Abstract
Objective:
The objective of this study was to examine the impact of dehydrated human amnion/chorion membrane (dHACM) allografts on prostate and bladder cancer growth in the setting of residual disease and positive surgical margins.
Materials and methods:
A commercially available version of dHACM was used. Cytokines were identified and quantified, followed by comparative analysis of cell growth in two different human cell lines: prostate cancer (LNCaP) and bladder cancer (UM-UC-3), in vitro and in vivo. Tumor growth between the two groups, membrane versus no membrane implant, was compared and immunohistochemistry studies were conducted to quantify CD-31, Ki-67, and vimentin. A Student’s unpaired t-test was used to determine statistical significance.
Results:
The UM-UC-3 and LNCaP cells grew quicker in medium plus 10% serum and dHACM extract than in the other media (p = 0.03). A total of 28 distinct cytokines were found in the extract, 11 of which had relatively high concentrations and are associated with prostate and bladder cancer tumor progression. In vivo LNCaP model, after 10 weeks, the median tumor volume in the membrane group was almost threefold larger than the partial resection alone (p = 0.01). Two weeks after resection, in the UM-UC-3 model, the membrane group reached fourfold larger than the partial resection without membrane group (p < 0.01). In both groups, the expression of CD-31 and Ki-67 markers were similar and showed no statistical significance (p > 0.05). It was only in the LNCaP tumors that vimentin expression was significantly higher in the group without membrane compared with the membrane group (p = 0.008).
Conclusion:
The use of dHACM after partial tumor resection is related to faster tumor relapse and growth in prostate and urothelial cancer in vivo models, showing a potential risk of rapid local recurrence in patients at high risk of positive margins.
Keywords: allografts, bladder cancer, positive margin resection, prostate cancer
Introduction
The therapeutic benefits of human amnion/chorion tissue grafts for wound healing has been studied and established in clinical practice over several decades.1 Native human amniotic membrane contains cytokines such as growth factors and interleukins that are known to play a central role in the physiological process of normal wound healing and tissue regeneration.2 Recently, standardized methods for tissue preparation and dehydration have been developed with the intent to preserve and maintain the biological activities inherent in the native amnion in the form of a shelf-stable commercialized product. This process is purported to retain the natural growth factors and interleukins contained in the amnion and chorion, allowing these membranes to be rehydrated for utilization as a biologic bandage or scaffold to promote localized tissue healing in a variety of surgical settings.2
In a recent study, Patel et al. suggested that important functional benefits, such as early recovery of continence and potency, could be significantly improved by using dehydrated human amnion/chorion membrane (dHACM) allografts wrapped around the prostatic neurovascular bundle at the time of robotic-assisted radical prostatectomy (RP).3 However, the use of a growth-promoting material in the oncologic surgery setting carries the potential elevated risk of local cancer recurrence. Certain growth factors and interleukins (e.g. IL-8) that may be present in dHACM and related to tissue healing have also been associated with tumor pathogenesis and disease progression.4 Although these substances are normally present at physiologic levels in surgically manipulated tissues, the effect of exogenous, unregulated factors in this environment, especially in the setting of positive surgical margins, could create a microenvironment that enables local disease persistence or recurrence.
To better understand the effect of these materials on cancer cells that might be present in the setting of residual disease and positive surgical margins, we examined the impact of dHACM on cancer growth characteristics in a series of experiments using both in vitro and in vivo models. Studies were replicated in two different urologic human cancers: prostate cancer and urothelial cancer.
Material and methods
Preparation of dHACM
All studies were performed with a commercially available version of dHACM (AmnioFix, MiMedx Group, Marietta, GA, USA). To make the dHACM extracts for the in vitro experiments, a 2 cm × 12 cm membrane sheet was minced and incubated in phosphate buffered saline (PBS) at a concentration of 10 mg/ml. The membrane was incubated at 4°C for 24 h and remaining membrane fragments were filtered out. To make the dHACM extracts for the in vivo studies, a 2 cm × 12 cm membrane sheet was rehydrated in PBS and cut in 10 mm × 10 mm squares for implantation in the tumor bed following partial tumor resection. Three samples of the membrane extract were submitted to Discovery Assay service (Eve Technologies Corporation, Calgary, AB, Canada) to identify and quantify the cytokines.
