with aLam showed increased diameter and myelin sheath thickness at 10wpi. The observed axon growth-promoting effects are in agreement with our previously published in vitro data showing that soluble aLam polymers promoted axon growth of cultured neurons from adult rat DRG [5]. Other studies showed that aLam promoted growth of axons from embryonic rat cortex [6] and postnatal day 1 rat retina [7]. While Menezes and colleagues [27] demonstrated that aLam injection resulted in axon growth promotion in the damaged spinal cord, we now successfully translated our earlier in vitro results [5] to an in vivo model of PNC. Future studies are necessary to develop and optimize aLam treatment for this and other types of peripheral nerve injuries. Our in vivo data support the idea that soluble aLam polymers can be used as an injectable therapeutic for the damaged peripheral nerve.
Administration of aLam to the crushed peripheral nerve accelerated the recovery of hind limb motor function as measured using the PFI. The effects of aLam polymers on both PFI and axon growth suggest an involvement of the growing and maturing axons in the observed hind limb motor recovery [28]. It should be noted that a number of control animals were unable and/or refused to walk on the treadmill after injury. As a consequence, in our assessment of hind limb function, the control group consisted of only high performing animals, whereas the aLam treatment group included animals across a spectrum of behavioral performance. The latter is a more accurate representation of a normal distribution within an experimental group. It is likely that the actual difference in hind limb function between groups is greater than the measured difference due to the non-compliance of only control animals. In order to test whether PBS injection was an appropriate control and not impairing endogenous recovery, we evaluated behavior in a cohort of animals with crush only or crush with PBS injection 2 days later and found no difference between the groups in the PFI at any of the time point (see Supplemental Figure 1). This further supports that aLam treatment accelerates functional recovery after PNC. For future studies, it will be important to include additional functional tests that are not reliant on compliance of the rats.
We found that aLam treatment of PNC does not aggravate sensory function. Previous studies revealed that strategies to promote axon regeneration after peripheral nerve damage could negatively impact sensory function [29]. Our data showed that administration of aLam into a crush promoted axon growth but this did not lead to changes in the thresholds for mechanical allodynia or thermal hypersensitivity compared to controls. The absence of adverse effect with aLam treatment on sensory function supports its possible clinical applications after peripheral nerve injuries.
We found that both experimental groups displayed similar hind limb function at 10 wpi, the latest time point of examination in this study. This finding corroborated with similar number of axons and numbers of traced motor neurons between groups. It is not atypical to observe recovery after a crush injury [30] resulting from natural axon growth, which in some cases can be as much as 5 mm/day [31]. It remains clinically relevant, however, that aLam treatment achieved a greater level of recovery in half the time of the control group. The severity and location of the crush are important determinants of the degree of endogenous recovery.
We evaluated autophagia and compliance for treadmill walking in our study. Autophagia and non-compliance to motor tests are common adverse side-effects after an injury to a peripheral nerve that may have detrimental effects on the recovery process. The data showed that autophagia decreased significantly in rats with aLam treatment compared with controls. This occurred while differences in mechanical allodynia and thermal hyperalgesia between groups were not observed. To our knowledge, there is no consensus in the field on the mechanisms underlying autophagy behavior after peripheral nerve injury. Because there were no differences in sensitivities measured in the Hargreaves and Von Frey filament tests, it seems less likely that these were involved in autophagia. It was shown that the strain of rat may be a determining factor in the occurrence of autophagy after peripheral nerve injury [32]. Clearly, future studies are needed to better understand this important phenomenon.
In addition, all rats with aLam treatment fully complied in the treadmill walking test. During the study, several control rats, but not aLam-treated rats, were non-compliant for treadmill walking. Autophagia and non-compliance to motor tests are common adverse side-effects after an injury to the peripheral nerve that may have detrimental effects on the recovery process. We found that axons in the crush site tended to have a greater diameter (p = 0.052) in compliant rats compared with noncompliant rats, which further suggests a contribution of larger diameter axons to motor function. Our finding that aLam treatment reduced autophagia and abolished non-compliance warrants future studies into the underlying mechanisms. Our results underscore the potential of aLam polymers for clinical application and participation in rehabilitation after peripheral nerve injury.
