Abstract
Lymphovascular invasion (LVI) by urothelial carcinoma of the upper urinary tract (UC-UUT) is associated with an unfavorable prognosis. However, a high proportion of patients with UC-UUT are unable to receive the recommended doses of cisplatin-based adjuvant chemotherapy due to advanced age or renal dysfunction resulting from nephroureterectomy. Tegafur-uracil is an oral form of 5-fluorouracil whose efficacy is influenced by the activities of enzymes associated with its metabolism, such as dihydropyrimidine dehydrogenase (DPD), orotatephosphoribosyltransferase (OPRT) and thymidylate synthase (TS). The aim of the present study was to investigate the efficacy of adjuvant 5-fluorouracil chemotherapy for UC-UUT with LVI, and to assess the expression of enzymes associated with 5-fluorouracil metabolism as promising biomarkers of therapy efficacy. The present study retrospectively investigated 52 cases of UC-UUT. Following nephroureterectomy, tegafur-uracil was administered to 15 out of 30 patients with LVI who were not eligible for cisplatin-based adjuvant chemotherapy. Levels of DPD, OPRT and TS expression in tumor specimens were determined by reverse transcription-quantitative polymerase chain reaction, and their associations with the efficacy of adjuvant 5-fluorouracil chemotherapy were analyzed. The levels of DPD, OPRT and TS expression were not associated with pathological factors or outcome, although a higher expression of TS was associated with a poorer outcome. Adjuvant 5-fluorouracil chemotherapy significantly improved the outcome of patients with lower DPD expression. However, the levels of OPRT and TS expression did not influence therapeutic efficacy. Adjuvant 5-fluorouracil chemotherapy appears to be effective for lymphovascular-invasive UC-UUT in patients with lower DPD expression.
Keywords: UC-UUT, 5-FU, DPD, tegafur-uracil, tegafur/gimeracil/oteracil
Introduction
Urothelial carcinoma of the upper urinary tract (UC-UUT) tends to be associated with intravesical recurrence, lymph node metastasis and distant metastasis, even after complete surgical resection, presumably due to occult micrometastasis present at the time of surgery and the thin wall and rich lymphatic drainage of the ureter (1,2). While intravesical recurrence can be controlled by transurethral resection, lymph node metastasis or distant metastasis tends to be refractory to chemotherapy, eventually leading to an unfavorable outcome (3). Advanced tumor stage, a higher nuclear grade and lymphovascular invasion (LVI) of UC-UUT are pathological factors conventionally associated with metastases and an unfavorable outcome (2,4–6). Among them, we have previously reported that LVI is associated with early recurrence and an unfavorable outcome after radical nephroureterectomy (1). Although gemcitabine- and cisplatin-based chemotherapy is frequently performed in an adjuvant setting for patients with such risk factors, its efficacy tends to be disappointing because of not only advanced age or renal dysfunction resulting from nephroureterectomy, but also the paucity of established biomarkers (7–10). Tegafur-uracil (UFT™, Taiho Pharmaceutical Co., Ltd., Tokyo, Japan) is an oral form of 5-fluorouracil (5-FU) that can be administered to a wide range of patients because of its lower incidence of severe adverse events (11,12). 5-FU is generally administered as adjuvant chemotherapy to patients with cancers of the lung and colon (13–17), and its antitumor effect is thought to be associated with the expression of enzymes related to 5-FU metabolism, such as dihydropyrimidine dehydrogenase (DPD), orotatephosphoribosyltransferase (OPRT) and thymidylate synthase (TS) (18). Intravenously or orally administered 5-FU is phosphorylated by OPRT and converted to 5-fluoro-2′-deoxyuridine 5′-monophosphate (FdUMP). It then inhibits TS, which is a rate-limiting enzyme for pyrimidine synthesis, and exerts an antitumor effect by inhibiting the synthesis of DNA. However, most of the administered 5-FU is broken down by DPD and thus unable to exert an antitumor effect (18). Therefore, underexpression of OPRT, overexpression of TS and overexpression of DPD are reported to be associated with 5-FU resistance in patients with urothelial carcinoma (19–21). Thus, the efficacy of 5-FU chemotherapy depends on inter-individual differences in the activity of these enzymes. Although it has been reported that the activity of these enzymes is associated with tumor stage or nuclear grade, their role in carcinogenesis has not yet been elucidated (19–22). On the other hand, there has been some controversy regarding the efficacy of 5-FU as adjuvant chemotherapy for UC-UUT. In the present study, we administered adjuvant 5-FU chemotherapy to UC-UUT patients with LVI who were at risk of poor outcome, and then we investigated the relationship between the efficacy of adjuvant 5-FU chemotherapy for lymphovascular-invasive UC-UUT and the expression of these enzymes, with the aim of detecting an effective biomarker.
