Abstract
Background:
The aim of this study was to perform a meta-analysis of randomized controlled trials (RCTs) that evaluate the efficacy of low-intensity extracorporeal shock wave therapy (LiESWT) for the treatment of erectile dysfunction (ED).
Materials and methods:
A comprehensive search of PubMed, Medline, and Cochrane databases was performed from November 2005 to July 2018. RCTs evaluating efficacy of LiESWT in the treatment of ED were selected. The primary outcomes were the mean difference between treatment and sham patients in the International Index of Erectile Function-Erectile Function (IIEF-EF) domain score 1 month after treatment, and the mean change in IIEF-EF from baseline to 1 month post-treatment. The secondary analysis considered the percentage of men whose erectile hardness score (EHS) changed from <2 at baseline to >3 after treatment. All analyses used a random effects method to pool study-specific results.
Results:
A total of seven RCTs provided data for 607 patients. The mean IIEF-EF 1 month post-treatment ranged from 12.8 to 22.0 in the treatment group versus 8.17–16.43 in the sham group. The mean difference between the treatment and sham groups at the 1 month follow up was a statistically significant increase in IIEF-EF of 4.23 (p = 0.012). Overall, five of the seven trials provided data on the proportion of patients with baseline EHS <2 who improved to EHS >3 at 1 month post-treatment. The proportions ranged from 3.5 to 90% in the treatment group versus 0–9% in the sham group and the pooled relative risk of EHS improvement for the treated versus sham group was 6.63 (p = 0.0095). No significant adverse events were reported.
Conclusions:
This is the first meta-analysis that evaluates RCTs exploring LiESWT as a treatment modality strictly for ED. This therapeutic strategy appears to be well tolerated with short-term benefits. However further studies exploring specific treatment regimens and long-term outcomes are needed.
Keywords: erectile dysfunction, low-intensity extracorporeal shock wave therapy, randomized trials, IIEF
Introduction
Erectile dysfunction (ED) is the consistent or recurrent inability to attain or maintain a penile erection that is sufficient for sexual satisfaction, including satisfactory sexual performance.1 The prevalence of ED in the general population ranges from 30 to 65% in men aged 40–80 years.2 Current medical treatments, including phosphodiesterase type 5 (PDE5) inhibitors have variable efficacies and there remains an ongoing need for well-tolerated and clinically durable therapeutic options for treatment-refractory men.
Emerging evidence has suggested that low-intensity extracorporeal shockwave therapy (LiESWT) may offer benefit for patients with ED.3–6 Shockwave therapy (SWT) relies on external energy sources depositing pulses of energy into a fluid environment, then propagating the harnessed energy until it meets the target tissue where the energy is deployed.7 Though SWT has played an important role in the treatment of urolithiasis for decades, recent advancements have allowed for broader applications. In a low-intensity state, SWT appears to induce angiogenesis and improve perfusion in target tissues.8 Cardiologists have employed SWT for patients with refractory angina, while orthopedic surgeons and physical therapists have explored its role in tendinitis and nonhealing bone fractures.9–11 The precise mechanism of neoangiogenesis is not completely understood; however, it appears that there is a release of vascular endothelial growth factor (VEGF) and fibroblast growth factor in response to cell membrane microtrauma and mechanical stress.12 VEGF is an important mediator of neoangiogenesis and collateral blood flow formation, and an increased concentration of this cytokine has been demonstrated in multiple basic science studies.13,14 Clinically, increased penile angiogenesis should demonstrate an increase in penile blood flow and erectile function.15 SWT has also been shown to increase brain-derived neurotrophic factor expression through activation of PERK/ATF4 signaling pathway,16 which offers a putative mechanism for a neuronal regenerative effect and may implicate this treatment in cavernous nerve injury models of ED.
Though several studies have examined the use of LiESWT in ED, most lack a placebo control, and have a heterogeneous design and data analysis, which render rigorous interpretation difficult. A handful of meta-analyses have examined LiESWT for ED. However, their validity is limited by heterogenous data that included extracorporeal shock wave therapy for conditions such as pelvic pain, nonrandomized prospective studies without controls, and inconsistent outcome measures.3–6 The purpose of this meta-analysis is to systematically clarify the role of LiESWT as it specifically pertains to the treatment of ED based on the current evidence from randomized controlled trials (RCTs).
Material and methods
Search strategy and inclusion criteria
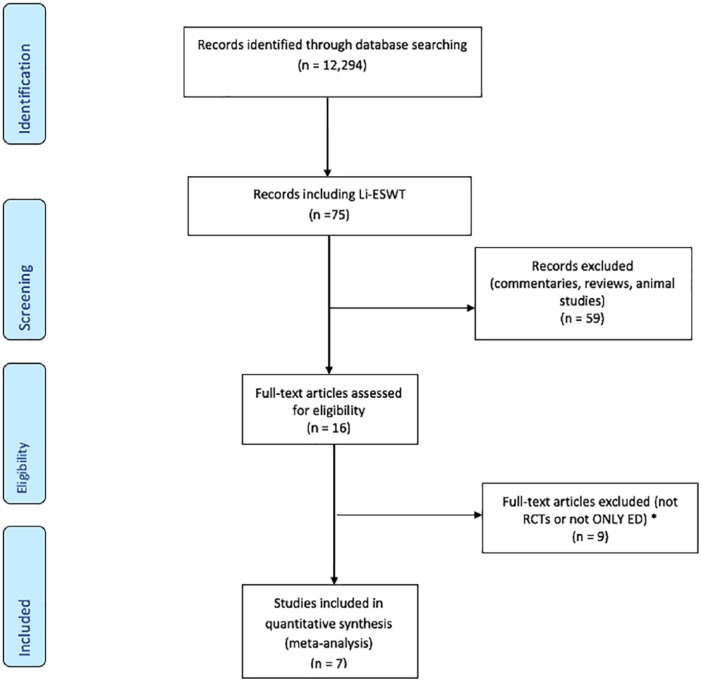
A systematic search of PubMed and Cochrane databases from November 2005 to May 2018 was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines17 using the search term ‘erectile dysfunction’. The search returned 12,294 articles. Animal studies were excluded. Figure 1 highlights our article evaluation process. A total of 16 articles met the criteria for further analysis. Exclusion criteria included: (1) non-English language articles, (2) articles on patients with Peyronie’s disease, and (3) nonrandomized studies. A total of seven RCTs were available for analysis. Overall, two investigators performed the searches and reviewed the studies (JDC, ARO or RAG). Discrepancies among the investigators were resolved by discussion.
