Abstract
Cell division cycle-associated protein 4 (CDCA4), also known as SEI-3/hematopoietic progenitor protein, is a target gene of transcription factor E2F and represses E2F-dependent transcriptional activation and cell proliferation. The present study investigated the effects of CDCA4 knockdown on the regulation of triple negative breast cancer (TNBC) cell proliferation in vitro and in vivo. Human TNBC MDA-MB-231 cells were subjected to CDCA4 expression knockdown using a lentiviral vector carrying CDCA4 or a negative control short hairpin RNA, and reverse transcription-quantitative polymerase chain reaction, MTT cell viability, cell growth, flow cytometric apoptosis, cell cycle and nude mouse tumorigenesis assays were conducted. The knockdown of CDCA4 expression effectively inhibited the growth of MDA-MB-231 cells by promoting apoptosis in vitro. Additionally, CDCA4 expression knockdown suppressed nude mouse tumor cell xenograft formation and growth in vivo. In conclusion, the data from the present study supported the hypothesis that CDCA4 may be involved in regulating human TNBC progression, and that targeting CDCA4 expression could be useful as a novel strategy in future TNBC treatment.
Keywords: triple negative breast cancer, cell division cycle-associated protein 4, cell proliferation, targeted therapy
Introduction
Breast cancer is the most significant health problem in women worldwide, which accounted for an estimated 1.7 million new cases and 521,900 cases of cancer-associated mortality globally in 2012 (1). Histologically, breast cancer can be classified into ductal carcinoma in situ, ductal and lobular carcinoma, and invasive breast cancer, and this classification is useful in selecting tumor lesions for surgical resection; however, there is no or limited value for the selection of targeted therapy. In addition, breast cancer can be molecularly subtyped according to the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor 2 receptor (HER2/neu) (2), or even according to gene signature (3). The three receptor-positive types of breast cancer can be effectively controlled by hormonal and targeted therapy, including tamoxifen or trastuzumab (4,5). However, triple negative breast cancer (TNBC), which does not express ER, PR or Her2/neu, is more difficult to treat (2). TNBC accounts for 15–20% of all breast cancer cases and has a high risk of early recurrence (6) due to poor response to conventional chemo- or radiotherapy; however, systemic chemotherapy is the only strategy currently available for recurrent or metastatic TNBC (7). The median survival of patients with metastatic TNBC following conventional chemotherapy is only 9–12 months (8). Therefore, the identification of novel and effective strategies to control TNBC is urgently required.
Cell division cycle-associated protein (CDCA) 4, also known as SEI-3/hematopoietic progenitor protein (HEPP), is a target gene of the transcription factor E2F that can repress E2F-dependent transcriptional activation and cell proliferation (9). CDCA4 was initially identified by Abdullah et al and termed HEPP due to its preferential expression in fetal and adult hematopoietic progenitor cells and mature blood cells (10). CDCA4 contains four highly conserved characteristic sequences: A cyclin A binding domain, C-terminal motif, SERTA domain and plant homeodomain (PHD)-bromine binding domain, which are closely associated with the functions of the SEI family (11–13). Therefore, CDCA4 is also referred to as SEI-3 or TRIP-Br3. Previous studies have demonstrated that SEI-1 and SEI-2 are involved in E2F-mediated cell cycle progression and tumorigenesis (14), while DNA damage induces the binding of E2F-1 and p53 to the CDCA4 cyclin A binding domain to promote apoptosis (15). In addition, the SEI family proteins, including CDCA4, can regulate p53-dependent transcriptional activity, and overexpression of the SEI family proteins inhibits proliferation of HeLa and U2OS cell lines (9) and suppresses c-JUN expression (16), while the association of CDCA4 with the formation and distribution of the spindle in early and mid-mitotic stages may serve as a main transcription factor in chromosome segregation and cytoplasmic division (17). Therefore, further studies concerning this family of proteins, including CDCA4, could provide an improved understanding of their role in tumorigenesis and may provide a novel target for the clinical control of TNBC.
The present study investigated the effects of CDCA4 knockdown, using CDCA4 short hairpin (sh)RNA (shCDCA4), on the regulation of TNBC cell proliferation in vitro and in vivo. This provided novel insights into the role of CDCA4 in TNBC MDA-MB-231 cells.
Materials and methods
Gene information
The online resource Metabolic gEne Rapid Visualizer (MERAV: http://merav.wi.mit.edu/, accessed by January 20, 2018) was used to generate boxplots of the expression levels of CDCA4 in normal breast tissue and primary breast tumors tissue (18).