Supplementary Material
Statement of Significance.
Incidence of peripheral nerve injury has been estimated to be as high as 5% of all cases entering a Level 1 trauma center and the majority of cases are young males. Peripheral nerves have some endogenous repair capabilities, but overall recovery of function remains limited, which typically has devastating effects on the individual, family, and society, as wages are lost and rehabilitation is extended until the nerves can repair. We report here that laminin polymers injected into a crush accelerated repair and recovery, had no adverse effects on sensory function, obliterated non-compliance for walking tests, and decreased the occurrence of autophagia. These data support the use of laminin polymers for safe and effective recovery after peripheral nerve injury.
Acknowledgements
The authors thank Ms. Tabitha Novosat, Ms. Doris Clay, Mr. Jonathan Franks, Dr. Donna Stolz for their contributions. This work was supported by the grants from the Wings for Life Foundation (WFL-US-004/12; AEH), Craig H. Neilsen Foundation (460461; MO), NIH (1R21NS101298; MO), and the Department of Veteran Affairs (5I01RX001807).
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Taylor CA, Braza D, Rice JB, Dillingham T, The incidence of peripheral nerve injury in extremity trauma, Am J Phys Med Rehabil 87(5) (2008) 381–5. [DOI] [PubMed] [Google Scholar]
- [2].Fawcett JW, Keynes RJ, Peripheral nerve regeneration, Annu Rev Neurosci 13 (1990) 43–60. [DOI] [PubMed] [Google Scholar]
- [3].Chen ZL, Strickland S, Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve, J Cell Biol 163(4) (2003) 889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Freire E, Barroso MM, Klier RN, Coelho-Sampaio T, Biocompatibility and structural stability of a laminin biopolymer, Macromolecular bioscience 12(1) (2012) 67–74. [DOI] [PubMed] [Google Scholar]
- [5].Haggerty AE, Al-Ali H, Oudega M, Soluble laminin polymers enhance axon growth of primary neurons in vitro, J Biomed Mater Res A (2018). [DOI] [PubMed] [Google Scholar]
- [6].Freire E, Gomes F, Linden R, Neto V, Coelho-Sampaio T, Structure of laminin substrates modulates cellular signaling for neuritogenesis, Journal of Cell Science 115 (2002) 4867–4876. [DOI] [PubMed] [Google Scholar]
- [7].Hochman-Mendez C, Lacerda de Menezes JR, Sholl-Franco A, Coelho-Sampaio T, Polylaminin recognition by retinal cells, J Neurosci Res 92(1) (2014) 24–34. [DOI] [PubMed] [Google Scholar]
- [8].Seddon H, Surgical disorders of the peripheral nerves, 2d ed., Churchill Livingstone, Edinburgh; New York, 1975. [Google Scholar]
- [9].Burchard W, Static and Dynamic Light-Scattering from Branched Polymers and Bio-Polymers, Adv Polym Sci 48 (1983) 1–124. [Google Scholar]
- [10].Ritfeld GJ, Rauck BM, Novosat TL, Park D, Patel P, Roos RAC, Wang YD, Oudega M, The effect of a polyurethane-based reverse thermal gel on bone marrow stromal cell transplant survival and spinal cord repair, Biomaterials 35(6) (2014) 1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mietto BS, Jurgensen S, Alves L, Pecli C, Narciso MS, Assuncao-Miranda I, Villa-Verde DM, de Souza Lima FR, de Menezes JR, Benjamim CF, Bozza MT, Martinez AM, Lack of galectin-3 speeds Wallerian degeneration by altering TLR and pro-inflammatory cytokine expressions in injured sciatic nerve, Eur J Neurosci 37(10) (2013) 1682–90. [DOI] [PubMed] [Google Scholar]
- [12].Bain JR, Mackinnon SE, Hunter DA, Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat, Plast Reconstr Surg 83(1) (1989) 129–38. [DOI] [PubMed] [Google Scholar]
- [13].Inserra MM, Bloch DA, Terris DJ, Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse, Microsurgery 18(2) (1998) 119–24. [DOI] [PubMed] [Google Scholar]