In vitro experiments
We performed a comparative analysis of cell growth in two different human cell lines (American Type Culture Collection): prostate cancer (LNCaP) and bladder cancer (UM-UC-3). LNCaP cells were cultured in RPMI (Roswell Park Memorial Institute) medium containing 2 mM L-glutamine and 1× penicillin/streptomycin. UM-UC-3 cells were cultured in EMEM (Eagles Modified Essential Media) containing 2 mM L-glutamine and 1× penicillin/streptomycin. Each cell line’s basal medium was supplemented with either fetal bovine serum (FBS) or dHACM extract in four different combinations to test the effect of the cytokines found within the dHACM membrane on cell proliferation. The four different culture conditions were (1) basal medium + 2%FBS (BM2%), (2) basal medium plus 10% dHACM membrane extract (dHACM extract), (3) basal medium plus 10% serum (BM10%), and (4) basal medium plus 10% serum + 10%dHACM extract. Cells were plated in a 24-well plate at a concentration of 100,000 cells per well. After 72 h, the plate was washed with PBS to remove unattached cells and the Cell Titer Glo 2.0 assay (Promega) was performed to quantify the cells, based on the relative light unit (RLU) measurement. This assay was repeated four times for statistical analysis.
In vivo experiments
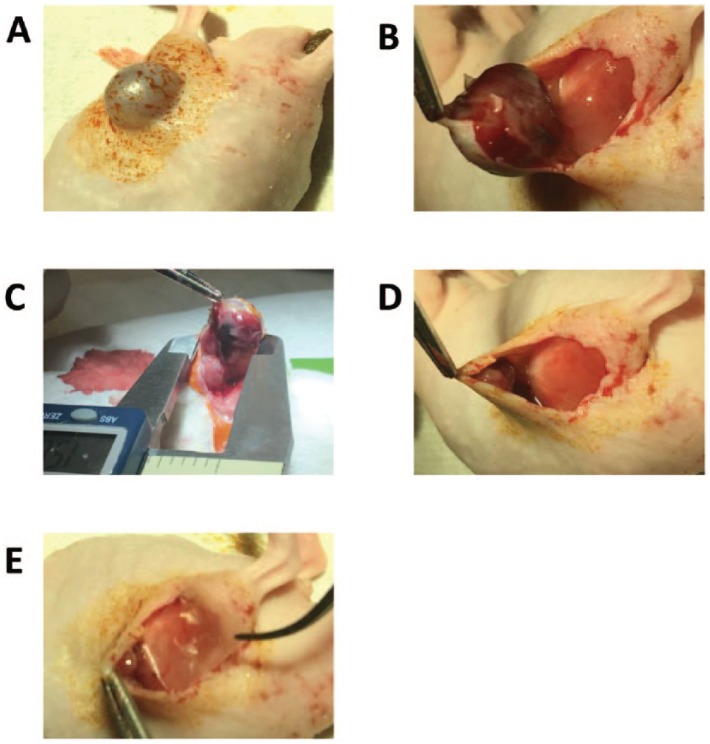
All animal work was performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center. After institutional approvals, we used 45 severe combined immunodeficiency (SCID) mice for the LNCaP flank injections and 30 nude mice for UM-UC-3 flank injections. The time between the injection and the surgical resection was 36 days for LNCaP and 21 days for UM-UC-3, with a tumor take rate and mean tumor volume of 55%/150 mm³ and 95%/411 mm³, respectively. Two groups were created: partial resection (n = 10) and partial resection plus membrane implant (n = 10). The surgery was performed under general anesthesia with isoflurane (Figure 1A). A 1 cm incision was made on the lateral border of the tumor for partial resection of the tumor and membrane onlay implant (Figure 1B). After dissection, the tumor length was measured (Figure 1C) and approximately 90% of the tumor was resected with the remaining part duly attached to collateral vessels (Figure 1D). The resected tumor was weighed in all surgical groups. In the membrane group, a 10 mm × 10 mm dHACM sheet was implanted on the tumor bed (Figure 1E). The skin was closed with staples, and a subcutaneous dose of meloxicam was used for local analgesia. From the first week following surgery, biweekly measurements of the tumors were performed. Mice who presented with no palpable tumors after 15 days were excluded from the study as they likely underwent a complete resection. Mice whose tumors reached 2000 mm3 were euthanized, and the tumors were harvested for histology assessment by a board-certificated veterinary pathologist (Sebastien Monette). Tumor growth speed (TGS) was analyzed and compared between the two groups: with membrane versus without membrane. Established TGS was defined by the formula: tumor volume median (mm³)/days after tumor injection.