Figure 1.
Preferred PRISMA flowchart depicting the search strategy and article selection process.
ED, erectile disfunction; LiESWT, low-intensity extracorporeal shock wave therapy; PRISMA, Reporting Items for Systematic Reviews and Meta-Analysis; RCT, randomized controlled trial.
Data extraction
Data were extracted from the seven available RCTs18–24 by two investigators (JDC and BJT). The accuracy of extracted data was cross checked and clarified with the manuscript authors as necessary. Extracted data from each study included age, treatment year, number of patients in each treatment arm, mean International Index of Erectile Function-Erectile Function (IIEF-EF) domain score at baseline and post-treatment and their respective standard deviation (SD), change in IIEF-EF post-treatment, and the proportion of patients increasing from erectile hardness score (EHS) ⩽2 at baseline to EHS ⩾3 at 1 month post-treatment. Table 1 describes data from each study used in the meta-analysis.
Table 1.
Characteristics and outcomes of seven randomized trials of LiESWT for treatment of erectile dysfunction.
| Study (country) |
Srini16
(India) |
Olsen17
(Denmark) |
Yee18
(China) |
Vardi19
(Israel) |
Kitrey20
(Israel) |
Kalyvianakis21
(Greece) |
Fojecki22
(Denmark) |
|---|---|---|---|---|---|---|---|
| Year(s) treated | 2009–2011 | 2012–2013 | 2011–2012 | 2009–2010 | ND | ND | 2014 |
| Age [median range; mean ± SD] | |||||||
| sham | ND | 60 (37–79) | 63.3 ± 6.4 | 57 (35–77) | 64 (29–81) | 55.1 (38–72) | 63.3 ± 9.5 |
| treatment | ND | 59 (41–80) | 58.9 ± 7.6 | 58 (27–72) | 60 (28–78) | 53.0 (31–72) | 65.4 ± 7.9 |
| Treatment regimen | 12 total tx with LiESWT or sham; 1, 3, 6, 9, 12 months post-tx measures | 5 weeks sham or LiESWT; 5, 12, 24-wk post-tx measures | 12 total tx LiESWT or sham; 1-month post-tx measures | 12 total tx with LiESWT or sham; 1 and 3-month post-tx measures | 12 total tx with either LiESWT or sham; 1, 6, 12, 18, 24-month post-tx measures | 12 total tx with LiESWT or sham; 1,3,6,9, 12 months post-tx measures | 10 total tx with sham or LiESWT; 1 month post-tx measure |
| # randomized | |||||||
| sham | 40 | 54 | 34 | 21 | 18 | 16 | 63 |
| treatment | 95 | 51 | 36 | 46 | 40 | 30 | 63 |
| # completed trial | |||||||
| sham | 17 | 54 | 28 | 20 | 18 | 16 | 60 |
| treatment | 60 | 51 | 30 | 40 | 37 | 30 | 58 |
| Baseline IIEF-EF | |||||||
| sham | 9.2 (SD 3.6*) | ND | 10.2 SD 3.8 | 11.5 ± 0.86 | 8.0 (SD 3.215) †† | 14.6 SD 3.4 | 11.5 SD 6.6 |
| treatment | 9.5 (SD 3.6*) | ND | 10.2 SD 3.8 | 12.6 ± 0.75 | 7.67 (SD 3.072) †† | 13.8 SD 3.6 | 10.9 SD 7.1 |
| Follow-up IIEF-EF | |||||||
| sham | 10.6 (SD 4.3*) | ND | 15.8 SD 6.1 | 14.5 ± 0.86† | 8.17 (SD 3.215) †† | 16.43 SD 3.5 | 13.0 SD 7.9 |
| treatment | 22.0 (SD 4.3*) | ND | 17.8 SD 4.8 | 19.3 ± 0.75† | 13.33 (SD 6.933) †† | 18.46 SD 3.6 | 12.8 SD 7.8## |
| Change in IIEF-EF | |||||||
| sham | 1.4 (SD 3.7*) | ND | 3.8 SD 3.6 | 3.0 ± 1.4 | 0.08 (SD 1.81) †† | 1.83 SD 3.45††† | 1.5 SD 7.3††† |
| treatment | 12.5 (SD 4.3*) | ND | 5.3 SD 5.5 | 6.7 ± 0.9 | 4.83 (SD 7.32) †† | 4.66 SD 3.6††† | 2.2 SD 7.5††† |
| % IIEF-EF 5-point improvement | |||||||
| sham | ND | 37.1 | ND | 20 | 0 | 12.5# | 38.3 |
| treatment | ND | 43.2 | ND | 65 | 40.5 | 56.7# | 37.9 |
| % increasing EHS from ⩽2 to ⩾3 | |||||||
| sham | 0 | 9 | ND | 0 (n = 12) | 0 | ND | 6.7### |
| treatment | 90 | 57 | ND | 68.0 (n = 28) | 54.1 | ND | 3.5### |
CI, confidence interval; EHS, erection hardness scale; IIEF-EF, International Index of Erectile Function-Erectile Function domain; IQR, interquartile range; LiESWT, low-intensity extracorporeal shockwave therapy; ND, not described; SD, standard deviation; SEM, standard error of the mean; tx, treatment.