Cell lines and culture
Human breast cancer (MDA-MB-231, MDA-MB-468 and T-47D) cell lines and the normal human mammary gland Hs578BST cell line were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life Sciences, Little Chalfont, UK) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and 1% antimycotic (Penicillin-Streptomycin-Amphotericin B solution; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified chamber with 5% CO2.
RNA interference
shCDCA4 constructs targeting the CDCA4 cDNA sequence (5′-CCTAGACCTAAGAGTAAATTA-3′) were synthesized and cloned into the GV115 lentiviral vector (Shanghai GeneChem Co., Ltd., Shanghai, China). Subsequently, 293T cells were co-infected with lentiviral vector carrying the shCDCA4 or negative control shRNA (shCtrl; 5′-TTCTCCGAACGTGTCACGT-3′; Shanghai GeneChem Co., Ltd.) and packaging plasmids. The lentiviruses were then harvested and the virus titer was determined. Additionally, the lentiviral vectors carried firefly luciferase and green fluorescent protein (GFP) genes to label tumor cells. The TNBC cell lines were transfected with lentivius (MOI=10) in a 24-well plate (5×104 cells/well). Following transfection for 24 h, the fresh complete medium was replaced and the cells were cultured for an additional 72 h at 37°C. These lentiviruses were used to stably knockdown CDCA4 expression in MDA-MB-231 and MDA-MB-468 breast cancer cell lines, and shRNA-infected cells were selected in puromycin (5 ug/ml)-containing DMEM (Clontech Laboratories, Inc., Mountainview, CA, USA). The images of infected cells were observed under a phase-contrast and fluorescence microscope (Olympus Corporation, Tokyo, Japan).
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The concentration of RNA samples was determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and reverse transcribed into cDNA using oligo (dT) primers and a reverse transcriptase from Moloney murine leukemia virus (Promega Corporation, Madison, WI, USA). The temperature protocol for RT was as follows: 42°C for 1 h, 70°C for 5 min followed by storage at 4°C. The resulting cDNA samples were subjected to qPCR amplification using the SYBR Premix Ex Taq kit (Takara Bio, Inc., Otsu, Japan) and the ABI 7500 apparatus (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. The optimized parameters for qPCR were set to an initial cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C for 5 sec, and then cooled to and maintained at 4°C. The primer sequences were: Human CDCA4, forward 5′-ATTTGAAACGCTGGAGACT-3′, reverse 5′-CCCATCATGCCTGTCAGTA-3′; and GAPDH, forward 5′-TGACTTCAACAGCGACACCCA-3′, and reverse 5′-CACCCTGTTGCTGTAGCCAAA-3′. GAPDH was used as the reference gene. The 2−ΔΔCq method was used to calculate the relative mRNA expression levels of CDCA4 as previously described (19).
MTT cell viability assay
MDA-MB-231 cells, infected with lentivirus carrying shCDCA4 or shCtrl, were seeded into 96-well plates at a density of 2,000 cells/well and grown for up to 5 days. Subsequently, 20 µl MTT (5 mg/ml; GenView, Tallahassee, FL, USA) was added to the cell culture, and the cells were cultured for an additional 4 h at 37°C. Subsequently, 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) replaced the cell culture medium to dissolve the formazan crystals for 15 min. Optical density values were measured using a microplate reader (Synergy H1; BioTek China, Beijing, China) at 490 nm. The experiments were performed in triplicate and repeated at least three times independently.
Cell counting assay
Logarithmic growth phase MDA-MB-231 cells, infected with lentivirus carrying shCDCA4 or shCtrl, were seeded into 96-well plates at a density of 1,500 cells/well and incubated at 37°C with 5% CO2 for up to 5 days. Subsequently, the cells were counted using the Celigo imaging cytometer (Nexcelom Bioscience, LLC, Lawrence, MA, USA). The experiments were performed in triplicate and repeated at least three times independently.
Flow cytometric cell cycle and Annexin V-allophycocyanin (APC) apoptosis assays
MDA-MB-231 cell cycle distribution and the levels of apoptosis were assessed using propidium iodide (PI; Sigma-Aldrich; Merck KGaA) and the Annexin V Apoptosis Detection kit APC (Thermo Fisher Scientific, Inc., Waltham, MA, USA), respectively, according to the manufacturers' protocols. Briefly, following infection with lentivirus carrying shCDCA4 or shCtrl, MDA-MB-231 cells were seeded into a 6-well plate at a density of 1×105 cells/well and grown for 5 days.
