Abstract
HIV-1 Tat is known to be neurotoxic and important for HIV/neuroAIDS pathogenesis. However, the overwhelming majority of the studies involved use of recombinant Tat protein. To understand the contributions of Tat protein to HIV/ neuroAIDS and the underlying molecular mechanisms of HIV-1 Tat neurotoxicity in the context of a whole organism and independently of HIV-1 infection, a doxycycline-inducible astrocyte-specific HIV-1 Tat transgenic mouse (iTat) was created. Tat expression in the brains of iTat mice was determined to be in the range of 1–5 ng/ml and led to astrocytosis, loss of neuronal dendrites, and neuroinflammation. iTat mice have allowed us to define the direct effects of Tat on astrocytes and the molecular mechanisms of Tat-induced GFAP expression/astrocytosis, astrocyte-mediated Tat neurotoxicity, Tat-impaired neurogenesis, Tat-induced loss of neuronal integrity, and exosome-associated Tat release and uptake. In this review, we will provide an overview about the creation and characterization of this model and its utilities for our understanding of Tat neurotoxicity and the underlying molecular mechanisms.
Keywords: HIV-1, Tat, Transgenic, Mouse model, Brain
Introduction
Tat protein is neurotoxic in vitro. A variety of mechanisms have been proposed and include neuron depolarization, increased intracellular calcium, pro-inflammatory cytokine production, immune cell infiltration, activation of excitatory amino acid receptors, and cell death (Kruman et al. 1998; New et al. 1998; Sabatier et al. 1991; Shi et al. 1998). Tat protein, when present in vivo, causes histological changes in the brain that are consistent with those observed in patients with HIV dementia (Jones et al. 1998; Rappaport et al. 1999). However, it is not clear whether there are high enough of Tat protein within the CNS of HIV-infected individuals (1 to 10 μM) to exhibit such an acute neurotoxicity. On the other hand, recombinant Tat protein was shown to alter gene expression and cell growth, adhesion, and morphology at lower concentrations (nM), at which no acute toxic effects were detected on target cells, including neurons (Ensoli et al. 1990; He et al. 1995; Helland et al. 1991; Marcuzzi et al. 1992; Milani et al. 1993).
Several HIV-1 Tat transgenic mouse models have been developed to study the role of HIV-1 Tat protein in HIV/ AIDS pathogenesis (Choi et al. 2000; Garza et al. 1996; Kundu et al. 1999; Nerenberg et al. 1987; Vellutini et al. 1995; Vogel et al. 1988). All of these models were designed to constitutively express Tat in most or all tissues from the beginning of development. Such a design made it difficult, if not entirely impossible, to discern Tat effects on the brain from those on other tissues or organs. Thus, to gain a better understanding of the role of Tat in HIV/neuroAIDS and the mechanisms of Tat neurotoxicity, if any, specifically resulting from Tat protein expression in the brain, the He lab combined the doxycycline (Dox)-regulated (rtTA) gene expression system (Gossen and Bujard 1992) with a brain-specific promoter, i.e., the glial fibrillary acid protein (GFAP) promoter (Mucke et al. 1995; Toggas et al. 1994). A similar strategy has been successfully used to study functions of a number of genes within the CNS at a specific time and in a specific concentration (Chen et al. 1998; Furth et al. 1994; Mansuy et al. 1998; Passman and Fishman 1994). The main reasons to select the GFAP promoter to target Tat to astrocytes in the CNS at that time were: (1) Tat expression in astrocytes would release Tat and allow Tat to interact with neurons in a way that is reminiscent of Tat in the HIV-infected brain; (2) Tat showed no apparent cytotoxicity to astrocytes (Zhou and He 2004); (3) Accumulating evidence suggested that astrocytes play important roles in HIV/neuroAIDS (Benos et al. 1994; Genis et al. 1992; Levi et al. 1993; Saito et al. 1994; Tornatore et al. 1991); (4) This strategy had been proven to be effective in delivering other proteins to the CNS, including HIV-1 gpl20 and AD-related factors such as ApoE4 (Campbell et al. 1993; Johnson et al. 2010; Mucke et al. 1995; Smith et al. 1998; Sun et al. 1998; Toggas et al. 1994); and (5) This strategy had successfully been used to implicate cytokines IL-3, IL-6, IL-12, TNF-α, and IFN-α in the pathogenesis of other CNS diseases (Brenner et al. 1994; Campbell 1998; Campbell et al. 1998; Carr et al. 1998; Raber et al. 1997).
Generation of iTat mouse model
The first step in the generation of the iTat mouse model was to prepare two transgene constructs that contained the essential components (Kim et al. 2003). One was the regulator, pTeton-GFAP, in which the CMV promoter was replaced by a 2.1-kb GFAP promoter (a gift from Dr. L. Mucke) (Fig. 1a). The other was the expressor, pTRE-Tat86, in which HIV-I HXB2 Tat cDNA (86 amino acids in length) was cloned under the control of the tetO promoter. The pTRE-CAT containing the CAT gene was created as an expressor control and used to determine the specificity of the GFAP promoter. pTeton-GFAP and pTRE-CAT DNA was transiently transfected into astroglial U87.MG cells, and non-astroglial HeLa cells to confirm the specificity of the GFAP promoter activity in astrocytes (Kim et al. 2003). To begin to create the iTat mice, the strategy was to generate two strains of transgenic mice, one for the GFAP-rtTA transgene (G-tg) and one for the TRE-Tat86 transgene (T-tg), and then crossbreed those two strains of mice to obtain the bigenic iTat mice. Both GFAP-rtTA and TRE-Tat86 transgenes were released by restriction digestion (Xho I and Pvu II) from their respective plasmid DNA pTeton-GFAP and pTRE-Tat86, purified, and microinjected into fertilized murine oocytes (C57BL6 xSJL). Five GFAP rtTA transgenic founders and seven TRE-Tat86 transgenic founders were obtained. Backcrossing with wild-type C57BL6 mice was performed to stabilize the GFAP-rtTA transgenic and TRE-Tat86 transgenic lines. Then, GFAP-rtTA mice were crossbred with TRE-Tat86 transgenic mice to obtain iTat mice carrying both GFAP-rtTA and TRE-Tat86 transgenes. The presence of transgenes was confirmed by PCR using genomic DNA from the mouse-tail with transgene-specific primers (Fig. 1b). Homozygous genotypes were confirmed by crossbreeding transgenic mice with wild-type C57/BL6 mice. After successfully obtaining four iTat mouse lines with expression levels from low to high (Kim et al. 2003), an iTat line with Tat expression levels close to that of HIV-infected brains was maintained (Fan et al. 2016; Westendorp et al. 1995; Xiao et al. 2000) and used in subsequent studies and distributed to many other laboratories.
Fig. 1.