Materials and methods
Patients and tissues
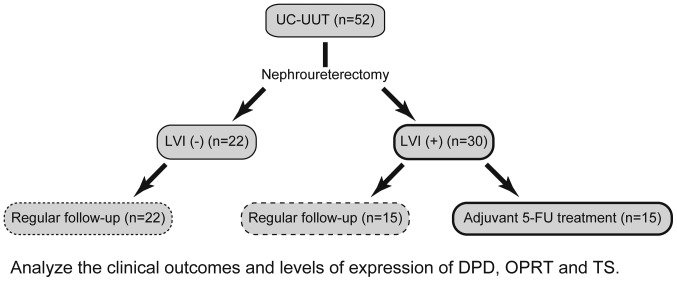
We retrieved archival formalin-fixed, paraffin-embedded (FFPE) tumor samples from 52 Japanese patients who had undergone nephroureterectomy for UC-UUT at Dokkyo Medical University Hospital (Tochigi, Japan) between 2002 and 2015. After surgical resection, UFT™ was administered at 200 mg/day to 15 of 30 patients with LVI who were ineligible for cisplatin-based adjuvant chemotherapy (Fig. 1). The median observation period was 44 months with a range of 1 to 145 months. Table I shows the adjuvant 5-FU treatment status and pathological data for the patients. Pathological factors were assessed in accordance with the TNM tumor classification (23). The sites of initial relapse and adjuvant 5-FU treatment status are shown in Table II. This study was conducted in accordance with the Helsinki Declaration and approved by the institutional ethics review board of Dokkyo Medical University Hospital (approval no. 24023). Each patient signed an informed consent form that had been approved by our institutional Committee on Human Rights in Research. All samples were anonymized before analysis to guarantee protection of patient privacy.
Figure 1.
Procedure for case classification and analysis. Nephroureterectomy was performed on 52 patients with UC-UUT. Following surgical resection, oral 5-FU was administered to 15 of 30 patients with LVI who were ineligible for cisplatin-based adjuvant chemotherapy. The remaining 37 patients underwent regular follow-up. Subsequently, the clinical outcomes and levels of expression of DPD, OPRT and TS were analyzed. UC-UUT, urothelial carcinoma of the upper urinary tract; 5-FU, 5-fluorouracil; LVI, lymphovascular invasion; DPD, dihydropyrimidine dehydrogenase; OPRT, orotatephosphoribosyltransferase; TS, thymidylate synthase.
Table I.
Conventional pathological factors and adjuvant 5-FU treatment status.
| pT | pN | Grade | ||||
|---|---|---|---|---|---|---|
| Factors | ≤2 | ≥3 | 0 or X | ≥1 | ≤2 | 3 |
| LVI (+), 5-FU (+) | 4 | 11 | 14 | 1 | 6 | 9 |
| LVI (+), 5-FU (−) | 2 | 13 | 11 | 4 | 4 | 11 |
| LVI (−), 5-FU (−) | 20 | 2 | 22 | 0 | 18 | 4 |
LVI, lymphovascular invasion; 5-FU, 5-fluorouracil.
Table II.