For the cell cycle assay, cells were harvested using trypsin, washed twice in D-Hanks buffer (pH 7.2–7.4), fixed with 70% ethanol for 30 min at 20°C, and stored at 4°C overnight. Subsequently, the cells were stained with 50 µg/ml PI solution containing 100 µg/ml RNase A (Sigma-Aldrich; Merck KGaA) and incubated for 1 h at room temperature in the dark. The cell cycle distribution was analyzed using a fluorescence-activated cell sorting (FACS) analyzer (EMD Millipore, Billerica, MA, USA).
For the apoptosis assay, cells were harvested using trypsin, washed twice in D-Hanks buffer (pH 7.2–7.4), and resuspended in the binding buffer from the kit. The cell suspension (990 µl) was then supplemented with 10 µl Annexin V-APC solution and incubated for 15 min at room temperature in the dark. The rate of cell apoptosis was analyzed using the FACS analyzer. Analysis of flow cytometery data was performed with the GuavaSoft foftware package for Guava easyCyte HT systems (version 2.5; EMD Millipore).
Animal experiments
To assess the effects of CDCA4 knockdown on the regulation of TNBC cell xenograft formation and growth in vivo, nude mouse MDA-MB-231 cell xenografts were established. A total of 20 female BALB/C nude mice (specific-pathogen free; age, 4 weeks; weight, 17–24 g) were purchased from the Shanghai Animal Laboratory Center (Shanghai, China) and randomized into two groups (n=10) receiving either shCDCA4- or shCtrl-infected MDA-MB-231 cells. The knockdown group of nude mice was subcutaneously injected with stable shCDCA4-infected MDA-MB-231 cells (1×107 cells in 200 µl) in the right axilla, while the negative control group of mice was subcutaneously injected with the same number and volume of MDA-MB-231 cells stably infected with shCtrl. The nude mice were housed in laminar flow cabinets under a specific pathogen-free environment with access to food and water ad libitum (temperature, 25±1°C; relative humidity, 40–60%; 12 h light/12 h dark cycle). Tumor xenograft formation and size were recorded every 3 days using a Vernier caliper. In addition, nude mice were anesthetized with pentobarbital (0.7%, 50 mg/kg; Sigma-Aldrich; Merck KGaA) and were treated with D-luciferin (10 µl/g; Shanghai Qcbio Science & Technologies Co., Ltd., Shanghai, China) to measure tumor cell fluorescence; the total tumor xenograft fluorescence radiant efficiency was measured on days 22, 29 and 36 using the IVIS Lumina LT (PerkinElmer, Inc., Waltham, MA, USA). After 2 months, the nude mice were sacrificed and tumor cell xenografts were isolated and weighed. All protocols were approved by the Ethics Review Committee of The First Affiliated Hospital of Guangxi Medical University (Nanning, China).
Statistical analysis
All data are expressed as the means ± standard deviation and were analyzed with SPSS v22.0 software (IBM Corp., Armonk, NY, USA). A Student's t-test was performed for two-group comparisons, and one-way analysis of variance and least significant difference post hoc test were performed for multiple-group comparisons. All the experiments were repeated in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
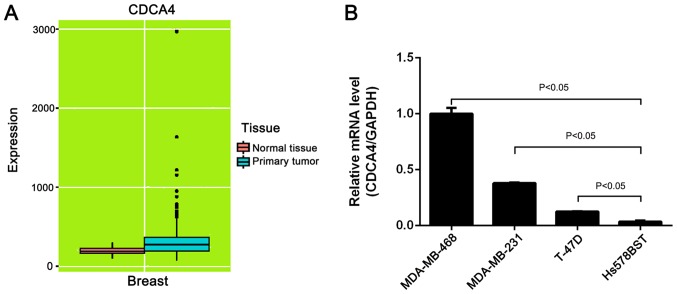
High expression of CDCA4 mRNA in breast cancer tissues and cell lines
In the present study, CDCA4 expression data were obtained from the online MERAV database (http://merav.wi.mit.edu/; accessed January 20, 2018) to identify CDCA4 expression in normal breast and breast tumor tissues (18). The boxplots of CDCA4 expression revealed that CDCA4 expression was higher in breast cancer tissues than in normal tissues (Fig. 1A). Additionally, the mRNA expression levels of CDCA4 in three breast cancer cell lines were higher than in a normal mammary gland cell line (Fig. 1B).
Figure 1.
