Abstract
Polydatin, extracted from Polygonum cuspidatum, is known for its anti-platelet aggregation and anti-inflammatory effects. However, studies on the association of polydatin with cancer are limited, particularly with regards to epithelial-mesenchymal transition (EMT)-associated migration and invasion of cancer cells. The purpose of the present study was to reveal the potential anticancer effects of polydatin on hepatocellular carcinoma (HCC) cells, particularly its effects on EMT. MTT assay was used to determine cell viability. Migration and invasion were evaluated through wound healing and transwell assays. Colony formation efficiency assay was conducted to detect proliferation. Flow cytometric analyses of apoptosis and cell cycle progression were performed following cells staining with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) and PI alone, respectively. Western blotting was used to investigate relevant molecular mechanisms. The results indicated that polydatin inhibited proliferation via G2/M arrest, suppressed migration and invasion of HCC cells, and promoted their apoptosis. In addition, phosphorylated (p)-protein kinase B (AKT), p-Janus kinase 1 and p-signal transducer and activator of transcription 3 (STAT3) levels were decreased as polydatin concentrations increased, and forkhead box protein O1 (FOXO1) expression was upregulated. Furthermore, the expression levels of various markers of EMT were reversed following treatment with polydatin. In conclusion, the present study validated that polydatin may inhibit proliferation via G2/M arrest, and suppressed EMT-associated migration and invasion of HCC cells. The results also suggested that polydatin may promote HCC cell apoptosis by blocking the AKT/STAT3-FOXO1 signaling pathway.
Keywords: polydatin, hepatocellular carcinoma, protein kinase B/signal transducer and activator of transcription 3-forkhead box protein O1, epithelial-mesenchymal transition, migration and invasion
Introduction
Hepatocellular carcinoma (HCC) is a common primary liver malignancy, which represents the fifth and seventh most common type of cancer worldwide in men and women, respectively (1). The malignancy of HCC is attributed to its fast progression, the rapid development of metastasis and the absence of efficient curative therapy, all leading to an unfavorable prognosis (2). Treatments available for HCC include surgical resection, regional ablation and liver transplantation; however, these options are not proposed to patients diagnosed at an advanced stage. Treatment with near infrared-induced hyperthermia exerts promising inhibitory effects on HCC, although the poor penetration of the light restricts its application (3). In addition, numerous patients with HCC present with tolerance to sorafenib, which is a common United States Food and Drug Administration-approved chemotherapeutic drug for patients at the advanced stage (4). Additionally, some novel tyrosine kinase inhibitors, including lenvatinib, cabozantinib and regorafenib, have demonstrated promising therapeutic effects in recent clinical trials. However, the efficacy, side effects and toxicity associated with these treatments still require further study (5–7). It is therefore crucial to explore novel anti-HCC drugs and therapeutic approaches.
Activation of epithelial-mesenchymal transition (EMT) is characterized by the progression of an epithelial phenotype to a mesenchymal phenotype. It is a crucial event for tumor metastasis, particularly in the early stages of disease (8). In addition, numerous EMT-related genes are associated with metastasis and the recurrence of cancer (9–11). In HCC, EMT is regulated by various oncogenes and tumor suppressor genes, and stimulates venous invasion and metastasis, leading to poor prognosis (12). Forkhead box O1 (FOXO1) belongs to the forkhead family of transcription factors characterized by a distinct forkhead domain. FOXO1 is considered a tumor suppressor gene that is regulated by the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and Janus kinase 1 (JAK1)/signal transducer and activator of transcription 3 (STAT3) signaling pathways, forming the AKT/STAT3-FOXO1 signaling pathway (13,14). Our previous studies have revealed that FOXO1 inhibits the invasion and metastasis of HCC by reversing zinc finger E-box-binding homeobox 2-induced EMT (15). Furthermore, various overexpressed oncogenes in HCC, including Epidermal growth factor receptor kinase substrate 8-like protein 3 and zinc finger and BTB domain-containing 20, are able to promote proliferation and inhibit apoptosis of HCC by repressing FOXO1, suggesting that FOXO1 may inhibit proliferation and stimulate apoptosis (16,17). These findings suggested that the AKT/STAT3-FOXO1 signaling pathway may participate in several processes involved in the progression of HCC, including proliferation, apoptosis, and EMT-associated migration and invasion.
Polydatin, also named pieceid, (E)-piceid, (E)-polydatin, trans-polydatin and 3,40,5-trihydroxystilbene-3-b-D-glucoside, is a monocrystalline compound originally extracted from the root and rhizome of Polygonum cuspidatum (Fig. 1). Polydatin is also detectable in grapes, peanuts, hop cones, red wine, hop pellets, cocoa-containing products and chocolate. Previous studies have demonstrated that polydatin has many biological functions, including the prevention of platelet aggregation (18), and cardioprotective (19) and anti-inflammatory (20) properties. In addition, polydatin stimulates apoptosis and cell cycle arrest in lung and colorectal cancers (21,22). However, the effects of polydatin on HCC are currently unknown.
Figure 1.
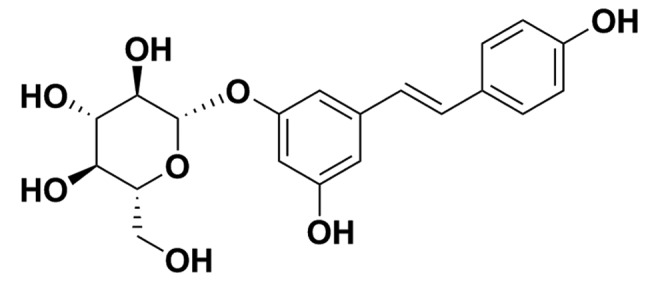
Chemical structure of polydatin.