Figure 1.
Partial tumor resection (>90%) and dehydrated human amnion/chorion membrane (dHACM) implantation technique: (A) tumor preparation, inhaled isoflurane was used for anesthesia; (B) a 1 cm incision was made on the lateral border of the tumor; (C) the tumor parameters were measured and (D) underwent partial resection (>90% of the tumor volume); (E) mice randomized to dHACM membrane implantation had a pre-prepared 1 cm × 1 cm amniotic membrane sheet (AmnioFix) square segment was implanted directly on the tumor bed.
All tumor specimens were fixed in 10% neutral buffered formalin, routinely processed in alcohol and xylene, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) and immunohistochemistry (IHC) at the Laboratory of Comparative Pathology at Memorial Sloan Kettering Cancer Center on a Leica Bond RX automated stainer (Leica Biosystems, Buffalo Grove, IL). Following heat-induced epitope retrieval at pH 9.0, the primary antibody against CD-31 (DIA-310, Dianova, Hamburg, Germany), Ki-67 (ab16667, Abcam, Cambridge, MA), or vimentin (5741, Cell Signaling, Danvers, MA) was applied at a concentration of 1:250, 1:100, and 1:250, respectively, followed by application of a polymer detection system (DS9800, Novocastra Bond Polymer Refine Detection, Leica Biosystems) adhering to the manufacturer’s instructions. For all IHC stains, the chromogen was 3,3′-diaminobenzidine tetrachloride (DAB), and sections were counterstained with hematoxylin. Whole-slide digital images were generated on a scanner (Panoramic 250 Flash III, 3DHistech, 20×/0.8NA objective, Budapest, Hungary) at a resolution of 0.2431 µm per pixel. Staining quantification was performed with QuPath 0.1.3.5 For CD-31 and Ki-67, the region of interest (ROI) was defined as viable tumor tissue excluding necrosis. For vimentin, the ROI was defined as viable tumor tissue and surrounding stroma, excluding necrosis. For CD-31 and vimentin, the positive area, defined as the ratio of DAB stained pixels to total ROI area, was measured using the positive pixel count algorithm. For Ki-67, the ratio of cells with positive nuclear staining to total cell number was measured with the positive cell detection algorithm. ROI selection, algorithm optimization, and validation and qualitative examination of H&E slides were performed by a board-certified veterinary pathologist (Sebastien Monette).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics v25 and reported with GraphPad Prism v7.01. Student’s unpaired t-test was used to determine statistical significance at the 95% confidence level for ROI analysis and two-way analysis of variance (ANOVA) test. The alpha level was set at 0.05 for tests of statistical significance.
Results
In vitro experiments
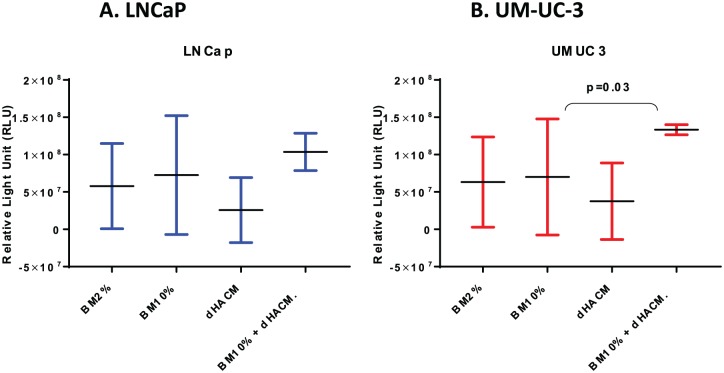
Twenty-eight cytokines were found in the extract, 13 of which presented with high concentrations and are associated with tumor progression (Table 1). The LNCaP and UM-UC-3 cell lines proliferated in similar patterns in each of the different media according to the RLU. In the LNCaP cell line (Figure 2A), the average luminescence increased 69% in the BM10% plus 10%dHACM extract (7.2 × 107 RLU) compared with BM2% alone (13 × 107 RLU) (p = 0.09). The difference of cell growth among the other three cell culture conditions (excluding BM10% plus 10%dHACM), was not statistically significant (p = 0.17). The UM-UC-3 cells (Figure 2B) in the medium containing BM10% plus 10%dHACM extract presented with an average luminescence of 1.3 × 108 RLU; compared with BM2%, this was an almost twofold increase in cell growth (p < 0.01). Once again, the results from the other cell culture conditions (BM2%, BM2% plus 10% dHACM extract, and BM10%) showed no statistical significance (p = 0.19).