Expression of CDCA4 in breast cancer tissues and cell lines. (A) mRNA expression levels of CDCA4 in normal breast vs. primary breast tumor tissues. CDCA4 expression data were obtained from the online MERAV database (http://merav.wi.mit.edu). (B) RT-qPCR. Relative mRNA expression levels of CDCA4 in MDA-MB-231, MDA-MB-468, T-47D and Hs578BST cells were assessed using RT-qPCR. The data were expressed as the means ± standard deviation. CDCA4, cell division cycle-associated protein 4; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Knockdown of CDCA4 expression in breast cancer cell lines using lentivirus carrying shCDCA4 or shCtrl
To investigate the effect of CDCA4 on breast cancer cells, lentiviruses carrying shCDCA4 or shCtrl were prepared and MDA-MB-231 and MDA-MB-468 breast cancer cells were infected. The present study demonstrated that shCDCA4 was able to effectively knockdown the mRNA expression levels of CDCA4 in TNBC MDA-MB-231 cells compared with the shCtrl; however, the knockdown efficiency in MDA-MB-468 cells was <50% and not suitable for subsequent experiments (Fig. 2A). Subsequently, MDA-MB-231 cells were screened with puromycin and subjected to fluorescence microscopy, which demonstrated that infection and GFP expression rates were >80% (Fig. 2B). Therefore, the human TNBC MDA-MB-231 cell line was selected as a model cell line to assess the effect of shCDCA4 on breast cancer cells in vitro and in vivo.
Figure 2.
mRNA expression levels of CDCA4 in TNBC cells. (A) RT-qPCR. TNBC cells were grown and infected with a lentivirus carrying shCDCA4 or shCtrl and then subjected to RT-qPCR. CDCA4 mRNA expression in MDA-MB-468 cells was slightly decreased compared with that in the shCtrl group, whereas MDA-MB-231 cells exhibited a significant knockdown of CDCA4 expression (P<0.05). (B) Fluorescence microscopy. MDA-MB-231 cells infected with lentivirus-shCtrl and lentivirus-shCDCA4, were observed under a fluorescence and phase-contrast microscope. Fluorescence microscopy demonstrated that infection and green fluorescent protein expression rates were >80%. Magnification, ×100. The data were expressed as the means ± standard deviation. CDCA4, cell division cycle-associated protein 4; Ctrl, negative control; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; sh, short hairpin RNA; TNBC, triple negative breast cancer.
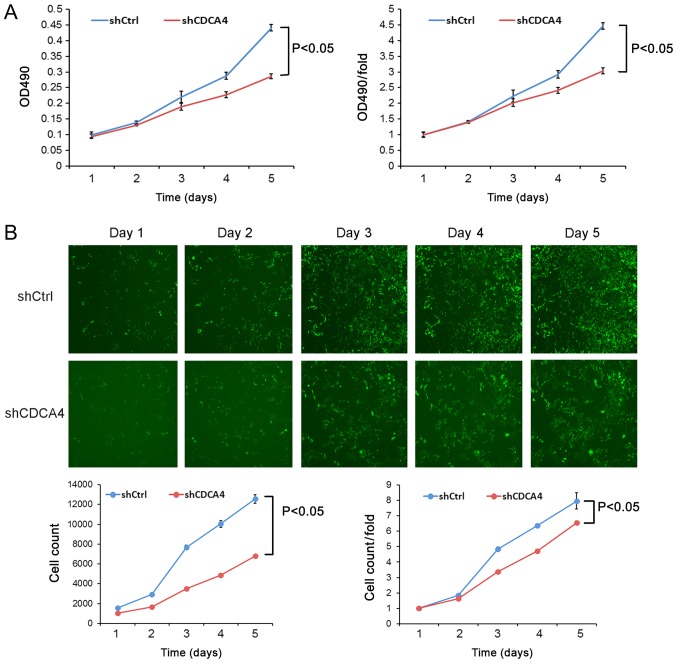
shCDCA4 reduces MDA-MB-231 cell proliferation in vitro
Following the knockdown of CDCA4 expression in TNBC MDA-MB-231 cells, cell viability and cell counting assays were performed. The cell viability following shCDCA4 infection was significantly reduced compared with in the shCtrl group (Fig. 3A). Similarly, cell-counting Celigo images revealed that the cell proliferation rate of the shCDCA4 group was significantly reduced (Fig. 3B). These data suggested that CDCA4 may enhance the proliferation of MDA-MB-231 cells.
Figure 3.