To the best of our knowledge, the present study suggested for the first time that polydatin may inhibit EMT-related migration and invasion, and proliferation (via G2/M arrest) of HCC cells, and may promote HCC cell apoptosis by limiting the AKT/STAT3-FOXO1 signaling pathway.
Materials and methods
Cells and reagents
The HCCLM3 human HCC cell line and LO2 normal hepatic cell line were purchased from the American Type Culture Collection (Manassas, VA, USA), and were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 100 IU/ml penicillin and 100 µg/ml streptomycin, which were all obtained from Gibco, Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Cells were incubated at 37°C in a humidified incubator containing 5% CO2. Polydatin (cat. no. P109977) was purchased from Aladdin Industrial Corporation (Shanghai, China). A stock solution of 350 mmol/l polydatin was prepared in dimethyl sulfoxide (DMSO) and freshly diluted in medium prior to experiments. MTT assay (cat. no. M2128) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and fluorescein isothiocyanate (FITC)-conjugated Annexin V/propidium iodide (PI) apoptosis detection kit was provided by 4A Beijing Biotech Co., Ltd. (Beijing, China). Primary and secondary antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The Bio-Rad protein assay kit II was supplied by Bio-Rad Laboratories, Inc. (Hercules, CA, USA) and the enhanced chemiluminescent western blot detection reagents (cat. no. RPN2106) were obtained from GE Healthcare (Chicago, IL, USA).
Cytotoxicity assay
The cytotoxicity of polydatin was measured using the MTT assay. Cells were seeded in 96-well plates at 4×103 cells/well for 24 h. HCCLM3 and LO2 cells were treated with increasing doses of polydatin (0–800 µmol/l) for various durations (24–72 h). MTT solution (5 mg/ml in DMEM medium) was added (20 µl/well) and plates were further incubated for 4 h at 37°C. A volume of 100 µl DMSO was added to each well to solubilize the formazan product prior to measuring the absorbance with a microplate reader at 490 nm. The assays were performed three times.
Colony formation efficiency assay
HCCLM3 cells were seeded in 6-well plates at a density of 1×103 cells/well for 24 h. Culture medium was replaced with DMEM containing different doses of polydatin (0–800 µmol/l) and cells were incubated for 24 h at 37°C. The supernatant was replaced with 2 ml regular DMEM containing 10% FBS, and cells were cultured for 2 weeks at 37°C until visible cell clones were formed. Once colonies were formed, cells were fixed with 4% paraformaldehyde (PFA) for 25 min and washed three times with PBS at room temperature. Cells were stained with crystal violet for 25 min and rinsed three times with PBS at room temperature. Colonies were counted in a double blind manner using a light microscope (Shanghai CSOIF Co., Ltd., Shanghai, China). Results were presented as the percentage of colony numbers (average colony numbers of each group compared with control), and the assays were replicated three times.
Wound healing assay
HCCLM3 cells were seeded into 6-well plates at 2.5×105 cells/well and cultured at 37°C until a monolayer was formed. Cells were scratched with a sterile micropipette tip and treated with 20 µg/ml mitomycin (Aladdin Industrial Corporation) for 20 min. Cells were washed with PBS to remove debris, and further cultivated with serum-free medium containing different doses of polydatin (0–150 µmol/l) for 24 and 48 h. The migration distance was measured and analyzed by Image J v1.8.0 (National Institutes of Health, Bethesda, MD, USA), and the assays were repeated three times.
Migration and invasion assays
Transwell chambers (pore size, 8 µm) were used to detect the migration and invasion of HCCLM3 cells pretreated with polydatin (0–150 µmol/l) for 48 h at 37°C. For the migration assay, 5×104 cells were seeded in serum-free DMEM (200 µl) into the upper chambers and 500 µl DMEM containing 10% FBS was added to the lower chamber. For the invasion assay, the upper polycarbonate membranes of the chambers were coated with 5 mg/ml Matrigel. Following a 48 h incubation, cells were fixed with 4% PFA for 20 min and stained with crystal violet for 20 min. Cells in three randomly chosen fields were assessed under a light microscope (Shanghai CSOIF Co., Ltd). Each assay was performed three times.
Analysis of apoptosis
Annexin V-FITC/PI double staining was conducted to examine the apoptosis of HCCLM3 cells following treatment with polydatin. Briefly, cells were seeded in 6-well plates at a density of 2.5×105 cells/well and were cultured overnight to allow adhesion. Following treatment with increasing doses of polydatin (0–800 µmol/l) for 48 h at 37°C, cells were stained with Annexin V-FITC/PI for 15 min in the dark, according to the manufacturer's protocol. Apoptotic cells were detected by flow cytometry using the FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The assays were carried out three times.
Cell cycle analysis
HCCLM3 cells were seeded in 6-well plates (2.5×105/well). At 24 h, cells were treated with 200 µmol/l polydatin for an additional 48 h at 37°C. Following trypsinization, cells were washed with PBS and fixed with 75% ethanol for 4 h at 4°C. Cells were washed twice with PBS and covered with 0.5 ml PBS containing 20 µl RNaseA and PI for 30 min at 4°C. The cell cycle progression of HCC cells was detected using the FACSCalibur flow cytometer with an excitation wavelength of 488 nm, and emission wavelength of at 670 nm. Cell cycle progression was analyzed using ModFit LT 4.1 (Verity Software House, Topsham, ME, USA) by gating live cells.