Table 1.
Cytokines detected in dHACM extract by Human Cytokine 42-Plex Discovery Assay. For each protein, seven standard levels were described based on the lowest (1) to highest (7) concentration expected in the medium solution (parameter logistic standard curve [SC]). We defined low concentration as SC < 4 (SC 2 or 3), intermediate as SC = 4, and high as SC > 4 (SC 5, 6, or 7).
| Low | Intermediate | High | |
|---|---|---|---|
| Growth factors | EGF, FGF-2, G-CSF, GROα, MCP-3, MDC, PDGF-AA | PDGF-BB | TGF-B1, TGF-B2, IP-10, MCP-1, MIP-1a, CCL5 |
| Interleukins | IL-1a, IL-4, IL-6, IL-18 | IL-15 | IL-1RA, IL-8 |
| MMP | MMP-12 | – | MMP-2, MMP-8, MMP-9, MMP-10 |
| TIMP | TIMP-4 | – | TIMP-1 |
CCL5, chemokine ligand 5; EGF, epidermal growth factor; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GROα, growth-regulated protein alpha; IP-10, interferon gamma-induced protein 10; MCP, monocyte chemotactic protein; MDC, mediator of DNA damage checkpoint; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinase; PDGF, platelet derived growth factors; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinases.
Figure 2.
Cell growth analysis by relative cells units (RLU) count of LNCaP and UM-UC-3 lines in vitro experiments. LNCap and UM-UC-3 cells demonstrated enhanced cellular proliferation in the presence of both dehydrated human amnion/chorion membrane (dHACM) extract and BS10% compared with BS10% only. For both cell lines, similar growth patterns were observed in the presence of BS2%only, BS10% only, and dHACM extract only.
In vivo experiments
No significant differences were found in the volume and weight of the tumors after partial resection between the two groups: membrane implantation and partial resection alone (Table 2). Local recurrence was observed in 70% and 90% of mice with positive surgical margins, LNCaP and UM-UC-3 tumors, respectively. Six mice (24%) with LNCaP cells and two mice (11%) with UM-UC-3 cells did not develop tumors after resection and were excluded from the study. At least 90% of the tumor’s mass was removed, based on preoperative measurement, and one tumor’s vessel branch was left intact (Figure 1A–E).
Table 2.
LNCaP and UM-UC-3 tumor volume and weight comparison after resection in the partial tumor resection model.
| Partial resection without dHACM | Partial resection with dHACM | p value | ||
|---|---|---|---|---|
| LNCaP | Number of mice | 9 | 10 | |
| Median resected tumor volume, mm (range) | 144 (58–382) | 154 (58–697) | 0.74 | |
| Median resected tumor weight, mg (range) | 80 (52–600) | 112 (14–590) | 0.67 | |
| UM-UC-3 | Number of mice | 8 | 8 | |
| Median resected tumor volume, mm (range) | 284 (137–756) | 344 (143–840) | 0.29 | |
| Median resected tumor weight, mg (range) | 35 (11–63) | 29 (10–83) | 0.64 |
dHACM, dehydrated human amnion/chorion membrane.
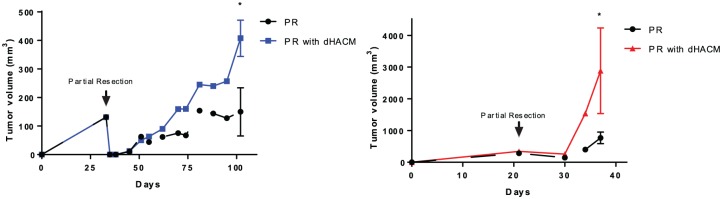
In the presence of dHACM, there was evidence of increased absolute tumor volumes compared with partial resection alone. In the LNCaP model, the tumor volumes 70 days after partial resection (day 106) in the dHACM group was larger compared with partial resection without dHACM group (408 mm³ versus 150 mm³, p = 0.01) (Figure 3A). Similar effects were observed in UM-UC-3 tumors. Fifteen days after partial resection (day 35), tumor volume in the dHACM group was fourfold larger compared with tumor volume in the partial resection alone group (2884 mm³ versus 767 mm³, p < 0.01) (Figure 3B).