Initial relapse site and adjuvant 5-FU treatment status.
| Variable | Urinary bladder | Lymph node | Lung | Bone | Liver | Ureter | Local recurrence | No relapse |
|---|---|---|---|---|---|---|---|---|
| LVI (+), 5-FU (+) | 4 | 5 | 0 | 0 | 0 | 0 | 0 | 6 |
| LVI (+), 5-FU (−) | 0 | 5 | 3 | 1 | 1 | 0 | 2 | 3 |
| LVI (−), 5-FU (−) | 6 | 2 | 0 | 0 | 0 | 1 | 0 | 13 |
LVI, lymphovascular invasion; 5-FU, 5-fluorouracil.
RNA extraction and quantitative RT-PCR
Tumor cells were collected from FFPE tissue samples using laser-capture microdissection. Total RNA was extracted from the cells using an RNeasy FFPE kit (Qiagen, Inc., Valencia, CA, USA), and cDNA was prepared using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in accordance with the manufacturer's instructions. The cDNA was then pre-amplified using a TaqMan PreAmp Master Mix kit (Applied Biosystems; Thermo Fisher Scientific, Inc.), and quantitative RT-PCR was performed using a TaqMan low-density array (LDA) (Applied Biosystems; Thermo Fisher Scientific, Inc.) for determining the relative levels of expression of mRNAs for DPD, OPRT and TS, as reported previously (24,25). Briefly, 2.5 µl of cDNA was pre-amplified using 2× TaqMan PreAmp Master Mix and a pool of 0.2× TaqMan Gene Expression Assays in a 10-µl PCR reaction volume (Applied Biosystems; Thermo Fisher Scientific, Inc.). Pre-amplification was performed under the following thermal cycling conditions: 95°C for 10 min followed by 14 cycles at 95°C for 15 sec, and 60°C for 4 min. A pre-amplified cDNA sample was diluted 20-fold in Tris-EDTA buffer, then 25 µl of pre-amplified cDNA was added to 25 µl of nuclease-free water and 50 µl of 2× TaqMan Gene Expression Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The mixture was then applied to the loading port of the TaqMan LDA. The LDA was centrifuged twice and PCR amplification was performed using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal cycling conditions for PCR amplification were as follows: 50°C for 2 min and 94.5°C for 10 min followed by 40 cycles at 97°C for 30 sec and 59.7°C for 1 min. The quantitative cycle (Cq) value detected was inversely proportional to the amount of cDNA. The Cq value for β-actin (ACTB) included in the LDA was used as a reference. The expression levels of the 3 genes relative to that of ACTB were calculated as the ratios between the differences in the Cq values (26).
Statistical analysis
Differences between two groups were analyzed by Mann-Whitney U test. Overall survival (OS) and progression-free survival (PFS) curves were drawn by the Kaplan-Meier method, and differences in survival were examined by log-rank test with Bonferroni correction for pairwise multiple comparisons. In all analyses, P<0.05 (Bonferroni adjusted P<0.0167) was considered to indicate a statistically significant difference. Data were analyzed using R version 3.2.2 (www.r-project.org).
Results
Levels of DPD, OPRT and TS expression are not associated with pathological factors
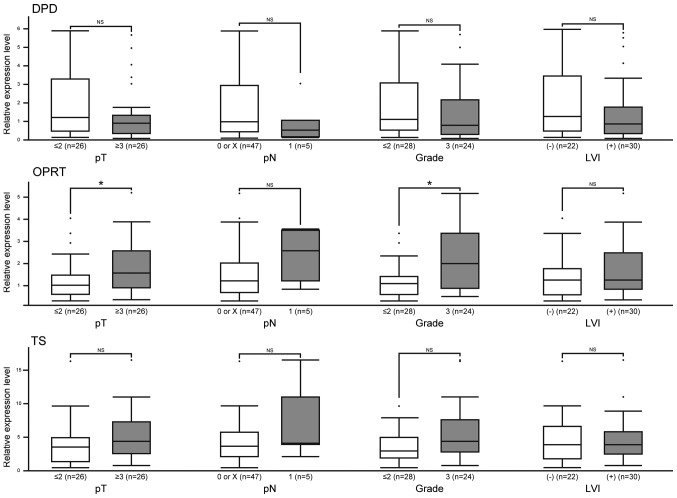
Clinicopathological characteristics of the patients are shown in Table SI. There were 39 male and 13 female patients with a mean age of 70 years (rage 45–85 years). None of them developed any severe adverse events. We first analyzed the relationship between conventional pathological factors and the levels of expression of enzymes related to 5-FU metabolism. The levels of DPD, OPRT and TS expression were not associated with pT stage, pN stage, nuclear grade or LVI; however, higher expression of OPRT was associated with high pT stage and nuclear grade (Fig. 2).