Western blotting
HCCLM3 cells were seeded into 6-well plates at 2.5×105 cells/well and incubated at 37°C overnight. The supernatant was replaced by fresh medium containing increasing concentrations of polydatin (0–800 µmol/l) for 48 h at 37°C. Following treatment, cells were lysed with RIPA buffer (Aladdin Industrial Corporation) and subjected to protein extraction. Protein concentrations were determined using the Pierce Micro BCA protein assay system (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein (25 µg) from each sample were separated via SDS-PAGE on a 10% gel, and transferred to nitrocellulose membranes that were further blocked with 5% non-fat milk (diluted in TBST containing 0.1% Tween-20) for 1 h at room temperature. Target proteins were immunoblotted with the appropriate primary and horseradish peroxidase (HRP)-conjugated secondary antibodies, which were all diluted at 1:1,000. The following antibodies were purchased from Wuhan Sanyang Biotechnology (Wuhan, China): Cyclin B1 (cat. no. 55004-1-AP) Cyclin D1 (cat. no. 60186-1-Ig) Bax (cat. no. 60267-1-Ig) Bcl-2 (cat. no. 60178-1-Ig) β-actin (cat. no. 20536-1-Ig) Vimentin (cat. no. 60,330-1-Ig) N-Cadherin (cat. no. 66219-1-Ig) E-Cadherin (cat. no. 60335-1-Ig), p-AKT (cat. no. 66444-1-Ig), AKT (cat. no. 60203-2-Ig) and FOXO1 (cat. no. 66457-1-Ig). The following antibodies were obtained from Abcam (Shanghai, China): p-JAK1 (cat. no. ab138005), JAK1 (cat. no. ab47435), p-STAT3 (cat. no. ab76315), STAT3 (cat. no. ab119352), p-p38 (cat. no. ab4822) and p38 (cat. no. ab31828). Primary antibodies were incubated at room temperature for 2 h. Following washing the membranes with PBST containing 0.1% Tween-20 three times, the bound antibodies were then detected using the secondary goat anti-rabbit or goat anti-mouse antibodies for 1 h at room temperature and protein signals were visualized by enhanced chemiluminescence using ECL Western blotting detection reagents (Beyotime Institute of Biotechnology, Haimen, China) for 1 min and exposed to Kodak Biomax XAR film. The experiments were repeated three times.
Statistical analysis
All data were analyzed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Results were expressed as the mean ± standard deviation. One-way analysis of variance was used to compare two groups of data. Multiple comparisons between the groups were performed using the Student-Newman-Keuls method. P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxic effects of polydatin on HCCLM3 cells
The associations between drug cytotoxicity and dose/time-dependent drug efficacy were the first parameters considered. In addition, an absence of toxicity of chemotherapeutic drugs on human normal cells is essential when developing a novel treatment, particularly for liver cancer, since the liver is the major organ involved in drug metabolism.
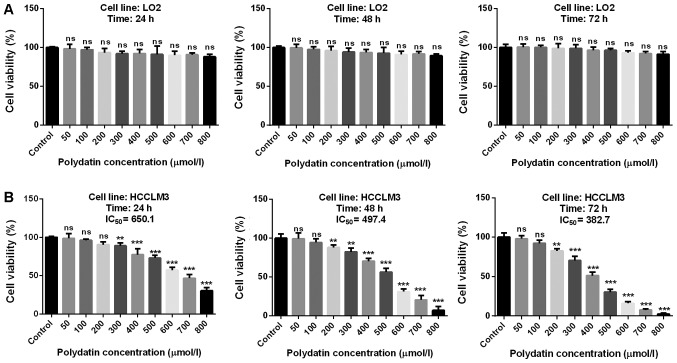
Results revealed that polydatin was not toxic for LO2 cells (Fig. 2A). Furthermore, polydatin was significantly toxic to HCCML3 cells, at concentrations of 300 and 200 µmol/l after 24 and 48 h, respectively. However, concentrations <200 µmol/l did not exhibit any cytotoxicity for all treatment durations. Furthermore, for concentrations >200 µmol/l, polydatin displayed toxicity in a dose-dependent manner. In combination with the drug half maximal inhibiting concentration illustrated in Fig. 2B, these results suggested that polydatin may effectively destroy HCC cells with low toxicity in healthy human hepatic cells.
Figure 2.
Effects of polydatin on cell viability of LO2 and HCCLM3 cells. Cytotoxicity of polydatin at various concentrations and treatment durations in (A) LO2 and (B) HCCLM3 cells. **P<0.01, ***P<0.001 vs. control. IC50, half maximal inhibitory concentration; ns, not significant.