Effects of CDCA4 knockdown on the regulation of triple negative breast cancer cell proliferation. (A) Cell viability assay. MDA-MB-231 cells infected with lentivirus-shCtrl and lentivirus-shCDCA4, were subjected to the MTT assay. (B) Tumor cell counting assay. MDA-MB-231 cells infected with lentivirus-shCtrl and lentivirus-shCDCA4 were subjected to 5 consecutive days of Celigo imaging cell counting. Magnification, ×40. The data were expressed as the means ± standard deviation. CDCA4, cell division cycle-associated protein 4; Ctrl, negative control; OD, optical density; sh, short hairpin RNA.
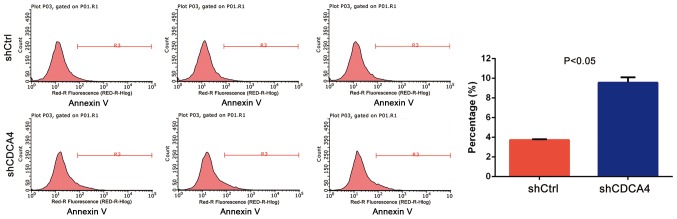
shCDCA4 induces MDA-MB-231 cell apoptosis in vitro
The effect of CDCA4 knockdown on the regulation of tumor cell apoptosis was assessed using FACS analysis. The data demonstrated that, after 5 days of lentiviral infection with shCtrl, the apoptosis rate of MDA-MB-231 cells was 3.72±0.09%, whereas the apoptosis rate of the shCDCA4 group was 9.56±0.53% (P<0.05, Fig. 4). These data suggested that CDCA4 may negatively regulate apoptosis of MDA-MB-231 cells.
Figure 4.
Effects of CDCA4 knockdown on the regulation of triple negative breast cancer cell apoptosis. MDA-MB-231 cells infected with lentivirus-shCtrl and lentivirus-shCDCA4, were subjected to a flow cytometric apoptosis assay. The data were expressed as the means ± standard deviation. CDCA4, cell division cycle-associated protein 4; Ctrl, negative control; sh, short hairpin RNA.
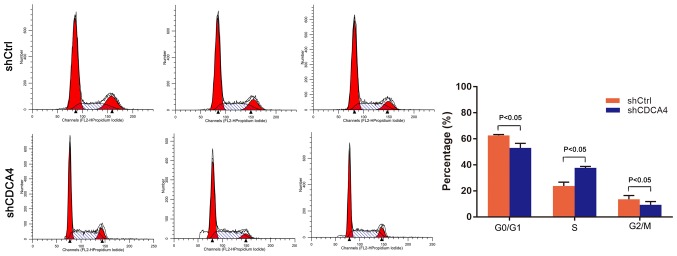
shCDCA4 induces regulation of the cell cycle
The effect of CDCA4 knockdown on regulation of the tumor cell cycle was assessed using FACS analysis. For the shCDCA4-infected cells, 53.05±3.51% of cells were in G0/G1 phase, while 37.67±1.10% were in S phase and 9.29±2.48% were in G2/M phase of the cell cycle, which was significantly different from the percentages of the shCtrl group (P<0.05, Fig. 5). Knockdown of CDCA4 led to increased accumulation of cells in the S phase of the cell cycle. These data indicated that CDCA4 altered the cell cycle progression of MDA-MB-231 cells. The inhibition of cell growth and proliferation following knockdown of CDCA4 may be achieved by preventing the transition between S and G2 phase.
Figure 5.
Effects of CDCA4 knockdown on the regulation of triple negative breast cancer cell cycle distribution. MDA-MB-231 cells, infected with lentivirus-shCtrl and lentivirus-shCDCA4, were subjected to a flow cytometric cell cycle distribution assay. The data were expressed as the means ± standard deviation. CDCA4, cell division cycle-associated protein 4; Ctrl, negative control; sh, short hairpin RNA.