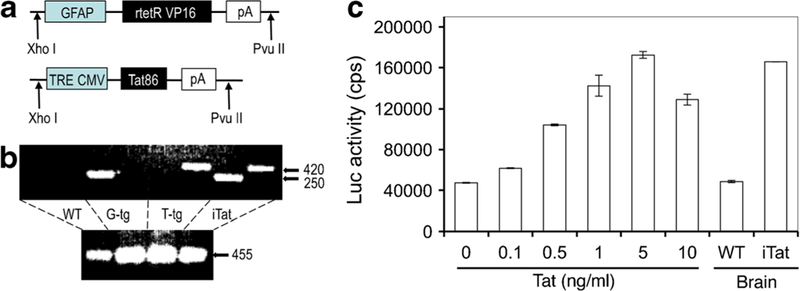
Generation of iTat mice and detection of Tat protein expression. a Two transgene constructs were constructed to make GFAP-rtTA transgenic mice (G-tg) and TRE-Tat86 transgenic mice (T-tg). b Genotyping of G-and T-tg mice and GT-tg (iTat) mice. Genomic DNA was isolated from the mouse tails and PCR was conducted using transgene-specific primers. GAPDH was included as a control. c Brain homogenates were prepared from Dox-treated iTat and wild-type (WT) mice and analyzed for the Tat protein using the LTR-driven luciferase reporter cell line TZM-bl and recombinant Tat standards. Modified from (Kim et al. 2003) with permission
Tat expression in the brain of iTat mice
To determine whether Dox would induce Tat expression in the brain of iTat mice, 21-day-old mice were provided different dosages of Dox-containing drinking water for different periods of time (Kim et al. 2003). Tat expression was initially confirmed using reverse transcriptase (RT)-PCR, and more recently real-time RT-PCR of the total RNA isolated from the brain with Tat gene-specific primers. There was a minimal (leaky) level of Tat mRNA in the brains of iTat mice without Dox treatment (Kim et al. 2003). To ensure that the designed constructs specifically targeted Tat expression within the brain, total RNA was isolated from various organs and tissues of the iTat mice, including the eye, heart, kidney, liver, lung, spleen, and thymus, and Tat expression was determined by RT-PCR. The results indicated that systematic exposure to Dox induced Tat expression only in the brain (Kim et al. 2003). Based on the level of Tat expression, 6 mg/ml Dox in drinking water for 7 days was chosen for the induction. Due to the inconsistency of Dox intake from the drinking water, Dox delivery route was subsequently changed from drinking water to i.p. injection at a dosage of 80 mg/kg/day for 7 days. To determine the expression level of Tat protein in the brain, the HIV LTR promoter-driven luciferase reporter cell line TZM-bl was utilized. TZM-bl cells were treated with serially diluted recombinant Tat protein and the luciferase reporter gene activity assay was performed to establish a linear regression between the level of Tat and the luciferase activity. Then, cells were treated with purified brain homogenates of Dox-treated iTat mice and the luciferase activity was determined. Based on the luciferase activity, Tat protein expression levels in the brains of Dox-treated iTat mice were calculated to be in the range of 1–5 ng/ml (Fan et al. 2016) (Fig. 1c), which is indeed close to the reported Tat levels in the brains of HIV-infected individuals (Fan et al. 2016; Westendorp et al. 1995; Xiao et al. 2000).
Neuropathologies of iTat mice
Among the features that are often observed in HIV-induced neuropathologies prior to and in the era of combination anti-retroviral therapy (cART) are reactive astrogliosis, loss of neuronal integrity (neuronal dendrites), and chronic neuroinflammation. Astrogliosis is a common manifestation of brain pathology in a variety of neurological diseases, although the precise cause(s) remain unknown. Therefore, we determined whether Tat expression in astrocytes would induce astrogliosis. Immunofluorescence labeling of the brain sections using an anti-GFAP antibody indicated that there was widespread reactive astrogliosis in the brains of the iTat mice treated with Dox (Kim et al. 2003; Zou et al. 2007) (Fig. 2), which was reflected by an increase in the number of GFAP-expressing astrocytes. In addition, astrocytes of iTat mice also had a typical reactive morphology, showing increases in both cell body size and cell processes. Moreover, microglia exhibited intense staining and morphological changes in the brains of iTat mice, both of which are typical characteristics of activated microglia (Zou et al. 2007). This finding suggests that microgllia activation may also contribute to Tat neurotoxicity in iTat mice.
Fig. 2.
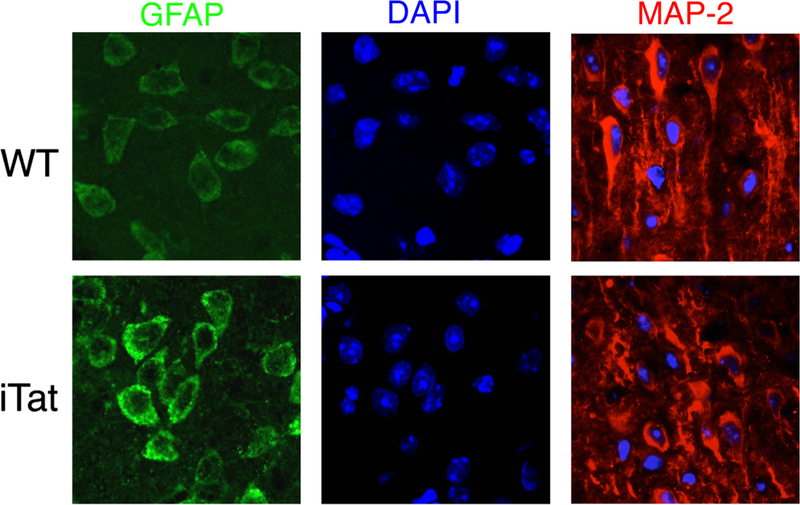
Increased GFAP expression and loss of neuronal dendrites in the brains of iTat mice. Wild-type (WT) and iTat mice were given Dox at 80 mg/kg/day for 7 days. The brains were collected for immunofluorescence labeling using anti-GFAP (green) and anti-MAP2 (red) antibodies. The sections were also counterstained with DAPI (blue)
Next, it was determined whether Tat expression in astrocytes was sufficient to cause neuronal damage. The integrity of neuronal dendrites in brain sections of the iTat mice treated with Dox was compared to age-matched wild-type control mice using an anti-MAP2 antibody. The results showed a significant loss of neuronal dendrites in the brains of the iTat mice treated with Dox, when compared with the age-matched wild-type controls (Fan and He 2016b; Kim et al. 2003; Rahimian and He 2016b; Zou et al. 2007) (Fig. 2).
Furthermore, the relationship between Tat expression and inflammatory response in the brain was assessed. Compared to the wild-type control mice treated with Dox, iTat mice showed increased infiltration of activated macrophages/monocytes and T lymphocytes into the brain (Kim et al. 2003; Zou et al. 2007). In agreement with those findings, there were increased levels of cytokines and chemokines in the brains of iTat mice including monocyte chemoattractant protein-1 and protein-2, macrophage inflammatory protein-1α and protein-1β, RANTES, inducing protein-10, and lymphotactin/single C motif-1α/activation-induced, and T cell-derived and chemokine-related cytokine (Kim et al. 2004).
Use of iTat mice in studies of Tat neurotoxicity and its molecular mechanisms
Tat expression inhibits astrocyte proliferation
Tat protein exerts various effects on host cells that include changes in the cell cycle (Kundu et al. 1998), T lymphocyte survival (Gibellini et al. 1995; Zauli et al. 1993), and growth of T lymphocytes and enterocytes (Canani et al. 2003; Viscidi et al. 1989). To determine effects of intracellular Tat expression on astrocytes, primary astrocytes were isolated from 16-day-old embryos of iTat mice and treated with Dox to induce Tat expression and then analyzed for proliferation using the [3H] thymidine incorporation assay. Compared to primary astrocytes from wild-type mice, iTat primary astrocytes exhibited significantly slower proliferation and the culture supernatants from iTat astrocytes were neurotoxic (Zhou and He 2004). The anti-proliferative effects of intracellular Tat on astrocytes did not appear to be due to premature cell senescence; instead, it could be attributed to Tat interactions with various cell cycle-related proteins including cyclin A, cyclin B, cyclin D3, cdk2, cdk, and cdk1/cdc2 (Fig. 3).
Fig. 3.