Effects of polydatin on HCCLM3 cell migration
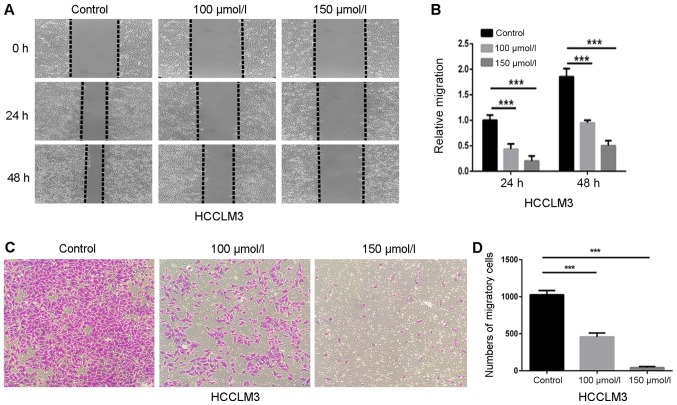
Wound healing and transwell chamber assays were performed to investigate the effects of polydatin on the migration of HCC cells. HCCLM3 cells were cultured with nontoxic doses of polydatin (0–150 µmol/l) to avoid interference with cell toxicity. Fig. 3A demonstrated that polydatin decreased the wound healing of HCCLM3 cells in a dose-dependent manner. In comparison with control cells, the relative migration level of HCCLM3 cells treated with 150 µmol/l polydatin was ~0.27-fold at 48 h (Fig. 3B). In addition, the migration assay demonstrated that migrated cells on the lower side of the membrane were reduced in a dose-dependent manner (Fig. 3C and D), which confirmed the results of the wound healing assay. These data suggested that polydatin may prevent HCCML3 migration in a dose-dependent manner.
Figure 3.
Polydatin inhibits migration of HCCLM3 cells. (A) Suppressive effects of polydatin on migration were assessed via wound healing assay (magnification, ×200). (B) Statistical analysis of the relative distances of cell migration (C) Migration assay of HCCLM3 cells following treatment with polydatin at non-cytotoxic concentrations (0–150 µmol/l) (magnification, ×200). (D) Statistical analysis of the number of cells crossing the membrane. ***P<0.001 vs. control.
Effects of polydatin on HCCLM3 cell invasion
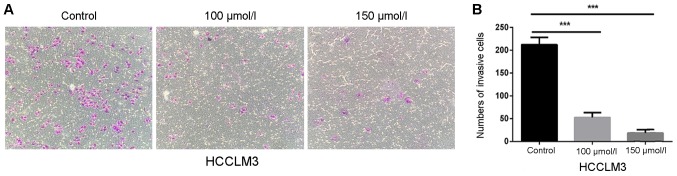
Compared with cell migration, which only involves mobility, cell invasion also involves the ability of cells to degrade the extracellular matrix. Therefore, Transwell chambers covered with Matrigel were used to detect the effects of polydatin on HCC cell invasion. Fig. 4A and B revealed that the number of invasive cells was significantly decreased following polydatin treatment in a dose-dependent manner. In comparison with control cells, the relative invasion of HCCLM3 cells treated with 150 µmol/l polydatin was ~0.09-fold. These findings suggested that polydatin may inhibit the invasive ability of HCCLM3 cells.
Figure 4.
Polydatin suppresses invasion of HCCLM3 cells. (A) Invasion assays of HCCLM3 cells following treatment with polydatin at non-cytotoxic concentrations (0–150 µmol/l) (magnification, ×200). (B) Statistical analysis of number of cells crossing the membrane ***P<0.001 vs. control.
Polydatin inhibits EMT in HCCLM3 cells
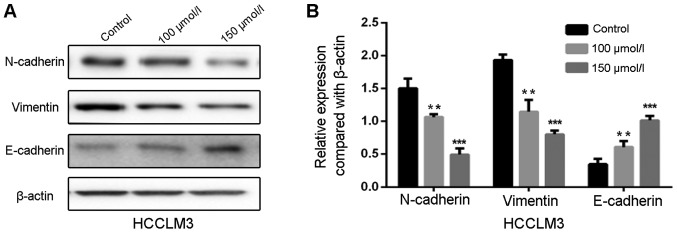
EMT is a crucial process for migration and invasion of cancer cells. In the present study, polydatin was suspected to suppress migration and invasion of HCCLM3 cells by inhibiting EMT. To further investigate the effects of polydatin on EMT, western blotting was used to detect the EMT-associated markers in HCCLM3 cells following treatment with polydatin for 48 h. The results suggested that the expression levels of the mesenchymal markers N-cadherin and vimentin were decreased by polydatin in a dose-dependent manner, whereas the expression levels of the epithelial marker E-cadherin were increased (Fig. 5A and B). These findings suggested that polydatin may prevent migration and invasion of HCCLM3 cells by reversing the EMT process.
Figure 5.
Polydatin represses epithelial-mesenchymal transition in HCCLM3 cells. (A) Protein expression of E-cadherin, N-cadherin and vimentin in HCCML3 cells following treatment with polydatin at non-toxic concentrations (0–150 µmol/l) evaluated by western blotting. (B) Statistical analysis of protein expression. **P<0.01, ***P<0.001 vs. control.