Figure 3.
Tumor volume growth in LNCaP and UM-UC-3 lines before and after partial resection in in vivo models. In both cell lines, mice who underwent dehydrated human amnion/chorion membrane (dHACM) implantation had larger tumors compared with those who did not receive dHACM allografts.
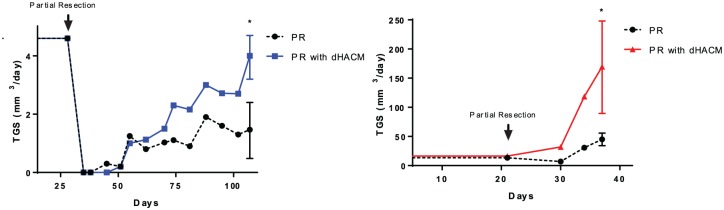
Prior to surgical resection, LNCaP tumors had the same median established tumor growth speed in both groups (established TGS = 4.6 mm³/day) (Figure 4A; Table 3). Twenty days after partial resection (day 56), the median TGS decreased around threefold in both groups to 1.1 mm³/day. The TGS of the LNCaP tumors that underwent partial resection with dHACM placement had a consistent increase (2.27 mm³/day at 28 days after surgery) and returned to the presurgery TGS (4.6 mm³/day) 70 days after partial resection. Over the same 70 days period, the TGS of the partial resection without dHACM model remained stable at 1.2 mm³/day and did not return to the presurgery TGS.
Figure 4.
LNCaP and UM-UC-3 tumor growth speed (TGS) analysis performed at three timepoints: (A) before resection (established TGS); (B) 1 week post-resection (early TGS); and (C) after 1 week post-resection (recurrent TGS). Over time in both LNCaP and UM-UC-3 models, TGS was faster in mice who underwent dehydrated human amnion/chorion membrane (dHACM) implantation compared with those who did not.
Table 3.
LNCaP and UM-UC-3 tumor volume measurements before and after partial resection.
| A: Established TGS (mm³/day) | B: Early TGS (mm³/day) |
C: Recurrent TGS (mm³/day) |
Recurrent TGS/Early TGS | ||
|---|---|---|---|---|---|
| LNCaP | Partial resection without dHACM | 4.6 | 1.1 | 1.4 | 127% |
| Partial resection with dHACM | 4.6 | 1.1 | 4 | 364% | |
| UM-UC-3 | Partial resection without dHACM | 13.5 | 14.2 | 45 | 317% |
| Partial resection with dHACM | 16.3 | 31.8 | 169.6 | 531% |
dHACM, dehydrated human amnion/chorion membrane; TGS, tumor growth speed.
Similar to the LNCaP model, in the UC-UM-3 tumors, no significant differences were seen in the established TGS between tumors that underwent partial resection with dHACM and those without dHACM (16.3 mm³/day versus 13.5 mm³/day; p = 0.4) (Figure 4B; Table 3). In contrast, UM-UC-3 tumors had important differences in TGS between the groups. In tumors with dHACM, the TGS 10 days after tumor resection (day 30) was twice as high compared with the presurgery established TGS (31.6 mm³/day versus 16.3 mm³/day; p < 0.05). However, in tumors without dHACM, early TGS was significantly lower compared with TGS before resection (6.8 mm³/day versus 13.5 mm³/day; p < 0.05). Fifteen days after surgery (day 36), the TGS of the group with dHACM increased fourfold more than the TGS of the group without dHACM (169.6 mm³/day versus 45 mm³/day; p < 0.01) (Table 3).
In the LNCaP and UM-UC-3 tumors, in both the partial resection plus membrane and the partial resection only group, almost all the differences in expressions of CD-31, Ki-67 and vimentin were minimal and showed no statistical significance (Table 4). However, vimentin expression was significantly higher only in the LNCaP tumors without the membrane group compared with the partial resection plus membrane group (p = 0.008).
Table 4.