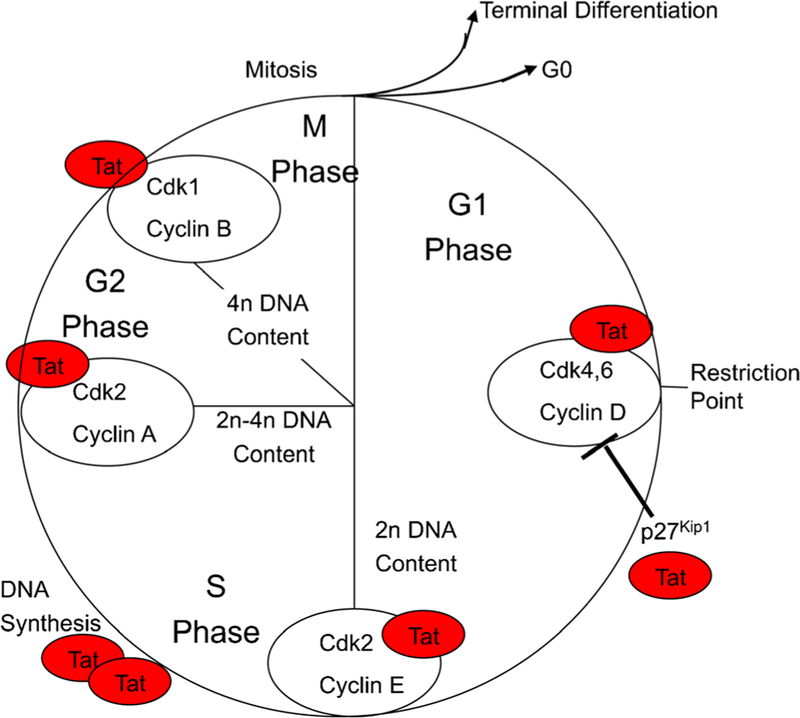
Interaction of Tat with cell cycle-related proteins. Cell lysates were prepared from Tat-expressing astrocytes and subjected to a protein-binding array analysis for cell cycle-related proteins. All Tat-binding proteins were identified as shown. Modified from (Zhou and He 2004) with permission
Tat transactivates GFAP expression
Astrogliosis has been noted in the brains of iTat mice. Meanwhile, intracellular Tat expression results in anti-proliferative effects on astrocytes. These findings raised the possibility that Tat could directly transactivate GFAP expression. To address this possibility, iTat mice or primary astrocytes isolated from iTat mice were used to define a cascade of transcriptional factors that formed the network through which Tat transactivates GFAP expression (Fan et al. 2015, 2011; Kim et al. 2003, 2004; Zhou et al. 2004; Zou et al. 2010) (Fig. 4). The first route is that Tat released from HIV-infected macrophages/microglia and astrocytes interacts with cell surface molecules, induces STAT3 phosphorylation and dimerization, and its subsequent nuclear translocation. The second route is that Tat directly translocates into the nucleus and transactivates STAT3 expression. In both scenarios, phosphorylated STAT3 transactivates Egr-1, transactivates p300 and GFAP, and contributes to increased GFAP expression in the brains of iTat mice and in the brains of HIV-infected individuals.
Fig. 4.
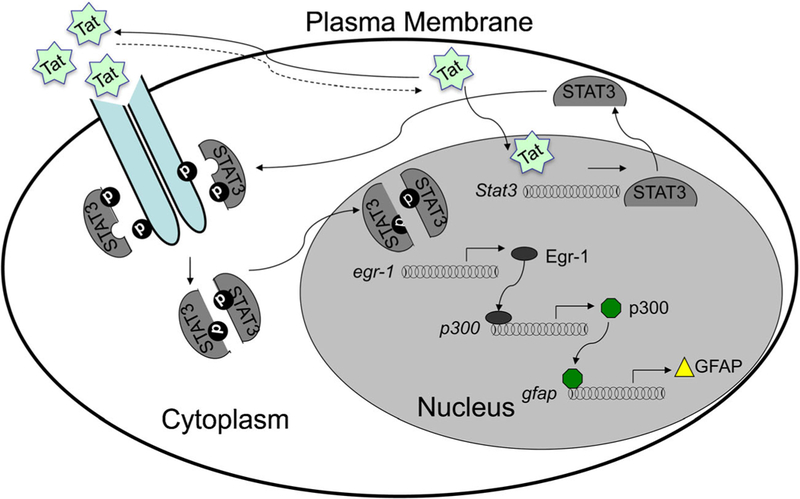
Transcriptional network for Tat-transactivated GFAP expression. Tat either transactivates STAT3 or interacts with cell surface receptors that lead to STAT3 phosphorylation and activation. Both routes result in sequential activation of Egr-1, p300 and GFAP. Modified from (Fan et al. 2015) with permission
GFAP activation and Tat neurotoxicity
Abnormal GFAP expression alone has been shown to be sufficient to cause neuropathologies (Gomi et al. 1995; Liedtke et al. 1996; Messing et al. 1998; Pekny et al. 1998). Astrogliosis or increased GFAP expression in astrocytes is one of the very few consistent hallmarks of HIV-1 infection of the CNS (Bell et al. 2006a; Price 1996). Astrocytes have a variety of functions, many of which are related to the CNS homeostasis [see reviews (Barres 1991; Eddleston and Mucke 1993)]. More recent evidence points to a highly dynamic and reciprocal relationship between astrocytes and neurons with a notion that astrocyte dysfunction contributes to neurological diseases. For example, viral infection-induced astrocyte dysfunctions contribute to several CNS diseases (Aksamit et al. 1986; Aubert et al. 1987; Carbone et al. 1991; Itoyama et al. 1991; Rinaman et al. 1993; Stowring et al. 1985). However, the direct link between GFAP activation, astrocytosis, and Tat neurotoxicity was not clear. Using iTat mice or primary astrocytes from iTat mice, studies showed that Tat expression in astrocytes led to marked impairment of glutamate uptake by astrocytes and importantly, that cell culture supernatants derived from Tat-expressing astrocytes induced neurotoxicity (Fitting et al. 2013; Kim et al. 2003; Zhou and He 2004; Zhou et al. 2004). Furthermore, it was shown that disruption and inhibition of GFAP expression abrogated astrocyte-mediated Tat neurotoxicity (Fan and He 2016a, b; Zou et al. 2007). These data show that Tat expression in astrocytes leads to astrocyte dysfunction and subsequent neurotoxicity, and support the notion that astrocyte dysfunction contributes, at least in part, to Tat neurotoxicity and subsequently to HIV/neuroAIDS.
Tat expression causes ER stress in astrocytes and enhances lysosomal exocytosis from astrocytes
Tat expression in astrocytes alone was sufficient to induce pathological changes such as astrocytosis, loss of neuronal dendrites, and neurobehavioral deficits including impaired motor and cognitive functions in the CNS reminiscent of HIV-associated minor cognitive motor disorder (MCMD) (Carey et al. 2012; Fan et al. 2011; Fitting et al. 2013; Kim et al. 2003; Paris et al. 2014c; Zhou et al. 2004) (see the review by McLaughlin, et al. in the same issue). However, the exact underlying molecular mechanisms were not clear. Using combined molecular, cellular, biochemical, and genetic approaches, Fan and He demonstrated that Tat expression led to formation of GFAP aggregates and activation of the unfolded protein response (UPR) and endoplasmic reticulum (ER) stress in astrocytes (Fan and He 2016a). In addition, they showed that UPR/ER stress activation promoted calcium-dependent lysosomal exocytosis from astrocytes and as a result, led to astrocyte-mediated Tat neurotoxicity (Fan and He 2016b). Lastly, these studies demonstrated that the chemical chaperone 4-phenylbutyrate significantly abrogated astrocyte-mediated Tat neurotoxicity through inhibition of Tat-induced UPR/ER stress (Fan and He 2016b). Taken together, these findings support a working model (Fig. 5): Tat from HIV-infected macrophages/microglia is taken up by astrocytes and/or Tat is expressed in HIV-infected astrocytes thereby activating GFAP expression and leading to GFAP aggregation. GFAP aggregation in turn activates UPR/ER stress, calcium release from ER storage into the cytoplasm, and subsequent lysosomal exocytosis from astrocytes and release of neurotoxic lysosomes and their neurotoxic components, such as cathepsins (Fan and He 2016b). These findings provide new and important insights not only about the roles of this critical and pervasive protein Tat in HIV/neuroAIDS, particularly in the era of cART, but also about the general roles of astrocytosis and GFAP up-regulation in contributing to neurodegenerative diseases.
Fig. 5.