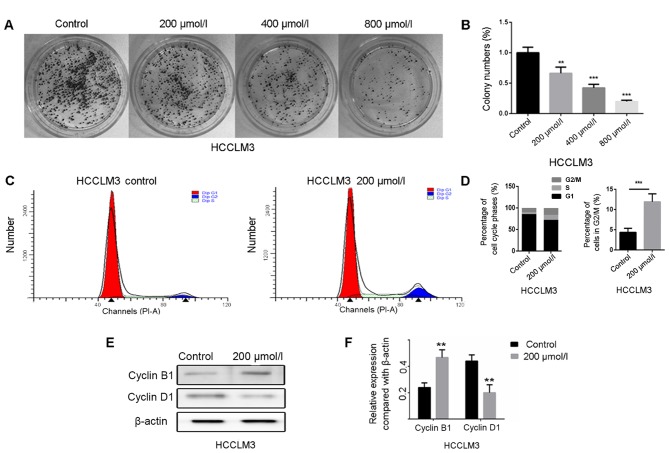
Anti-proliferative effects of polydatin on HCCLM3 cells
Proliferation is an essential process for tumorigenesis and development. In the present study, the colony formation assay was performed to evaluate the proliferative ability of HCCLM3 cells following treatment with polydatin. The results demonstrated that polydatin significantly inhibited HCCLM3 cell proliferation in a dose-dependent manner (Fig. 6A and B). It is generally accepted that cell proliferation is controlled by the cell cycle. The effect of polydatin on cell cycle progression was therefore assessed. As presented in Fig. 6C and D, polydatin arrested the HCCLM3 cell cycle at the G2/M phase. To further confirm this result, the expression levels of the proteins cyclin D1 and B1, which regulate the G0/G1 and G2/M phases, respectively, were analyzed by western blotting. The results revealed that the expression levels of cyclin B1 were markedly increased following polydatin treatment, whereas a decreased expression of cyclin D1 was observed (Fig. 6E and F). These results suggested that polydatin may arrest the cell cycle at the G2/M phase, combined with a significantly reduced proportion of HCCML3 cells in the G0/G1 phase. Taken together, these data suggested that polydatin may inhibit HCCLM3 cell proliferation by inducing cell cycle arrest at the G2/M phase.
Figure 6.
Polydatin inhibits proliferation of HCCLM3 cells. (A) Representative images of colony formation of HCCLM3 cells following treatment with polydatin at various concentrations. (B) Statistical analysis of the colony formation assay. (C) Cell cycle analysis results of HCCLM3 cells following treatment with or without 200 mol/l polydatin. (D) Statistical analysis of the percentage of cells in the G2/M phase. (E) Alterations in protein expression levels of cyclin B1 and cyclin D1 in HCCML3 cells, following treatment with or without polydatin (200 µmol/l) evaluated by western blotting. (F) Statistical analysis of protein expression. **P<0.01, ***P<0.001 vs. control.
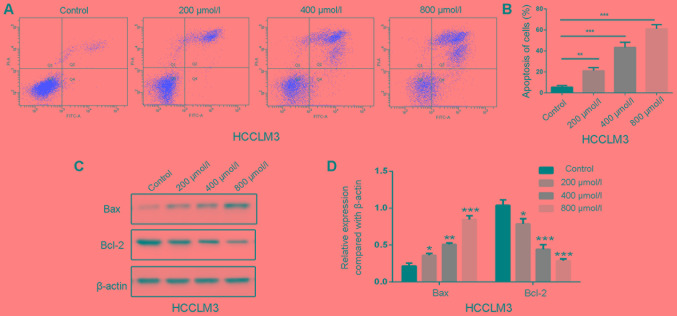
Polydatin induces apoptosis in HCCLM3 cells
Apoptosis is a primary type of cell death induced by chemotherapeutic drugs, and the effects of polydatin on apoptosis have been reported in human lung cancer cells (21). The effects of 48 h treatment of polydatin on HCCML3 cell apoptosis were therefore evaluated. As shown in Fig. 7A, polydatin induced HCCML3 cell apoptosis in a dose-dependent manner. At a concentration of 800 µmol/l, the percentage of apoptotic cells reached ~60% compared with untreated cells (Fig. 7B).
Figure 7.
Polydatin induces apoptosis of HCCLM3 cells. (A) Apoptosis assays of HCCLM3 cells following treatment with polydatin at various concentrations. (B) Statistical analysis of cell apoptosis. (C) Changes in the protein expression levels of Bax and Bcl-2 in HCCML3 cells following treatment with polydatin at different concentrations evaluated by western blotting. (D) Statistical analysis of protein expression. *P<0.05, **P<0.01, ***P<0.001 vs. control. Bax, Bcl-2-associated X; Bcl-2, B-cell lymphoma 2.
Apoptosis is mediated by death receptors, mitochondria and the endoplasmic reticulum (23). In the present study, the expression levels of the pro-apoptotic factor B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) increased following polydatin treatment, whereas the expression levels of the anti-apoptotic factor Bcl-2 were decreased (Fig. 7C and D). These results suggested that polydatin may induce HCCML3 cell apoptosis by regulating the expression of Bax and Bcl-2 in a dose-dependent manner.