Histology analysis by H&E and IHC. CD-31, Ki-67 and vimentin immunohistochemistry expressions in LNCaP and UM-UC-3 tumor models among mice who underwent dHACM implantation (P+M) and those who did not (Partial). Vimentin expression in the LNCaP tumors was lower in mice underwent dHACM implantation compared to those who did not.
| Tumor Cell/Group | H&E | CD31 | Ki67 | Vimentin |
|---|---|---|---|---|
| LNCap/Partial |

|
|||
| LNCap/P+M |
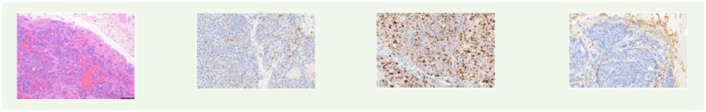
|
|||
| UMUC3/Partial |

|
|||
| UMUC3/P+M |
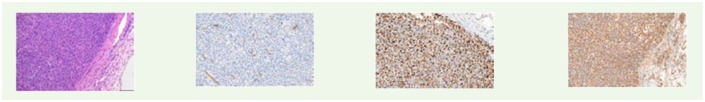
|
|||
| Partial resection without dHACM |
Partial resection with dHACM(P+M) |
p value | ||
| LNCaP | CD-31 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.752 |
| Ki-67 | 33.0 ± 3.0 | 31.1 ± 3.2 | 0.671 | |
| vimentin | 7.7 ± 1.0 | 4.3 ± 0.5 | 0.008 | |
| UM-UC-3 | CD-31 | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.361 |
| Ki-67 | 69.2 ± 2.5 | 65.3 ± 2.0 | 0.272 | |
| vimentin | 49.2 ± 2.6 | 50.2 ± 1.1 | 0.740 | |
dHACM, dehydrated human amnion/chorion membrane; H&E, hematoxylin and eosin; IHC, immunohistochemistry.
Discussion
Although the application of amniotic membrane allografts as a therapy following procedures for cancer treatment is a relatively recent concept, it has been regularly used in other fields including dermatology and ophthalmology. Much research on dHACM has focused on its healing capacity in benign cases, which has allowed it to be used for some cancer treatments. However, there is still a minimal understanding of the effects of dHACM on cancer cells. In fact, there are very few reports, and these have divergent findings, on cancer cell interactions and behavior after dHACM exposure.2,6,7
In the present study, the in vitro experiment showed that the use of dHACM allografts had a modest impact on tumor cell growth. Although the dHACM extract alone did not present as a significant cell stimulator compared with the other mediums, the combination of dHACM extract with 10%FBS resulted in accelerated tumor cell growth in both LNCaP and UMUC3 cell lines. A reasonable explanation is that the effects of dHACM are more pronounced in tumor cells that are in a favorable environment (e.g. with 10% FBS) with less direct activity in a poor cell culture condition (e.g. 2%FBS). Mamede et al.8 conducted in vitro experiments to evaluate the different metabolic activity (MA) responses of 21 types of human cancer cells when cultivated with fresh human amniotic membrane protein extracts. Respecting the genetic profile differences of each cell line,9 MA reactions were distinctive. For example, PC3 cells had a 54% decrease in the MA whereas LNCaP cells did not show any changes in MA. Our in vitro findings align with the findings of Mamede et al.’s experiment, which showed that dHACM alone did not result in increased growth in LNCaP. However, this suggests that the major role of these cytokines is related to microenvironment changes and less directly on cellular stimulation.10
In our in vivo experiments, after partial resection, LNCaP and UM-UC-3 tumors exposed to dHACM were larger and showed increased TGS compared with those not exposed to dHACM. This observation represents a concern related to the use of dHACM after surgical procedures such as RP where the potential benefits of functional improvement, faster recovery of both urinary continence and/or erectile function must be counterpointed with the possible risk of faster relapse. It has been demonstrated that positive surgical margins are not uncommon and could be implicated in biochemical recurrence after RP. Silberstein et al. published a study involving 2442 patients with clinical stage T1–T3 prostate cancer treated with RP with a positive margin rate of 11%; the rate was as high as 48% in other studies.11 The same concern is reported after radical cystectomy, with positive margins rates ranging from 5% to 10.2% depending on the institution.12,13 In patients with positive surgical margins, the use of materials that contain growth factor substances, such as dHACM, could be implicated in early relapse and thereby necessitate earlier salvage treatments such as radiotherapy, chemotherapy, and androgen deprivation therapy.