- [14].Von Frey M, Untersuchungen über die sinnesfunctionen der menschlichen haut: 1. abhandlung: Druckempfindung und schmerz, S. Hirzel 1896. [Google Scholar]
- [15].Tena B, Escobar B, Arguis MJ, Cantero C, Rios J, Gomar C, Reproducibility of electronic von Frey and von Frey monofilaments testing, The Clinical journal of pain 28(4) (2012) 318–323. [DOI] [PubMed] [Google Scholar]
- [16].Hargreaves K, Dubner R, Brown F, Flores C, Joris J, A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia, Pain 32(1) (1988) 77–88. [DOI] [PubMed] [Google Scholar]
- [17].Oudega M, Hagg T, Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord, Experimental neurology 140(2) (1996) 218–29. [DOI] [PubMed] [Google Scholar]
- [18].Oudega M, Varon S, Hagg T, Distribution of corticospinal motor neurons in the postnatal rat: quantitative evidence for massive collateral elimination and modest cell death, J Comp Neurol 347(1) (1994) 115–26. [DOI] [PubMed] [Google Scholar]
- [19].Schneider CA, Rasband WS, Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis, Nat Methods 9(7) (2012) 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA, Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination, The Journal of neuroscience : the official journal of the Society for Neuroscience 30(26) (2010) 8953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vogelaar CF, Vrinten DH, Hoekman MF, Brakkee JH, Burbach JP, Hamers FP, Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome, Brain Res 1027(1–2) (2004) 67–72. [DOI] [PubMed] [Google Scholar]
- [22].Kalender AM, Dogan A, Bakan V, Yildiz H, Gokalp MA, Kalender M, Effect of Zofenopril on regeneration of sciatic nerve crush injury in a rat model, J Brachial Plex Peripher Nerve Inj 4 (2009) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR, c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration, Neuron 75(4) (2012) 633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bunge RP, Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration, Curr Opin Neurobiol 3(5) (1993) 805–9. [DOI] [PubMed] [Google Scholar]
- [25].Feneley MR, Fawcett JW, Keynes RJ, The role of Schwann cells in the regeneration of peripheral nerve axons through muscle basal lamina grafts, Experimental neurology 114(3) (1991) 275–85. [DOI] [PubMed] [Google Scholar]
- [26].Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S, Schwann cell basal lamina and nerve regeneration, Brain Res 288(1–2) (1983) 61–75. [DOI] [PubMed] [Google Scholar]
- [27].Menezes K, de Menezes JR, Nascimento MA, Santos Rde S, Coelho-Sampaio T, Polylaminin, a polymeric form of laminin, promotes regeneration after spinal cord injury, FASEB J 24(11) (2010) 4513–22. [DOI] [PubMed] [Google Scholar]
- [28].Brushart TM, Preferential reinnervation of motor nerves by regenerating motor axons, The Journal of neuroscience : the official journal of the Society for Neuroscience 8(3) (1988) 1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zimmermann M, Herdegen T, Plasticity of the nervous system at the systemic, cellular and molecular levels: a mechanism of chronic pain and hyperalgesia, Progress in brain research 110 (1996) 233–259. [DOI] [PubMed] [Google Scholar]
- [30].Burnett MG, Zager EL, Pathophysiology of peripheral nerve injury: a brief review, Neurosurgical focus 16(5) (2004) 1–7. [DOI] [PubMed] [Google Scholar]
- [31].Seddon H, Medawar P, Smith H, Rate of regeneration of peripheral nerves in man, The Journal of physiology 102(2) (1943) 191–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Berrocal YA, Almeida VW, Gupta R, Levi AD, Transplantation of Schwann cells in a collagen tube for the repair of large, segmental peripheral nerve defects in rats, J Neurosurg 119(3) (2013) 720–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.