shCDCA4 reduces the growth of MDA-MB-231 cell xenografts in nude mice
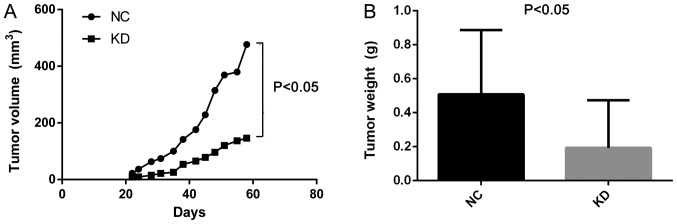
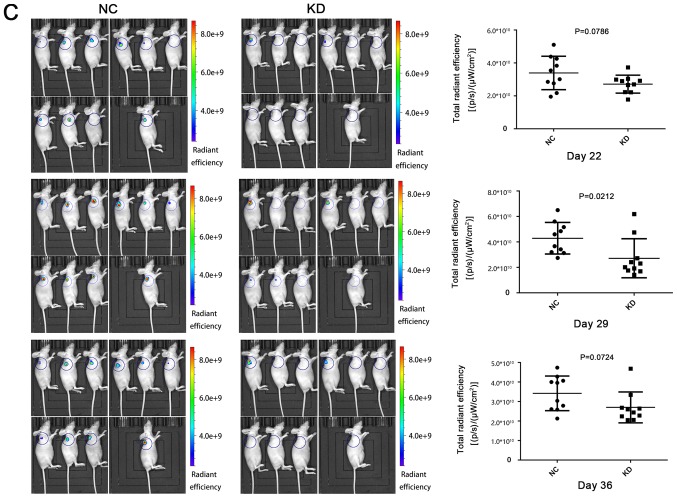
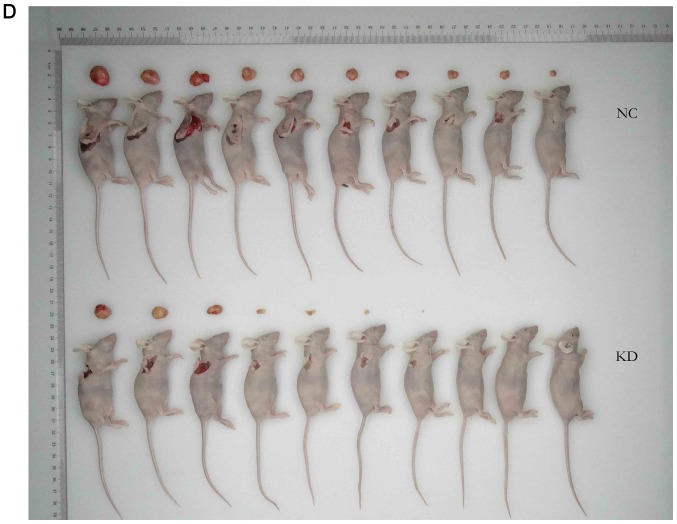
Additionally, the effect of CDCA4 knockdown on the regulation of breast cancer xenograft growth in vivo was assessed by injecting MDA-MB-231 cells into nude mice following stable infection with shCDCA4 or negative control shRNA. Tumor volume and weight were significantly smaller in the knockdown group compared with in the negative control group (Fig. 6A and B). In vivo small animal imaging data also demonstrated smaller mean values for the knockdown group, with the difference on days 29 being statistically significant (Fig. 6C). Tumors isolated from the nude mice were markedly smaller in the knockdown group (Fig. 6D). The results of the present study demonstrated that the knockdown of CDCA4 expression suppressed the growth of MDA-MB-231 cell xenografts in vivo.
Figure 6.
Effects of cell division cycle-associated protein 4 KD on the regulation of triple negative breast cancer cell xenograft growth in vivo. (A) Curves of tumor xenograft growth over time in nude mice. (B) Tumor xenograft weight. Differences in tumor xenograft weights between the NC and KD groups (P<0.05). (C) In vivo small animal imaging. Tumor cell xenografts were measured using fluorescence total radiant efficiency on days 22 (P>0.05), 29 (P<0.05) and 36 (P>0.05). (D) Tumor xenograft size. The xenografts were dissected from the subcutaneous tissues of the mice, and size was compared between the NC and KD groups. The data were expressed as the means ± standard deviation. KD, knockdown; NC, negative control.
Discussion
Due to a lack of treatment options, TNBC is a highly invasive and metastatic malignancy (4,20). The present study investigated the effects of CDCA4 knockdown on the regulation of TNBC cell growth, apoptosis and xenograft growth in vitro and in vivo. CDCA4 is a protein of the SEI family, which contains common protein features, including the cyclin A binding domain, C-terminal motif, SERTA domain and PHD-bromine binding domain. MERAV database (18) analysis revealed that CDCA4 was highly expressed in breast cancer tissue, indicating that CDCA4 may be closely associated with breast cancer development and progression. Our previous study demonstrated that the downregulation of CDCA4 expression significantly inhibited the proliferation of human breast cancer doxorubicin-resistant MCF-7/ADM cells in vitro (21). The present study revealed that knockdown of CDCA4 expression significantly reduced the growth of MDA-MB-231 cells and promoted their apoptosis in vitro. Additionally, knockdown of CDCA4 expression suppressed nude mouse MDA-MB-231 cell xenograft growth in vivo. In conclusion, the results of the present study supported the hypothesis that CDCA4 overexpression in breast cancer tissues and cells contributes to TNBC progression, and that targeting CDCA4 expression may be a novel strategy in the future control of TNBC.