Our membrane extract analysis showed the expression of many cytokines including the following, which had the highest concentration: IP-10, MCP-1, MIP-1a, CCL5, TGF-B1, MMP-2, MMP-8, MMP-9, MMP-10, IL-1RA, and IL-8. These substances have been previously reported as significant factors in tumor progression in different types of tumors, including prostate and bladder.14–17 These cytokines have been implicated in issues such as increased angiogenesis and cell proliferation/invasion activities. Neveu et al. recently published the correlation between IL-8 secretion from normal prostate epithelial cells and cancer aggressiveness, suggesting a potential role in prostate carcinogenesis.18 Gong et al. also demonstrated in an in vitro study that increased TIMP-1 expression stimulates accumulation of CAF, providing a favorable pro-tumor microenvironment for prostate cancer progression.19,20 A review by Aldinucci et al.21 showed the association of the high expressions of IP-10 and CCL5 with tumor progression and metastasis. In another recent publication, Kumari et al. demonstrated the correlation of expressions of IL-2, IL-4, IL-8, IL-10, GM-CSF, IFN-γ, IP-10, MIP-1a, PDGF, MIP-1b, CCL5, and VEGF with poor bladder cancer outcomes.22 Many of these cytokines have been proposed as potential targets for anticancer therapy. Thus, the use of any device presenting higher levels of these substances, such as dHACM, must be done with careful consideration and restrictive criteria should be established.
Our histological results demonstrated that tumor expression of vessels (CD-31) and cellular proliferation (Ki-67) did not change with dHACM implantation. This interesting finding supports our in vitro results that dHACM has a small, yet direct effect in the LNCaP and UM-UC-3 tumor cells and, probably, much more effect in a normal tissue setting. Presumably, dHACM healing properties are responsible for faster tissue remodeling and tumor microenvironment recomposition after partial resection and, consequently, rapid tumor regrowth. Recently, Jie et al. showed that tissue remodeling after amniotic membrane implantation in a rabbit ocular injury is directly related to faster regeneration of normal epithelium without any difference in epithelial cell phenotype.7
The lower levels of vimentin expression in LNCaP tumors after dHACM implantation is another noteworthy finding in our study. Vimentin expression has been described as a significant factor of invasiveness and aggressiveness in prostate cancer, but has not been shown to correlate with tumor size.23 Similar to our observation, Niknami et al.24 showed the correlation of low vimentin expression and larger tumor sizes in colorectal cancer.
Our study has several limitations, especially related to the animal model chosen. In addition to the absence of a model to measure metastasic progression mentioned previously, the use of human cancer cell lines and a human directed device (e.g. dHACM) obligated the use of immunocompromised mice. The lack of immune system could misrepresent the dHACM effect on tumor behavior in the setting of an immunocompetent patient. The highly complex and not well-understood relationship between growth factors, interleukins, and macrophage inflammatory proteins in dHACM and the epithelial-to-mesenchymal transition mechanism is directly influenced by immune cells. Furthermore, these findings are limited to resections where there is gross positive disease remaining. Compared with microscopic positive margins, gross positive margins are rare and extrapolation of our results to microscopic positive margins is unwise without further investigation. Moreover, the results of this study only apply to the dHACM allografts used in this study; there are likely different cytokines in other commercially available versions of dHACM. Nonetheless, as far as we know, this study is the first preclinical model using dHACM after cancer resection. Our results suggest that it is necessary reevaluate the use dHACM in settings where there is a risk of a positive surgical margin.
Conclusion
The use of dHACM after partial tumor resection is related to faster tumor relapse and tumor growth speed in our prostate cancer and urothelial cancer models. The healing benefits of dHACM must be counterbalanced with the potential harm of rapid local recurrence in patients at high risk of gross positive margins.