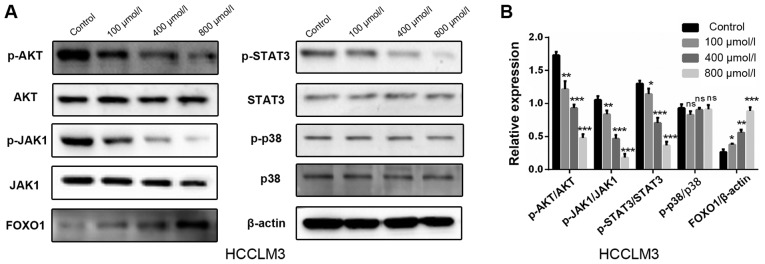
Polydatin inhibits HCC via the AKT/STAT3-FOXO1 signaling pathway
Results from the present study demonstrated that polydatin affected proliferation, apoptosis and EMT-associated migration and invasion in HCCLM3 cells. The possible underlying mechanism involved in these processes were therefore further investigated. Numerous signaling pathways, including PI3K/AKT, JAK1/STAT3 and p38, are known to serve important roles in the progression of HCC, including the EMT process (24–27). The effect of polydatin on these signaling pathways was thus evaluated. Following treatment with increasing doses of polydatin for 48 h, the expression levels of the central proteins involved in the aforementioned pathways were evaluated in HCCLM3 cells by western blotting. The following were detected: Activated AKT [phosphorylated (p)-AKT, Ser 473], AKT, activated JAK1 (p-JAK1, Tyr 1034/1035), JAK1, activated STAT3 (p-STAT3, Tyr 705), STAT3, activated p38 (p-p38, Thr 180/Tyr 182) and p38. The results demonstrated that polydatin induced a downregulation of p-AKT, p-JAK1 and p-STAT3 as compared with the control group; however, no significant changes were observed in p-p38 levels (Fig. 8A and B). These results suggested that polydatin may suppress HCC via the regulation of the PI3K/AKT and JAK1/STAT3 signaling pathways. As a downstream gene of these two signaling pathways, FOXO1 has garnered interest for its involvement in EMT, in addition to its well-known involvement in cell proliferation and apoptosis. The expression of FOXO1 was therefore evaluated. The results revealed that following treatment of HHCML3 cells with polydatin, the expression levels of FOXO1 were upregulated in a dose-dependent manner (Fig. 8A and B). These results suggested that polydatin may exert anti-HCC effects via the AKT/STAT3-FOXO1 signaling pathway.
Figure 8.
Polydatin inhibits hepatocellular carcinoma via the AKT/STAT3-FOXO1 signaling pathway. (A) Alterations in the expression levels of proteins involved in common signaling pathways in HCCML3 cells following treatment with polydatin at various concentrations, were evaluated by western blotting. (B) Statistical analysis of protein expression. All protein expression levels have been normalized to β-actin prior to further normalization with total protein level. *P<0.05, **P<0.01, ***P<0.001 vs. control. AKT, protein kinase B; FOXO1, forkhead box protein O1; ns, not significant; STAT3, signal transducer and activator of transcription 3.
Discussion
Natural herbal medicines have gained increasing attention in the development of chemotherapeutic regimens. Polydatin is a glycoside of resveratrol, in which the glycoside group is bound in the C-3 position, leading to alterations in its biological properties. Compared with resveratrol, polydatin is more efficiently absorbed by the digestive system via an active mechanism using glucose carriers (28–30). Polydatin is also more resistant to enzymatic oxidation. In addition, polydatin preserves the active groups of resveratrol, including the two benzene rings with a hydroxyl group and double bonds between the two rings, whose anticancerous properties have previously been demonstrated (31,32). Polydatin may therefore have some anticancer potential. To the best of our knowledge, studies investigating the anticancer potential of polydatin are lacking (21,22), with none suggesting an anti-EMT effect on HCC cells.
Tumor metastasis is a characteristic feature of malignant tumors, which is primarily responsible for cancer-associated mortality. Migration and invasion are essential conditions for tumor metastasis. In the present study, polydatin was demonstrated to inhibit the migration and invasion of HCCLM3 cells in a dose-dependent manner. In order to evaluate the anti-HCC effects of polydatin, numerous proliferation-associated experiments were performed, including colony formation efficiency, cell cycle analysis and apoptosis analysis. Results revealed that polydatin significantly inhibited proliferation and promoted apoptosis of HCCML3 cells in a dose-dependent manner.
Previous studies have revealed that EMT leads to increased cell migration and invasion in various types of cancer (33,34). Subsequently, the present study further explored the influences of polydatin on EMT. The results revealed that treatment with polydatin increased the expression levels of E-cadherin, and decreased expression levels of N-cadherin and vimentin. These results suggested that polydatin may promote EMT in HCCML3 cells in order to block their migration and invasion.
Three common signaling pathways have proven critical in the progression of HCC, including PI3K/AKT, JAK1/STAT3 and p38, and are closely associated with EMT in cancer metastasis (35). These pathways were therefore evaluated in the present study in HCCML3 cells following treatment with polydatin. The activation of AKT leads to the loss of tumor cell-cell junctions, disruption of tumor cell polarity and morphological alterations of tumor cells, thus stimulating tumor cell motility (36,37). Furthermore, activated AKT inhibits the transcription of E-cadherin (24). Similarly, activation of STAT3 increases the mesenchymal level in various types of cancer cells (25,26), whereas p38 participates in EMT in colorectal cancer progression (27). The results of the present study indicated that the levels of phosphorylated AKT, JAK1 and STAT3 were markedly decreased following treatment of HCCML3 cells with polydatin; however, no change was observed in p38 and p-p38 expression. The protein p38 is involved in oxidative stress regulation, suggesting that polydatin may not increase intracellular reactive oxygen species, which is consistent with the findings of previous studies (38,39). FOXO1 belongs to the forkhead family and serves numerous roles, including cellular differentiation, proliferation, cell cycle progression, apoptosis and glucose metabolism (40). Previous studies have demonstrated that the expression levels of FOXO1 are closely associated with the EMT process in HCC (15,41). By searching the Kyoto Encyclopedia of Genes and Genomes Pathway Database, FOXO1 was revealed to be a downstream gene of PI3K/AKT and JAK1/STAT3 signaling pathways (42,43). This suggested that polydatin may control cell proliferation (G2/M arrest) and EMT-associated migration and invasion, and promote apoptosis of HCC cells via FOXO1 regulation. The expression of FOXO1 was therefore assessed and was increased in HCCLM3 cells following polydatin treatment. These results suggested that polydatin may serve therapeutic roles via increasing FOXO1 expression, which was regulated by PI3K/AKT and JAK1/STAT3 signaling pathways.