Figure 2.
Association between the levels of expression of enzymes associated with 5-FU metabolism and conventional pathological factors. The expression levels of DPD, OPRT and TS were not associated with conventional pathological factors, although a higher expression of OPRT was associated with high pT stage and nuclear grade. Reverse transcription-quantitative polymerase chain reaction analyses were performed for 52 cases of UC-UUT. The y-axis displays the level of expression relative to β-actin. *P<0.05, as indicated. NS, not significant; LVI, lymphovascular invasion; DPD, dihydropyrimidine dehydrogenase; OPRT, orotatephosphoribosyltransferase; TS, thymidylate synthase.
Levels of DPD and OPRT expression are not associated with clinical outcomes
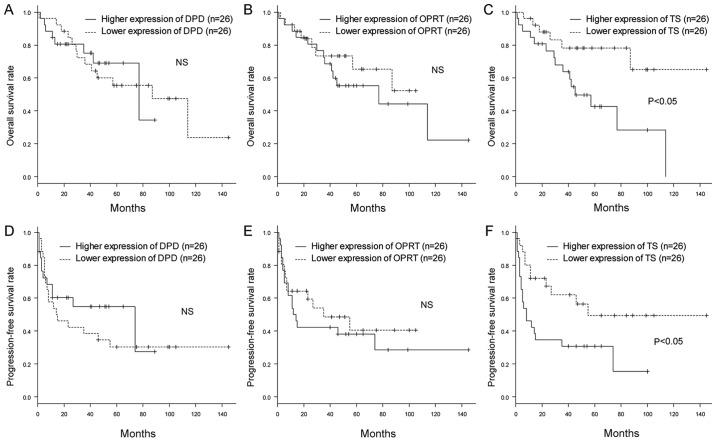
We then investigated the influences of DPD, OPRT and TS expression on OS and PFS in UC-UUT patients. We divided the patients into two groups according to the median level of expression of each gene, and compared the OS and PFS rates between them. Kaplan-Meier plots showed that patients with higher expression of TS had poorer OS and PFS rates than those with lower expression. On the other hand, the levels of DPD and OPRT expression were not associated with the OS and PFS rates (Fig. 3). Furthermore, to exclude the influence of adjuvant 5-FU chemotherapy on outcome, we excluded the patients who had received 5-FU and also compared the OS and PFS rates between them according to the expression of each gene. The patients with lower expression of DPD and higher expression of TS had poorer PFS rates. However, the level of TS expression was not associated with OS rate (Fig. S1).
Figure 3.
Association between the expression levels of 5-FU metabolism-associated enzymes and clinical outcomes. The overall survival rates (shown by Kaplan-Meier curves) for patients with higher expression levels of (A) DPD, (B) OPRT and (C) TS in their primary tumors were compared with those exhibiting lower expression. The progression-free survival rates (shown by Kaplan-Meier curves) for patients with higher expression levels of (D) DPD, (E) OPRT and (F) TS were also compared. The patients with a higher expression of TS exhibited lower rates of overall survival and progression-free survival than those with lower expression (P<0.05 determined by log rank test). The patients with higher expression levels of DPD and OPRT showed no significant differences in the survival curves. NS, not significant; 5-FU, 5-fluorouracil; LVI, lymphovascular invasion; DPD, dihydropyrimidine dehydrogenase; OPRT, orotatephosphoribosyltransferase; TS, thymidylate synthase.