Footnotes
Funding: This research was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and Thompson Family Foundation.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Ricardo G. Alvim  https://orcid.org/0000-0002-9774-1723
https://orcid.org/0000-0002-9774-1723
Contributor Information
Ricardo G. Alvim, Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Christopher Hughes, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Alexander Somma, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Karan K. Nagar, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Nathan C. Wong, Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Stephen La Rosa, Department of Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Sebastien Monette, Laboratory of Comparative Pathology and the Genetically Modified Animal Phenotyping Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Kwanghee Kim, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Jonathan A. Coleman, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
References
- 1. Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 2000; 20: 173–177. [PubMed] [Google Scholar]
- 2. Koob TJ, Lim JJ, Massee M, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell 2014; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel VR, Samavedi S, Bates AS, et al. Dehydrated human amnion/chorion membrane allograft nerve wrap around the prostatic neurovascular bundle accelerates early return to continence and potency following robot-assisted radical prostatectomy: propensity score-matched analysis. Eur Urol 2015; 67: 977–980. [DOI] [PubMed] [Google Scholar]
- 4. Sharma J, Gray KP, Harshman LC, et al. Elevated IL-8, TNF-alpha, and MCP-1 in men with metastatic prostate cancer starting androgen-deprivation therapy (ADT) are associated with shorter time to castration-resistance and overall survival. Prostate 2014; 74: 820–828. [DOI] [PubMed] [Google Scholar]
- 5. Bankhead P, Loughrey MB, Fernandez JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep 2017; 7: 16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim SH, Bang SH, Kang SY, et al. Human amniotic membrane-derived stromal cells (hAMSC) interact depending on breast cancer cell type through secreted molecules. Tissue Cell 2015; 47: 10–16. [DOI] [PubMed] [Google Scholar]
- 7. Jie J, Yang J, He H, et al. Tissue remodeling after ocular surface reconstruction with denuded amniotic membrane. Sci Rep 2018; 8: 6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mamede AC, Laranjo M, Carvalho MJ, et al. Effect of amniotic membrane proteins in human cancer cell lines: an exploratory study. J Membr Biol 2014; 247: 357–360. [DOI] [PubMed] [Google Scholar]
- 9. Higgins LH, Withers HG, Garbens A, et al. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim Biophys Acta 2009; 1787: 1433–1443. [DOI] [PubMed] [Google Scholar]
- 10. Predina JD, Judy B, Fridlender ZG, et al. A positive-margin resection model recreates the postsurgical tumor microenvironment and is a reliable model for adjuvant therapy evaluation. Cancer Biol Ther 2012; 13: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silberstein JL, Eastham JA. Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J Urol 2014; 30: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dotan ZA, Kavanagh K, Yossepowitch O, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol 2007; 178: 2308–2312. [DOI] [PubMed] [Google Scholar]
- 13. Luchey AM, Lin HY, Yue B, et al. Implications of definitive prostate cancer therapy on soft tissue margins and survival in patients undergoing radical cystectomy for bladder urothelial cancer. J Urol 2015; 194: 1220–1225. [DOI] [PubMed] [Google Scholar]
- 14. Blanco-Prieto S, Barcia-Castro L, Paez de, la Cadena M, et al. Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. BMC Cancer 2017; 17: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corn PG, Wang F, McKeehan WL, et al. Targeting fibroblast growth factor pathways in prostate cancer. Clin Cancer Res 2013; 19: 5856–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ridge SM, Bhattacharyya D, Dervan E, et al. Secreted factors from metastatic prostate cancer cells stimulate mesenchymal stem cell transition to a pro-tumourigenic ‘activated’ state that enhances prostate cancer cell migration. Int J Cancer 2018; 142: 2056–2067. [DOI] [PubMed] [Google Scholar]
- 17. Xu Y, Hou R, Lu Q, et al. MiR-491–5p negatively regulates cell proliferation and motility by targeting PDGFRA in prostate cancer. Am J Cancer Res 2017; 7: 2545–2553. [PMC free article] [PubMed] [Google Scholar]
- 18. Neveu B, Moreel X, Deschenes-Rompre MP, et al. IL-8 secretion in primary cultures of prostate cells is associated with prostate cancer aggressiveness. Res Rep Urol 2014; 6: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong Y, Chippada-Venkata UD, Galsky MD, et al. Elevated circulating tissue inhibitor of metalloproteinase 1 (TIMP-1) levels are associated with neuroendocrine differentiation in castration resistant prostate cancer. Prostate 2015; 75: 616–627. [DOI] [PubMed] [Google Scholar]
- 20. Gong Y, Scott E, Lu R, et al. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One 2013; 8: e77366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm 2014; 2014: 292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumari N, Agrawal U, Mishra AK, et al. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumour Biol 2017; 39: 1010428317697552. [DOI] [PubMed] [Google Scholar]
- 23. Lo UG, Lee CF, Lee MS, et al. The role and mechanism of epithelial-to-mesenchymal transition in prostate cancer progression. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niknami Z, Eslamifar A, Emamirazavi A, et al. The association of vimentin and fibronectin gene expression with epithelial–mesenchymal transition and tumor malignancy in colorectal carcinoma. Excli J 2017; 16: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]