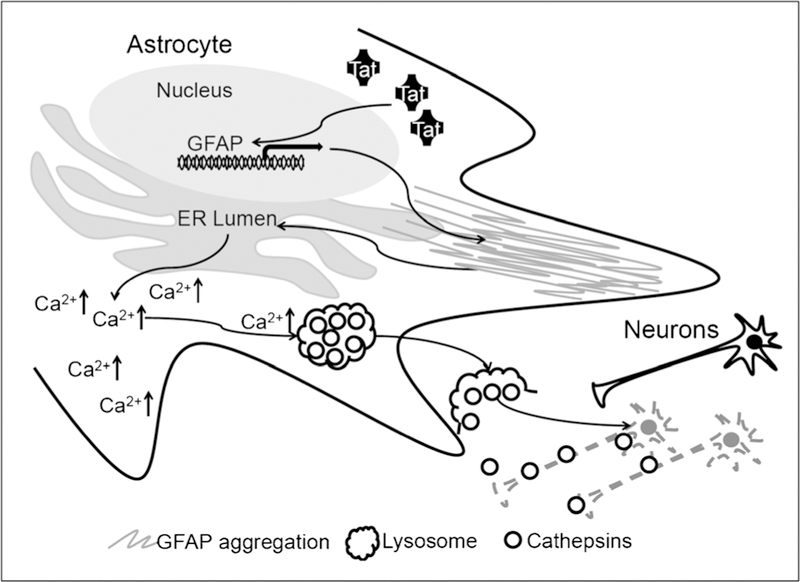
Astrocyte-mediated Tat neurotoxicity. Tat expression or uptake by astrocytes transactivates GFAP expression and causes GFAP aggregation and ER stress, which in turn triggers release of calcium from the ER and excessive lysosomal exocytosis and cathepsin-dependent neurotoxicity. Modified from (Fan and He 2016b) with permission
Tat expression impaired neurogenesis
HIV infection of the CNS often leads to cognitive, motor, and neurobehavioral dysfunction (Dube et al. 2005; Epstein et al. 1986; Valcour et al. 2004). Adult neurogenesis in the hippo-campus is critical for the maintenance of intact cognitive functions, such as learning and memory, and is regulated by physiological and pathological stimuli (Fuchs and Gould 2000; Leuner et al. 2006; Ming and Song 2005). Impaired neurogenesis has been noted in the hippocampus of HIV-infected individuals, SIV-infected macaques, and severe combined immunodeficiency mice injected with HIV-infected human macrophages/microglia (Curtis et al. 2014; Krathwohl and Kaiser 2004a, b; Poluektova et al. 2005). HIV could have effects on neuron progenitor cells (NPC) through direct infection (Lawrence et al. 2004; Schwartz and Major 2006; Tran et al. 2005; Whitney et al. 2009), proliferation (Krathwohl and Kaiser 2004b; Okamoto et al. 2007), and migration (Belmadani et al. 2005, 2006). Tat protein has been shown to affect mature neurons in a variety of ways (Chen et al. 1997; Cheng et al. 1998; Conant et al. 1998; Hofman et al. 1999; Jones et al. 1998; Zidovetzki et al. 1998) and to inhibit NPC proliferation and differentiation in vitro (Mishra et al. 2010; Yao et al. 2012). Given the recent findings that Tat is persistently expressed in the brain of HIV-infected individuals treated with cART (Johnson et al. 2013), it was imperative to determine the effects of Tat expression on neurogenesis and HIV-associated minor cognitive motor disorder (MCMD). Taking advantage of the iTat mice, researchers showed that Tat inhibited NPC proliferation and migration and altered NPC differentiation to favor astrogliogenesis over neurogenesis through Tat binding to Notch signaling factors (Fan et al. 2016) (Fig. 6). These findings point to the potential of developing Notch signaling inhibitors as HIV/neuroAIDS therapeutics.
Fig. 6.
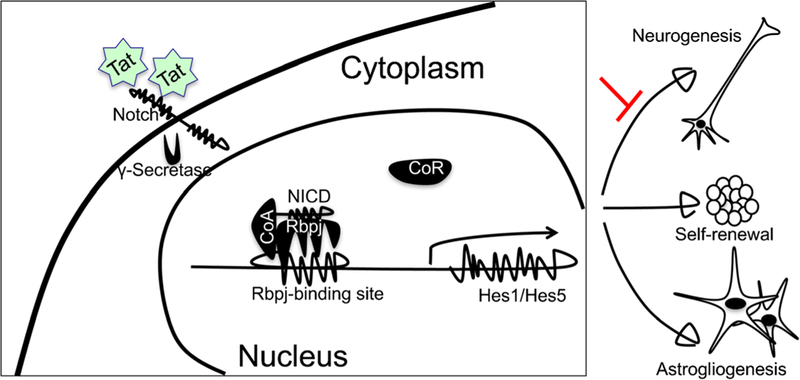
Tat promotes astrogliogenesis over neurogenesis through its binding to Notch to activate the Notch signaling pathway. Tat binds to the Notch extracellular domain and leads to cleavage of Notch intracellular domain (NICD) by γ-secretase. NICD translocates to the nucleus and binds to the Rbpj binding site on the Hes1 promoter, dissociates the co-repressor (CoR), and recruits the co-activator (CoA) to transactivate downstream molecular Hes1 expression. Activation of Notch signaling is sufficient to favor astrogliogenesis over neurogenesis
Tat-mediated miR-132 expression and its effects on neuronal dendrites
Successful suppression of active HIV replication by cART has resulted in dramatic decreases of the incidence of HIV-associated dementia from 15 to 2% (Hult et al. 2008) (Ghafouri et al. 2006). However, up to 50% of HIV-infected individuals exhibit a less severe form of HIV-associated neurocognitive disorders (HAND), MCMD (Bell et al. 2006b). Synaptodendritic injury is a positive correlate of MCMD, neuroinflammation, and cognitive impairments of HIV-infected individuals (Adle-Biassette et al. 1999; Masliah et al. 1992, 1997; Moore et al. 2006; Rao et al. 2012, 2011). However, the exact underlying molecular mechanisms of HIV-associated synaptodendritic injury and synaptic loss were largely unknown. Using a combined molecular, cellular, and genetic approach, including iTat mice, Rahimian and He showed that Tat-induced miR-132 expression and that exosomes associated with miR-132 from astrocytes caused neurite shortening, likely through down-regulation of miR-132 target genes such as brain-derived neurotrophic factor (BDNF) and methyl CpG-binding protein 2 (MeCP2) (Rahimian and He 2016b) (Fig. 7). These findings showed that Tat-induced miR-132 expression contributes to both direct and astrocyte-mediated Tat neurotoxicity, and supports the important roles of miR-132 in regulation of neurite outgrowth.
Fig. 7.