Poor outcome of UC-UUT with LVI and efficacy of adjuvant 5-FU chemotherapy
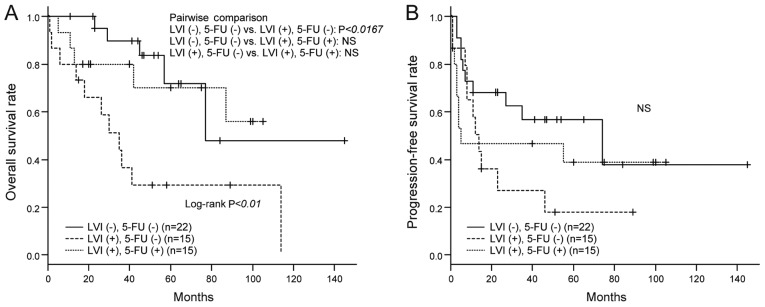
To evaluate the efficacy of adjuvant 5-FU chemotherapy, we compared the OS and PFS rates among the three groups of patients divided according to LVI and 5-FU treatment status. As shown in Fig. 4A, UC-UUT patients with LVI had poorer OS than those without LVI. However, there were no significant inter-group differences between UC-UUT patients with LVI and those without LVI when adjuvant 5-FU was administered to the former. We also investigated the improvement of OS rates resulting from adjuvant 5-FU administration in UC-UUT patients with LVI, but the degree of improvement did not reach a statistically significant level. The PFS rates showed no significant inter-group differences (Fig. 4B).
Figure 4.
Influence of LVI and adjuvant 5-FU chemotherapy on clinical outcome. (A) The overall survival rates (shown by Kaplan-Meier curves) of patients without LVI who did not receive adjuvant 5-FU chemotherapy, those of patients with LVI who did not receive 5-FU and those of patients with LVI who received 5-FU were compared. The patients with LVI who did not receive 5-FU exhibited lower overall survival rates than those without LVI (P<0.0167 determined by Bonferroni correction for pairwise multiple comparisons). No significant differences were observed between the patients with LVI who received 5-FU and the patients without LVI. (B) Progression-free survival rates (shown by Kaplan-Meier curves) were also compared, but no significant differences were observed. NS, not significant; LVI, lymphovascular invasion; 5-FU, 5-fluorouracil.
Adjuvant 5-FU chemotherapy improves the OS and PFS rates of patients with lymphovascular-invasive UC-UUT and lower DPD expression
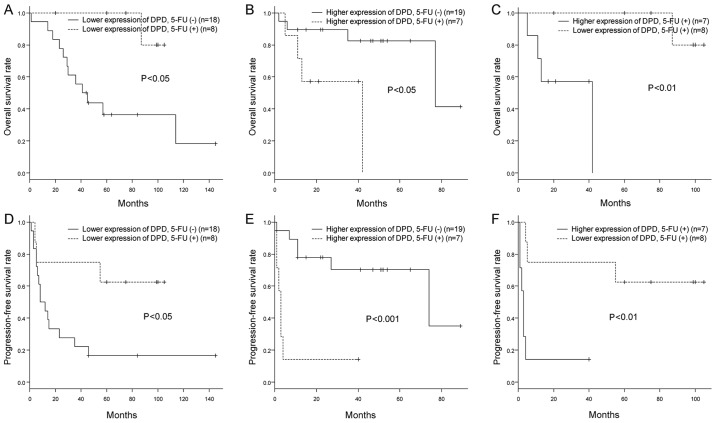
We further investigated the relationship between the efficacy of adjuvant 5-FU chemotherapy for lymphovascular-invasive UC-UUT and the levels of expression of enzymes related to 5-FU metabolism. We classified the patients according to their median level of expression of DPD, OPRT or TS, and then analyzed their OS and PFS rates in relation to 5-FU administration status. Interestingly, patients who had received 5-FU, especially those whose primary tumors had lower levels of DPD expression, had better OS and PFS rates (Fig. 5A and D). On the other hand, patients with higher expression of DPD had rather poor OS and PFS rates, regardless of 5-FU administration (Fig. 5B and E). Furthermore, among the patients who had received 5-FU, the OS and PFS rates were better for those who had lower expression of DPD than for those who had higher expression (Fig. 5C and F). No significant relationships were found between the efficacy of adjuvant 5-FU chemotherapy and the levels of OPRT and TS expression (Figs. S2 and S3).