Notably, shCDCA4 lentivirus was infected into two TNBC cell lines, MDA-MB-231 and MDA-MB-468. However, the silencing efficiency of shCDCA4 in MDA-MB-468 cells was unsatisfactory (knockdown ratio, 25.0% compared with shCtrl). Therefore, only MDA-MB-231 cells (knockdown ratio, 68.9%) were utilized in the present study. A possible reason for this difference may be the poor infection efficiency of the RNA interference sequence selected for the MDA-MB-468 cell line (20,22). In the present study, a straightforward study design was followed by assessing alterations in cell viability, proliferation and apoptosis in vitro, and tumor cell xenograft growth in a nude mouse model in vivo. The data indicated that the knockdown of CDCA4 expression inhibited the proliferation of TNBC cells in vitro and in vivo. These data were consistent with those of previous studies (23), including our previous study (21).
A previous study reported that CDCA4 is an E2F transcription factor-induced nuclear factor that regulates E2F-dependent transcription as an E2F-downstream gene (24). CDCA4 protein is expressed in different human cancer cell lines and induces progression of the G1/S phase of the cell cycle (24). MicroRNA-15a-induced inhibition of growth and invasiveness of malignant melanoma occurs by directly targeting CDCA4 expression (23). Furthermore, another previous study reported that the mRNA expression levels of CDCA2, CDCA3, CDCA4, CDCA5, CDCA7 and CDCA8 are significantly higher in clinical tumor samples and cancer cell lines compared with the control samples. Among them, the overexpression of CDCA3, CDCA5 and CDCA8 genes is negatively associated with the survival of patients with breast cancer (25). Although this previous study did not confirm the role of CDCA4 in breast cancer survival, there are a number of factors contributing to survival of patients with cancer. Therefore, joint survival analysis evaluating the co-expression of multiple genes for patients with breast cancer should be performed in future studies. Our recent study demonstrated that CDCA4 is a downstream gene of the nuclear factor erythroid 2 like 2 signaling pathway and that it upregulates the proliferation of breast cancer MCF7/ADM cells (21). The present study did not explore the underlying molecular events of CDCA4 action in TNBC cells due to limited funding and time.
In conclusion, the present study demonstrated that the downregulation of CDCA4 expression was able to inhibit the proliferation and promote the apoptosis of MDA-MB-231 cells in vitro, and the in vivo data supported the in vitro data, demonstrating that knockdown of CDCA4 expression suppressed the growth of MDA-MB-231 cell xenografts in vivo. Combined with our recent study, it has been demonstrated that CDCA4 expression was not only associated with breast cancer drug resistance but also promoted the growth of TNBC cells. Therefore, a future study will investigate whether targeting CDCA4 expression using shCDCA4 could be a novel strategy for treating TNBC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CDCA4
cell division cycle-associated protein 4
- Ctrl
control
- shRNA
short hairpin RNA
- TNBC
triple negative breast cancer
Funding
This study was supported in part by a grant from the National Natural Science Foundation of China (grant no. 81260341).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XW contributed to the study design and data analysis, and the review and revision of the manuscript. SP contributed to research design, data collection and analysis, and drafting and revision of the manuscript. YX and JC assisted with the statistical analysis and data interpretation. GL and JH participated in the revision of the manuscript and statistical analysis. All authors approved the final manuscript.