SD estimated using the relationship of IIEF-EF score per measured unit length (mm) on the y-axis to scale the length of the SD bars in Figure 2(b) (baseline and follow-up IIEF-EF), or Figure 2(a) (change in IIEF-EF).
SEM not described, assume values are the same as for baseline based on similar error bars in Figure 4.
mean and SD estimated from median and IQR using the method of Wan and colleagues6
SD of difference calculated with means and SDs from baseline and follow up for each treatment group
Percentage achieving ‘minimal clinically important difference’ with the size of difference not specified
Incorrect IIEF-EF score and 95% CI in published paper. First author provided corrected score and SD in email.
Results concerning the change in EHS were only available on a subset (n = 83), but the number within each group was not provided. We assumed that the ratio of sample sizes of sham:treatment for this endpoint would be the same as the corresponding ratio in all patients who completed the trial, (60:58 = 1.034). We estimated the number in the treatment group (nT) and sham group (np) by solving the following system of equations:
This gave an estimate of nT = 41, so np was estimated as 83 – 41 = 42.
Risk of bias assessment
A risk of bias assessment was performed using the tool for RCTs developed by the Cochrane Collaboration. This tool evaluates the potential risk of bias as low, unclear, or high in each of seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias, that is, sources of potential bias not addressed by the other six categories.25
Statistical analysis
Analysis was performed on outcomes available from at least five trials. The primary analysis focused on the IIEF-EF, pooling estimates of the mean scores at the 1 month post-treatment measurement, and also pooling the change from baseline to 1 month post-treatment. The secondary analysis considered the percentage of men whose EHS changed from ⩽2 at baseline to ⩾3 at the post-treatment measure, and the percentage of men with at least a 5 point improvement on the IIEF-EF. Several of the studies lacked a number of details of the data so a number of assumptions were required. These are indicated by footnotes to Table 1, and include the following:
Not providing standard error of the mean (SEM) for follow-up IIEF-EF scores.21 For that study we assumed that the SEM values at follow up were the same as those at baseline, based on similar length of error bars in Figure 4 from the publication.
Standard deviations (SDs) for baseline, follow up, and change in IIEF-EF were not described.18 For that study SD values were estimated using the relationship of IIEF-EF score per measured unit length (mm) on the y-axis to scale the length of the SD bars in Figure 2(a and b) from the publication.
Means and SDs for baseline, follow up, and change in IIEF-EF in Kitrey and colleagues22 were estimated from median and interquartile range, respectively using the method described by Wan and colleagues.26
The SD of the difference between baseline and follow-up measures was not provided,23,24 so it was calculated based on the means and SDs at baseline and follow up using established formulae for the SD of a difference.27
Results concerning the change in EHS in Fojecki and colleagues24 were only available on a subset (n = 83), but the number within each treatment group was not provided. We assumed that the ratio of sample sizes of sham:treatment for this endpoint would be the same as the corresponding ratio in all patients who completed this trial, (60:58 = 1.034). We estimated the number in the treatment group (nT) and sham group (np) by solving the following simple system of equations:
Figure 4.
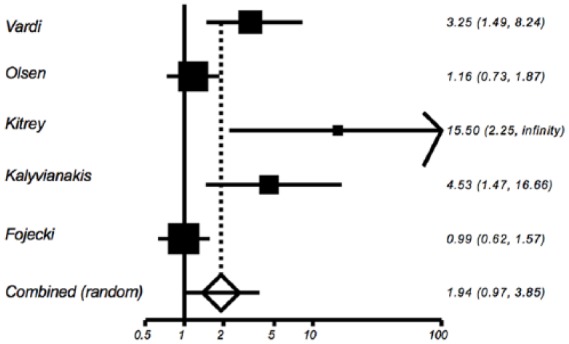
Relative risk of treatment versus placebo for percentage with International Index of Erectile Function-Erectile Function domain score increase of 5 points or greater at post-treatment follow up.
Figure 2.
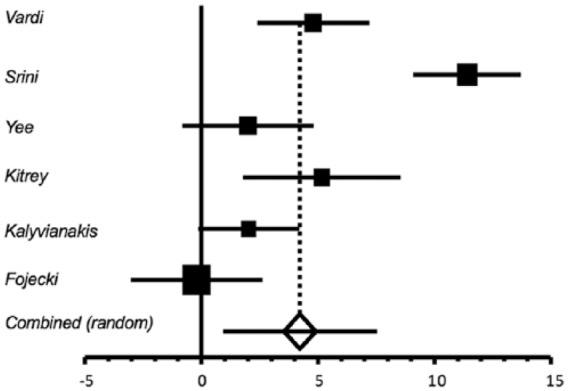
Difference in International Index of Erectile Function-Erectile Function domain score at 1 month post-treatment follow up between treated versus placebo patients.
In addition, some of the means and SDs for one study were incorrect,24 and corrected numbers were obtained by contacting the first author.
These assumptions were evaluated in sensitivity analyses. Analyses of pooled differences in means were performed using the method of Sutton28 as implemented in NCSS 2007 (NCSS Software, Kaysville, UT, USA), and pooled relative risks were performed using Mantel-Haenszel methods29,30 as implemented in StatsDirect version 2.8.0 (StatsDirect, Cheshire, UK); all pooled estimates were derived using a random effects model.31
Results
The seven RCTs in this analysis are summarized in Table 1.18–24 The studies encompassed patients from five different countries, including Israel, India, China, Denmark, and Greece. Mean ages ranged from 57–65 years across the seven trials. There was a total of 607 patients randomized, 519 (86%) of whom completed the trial. Treatment time periods were similar across studies, ranging from 2009 to 2014. All trials enrolled men with history of organic ED for at least 6 months and excluded men with ED associated with prostate surgery, pelvic radiation, penile abnormalities, and hormonal or neurological conditions.