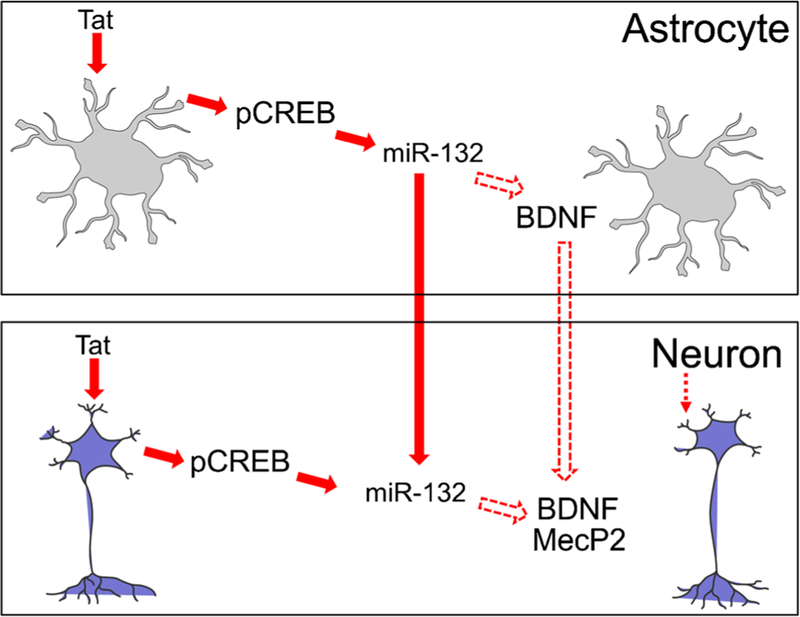
Tat, miR-132, and neurite growth. Tat expression leads to phosphorylation of CREB, which in turn transactivates miR-132 expression in both astrocytes and neurons. miR-132 down-regulates BDNF expression, while it is also taken up into neurons via exosomes and down-regulates MeCP2 expression in neurons. Both BDNF down-regulation in astrocytes and MeCP2 down-regulation in neurons contribute to Tat-induced neurite shortening. Reproduced from (Rahimian and He 2016b) with permission
Exosome-associated Tat release and uptake
Biologically active and intact Tat protein is secreted from HIV-infected cells (Westendorp et al. 1995; Xiao et al. 2000), Tat-expressing cells (Albini et al. 1998; Chang et al. 1997; Ensoli et al. 1990; Milani et al. 1993; Morgavi et al. 1997; Zauli et al. 1993, 1995), and is detected in HIV-infected brains (Hudson et al. 2000). Tat does not contain an export signal sequence (Chang et al. 1997; Morgavi et al. 1997). Thus, its secretion occurs through unconventional secretory pathways. Tat or Tat-derived peptides are capable of entering cultured cells and transactivating the HIV LTR promoter (Bonifaci et al. 1995; Frankel and Pabo 1988; Green et al. 1989; Liu et al. 2000; Mann and Frankel 1991; Viscidi et al. 1989). The basic domain of Tat protein has also been utilized to successfully deliver protein cargo into cells (Fawell et al. 1994; Schwarze et al. 1999; Vives et al. 1997). We have demonstrated that Tat specifically binds to low-density lipoprotein receptors on neurons, which leads to Tat neuronal uptake in its biologically active form (Liu et al. 2000). Using primary astrocytes from iTat mice, a HIV-1 LTR-driven luciferase reporter-based cell system and a well-defined OptiPrep-based exosome purification protocol, researchers showed that Tat was secreted in exosomes, which were potently neurotoxic (Rahimian and He 2016a). These findings indicated that a significant fraction of Tat is secreted in the form of exosomes and may contribute to the stability of extracellular Tat and broaden the spectrum of its target cells.
Use of iTat mice in other studies
While many studies in the HIV/neuroAIDS field focus on astrocytosis, neuronal integrity, and neuroinflammation as the outcome measures, there are many other processes that occur within the brains of HAND patients. The iTat mouse has been utilized to investigate some of these processes. Among the many reports utilizing the iTat model just within the last decade are studies addressing Tau processing (Kadri et al. 2015), host cell cycle regulation (Fields et al. 2015b), cell survival (Fan and He 2016b), differentiation (Perry et al. 2010; Wheeler et al. 2008; Yao et al. 2012) and glial activation (Kiebala et al. 2010), substance abuse (Fitting et al. 2012, 2010; Hauser et al. 2009; Mediouni et al. 2015; Paris et al. 2014a; Zou et al. 2011), sex differences (Hahn et al. 2015a, b), behavior (Hahn et al. 2016; Paris et al. 2014b, c, 2015), memory and learning (Carey et al. 2012; Fitting et al. 2013) and brain changes observed via imaging (Carey et al. 2015, 2013). Table 1, while not comprehensive by any means, provides examples of the diversity of studies that have utilized the iTat mouse model. Continuing studies using the iTat model will open new opportunities for addressing important processes such as cART effects, aging, epigenetics, and therapy development in the context of the Tat protein.
Table 1.
Use of iTat Mice in other studies
| Mechanism(s) studied | References |
|---|---|
| Substance abuse | (Fitting et al. 2012, 2010; Hauser et al. 2009; Mediouni et al. 2015; Paris et al. 2014a; Zou et al. 2011) |
| Sex differences | (Hahn et al. 2015a, b) |
| Behavior | (Hahn et al. 2016; Paris et al. 2014b, c, 2015) |
| Learning and memory | (Carey et al. 2012; Fitting et al. 2013) |
| Structural changes | (Carey et al. 2015, 2013) |
| Lysosome/autophagosome | (Fan and He 2016b; Fields et al. 2015a) |
| Cell cycle | (Fields et al. 2015b) |
| Tau processing | (Kadri et al. 2015) |
| Enteric neuropathogenesis | (Ngwainmbi et al. 2014) |
| Cell survival/growth factors/differentiation | (Perry et al. 2010; Wheeler et al. 2008; Yao et al. 2012) |
| Glial activation | (Kiebala et al. 2010) |
| Microvascular changes | (Silva et al. 2014) |
Reversible changes of Tat-induced neuropathologies in iTat mice
cART effectively suppresses HIV replication in the periphery. With a better ability to cross the blood-brain barrier, cART can also suppress HIV replication in the central nervous system and lead to decreases in HAND. However, neuroinflammation rather than viral load appears to be more important to HAND, and there have been no HAND-specific therapeutics. As discussed above, iTat mice have consistently recapitulated astrocytosis, compromised neuronal integrity, and neuroinflammation, three consistent hallmarks of HAND in the era of cART. EGb 761, a standardized formulation of Ginkgo biloba extract, has the ability to protect iTat mice from Tat-induced astrocytosis and neuroinflammation through down-regulation of GFAP expression (Zou et al. 2007). Chemical chaperone 4-phenylbutyrate inhibits Tat- or GFAP-induced unfold protein response/endoplasmic reticulum stress and alleviates astrocyte-mediated Tat neurotoxicity in vitro and in the brains of iTat mice (Fan and He 2016a). Treatment of conditioned medium of Tat-expressing astrocytes with anti-cathepsin B antibody led to marked decreases in the neurotoxicity of Tat-expressing astrocytes (Fan and He 2016b). Notch signaling inhibitor greatly improved Tat-impaired NPC differentiation and neurogenesis in iTat mice (Fan et al. 2016). miR-132 inhibitor inhibits astrocyte-derived Tat neurotoxicity, specifically neuron dendritic damage and loss of and synapse formation (Rahimian and He 2016b). Taken together, these findings demonstrate that Tat-induced neuropathologies (likely neurocognitive impairments as well) are reversible and suggest the utility of this model for development of HIV/neuroAIDS therapeutics, particularly those targeted at the early stages of HIV infection of the CNS.
Limitations of the iTat mouse model
Even though transgenic mouse models were among the first developed to study HIV infection, the use of these models has posed numerous challenges since rodents are not natural hosts of HIV and do not support viral replication (Jaeger and Nath 2012). As more knowledge was gained regarding the specific effects of certain viral proteins including gp120 and Tat, and in answer to these biological barriers, viral protein-specific transgenic animals were created.
Recombinant Tat protein when present in the brain causes histological changes that are consistent with those seen in patients with HIV dementia (Jones et al. 1998; Rappaport et al. 1999). However, it is still unclear whether Tat is present in the CNS or cerebrospinal fluid in HIV-infected individuals at sufficient concentrations to directly cause acute neurotoxicity that is observed in the transgenic models. However, the GFAP-driven expression of Tat protein by astrocytes in the brains of iTat mice was close to actual levels in the brains of HAND patients (Fan et al. 2016; Westendorp et al. 1995; Xiao et al. 2000). In vitro cell culture and in vivo studies show that HIV infects astrocytes, but the contribution that this population of cells makes to HAND is unclear (Jaeger and Nath 2012; Kramer-Hammerle et al. 2005). A recent study shows that in individuals with HAND, up to 19% of perivascular astrocytes are infected by HIV (Churchill et al. 2009), which may represent a considerable percentage of infected cells in the CNS. On the other hand, astrocytes are not believed to generate significant amounts of virus, but studies have shown that even in the absence of productive viral replication, Tat is still produced. Moreover, studies have shown that even during successful cART, Tat is generated (Jaeger and Nath 2012).