Ethics approval and consent to participate
All protocols were approved by the Ethics Review Committee of The First Affiliated Hospital of Guangxi Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137:183–192. doi: 10.1007/s00432-010-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ, Gradishar WJ, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 6.Rastelli F, Biancanelli S, Falzetta A, Martignetti A, Casi C, Bascioni R, Giustini L, Crispino S. Triple-negative breast cancer: Current state of the art. Tumori. 2010;96:875–888. doi: 10.1177/548.6505. [DOI] [PubMed] [Google Scholar]
- 7.Lee A, Djamgoz MBA. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110–122. doi: 10.1016/j.ctrv.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, Fralick M, Kumar R, Clemons M. Survival outcomes for patients with metastatic triple-negative breast cancer: Implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe-Fukunaga R, Iida S, Shimizu Y, Nagata S, Fukunaga R. SEI family of nuclear factors regulates p53-dependent transcriptional activation. Genes Cells. 2005;10:851–860. doi: 10.1111/j.1365-2443.2005.00881.x. [DOI] [PubMed] [Google Scholar]
- 10.Abdullah JM, Jing X, Spassov DS, Nachtman RG, Jurecic R. Cloning and characterization of Hepp, a novel gene expressed preferentially in hematopoietic progenitors and mature blood cells. Blood Cells Mol Dis. 2001;27:667–676. doi: 10.1006/bcmd.2001.0434. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SI, Yang CM, Sim KG, Hentschel DM, O'Leary E, Bonventre JV. TRIP-Br: A novel family of PHD zinc finger- and bromodomain-interacting proteins that regulate the transcriptional activity of E2F-1/DP-1. EMBO J. 2001;20:2273–2285. doi: 10.1093/emboj/20.9.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugimoto M, Nakamura T, Ohtani N, Hampson L, Hampson IN, Shimamoto A, Furuichi Y, Okumura K, Niwa S, Taya Y, Hara E. Regulation of CDK4 activity by a novel CDK4-binding protein, p34(SEI-1) Genes Dev. 1999;13:3027–3033. doi: 10.1101/gad.13.22.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calgaro S, Boube M, Cribbs DL, Bourbon HM. The Drosophila gene taranis encodes a novel trithorax group member potentially linked to the cell cycle regulatory apparatus. Genetics. 2002;160:547–560. doi: 10.1093/genetics/160.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheong JK, Gunaratnam L, Zang ZJ, Yang CM, Sun X, Nasr SL, Sim KG, Peh BK, Rashid SB, Bonventre JV, et al. TRIP-Br2 promotes oncogenesis in nude mice and is frequently overexpressed in multiple human tumors. J Transl Med. 2009;7:8. doi: 10.1186/1479-5876-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh JK, Yap D, O'Connor DJ, Fogal V, Fallis L, Chan F, Zhong S, Lu X. Novel function of the cyclin A binding site of E2F in regulating p53-induced apoptosis in response to DNA damage. Mol Cell Biol. 2002;22:78–93. doi: 10.1128/MCB.22.1.78-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tategu M, Nakagawa H, Hayashi R, Yoshida K. Transcriptional co-factor CDCA4 participates in the regulation of JUN oncogene expression. Biochimie. 2008;90:1515–1522. doi: 10.1016/j.biochi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zhu G, Yang D, Li Q, Li Y, Xu X, He D, Zeng C. The spindle function of CDCA4. Cell Motil Cytoskeleton. 2008;65:581–593. doi: 10.1002/cm.20286. [DOI] [PubMed] [Google Scholar]
- 18.Shaul YD, Yuan B, Thiru P, Nutter-Upham A, McCallum S, Lanzkron C, Bell GW, Sabatini DM. MERAV: A tool for comparing gene expression across human tissues and cell types. Nucleic Acids Res. 2016;44:D560–D566. doi: 10.1093/nar/gkv1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Lenz G. The RNA interference revolution. Braz J Med Biol Res. 2005;38:1749–1757. doi: 10.1590/S0100-879X2005001200003. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Wu X, Li F, Huang D, Zhu W. CDCA4, a downstream gene of the Nrf2 signaling pathway, regulates cell proliferation and apoptosis in the MCF7/ADM human breast cancer cell line. Mol Med Rep. 2018;17:1507–1512. doi: 10.3892/mmr.2017.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima S, Borisy GG. An image-based, dual fluorescence reporter assay to evaluate the efficacy of shRNA for gene silencing at the single-cell level. F1000Res. 2014;3:60. doi: 10.12688/f1000research.3-60.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alderman C, Sehlaoui A, Xiao Z, Yang Y. MicroRNA-15a inhibits the growth and invasiveness of malignant melanoma and directly targets on CDCA4 gene. Tumour Biol. 2016;37:13941–13950. doi: 10.1007/s13277-016-5271-z. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi R, Goto Y, Ikeda R, Yokoyama KK, Yoshida K. CDCA4 is an E2F transcription factor family-induced nuclear factor that regulates E2F-dependent transcriptional activation and cell proliferation. J Biol Chem. 2006;281:35633–35648. doi: 10.1074/jbc.M603800200. [DOI] [PubMed] [Google Scholar]
- 25.Phan NN, Wang CY, Li KL, Chen CF, Chiao CC, Yu HG, Huang PL, Lin YC. Distinct expression of CDCA3, CDCA5, and CDCA8 leads to shorter relapse free survival in breast cancer patient. Oncotarget. 2018;9:6977–6992. doi: 10.18632/oncotarget.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.