A total of five trials limited eligibility to men who had previously responded to phosphodiesterase type-5 inhibitors (PDE5is).18,19,21–23 Only two trials stated that all men had baseline EHS ⩽2.19,22 A total of five studies required a PDE5i ‘washout’ period of 4 weeks,18,19,21,23,24 one study required 2 weeks,20 and one study used a 4 week run-in period with PDE5i therapy and kept patients on this medication to evaluate whether there was an improvement in response.22
In five studies, LiESWT was delivered by a focused electrohydraulic unit (Omnispec ED1000, Medispec, Germantown, MD, USA) to either three23 or five18,20–22 locations on the penis (distal, mid, proximal, left and right crura) for a total of 1500 shocks per session (energy density 0.09 mJ/mm2, frequency 120/min). One study delivered LiESWT using an electromagnetic unit (Duolith SD1, Storz, Tagerwilen, Switzerland) to six locations on the penis (distal, center, and proximal part of each corpus cavernosum); settings were 0.15 mJ/mm2, 25 Hz, 3000 total impulses and total energy of 12.8 J per treatment.19 The final study used a piezoelectric linear therapy source (FBL10, Richard-Wolf GmbH, Knitlingen, Germany) to deliver a total of 600 shocks per session to three locations (shaft, bilateral crus).24
A total of five trials used the same treatment protocol, which was outlined as the treatment and placebo groups receiving actual or sham treatment twice a week for 3 weeks, then had a 3 week period without treatment, followed by a second 3-week period with twice-weekly treatment. Primary outcome measures were made 1 month after the end of the 9-week treatment period.18,20–23 Overall, one trial provided patients with two rounds of 5-weekly treatment sessions with a 4-week interval and measured primary outcomes at week 9 (4 weeks after final treatment).24 The seventh trial administered actual or sham treatment over a 5-week period, but did not describe the treatment frequency and measured their primary outcomes at the end of the 5-week period.19
All studies evaluated adverse events.
IIEF-EF outcomes
A total of six of the seven trials provided data on the mean IIEF-EF at 1 month post-treatment follow up, and also on the change from baseline at 1 month follow up.18,20–24 Table 1 shows that mean IIEF-EF 1 month post-treatment ranged from 12.8 to 22.0 in the treatment group versus 8.17–16.43 in the sham group. The mean difference between treatment and sham at 1 month follow up is a statistically significant increase in IIEF-EF of 4.23 [95% confidence interval (CI): 0.94, 7.53], p = 0.012 (Figure 2). There was significant heterogeneity among the six trials, Cochran’s Q = 53.06, p < 0.0001, I2 = 90.6%. The heterogeneity reflects variation in magnitude of the difference between treatment and sham, but not in the direction of difference, that is, IIEF-EF was greater in the treated versus sham patients in five of the six studies.18,20–23 Owing to the several assumptions that needed to be made to estimate the SDs for three of the trials,18,21,22 sensitivity analyses were performed that increased by 50% or decreased by 50% the SDs for these trials. This produced only minimal changes in the pooled estimate of change from baseline, and the results remained statistically significant (data not shown).
The mean change from baseline to 1 month post-treatment ranged from 2.2 to 12.5 in the treatment group versus 0.08 to 3.8 in the sham group (Table 1). The mean difference between treatment and sham groups in the change from baseline to 1 month post-treatment follow up was statistically significant, mean change in IIEF-EF = 4.13 (95% CI: 0.80, 7.47), p = 0.015 (Figure 3). There was significant heterogeneity among the six trials, Cochran’s Q = 55.93, p < 0.0001, I2 = 91.1%. Again, the heterogeneity reflects the magnitude but not the direction of the difference in change from baseline to follow up, because the change was greater in treatment than in control for all six studies. Sensitivity analyses were again performed to either increase or decrease by 50% the SDs for the Srini18 and Kitrey22 trials. This produced only minimal changes in the pooled estimate of change from baseline, and the results remained statistically significant (data not shown).
Figure 3.
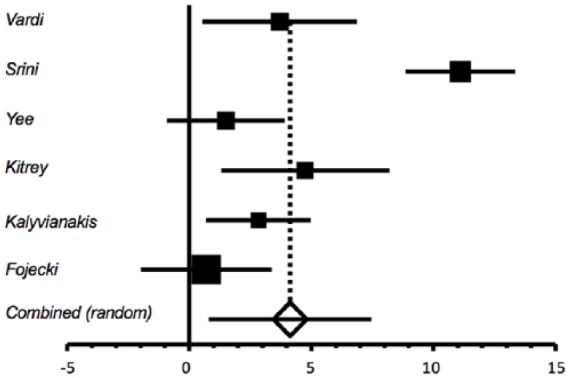
Mean change in International Index of Erectile Function-Erectile Function domain score from baseline to post-treatment follow up in treated versus placebo patients.