Another main limiting factor in using iTat mice to study HAND is the fact that Tat is only one factor involved in the neuropathogenic processes in CNS infection with HIV. Many viral proteins including Tat, gp120, gp140, Nef, Vpr, and Rev are also toxic to neurons (Nath 2002), and the iTat mouse model considers only one of these. In this context, different viral proteins target different host cell pathways can, therefore, dysregulate a variety of downstream signaling cascades that may lead to aberrant cell signaling.
One other question remains: How does the iTat model relate to the neuropathology of HAND patients without HIVE treated with cART? With cART, no specific neuropathology has been reported to be linked to HAND (Gelman 2015; Gelman et al. 2013). HAND persists in up to 50% of virally suppressed HIV patients (Heaton et al. 2010) and it could involve other neuronal dysfunctions (Ellis et al. 2007; Gelman 2015). Some studies point to neurovascular unit damage as a possible pathology associated with HAND in the absence of HIVE (Gelman et al. 2012; Wolf et al. 2002). Disturbances in bioenergetics and in glutamate homeostasis are reported in cART-treated HIV patients [see review (Saylor et al. 2016)]. Executive dysfunction, memory impairment with disruptions in attention, multi-tasking, impulse control, judgment and memory encoding, and retrieval and motor dysfunction are associated with HAND (Heaton et al. 2011; Saylor et al. 2016). Taking these findings into account, the iTat mouse model provides some but not all characteristics of patients with HAND and its use remains relevant to HAND studies.
Acknowledgments
Funding information This work was supported in part by the grants NIH/NINDS R01NS090960 and NIH/NIDA R01DA043162 (to JJH), and NIH/NIMH R01MH107340 and NIH/NIDA P01DA037830 (to DL and KK).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F (1999) Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol 25:123–133 [DOI] [PubMed] [Google Scholar]
- Aksamit AJ, Sever JL, Major EO (1986) Progressive multifocal leukoencephalopathy: JC virus detection by in situ hybridization compared with immunohistochemistry. Neurology 36:499–504 [DOI] [PubMed] [Google Scholar]
- Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, Rabin RL, Farber JM, Noonan DM (1998) HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci USA 95:13153–13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert C, Chamorro M, Brahic M (1987) Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog 3:319–326 [DOI] [PubMed] [Google Scholar]
- Barres BA (1991) New roles for glia. J Neurosci 11:3685–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Anthony IC, Simmonds P (2006a) Impact of HIVon regional & cellular organisation of the brain. Curr HIV Res 4:249–257 [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC (2006b) Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J NeuroImmune Pharmacol 1:182–191 [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ (2005) The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci 25:3995–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ (2006) Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci 26:3182–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos DJ, Hahn BH, Shaw GM, Bubien JK, Benveniste EN (1994) gp120-mediated alterations in astrocyte ion transport. Adv Neuroimmunol 4:175–179 [DOI] [PubMed] [Google Scholar]
- Bonifaci N, Sitia R, Rubartelli A (1995) Nuclear translocation of an exogenous fusion protein containing HIV Tat requires unfolding. AIDS 9:995–1000 [DOI] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A (1994) GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci 14:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL (1998) Transgenic mice and cytokine actions in the brain: bridging the gap between structural and functional neuropathology. Brain Res Brain Res Rev 26:327–336 [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L (1993) Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA 90:10061–10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IL, Stalder AK, Akwa Y, Pagenstecher A, Asensio VC (1998) Transgenic models to study the actions of cytokines in the central nervous system. Neuroimmunomodulation 5:126–135 [DOI] [PubMed] [Google Scholar]
- Canani RB, Cirillo P, Mallardo G, Buccigrossi V, Secondo A, Annunziato L, Bruzzese E, Albano F, Selvaggi F, Guarino A (2003) Effects of HIV-1 Tat protein on ion secretion and on cell proliferation in human intestinal epithelial cells. Gastroenterology 124:368–376 [DOI] [PubMed] [Google Scholar]
- Carbone KM, Moench TR, Lipkin WI (1991) Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol 50:205–214 [DOI] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP (2012) Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 229:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, Kaufman MJ (2013) Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuro-Psychopharmacol Biol Psychiatry 43:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Liu X, Mintzopoulos D, Paris JJ, McLaughlin JP, Kaufman MJ (2015) Conditional Tat protein brain expression in the GT-tg bigenic mouse induces cerebral fractional anisotropy abnormalities. Curr HIV Res 13:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Veress LA, Noisakran S, Campbell IL (1998) Astrocyte-targeted expression of IFN-alpha1 protects mice from acute ocular herpes simplex virus type 1 infection. J Immunol 161:4859–4865 [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11:1421–1431 [DOI] [PubMed] [Google Scholar]
- Chen P, Mayne M, Power C, Nath A (1997) The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem 272: 22385–22388 [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ (1998) Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol 54:495–503 [DOI] [PubMed] [Google Scholar]
- Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS (1998) Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience 82:97–106 [DOI] [PubMed] [Google Scholar]
- Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W, Maxson R, Forman HJ (2000) Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J Biol Chem 275:3693–3698 [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR (2009) Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol 66:253–258 [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO (1998) Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA 95:3117–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis K, Rollins M, Carryl H, Bradshaw K, Van Rompay KK, Abel K, Burke MW (2014) Reduction of pyramidal and immature hippocampal neurons in pediatric simian immunodeficiency virus infection. Neuroreport 25:973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube B, Benton T, Cruess DG, Evans DL (2005) Neuropsychiatric manifestations of HIV infection and AIDS. J Psychiatry Neurosci 30: 237–246 [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Mucke L (1993) Molecular profile of reactive astrocytes— implications for their role in neurologic disease. Neuroscience 54: 15–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8:33–44 [DOI] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F (1990) Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature 345:84–86 [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Oleske JM, Connor EM, Goudsmit J, Bagdon L, Robert-Guroff M, Koenigsberger MR (1986) Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics 78:678–687 [PubMed] [Google Scholar]
- Fan Y, He JJ (2016a) HIV-1 Tat induces unfolded protein response and endoplasmic reticulum stress in astrocytes and causes neurotoxicity through glial Fibrillary acidic protein (GFAP) activation and aggregation. J Biol Chem 291:22819–22829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, He JJ (2016b) HIV-1 Tat promotes Lysosomal exocytosis in astrocytes and contributes to astrocyte-mediated Tat neurotoxicity. J Biol Chem 291:22830–22840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zou W, Green LA, Kim BO, He JJ (2011) Activation of Egr-1 expression in astrocytes by HIV-1 Tat: new insights into astrocyte-mediated Tat neurotoxicity. J NeuroImmune Pharmacol 6:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Timani KA, He JJ (2015) STAT3 and its phosphorylation are involved in HIV-1 Tat-induced transactivation of glial fibrillary acidic protein. Curr HIV Res 13:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Gao X, Chen J, Liu Y, He JJ (2016) HIV Tat impairs neurogenesis through functioning as a notch ligand and activation of notch signaling pathway. J Neurosci 36:11362–11373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J (1994) Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA 91:664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Eleuteri S, Campos S, Serger E, Trejo M, Kosberg K, Adame A, Spencer B, Rockenstein E, He JJ, Masliah E (2015a) HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci 35:1921–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Dumaop W, Crews L, Adame A, Spencer B, Metcalf J, He J, Rockenstein E, Masliah E (2015b) Mechanisms of HIV-1 Tat neurotoxicity via CDK5 translocation and hyper-activation: role in HIV-associated neurocognitive disorders. Curr HIV Res 13:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF (2010) Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol 177:1397–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, Hauser KF (2012) Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur J Pharmacol 689:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF (2013) Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 73:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189–1193 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Gould E (2000) Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Eur J Neurosci 12: 2211–2214 [DOI] [PubMed] [Google Scholar]