In conclusion, the present study demonstrated that polydatin may inhibit proliferation via G2/M arrest, suppress migration and invasion of HCC cells and promote HCC cell apoptosis in a dose dependent-manner. Overall, polydatin exhibited some therapeutic potential for HCC, and induced no toxicity in normal human cells. The study suggested that polydatin may exert therapeutic effects by inhibiting the AKT/STAT3-FOXO1 signaling pathway, and reducing proliferation, apoptosis and EMT-related migration and invasion. These findings may provide a novel theoretical foundation for the development of polydatin-based chemotherapeutic treatment for HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grants no. 81771894) and the Fund of Scientific Research Innovation of The First Affiliated Hospital of Harbin Medical University (grant no. 2018B009).
Availability of data and materials
The datasets used and/or data analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JJ, YC, PL and XY conceived this study. JJ and YC designed the experiments. JJ, YC, TD, MY and YZ performed the experiments. TA and JZ conducted the statistical analysis. JJ and YC wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei KR, Yu X, Zheng RS, Peng XB, Zhang SW, Ji MF, Liang ZH, Ou ZX, Chen WQ. Incidence and mortality of liver cancer in China, 2010. Chin J Cancer. 2014;33:388–394. doi: 10.5732/cjc.014.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang CY, Chen YD, Guo W, Gao Y, Song CQ, Zhang Q, Zheng NN, Han XJ, Guo CS. Bismuth ferrite-based nanoplatform design: An ablation mechanism study of solid tumor and NIR-triggered photothermal/photodynamic combination cancer therapy. Adv Funct Mater. 2018:1706827. doi: 10.1002/adfm.201706827. [DOI] [Google Scholar]
- 4.Raza A, Sood GK. Hepatocellular carcinoma review: Current treatment, and evidencebased medicine. World J Gastroenterol. 2014;20:4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Park JW, Blanc JF, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36:207. doi: 10.1200/JCO.2018.36.15_suppl.4019. [DOI] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al. Lenvatinib versus sorafenib in frst-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 9.Yopp AC, Singal AG. Epithelial to mesenchymal transition expression profiles as predictive biomarkers of recurrence following resection of HCC: Implications for current clinical use and future stratification for systemic therapy. Ann Surg Oncol. 2014;21:3723–3724. doi: 10.1245/s10434-014-3790-7. [DOI] [PubMed] [Google Scholar]
- 10.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557–1566. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 11.Yamada S, Okumura N, Wei L, Fuchs BC, Fujii T, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y. Epithelial to mesenchymal transition is associated with shorter disease-free survival in hepatocellular carcinoma. Ann Surg Oncol. 2014;21:3882–3890. doi: 10.1245/s10434-014-3779-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang CH, Guo ZY, Chen ZT, Zhi XT, Li DK, Dong ZR, Chen ZQ, Hu SY, Li T. TMPRSS4 facilitates epithelial-mesenchymal transition of hepatocellular carcinoma and is a predictive marker for poor prognosis of patients after curative resection. Sci Rep. 2015;5:12366. doi: 10.1038/srep12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghazadeh S, Yazdanparast R. Mycophenolic acid potentiates HER2-overexpressing SKBR3 breast cancer cell line to induce apoptosis: Involvement of AKT/FOXO1 and JAK2/STAT3 pathways. Apoptosis. 2016;21:1302–1314. doi: 10.1007/s10495-016-1288-4. [DOI] [PubMed] [Google Scholar]
- 15.Dong T, Zhang Y, Chen Y, Liu P, An T, Zhang J, Yang H, Zhu W, Yang X. FOXO1 inhibits the invasion and metastasis of hepatocellular carcinoma by reversing ZEB2-induced epithelial-mesenchymal transition. Oncotarget. 2017;8:1703–1713. doi: 10.18632/oncotarget.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng CX, Tang LY, Xie CY, Li FX, Zhao JY, Jiang N, Tong Z, Fu SB, Wen FJ, Feng WS. Overexpression of EPS8L3 promotes cell proliferation by inhibiting the transactivity of FOXO1 in HCC. Neoplasma. 2018;65:701–707. doi: 10.4149/neo_2018_170725N503. [DOI] [PubMed] [Google Scholar]
- 17.Kan H, Huang Y, Li X, Liu D, Chen J, Shu M. Zinc finger protein ZBTB20 is an independent prognostic marker and promotes tumor growth of human hepatocellular carcinoma by repressing FoxO1. Oncotarget. 2016;7:14336–14349. doi: 10.18632/oncotarget.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu LT, Guo G, Wu M, Zhang WG. The progress of the research on cardio-vascular effects and acting mechanism of polydatin. Chin J Integr Med. 2012;18:714–719. doi: 10.1007/s11655-012-1060-8. [DOI] [PubMed] [Google Scholar]