A total of five of the seven trials provided data on the proportion of patients with a ⩾5 point increase in IIEF-EF after treatment.19,21–24 The proportions ranged from 37.9 to 65.0% in the treatment group versus 0 to 38.3% in the sham group. The pooled relative risk of a 5-point or greater increase in IIEF-EF for treated versus sham patients is 1.94 (95% CI: 0.97, 3.85), p = 0.0595, which was not statistically significant (Figure 4). There was significant heterogeneity among the five relative risk estimates, Cochran’s Q = 14.03, p = 0.0072, I2 = 71.5%; again, there was consistency in the direction of effect, with four of the five studies exhibiting a higher percentage with a ⩾5-point increase in the treated group.19,21–23
EHS outcomes
Overall five of the seven trials provided data on the proportion of patients with baseline EHS ⩽2 who improved to EHS ⩾3 at 1 month post-treatment,18,21,22 or after 5 weeks of treatment.19,24 The proportions ranged from 3.5 to 90.0% in the treatment group versus 0–9.0% in the sham group (Table 1). The pooled relative risk of EHS improvement for the treated versus sham group is 6.63 (95% CI: 1.59, 27.71), p = 0.0095 (Figure 5). This analysis suggests that patients receiving LiESWT were approximately six times more likely than sham patients to improve to an EHS ⩾3. There was significant heterogeneity among the five relative risk estimates, Cochran’s Q = 9.82, p = 0.044, I2 = 59.3%. This again reflected the magnitude of increase in the treatment versus sham group; four of the five studies exhibited a higher percentage with EHS ⩾3 in the treated group.18,19,21,22 Because the number of patients in treatment and sham group in the Fojecki trial24 was estimated based on the total number, sensitivity analyses were again performed to either increase the number of treated patients by 20% and decrease the number of sham patients by 20%, or vice versa. This produced only minimal changes in the pooled relative risk estimate, and the results remained statistically significant (data not shown).
Figure 5.
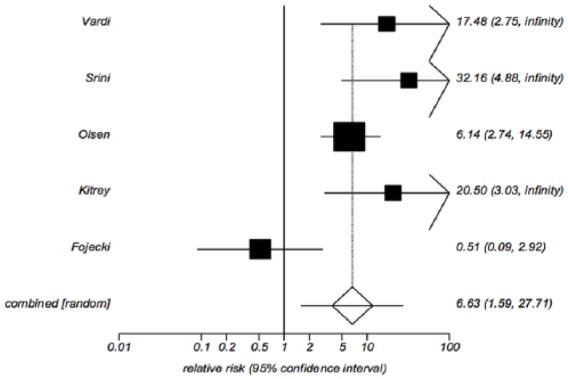
Relative risk of treatment versus placebo for increase in erectile hardness score to 3 or greater at post-treatment follow up.
Adverse events
All studies evaluated for adverse events. Overall, five studies reported no adverse events or side effects,18,20–23 while two studies reported that some patients in both groups had a slight burning sensation19 or local irritation24 after treatment.
Risk of bias assessment
Risk of bias was evaluated according to the seven domains incorporated into the tool developed by the Cochrane Collaboration and presented in Table 2.25 Random sequence generation was considered to have low risk of bias for four of seven studies, and unclear risk of bias in three studies that provided no information on the method of producing random assignments. Allocation concealment was considered to have low risk of bias in two trials, but unclear risk of bias in five trials that did not clarify whether the randomization list was generated by and implemented by someone other than the physicians enrolling the patients and performing the procedure. All trials had low risk of bias regarding blinding patients and physicians to the procedure because all used a sham procedure that was indistinguishable from the treatment procedure, and the operators were unaware of which was being used. Blinding of outcome assessment was also considered low risk of bias in all trials because the outcome questionnaires were completed independently by the patients rather than the physicians. Incomplete outcome data was considered a low risk of bias for six of the trials, but high risk of bias for one trial where only 43% of controls and 63% of treated patients completed the trial. Selective reporting was considered to have a low risk of bias for six trials, but high risk of bias for one trial where statistical comparison of the primary endpoints between treatment and sham were unclear. Other bias was considered low risk for six trials and high risk for one trial18 that did not adjust for imbalance in potential confounding factors that remained after randomization and was subject to selection bias due to the high dropout rate. Overall, the pooled trial outcome data are considered to have a low risk of bias. Although one trial was judged to have high risk of bias for three categories and unclear risk of bias for two categories, plots of IIEF-ES for sham and treatment groups at all time points show clear improvement in the treatment group, and EHS increased only in the treatment group.
Table 2.
Risk of bias assessment for the seven included randomized controlled trials according to the Cochrane Collaboration tool.
| Random sequence | Allocation concealment | Blinding pts & personnel | Blinding outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
|---|---|---|---|---|---|---|---|
| Vardi | + | ? | + | + | - | + | + |
| Srini | ? | ? | + | + | + | + | - |
| Yee | + | ? | + | + | ? | + | + |
| Olsen | ? | + | + | + | + | + | + |
| Kitrey | ? | ? | + | + | + | + | + |
| Kalyvianakis | + | ? | + | + | + | + | + |
| Fojecki | + | + | + | + | + | + | + |
Discussion
Our analysis of the available RCTs supports a clinically significant improvement in short-term IIEF-EF and EHS with a favorable adverse event profile. Pooled analysis of the seven RCTs included 607 randomized patients and demonstrated a significantly higher IIEF-EF and EHS 1 month after treatment with LiESWT compared with placebo. Mean difference in IIEF-EF from baseline to 1 month after intervention was statistically greater after LiESWT compared with placebo. In the studies that reported percentage of patients with at least a 5-point IIEF improvement from baseline, there was a higher number of patients that improved after LiESWT. Impressively, LiESWT was associated with an approximate six-fold increase compared with placebo in proportion of patients whose EHS increased to 3 or greater (p < 0.0001). Few adverse events were noted in any of the trials.
At the time of manuscript preparation, there were four meta-analyses published in the English literature that evaluated the efficacy of LiESWT on erectile function.3–6 While these studies corroborated short-term efficacy and safety of LiESWT for treatment of ED, these studies were limited by enrolling heterogenous populations, omitting the most recent RCTs,23,24 and citing studies that did not specify erectile function as the primary outcome. Overall, three of the four meta-analyses only included four or five of the seven currently published RCTs.4–6 Although one these meta-analyses included a larger number of studies than our analysis, they incorporated data gathered outside of RCTs. Owing to this heterogenous accumulation of data, these studies have yielded different outcomes. In a recently published study, Fojecki and colleagues, performed a systematic review of extracorporeal shock wave therapy in Peyronie’s disease, chronic pelvic pain and ED.32 Interestingly, their study search and systematic review for the ED subsection included four of the earlier five RCTs also selected for our study, but they did not perform a meta-analysis of these data.