- Furth PA, St Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L (1994) Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci USA 91:9302–9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza HH Jr, Prakash O, Carr DJ (1996) Aberrant regulation of cytokines in HIV-1 TAT72-transgenic mice. J Immunol 156:3631–3637 [PubMed] [Google Scholar]
- Gelman BB (2015) Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Curr HIV/AIDS Rep 12:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, Singer EJ, Levine AJ, Miller J, Winkler JM, Fox HS, Luxon BA, Morgello S, National Neuro ATC (2012) The national NeuroAIDS tissue consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One 7:e46178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Morgello S, Masliah E, Commins D, Achim CL, Fox HS, Kolson DL, Grant I, Singer E, Yiannoutsos CT, Sherman S, Gensler G, Moore DJ, Chen T, Soukup VM (2013) Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr 62:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ et al. (1992) Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med 176: 1703–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri M, Amini S, Khalili K, Sawaya BE (2006) HIV-1 associated dementia: symptoms and causes. Retrovirology 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini D, Caputo A, Celeghini C, Bassini A, La Placa M, Capitani S, Zauli G (1995) Tat-expressing Jurkat cells show an increased resistance to different apoptotic stimuli, including acute human immuno-deficiency virus-type 1 (HIV-1) infection. Br J Haematol 89:24–33 [DOI] [PubMed] [Google Scholar]
- Gomi H, Yokoyama T, Fujimoto K, Ikeda T, Katoh A, Itoh T, Itohara S (1995) Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 14:29–41 [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89:5547–5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Ishino M, Loewenstein PM (1989) Mutational analysis of HIV-1 Tat minimal domain peptides: identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell 58: 215–223 [DOI] [PubMed] [Google Scholar]
- Hahn YK, Masvekar RR, Xu R, Hauser KF, Knapp PE (2015a) Chronic HIV-1 Tat and HIV reduce Rbfox3/NeuN: evidence for sex-related effects. Curr HIV Res 13:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE (2015b) Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct 220:605–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Paris JJ, Lichtman AH, Hauser KF, Sim-Selley LJ, Selley DE, Knapp PE (2016) Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and beta-arrestin 2 activity in the forebrain. Neurobiol Dis 92:124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE (2009) HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia 57:194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR (1995) Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 69:6705–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neuro-Oncol 17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland DE, Welles JL, Caputo A, Haseltine WA (1991) Transcellular transactivation by the human immunodeficiency virus type 1 tat protein. J Virol 65:4547–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman FM, Chen P, Incardona F, Zidovetzki R, Hinton DR (1999) HIV-1 tat protein induces the production of interleukin-8 by human brain-derived endothelial cells. J Neuroimmunol 94:28–39 [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I (2000) Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neuro-Oncol 6:145–155 [DOI] [PubMed] [Google Scholar]
- Hult B, Chana G, Masliah E, Everall I (2008) Neurobiology of HIV. Int Rev Psychiatry 20:3–13 [DOI] [PubMed] [Google Scholar]
- Itoyama Y, Sekizawa T, Openshaw H, Kogure K, Goto I (1991) Early loss of astrocytes in herpes simplex virus-induced central nervous system demyelination. Ann Neurol 29:285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Nath A (2012) Modeling HIV-associated neurocognitive disorders in mice: new approaches in the changing face of HIV neuropathogenesis. Dis Model Mech 5:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, Ding J, McCluskey L, Martinez-Lage M, Falcone D, Hernandez DG, Arepalli S, Chong S, Schymick JC, Rothstein J, Landi F, Wang YD, Calvo A, Mora G, Sabatelli M, Monsurro MR, Battistini S, Salvi F, Spataro R, Sola P, Borghero G, Consortium I, Galassi G, Scholz SW, Taylor JP, Restagno G, Chio A, Traynor BJ (2010) Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A (2013) Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci USA 110:13588–13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A (1998) Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol 57:563–570 [DOI] [PubMed] [Google Scholar]
- Kadri F, Pacifici M, Wilk A, Parker-Struckhoff A, Del Valle L, Hauser KF, Knapp PE, Parsons C, Jeansonne D, Lassak A, Peruzzi F (2015) HIV-1-Tat protein inhibits SC35-mediated tau exon 10 inclusion through up-regulation of DYRK1A kinase. J Biol Chem 290: 30931–30946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebala M, Polesskaya O, Yao Z, Perry SW, Maggirwar SB (2010) Nuclear factor-kappa B family member RelB inhibits human immunodeficiency virus-1 Tat-induced tumor necrosis factor-alpha production. PLoS One 5:e11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, ZC X, Schantz L, He JJ (2003) Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 162:1693–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Zhou BY, He JJ (2004) Induction of C chemokine XCL1 (lymphotactin/single C motif-1 alpha/activation-induced, T cell-derived and chemokine-related cytokine) expression by HIV-1 Tat protein. J Immunol 172:1888–1895 [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R (2005) Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res 111:194–213 [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL (2004a) Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells 22:109–118 [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL (2004b) HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis 190:216–226 [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP (1998) HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol 154: 276–288 [DOI] [PubMed] [Google Scholar]
- Kundu M, Sharma S, De Luca A, Giordano A, Rappaport J, Khalili K, Amini S (1998) HIV-1 Tat elongates the G1 phase and indirectly promotes HIV-1 gene expression in cells of glial origin. J Biol Chem 273:8130–8136 [DOI] [PubMed] [Google Scholar]
- Kundu RK, Sangiorgi F, LY W, Pattengale PK, Hinton DR, Gill PS, Maxson R (1999) Expression of the human immunodeficiency virus-Tat gene in lymphoid tissues of transgenic mice is associated with B-cell lymphoma. Blood 94:275–282 [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO (2004) Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol 78:7319–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ (2006) Is there a link between adult neurogenesis and learning? Hippocampus 16:216–224 [DOI] [PubMed] [Google Scholar]
- Levi G, Patrizio M, Bernardo A, Petrucci TC, Agresti C (1993) Human immunodeficiency virus coat protein gp120 inhibits the beta-adrenergic regulation of astroglial and microglial functions. Proc Natl Acad Sci USA 90:1541–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS (1996) GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 17:607–615 [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ (2000) Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med 6:1380–1387 [DOI] [PubMed] [Google Scholar]
- Mann DA, Frankel AD (1991) Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J 10:1733–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy IM, Winder DG, Moallem TM, Osman M, Mayford M, Hawkins RD, Kandel ER (1998) Inducible and reversible gene expression with the rtTA system for the study of memory. Neuron 21:257–265 [DOI] [PubMed] [Google Scholar]
- Marcuzzi A, Lowy I, Weinberger OK (1992) Transcellular activation of the human immunodeficiency virus type 1 long terminal repeat in T lymphocytes requires CD4-gp120 binding. J Virol 66:4536–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA (1992) Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol 32:321–329 [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I (1997) Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV neurobehavioral research center. Ann Neurol 42:963–972 [DOI] [PubMed] [Google Scholar]
- Mediouni S, Jablonski J, Paris JJ, Clementz MA, Thenin-Houssier S, McLaughlin JP, Valente ST (2015) Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res 13:64–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A, Head MW, Galles K, Galbreath EJ, Goldman JE, Brenner M (1998) Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol 152:391–398 [PMC free article] [PubMed] [Google Scholar]
- Milani D, Zauli G, Neri LM, Marchisio M, Previati M, Capitani S (1993) Influence of the human immunodeficiency virus type 1 Tat protein on the proliferation and differentiation of PC12 rat pheochromocytoma cells. J Gen Virol 74(Pt 12):2587–2594 [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250 [DOI] [PubMed] [Google Scholar]
- Mishra M, Taneja M, Malik S, Khalique H, Seth P (2010) Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in NeuroAIDS. J Neuro-Oncol 16:355–367 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I, Group H (2006) Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20:879–887 [DOI] [PubMed] [Google Scholar]
- Morgavi P, Bonifaci N, Pagani M, Costigliolo S, Sitia R, Rubartelli A (1997) The association of HIV-1 Tat with nuclei is regulated by Ca2+ ions and cytosolic factors. J Biol Chem 272:11256–11260 [DOI] [PubMed] [Google Scholar]