- 19.Gao JP, Chen CX, Gu WL, Wu Q, Wang Y, Lü J. Effects of polydatin on attenuating ventricular remodeling in isoproterenol-induced mouse and pressure-overload rat models. Fitoterapia. 2010;81:953–960. doi: 10.1016/j.fitote.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Shi YW, Wang CP, Liu L, Liu YL, Wang X, Hong Y, Li Z, Kong LD. Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Mol Nutr Food Res. 2012;56:1433–1444. doi: 10.1002/mnfr.201100828. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhuang Z, Meng Q, Jiao Y, Xu J, Fan S. Polydatin inhibits growth of lung cancer cells by inducing apoptosis and causing cell cycle arrest. Oncol Lett. 2014;7:295–301. doi: 10.3892/ol.2013.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Maria S, Scognamiglio I, Lombardi A, Amodio N, Caraglia M, Cartenì M, Ravagnan G, Stiuso P. Polydatin, a natural precursor of resveratrol, induces cell cycle arrest and differentiation of human colorectal Caco-2 cell. J Transl Med. 2013;11:264. doi: 10.1186/1479-5876-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 25.Chen YD, Zhang Y, Dong TX, Xu YT, Zhang W, An TT, Liu PF, Yang XH. Hyperthermia with different temperatures inhibits proliferation and promotes apoptosis through the EGFR/STAT3 pathway in C6 rat glioma cells. Mol Med Rep. 2017;16:9401–9408. doi: 10.3892/mmr.2017.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luwor RB, Baradaran B, Taylor LE, Iaria J, Nheu TV, Amiry N, Hovens CM, Wang B, Kaye AH, Zhu HJ. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene. 2013;32:2433–2441. doi: 10.1038/onc.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Zhang L, Zhao L, Zhou R, Ding Y, Li G, Zhao L. LASP2 suppresses colorectal cancer progression through JNK/p38 MAPK pathway meditated epithelial-mesenchymal transition. Cell Commun Signal. 2017;15:21. doi: 10.1186/s12964-017-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 29.Paganga G, Rice-Evans CA. The identifcation of flavonoids as glycosides in human plasma. FEBS Lett. 1997;401:78–82. doi: 10.1016/S0014-5793(96)01442-1. [DOI] [PubMed] [Google Scholar]
- 30.Krasnow MN, Murphy TM. Polyphenol glucosylating activity in cell suspensions of grape (Vitis vinifera) J Agric Food Chem. 2004;52:3467–3472. doi: 10.1021/jf035234r. [DOI] [PubMed] [Google Scholar]
- 31.Delmas D, Aires V, Limagne E, Dutartre P, Mazué F, Ghiringhelli F, Latruffe N. Transport, stability, and biological activity of resveratrol. Ann N Y Acad Sci. 2011;1215:48–59. doi: 10.1111/j.1749-6632.2010.05871.x. [DOI] [PubMed] [Google Scholar]
- 32.Chalal M, Klinguer A, Echairi A, Meunier P, Vervandier-Fasseur D, Adrian M. Antimicrobial activity of resveratrol analogues. Molecules. 2014;19:7679–7688. doi: 10.3390/molecules19067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6:10697–10711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu CY, Lin CH, Jan YH, Su CY, Yao YC, Cheng HC, Hsu TI, Wang PS, Su WP, Yang CJ, et al. Huntingtin-interacting protein-1 is an early-stage prognostic biomarker of lung adenocarcinoma and suppresses metastasis via AKT-mediated epithelial-mesenchymal transition. Am J Respir Crit Care Med. 2016;193:869–880. doi: 10.1164/rccm.201412-2226OC. [DOI] [PubMed] [Google Scholar]
- 36.Attoub S, Arafat K, Hammadi NK, Mester J, Gaben AM. AKT2 knock-down reveals its contribution to human lung cancer cell proliferation, growth, motility, invasion and endothelial cell tube formation. Sci Rep. 2015;5:12759. doi: 10.1038/srep12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang Z, Lu W, Wang XA, Zhang F, Cao Y, et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett. 2016;375:179–189. doi: 10.1016/j.canlet.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Chang F, Li F, Fu H, Wang J, Zhang S, Zhao J, Yin D. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun. 2015;463:262–267. doi: 10.1016/j.bbrc.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 39.Ince S, Avdatek F, Demirel HH, Arslan-Acaroz D, Goksel E, Kucukkurt I. Ameliorative effect of polydatin on oxidative stress-mediated testicular damage by chronic arsenic exposure in rats. Andrologia. 2016;48:518–524. doi: 10.1111/and.12472. [DOI] [PubMed] [Google Scholar]
- 40.Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress, Exp. Diabetes Res. 2012;2012:939751. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du M, Wang Q, Li W, Ma X, Wu L, Guo F, Zhao S, Huang F, Wang H, Qin G. Overexpression of FOXO1 ameliorates the podocyte epithelial-mesenchymal transition induced by high glucose in vitro and in vivo. Biochem Biophys Res Commun. 2016;471:416–422. doi: 10.1016/j.bbrc.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 42.Duan S, Huang W, Liu X, Liu X, Chen N, Xu Q, Hu Y, Song W, Zhou J. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Cancer Res. 2017;37:304. doi: 10.1186/s13046-018-0980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang G, Huang C, Li J, Huang H, Jin H, Zhu J, Wu XR, Huang C. Role of STAT3 and FOXO1 in the divergent therapeutic responses of non-metastatic and metastatic bladder cancer cells to miR-145. Mol Cancer Ther. 2017;16:924–935. doi: 10.1158/1535-7163.MCT-16-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or data analyzed during the present study are available from the corresponding author on reasonable request.