In the present meta-analysis, we opted to incorporate only RCTs because this study design methodology provides the highest level of scientific evidence. We excluded data on Peyronie’s disease, renal transplant patients, and chronic pelvic pain since these conditions represent very different disease entities which encompass their own unique pathophysiology with treatment strategies that differ from typical organic ED. While the number of available RCTs and the sample size of each trial was modest, we nevertheless consistently observed greater improvement with LiESWT, yielding statistically significant pooled effects for all outcomes we evaluated. A recent RCT excluded from this analysis, explored the specific patient population of post-renal transplant ED. The 10 patients in the treatment group of this study had subjective improvement in erectile function after 3 weeks of treatment, but there was no demonstrated improvement in follow up penile Doppler studies.33
Overall our analysis was unable to report on objective endpoints such as ultrasound Doppler findings, nocturnal penile tumescence (NPT) or flow-mediated dilatation (FMD) because they were not uniformly reported by the RCTs. Overall, two RCTs have evaluated penile Doppler ultrasound findings but have variable outcomes and therefore further studies are needed to help clarify if there is indeed an improvement in penile blood flow associated with LiESWT for ED.23,33 Some prospective observational studies provide information about the effect of LiESWT on these outcome measures, but these were not included in our analysis due to their heterogeneity of outcomes and methods, and lack of randomized placebo control. Vardi and colleagues evaluated 20 men with ED and abnormal NPT and reported that all NPT parameters improved on 1 month of follow up.34 They also investigated changes in FMD, a previously described technique,35 which uses veno-occlusive strain gauge plethysmography to compare penile and forearm blood flow after 5 minutes of ischemia. They found that after LiESWT, penile basal flow significantly improved from 7.3 ml/min per deciliter to 17.8 ml/min per deciliter and maximal flow significantly increased from 12.0 ml/min per deciliter to 28.9 ml/min/dl, while no significant changes were noted in forearm flow parameters.
Animal studies have attempted to elucidate the mechanism by which LiESWT exerts its effect on erectile tissues. While evaluating a diabetic rat model, Qiu and colleagues found that SWT partially ameliorated diabetes-associated ED by promoting the regeneration of neuronal nitric oxide synthase (nNOS)-positive nerves, endothelium, and smooth muscles of the penis;36 these effects appeared to be through the recruitment of endogenous mesenchymal stem cells. Recently, Assaly-Kaddoum and colleagues reported that LiESWT’s impact on ED was not mediated by the NO/cyclic guanosine monophosphate-dependent pathway, the mechanism by which most nonsurgical treatment currently improves erectile function.37 Interestingly, they noted that sildenafil may augment the action of LiESWT, raising interest in multimodal therapy for ED. Other studies have evaluated patient factors that predict response to LiESWT. For instance, Hisasue and colleagues recently found that age and comorbidity were negative predictors of the therapeutic efficacy of LiESWT.38 Future studies are needed to investigate other prognostic patient variables, sensitizing therapies to augment effect of LiESWT, and the exact schedule of extracorporeal shock wave therapy delivery that will maximize treatment benefits.
Of the seven trials included in our analysis, five used a similar treatment protocol,18,20–23 which was derived from the cardiology literature.39 These studies had a similar schedule, treated the same locations of the penis, and used the same device with similar energy delivery parameters. Overall, two of the other studies differed in their treatment modality and protocols.19,24 The device most commonly used in these RCTs was a focused electrohydraulic unit;18,20–23 however, an electromagnetic unit19 or a piezoelectric linear therapy source24 was also included for comparison. All lithotripters have three basic components, including a shock wave generating system, a localization system to identify and target the tissue, and a positioning system.40 In the standard electrohydraulic unit, a spark plug and ellipsoidal reflector are used to create a focused shock wave in the focal zone. In contrast, an electromagnetic system uses high voltage electric pulses from an electromagnetic shock wave emitter to generate a planar acoustic pulse that is focused by an acoustic lens. The piezoelectric lithotripter uses piezoelectric crystals which synchronously get excited and rapidly expand to create a high voltage electrical pulse. Despite the differences in physics between these devices, all have clinical application in the urologic literature for treatment of stones, and have potential to produce a clinical benefit for treatment of ED.40 Our study was not designed to compare treatment protocols, and head-to-head comparisons of treatment parameters should be addressed in future studies to determine the optimal treatment protocol for ED. Interestingly, despite the difference in treatment protocol, the Olsen19 trial showed a positive effective of LiESWT for ED treatment. In contrast, the Fojecki24 protocol did not produce a clinical benefit to support the use of LiESWT for ED.
Readers must be cautioned that there are a number of limitations with the existing studies available for analysis. Long-term results cannot be included because most study endpoints are assessed at only 1 month after treatment and later outcomes are not consistently reported in these RCTs. All trials comprise patients who were randomized but did not complete the trial and therefore they do not represent comprehensive data. None of the trials performed intention to treat analyses; all reported only results of the evaluable patients. This omission may overstate the efficacy of intervention if patients dropped out because intervention was unacceptable. Finally, some trials had an incomplete reporting of SD values required for meta-analysis calculations, and thus we had to derive estimated values, as described in the Materials and Methods section. However, sensitivity testing indicated that varying the assumptions used to derive estimates had little effect on analysis results. Our risk of bias assessment confirms that the RCTs were compliant with their randomization process and blinding, however even these well-designed studies have some elements of bias. The most recent RCTs have limited their potential sources of bias23,24 compared with their predecessors and additional, properly designed studies will help confer these results in the future.