- Mucke L, Abraham CR, Ruppe MD, Rockenstein EM, Toggas SM, Mallory M, Alford M, Masliah E (1995) Protection against HIV-1 gp120-induced brain damage by neuronal expression of human amyloid precursor protein. J Exp Med 181:1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A (2002) Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 186(Suppl 2): S193–S198 [DOI] [PubMed] [Google Scholar]
- Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G (1987) The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324–1329 [DOI] [PubMed] [Google Scholar]
- New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA (1998) HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-D-aspartate receptors by a NFkappaB-independent mechanism. J Biol Chem 273:17852–17858 [DOI] [PubMed] [Google Scholar]
- Ngwainmbi J, De DD, Smith TH, El-Hage N, Fitting S, Kang M, Dewey WL, Hauser KF, Akbarali HI (2014) Effects of HIV-1 Tat on enteric neuropathogenesis. J Neurosci 34:14243–14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA (2007) HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell 1:230–236 [DOI] [PubMed] [Google Scholar]
- Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP (2014a) Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology 39:380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Fenwick J, McLaughlin JP (2014b) Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Horm Behav 65:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP (2014c) Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology 231:2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Carey AN, McLaughlin JP (2015) Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res 291:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passman RS, Fishman GI (1994) Regulated expression of foreign genes in vivo after germline transfer. J Clin Invest 94:2421–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Eliasson C, Chien CL, Kindblom LG, Liem R, Hamberger A, Betsholtz C (1998) GFAP-deficient astrocytes are capable of stellation in vitro when cocultured with neurons and exhibit a reduced amount of intermediate filaments and an increased cell saturation density. Exp Cell Res 239:332–343 [DOI] [PubMed] [Google Scholar]
- Perry SW, Barbieri J, Tong N, Polesskaya O, Pudasaini S, Stout A, Lu R, Kiebala M, Maggirwar SB, Gelbard HA (2010) Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax. J Neurosci 30:14153–14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE (2005) Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia 52:344–353 [DOI] [PubMed] [Google Scholar]
- Price RW (1996) Neurological complications of HIV infection. Lancet 348:445–452 [DOI] [PubMed] [Google Scholar]
- Raber J, O’Shea RD, Bloom FE, Campbell IL (1997) Modulation of hypothalamic-pituitary-adrenal function by transgenic expression of interleukin-6 in the CNS of mice. J Neurosci 17:9473–9480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian P, He JJ (2016a) Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. J Neuro-Oncol 22:774–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian P, He JJ (2016b) HIV-1 Tat-shortened neurite outgrowth through regulation of microRNA-132 and its target gene expression. J Neuroinflammation 13:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Kim HW, Kellom M, Greenstein D, Chen M, Kraft AD, Harry GJ, Rapoport SI, Basselin M (2011) Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflammation 8:101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rao JS, Kellom M, Kim HW, Rapoport SI, Reese EA (2012) Neuroinflammation and synaptic loss. Neurochem Res 37:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K (1999) Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, tat. J Leukoc Biol 65:458–465 [DOI] [PubMed] [Google Scholar]
- Rinaman L, Card JP, Enquist LW (1993) Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci 13:685–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E (1991) Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol 65:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM (1994) Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology 44:474–481 [DOI] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L, Major EO (2006) Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res 4:319–327 [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569–1572 [DOI] [PubMed] [Google Scholar]
- Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D (1998) Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neuro-Oncol 4:281–290 [DOI] [PubMed] [Google Scholar]
- Silva JN, Polesskaya O, Wei HS, Rasheed IY, Chamberlain JM, Nishimura C, Feng C, Dewhurst S (2014) Chronic central nervous system expression of HIV-1 Tat leads to accelerated rarefaction of neocortical capillaries and loss of red blood cell velocity heterogeneity. Microcirculation 21:664–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Sikes J, Levin JA (1998) Human apolipoprotein E allele-specific brain expressing transgenic mice. Neurobiol Aging 19: 407–413 [DOI] [PubMed] [Google Scholar]
- Stowring L, Haase AT, Petursson G, Georgsson G, Palsson P, Lutley R, Roos R, Szuchet S (1985) Detection of visna virus antigens and RNA in glial cells in foci of demyelination. Virology 141:311–318 [DOI] [PubMed] [Google Scholar]
- Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, Fagan AM, Holtzman DM (1998) Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci 18:3261–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L (1994) Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367:188–193 [DOI] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K, Major EO (1991) Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol 65:6094–6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Ren D, Miller RJ (2005) The HIV-1 coat protein gp120 regulates CXCR4-mediated signaling in neural progenitor cells. J Neuroimmunol 160:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Watters MR, Sacktor NC (2004) Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS 18(Suppl 1):S79–S86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellutini C, Horschowski N, Philippon V, Gambarelli D, Nave KA, Filippi P (1995) Development of lymphoid hyperplasia in transgenic mice expressing the HIV tat gene. AIDS Res Hum Retrovir 11:21–29 [DOI] [PubMed] [Google Scholar]
- Viscidi RP, Mayur K, Lederman HM, Frankel AD (1989) Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 246:1606–1608 [DOI] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B (1997) A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 272:16010–16017 [DOI] [PubMed] [Google Scholar]
- Vogel J, Hinrichs SH, Reynolds RK, Luciw PA, Jay G (1988) The HIV tat gene induces dermal lesions resembling Kaposi’s sarcoma in transgenic mice. Nature 335:606–611 [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH (1995) Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375: 497–500 [DOI] [PubMed] [Google Scholar]
- Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ (2008) A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 131:3092–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC (2009) Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem 108:1343–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M, Swiss HIVCS (2002) Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis 185:456–462 [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT (2000) Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA 97:11466–11471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Yang L, Buch S (2012) Platelet-derived growth factor-BB restores human immunodeficiency virus Tat-cocaine-mediated impairment of neurogenesis: role of TRPC1 channels. J Neurosci 32:9835–9847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G, Gibellini D, Milani D, Mazzoni M, Borgatti P, La Placa M, Capitani S (1993) Human immunodeficiency virus type 1 Tat protein protects lymphoid, epithelial, and neuronal cell lines from death by apoptosis. Cancer Res 53:4481–4485 [PubMed] [Google Scholar]
- Zauli G, La Placa M, Vignoli M, Re MC, Gibellini D, Furlini G, Milani D, Marchisio M, Mazzoni M, Capitani S (1995) An autocrine loop of HIV type-1 Tat protein responsible for the improved survival/ proliferation capacity of permanently Tat-transfected cells and required for optimal HIV-1 LTR transactivating activity. J Acquir Immune Defic Syndr Hum Retrovirol 10:306–316 [PubMed] [Google Scholar]
- Zhou BY, He JJ (2004) Proliferation inhibition of astrocytes, neurons, and non-glial cells by intracellularly expressed human immunodeficiency virus type 1 (HIV-1) Tat protein. Neurosci Lett 359:155–158 [DOI] [PubMed] [Google Scholar]
- Zhou BY, Liu Y, Kim B, Xiao Y, He JJ (2004) Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci 27:296–305 [DOI] [PubMed] [Google Scholar]
- Zidovetzki R, Wang JL, Chen P, Jeyaseelan R, Hofman F (1998) Human immunodeficiency virus Tat protein induces interleukin 6 mRNA expression in human brain endothelial cells via protein kinase C- and cAMP-dependent protein kinase pathways. AIDS Res Hum Retrovir 14:825–833 [DOI] [PubMed] [Google Scholar]
- Zou W, Kim BO, Zhou BY, Liu Y, Messing A, He JJ (2007) Protection against human immunodeficiency virus type 1 Tat neurotoxicity by Ginkgo biloba extract EGb 761 involving glial fibrillary acidic protein. Am J Pathol 171:1923–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Wang Z, Liu Y, Fan Y, Zhou BY, Yang XF, He JJ (2010) Involvement of p300 in constitutive and HIV-1 Tat-activated expression of glial fibrillary acidic protein in astrocytes. Glia 58:1640–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE (2011) Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia. Brain 134: 3616–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]