The multiple recent publications on the role of LiESWT in ED highlight the ongoing quest for new therapies for ED that may add to our armamentarium and fill gaps in current treatment algorithms. The suggestion that LiESWT may be used to salvage PDE5i nonresponders suggests a role in patients who would traditionally be offered more invasive treatments such as intracavernosal injection, intraurethral suppositories, or inflatable penile prosthesis implantation. The proposed rehabilitative nature of this therapy makes it attractive in urologic research. Long-term data are necessary before promoting this therapy. In fact, Fojecki and colleagues have 1 year of data in their ED patients, but only showed a 50% success rate 1 year after treatment, indicating that the proposed benefit of this treatment is short lived.41 As it stands, the American Urology Association guideline on ED does not include LiESWT as an approved treatment option.1 In addition, the Sexual Medicine Society of North America recently published a consensus statement that only recommends the use of LiESWT under the guidance of an Institutional Review Board approved clinical trial.42 Despite these authorities’ recommendations, LiESWT is widely being used as a rejuvenating ED treatment without regulatory agency approval. In the next decade we can expect an increase in the quality of published data on LiESWT in ED by accruing long-term data, using animal models to decipher the underlying pathophysiology, and defining appropriate treatment protocols.
Conclusion
Our meta-analysis is the first to evaluate the efficacy of LiESWT for the treatment of ED in RCTs, which are considered the gold standard of clinical research. Unlike previous meta-analyses, our analysis is not confounded by the inclusion of nonrandomized studies or those evaluating treatment of other urologic conditions, such as chronic pelvic pain and Peyronie’s disease. Our findings indicate an improvement in both IIEF-EF and EHS, which lends support for the role of LiESWT in the treatment of organic ED. Future high quality RCTs with uniform data reporting and long-term follow up are needed. In addition, studies evaluating the dose dependency of LiESWT and optimal treatment schedule are needed to determine the appropriate regimen for maximizing the benefits of LiESWT.
Acknowledgments
The following author contributions are acknowledged. J.D. Campbell: data collection, manuscript writing and editing; A.R. Oppenheim: data collection, manuscript writing; B.J. Trock: data analysis; I. Anusionwu: project development, manuscript editing; R. Gor: data collection, manuscript editing; and A.L. Burnett: project development, manuscript editing.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, or publication of this article: Medispec Ltd. for research support.
Conflict of interest statement: Authors I. Anusionwu and A.L. Burnett have received research grants from Medispec Ltd.
The remaining authors declare no conflict of interest
ORCID iD: Jeffrey D Campbell  https://orcid.org/0000-0001-8129-3260
https://orcid.org/0000-0001-8129-3260
Contributor Information
Jeffrey D. Campbell, The James Buchanan Brady Urological Institute and Department of Urology, Johns Hopkins Medical Institutions, 600 N. Wolfe Street, Marburg 405, Baltimore, MD 21287, USA; Western University, Department of Surgery, Division of Urology, London, ON, Canada.
Bruce J. Trock, The James Buchanan Brady Urological Institute and Department of Urology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Adam R. Oppenheim, Department of Urology, Einstein Healthcare Network, Philadelphia, PA, USA
Ifeanyichukwu Anusionwu, Department of Urology, Hahnemann University Hospital, Philadelphia, PA, USA.
Ronak A. Gor, The James Buchanan Brady Urological Institute and Department of Urology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Arthur L. Burnett, The James Buchanan Brady Urological Institute and Department of Urology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- 1. Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol 2018; 200: 633–641. [DOI] [PubMed] [Google Scholar]
- 2. Corona G, Lee DM, Forti G, et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med 2010; 7: 1362–1380. [DOI] [PubMed] [Google Scholar]
- 3. Clavijo RI, Kohn TP, Kohn JR, et al. Effects of low-intensity extracorporeal shockwave therapy on erectile dysfunction: a systematic review and meta-analysis. J Sex Med 2017; 14: 27–35. [DOI] [PubMed] [Google Scholar]
- 4. Lu Z, Lin G, Reed-Maldonado A, et al. Low-intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis. Eur Urol 2017; 71: 223–233. [DOI] [PubMed] [Google Scholar]
- 5. Man L, Li G. Low-intensity extracorporeal shock wave therapy for erectile dysfunction: a systematic review and meta-analysis. Urology 2018; 119: 97–103. [DOI] [PubMed] [Google Scholar]
- 6. Zou ZJ, Tang LY, Liu ZH, et al. Short-term efficacy and safety of low-intensity extracorporeal shock wave therapy in erectile dysfunction: a systematic review and meta-analysis. Int Braz J Urol 2017; 43: 805–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wein AJ, Kavoussi LR, Novick AC, et al. Campbell-Walsh Urology. 10th ed. Chapt 24. Philadelphia: Elsevier Health Sciences, 2011, pp. 721–747. [Google Scholar]
- 8. Alunni G, Marra S, Meynet I, et al. The beneficial effect of extracorporeal shockwave myocardial revascularization in patients with refractory angina. Cardiovasc Revasc Med 2015; 16: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zuoziene G, Leibowitz D, Celutkiene J, et al. Multimodality imaging of myocardial revascularization using cardiac shock wave therapy. Int J Cardiol 2015; 187: 229–230. [DOI] [PubMed] [Google Scholar]
- 10. Wang CJ, Cheng JH, Kuo YR, et al. Extracorporeal shockwave therapy in diabetic foot ulcers. Int J Surg 2015; 24: 207–209. [DOI] [PubMed] [Google Scholar]
- 11. Cacchio A, Rompe JD, Furia JP, et al. Shockwave therapy for the treatment of chronic proximal hamstring tendinopathy in professional athletes. Am J Sports Med 2011; 39: 146–153. [DOI] [PubMed] [Google Scholar]
- 12. Pan MM, Raees A, Kovac JR. Low-Intensity Extracorporeal Shock Wave as a Novel Treatment for Erectile Dysfunction. Am J Mens Health 2016; 10: 146–148. [DOI] [PubMed] [Google Scholar]
- 13. Yamaya S, Ozawa H, Kanno H, et al. Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury. J Neurosurg 2014; 121: 1514–1525. [DOI] [PubMed] [Google Scholar]
- 14. Ito K, Fukumoto Y, Shimokawa H. Extracorporeal shock wave therapy as a new and non-invasive angiogenic strategy. Tohoku J Exp Med 2009; 219: 1–9. [DOI] [PubMed] [Google Scholar]
- 15. Gruenwald I, Appel B, Vardi Y. Low-intensity extracorporeal shock wave therapy–a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J Sex Med 2012; 9: 259–264. [DOI] [PubMed] [Google Scholar]
- 16. Wang B, Ning H, Reed-Maldonado AB, et al. Low-intensity extracorporeal shock wave therapy enhances brain-derived neurotrophic factor expression through PERK/ATF4 signaling pathway. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srini VS, Reddy RK, Shultz T, et al. Low intensity extracorporeal shockwave therapy for erectile dysfunction: a study in an Indian population. Can J Urol 2015; 22: 7614–7622. [PubMed] [Google Scholar]
- 19. Olsen AB, Persiani M, Boie S, et al. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol 2015; 49: 329–333. [DOI] [PubMed] [Google Scholar]
- 20. Yee CH, Chan ES, Hou SS, et al. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol 2014; 21: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 21. Vardi Y, Appel B, Kilchevsky A, et al. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol 2012; 187: 1769–1775. [DOI] [PubMed] [Google Scholar]
- 22. Kitrey ND, Gruenwald I, Appel B, et al. Penile low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: a double-blind, sham controlled study. J Urol 2016; 195: 1550–1555. [DOI] [PubMed] [Google Scholar]
- 23. Kalyvianakis D, Hatzichristou D. Low-intensity shockwave therapy improves hemodynamic parameters in patients with vasculogenic erectile dysfunction: a triplex ultrasonography-based sham-controlled trial. J Sex Med 2017; 14: 891–897. [DOI] [PubMed] [Google Scholar]
- 24. Fojecki GL, Tiessen S, Osther PJ. Effect of low-energy linear shockwave therapy on erectile dysfunction-a double-blinded, sham-controlled, randomized clinical trial. J Sex Med 2017; 14: 106–112. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Snedecor GW, Cochran WG. Statistical methods. Ames: Iowa State University Press, 1976. [Google Scholar]
- 28. Sutton AJ, Abrams KR, Jones DR, et al. Methods for meta-analysis in medical research. New York: John Wiley & Sons, 2000. [Google Scholar]
- 29. Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia: Lippincott-Raven, 1998. [Google Scholar]
- 30. Greenland S, Robins JM. Estimation of common effect parameter from sparse follow up data. Biometrics 1985; 41: 55–68. [PubMed] [Google Scholar]
- 31. Der Simonian R, Laird N. Meta-analysis in clinical trials. Contemp Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 32. Fojecki GL, Tiessen S, Osther PJ. Extracorporeal shock wave therapy (ESWT) in urology: a systematic review of outcome in Peyronie’s disease, erectile dysfunction and chronic pelvic pain. World J Urol 2017; 35: 1–9. [DOI] [PubMed] [Google Scholar]
- 33. Yamaçake KGR, Carneiro F, Cury J, et al. Low-intensity shockwave therapy for erectile dysfunction in kidney transplant recipients. A prospective, randomized, double blinded, sham-controlled study with evaluation by penile Doppler ultrasonography. Int J Impot Res. Epub ahead of print 14 August 2018. DOI: 10.1038/s41443-018-0062-2 [DOI] [PubMed] [Google Scholar]
- 34. Vardi Y, Appel B, Jacob G, et al. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol 2010; 58: 243–248. [DOI] [PubMed] [Google Scholar]
- 35. Vardi Y, Dayan L, Apple B, et al. Penile and systemic endothelial function in men with and without erectile dysfunction. Eur Urol 2009; 55: 979–985. [DOI] [PubMed] [Google Scholar]
- 36. Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 2013; 10: 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Assaly-Kaddoum R, Giuliano F, Laurin M, et al. Low intensity extracorporeal shock wave therapy improves erectile function in a model of type II diabetes independently of NO/cGMP pathway. J Urol 2016; 196: 950–956. [DOI] [PubMed] [Google Scholar]
- 38. Hisasue S, China T, Horiuchi A, et al. Impact of aging and comorbidity on the efficacy of low-intensity shock wave therapy for erectile dysfunction. Int J Urol 2016; 23: 80–84. [DOI] [PubMed] [Google Scholar]
- 39. Khattab AA, Brodersen B, Schuermann-Kuchenbrandt D, et al. Extracorporeal cardiac shock wave therapy: first experience in the everyday practice for treatment of chronic refractory angina pectoris. Int J Cardiol 2007; 121: 84–85. [DOI] [PubMed] [Google Scholar]
- 40. Chung E, Wang J. A state-of-art review of low intensity extracorporeal shock wave therapy and lithotripter machines for the treatment of erectile dysfunction. Expert Rev Med Devices 2017; 14: 929–934. [DOI] [PubMed] [Google Scholar]
- 41. Fojecki GL, Tiessen S, Osther PJS. Effect of linear low-intensity extracorporeal shockwave therapy for erectile dysfunction-12-month follow-up of a randomized, double-blinded, sham-controlled study. Sex Med 2018; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. SMSNA Executive Office. SMSNA position statement on restorative therapies for ED. Available at: www.smsna.org/V1/news/433-smsna-position-statement-on-restorative-therapies-for-ed (accessed 2 May 2018). [Google Scholar]