Figure 5.
Association between efficacy of adjuvant 5-FU chemotherapy and expression of DPD. (A) The overall survival rates (shown by Kaplan-Meier curves) of patients with lower DPD expression who received adjuvant 5-FU chemotherapy were compared with those of patients who did not. The UC-UUT patients who received 5-FU all had LVI, and among those who did not receive 5-FU, some had LVI and some did not. The patients with lower expression levels of DPD who received 5-FU showed higher overall survival rates than those who did not (P<0.05 determined by log rank test). (B) The overall survival rates (shown by Kaplan-Meier curves) of patients with higher DPD expression who received adjuvant 5-FU chemotherapy were compared with those of patients who did not. The UC-UUT patients who received 5-FU all had LVI, and among those who did not receive 5-FU, some had LVI and some did not. The patients with higher expression of DPD who received 5-FU showed lower overall survival rates than those who did not (P<0.05 determined by log rank test). (C) The overall survival rates (shown by Kaplan-Meier curves) of patients with lower expression levels of DPD who received adjuvant 5-FU chemotherapy were compared with those of patients with higher expression levels of DPD who also received the therapy. The two groups were comprised of UC-UUT patients with LVI. The patients with a lower expression of DPD showed higher overall survival rates than those with higher expression (P<0.01 determined by log rank test). (D-F) Progression-free survival rates were also investigated for these 3 different group comparisons, and similar results were obtained. LVI, lymphovascular invasion; 5-FU, 5-fluorouracil; UC-UUT, urothelial carcinoma of the upper urinary tract; DPD, dihydropyrimidine dehydrogenase.
Discussion
Complete surgical resection is one of the most important factors for eradication of UC-UUT. However, even when this has been achieved, some patients, particularly those with LVI, suffer early recurrence and metastasis, and have poorer clinical outcomes (1,3–5). In this study also, patients with LVI showed poorer OS rates than those without LVI. It is difficult to control distant metastasis of UC-UUT by surgical resection (27), and therefore the efficacy of chemotherapy is limited (3,28). Considering these characteristics of UC-UUT, it can be concluded that reducing the incidence of recurrence and distant metastasis is the most important consideration if clinical outcomes are to be improved. Although cisplatin-based adjuvant chemotherapy is often performed for UC-UUT patients with risk factors for recurrence or distant metastasis, its efficacy is debatable due to the nephrotoxicity of cisplatin and the impairment of renal function caused by nephroureterectomy (7–10). Furthermore, no biomarkers that can predict the efficacy of chemotherapy have yet been established.
UFT™ is an oral prodrug of 5-FU that is associated with less severe adverse events, and can be used for a wider variety of patients (11,12). In fact, adjuvant chemotherapy with this oral form of 5-FU, which contains a DPD inhibitor, is commonly used for patients with lung and colon cancer, and helps to improve their prognosis (13–17). Some groups have reported the efficacy of adjuvant chemotherapy with oral 5-FU for urothelial carcinoma of the urinary bladder, although no unified view has yet emerged (29–31). Also, the efficacy of adjuvant 5-FU chemotherapy for UC-UUT has not yet been investigated (21). The present study was designed to assess the efficacy of 5-FU for reducing the rate of recurrence and prolonging the survival of UC-UUT patients undergoing radical nephroureterectomy, especially those with LVI. None of the patients included developed any severe adverse events associated with the oral 5-FU agent, suggesting that this form of adjuvant chemotherapy may be an effective option, especially for UC-UUT patients with LVI who are not eligible for cisplatin-based adjuvant chemotherapy. Even though UC-UUT patients with LVI may be at risk of poor outcome, we found no significant difference in OS rates after 5-FU administration between them and UC-UUT patients without LVI. However, despite the importance of reducing the rates of recurrence and distant metastasis in patients with UC-UUT, we found that adjuvant 5-FU chemotherapy did not significantly improve the PFS. This might have been attributable to the small number of patients we analyzed, and the fact that intravesical recurrence can be treated easily by transurethral resection. In fact, Harada et al (31) have reported that adjuvant chemotherapy using oral 5-FU for urothelial carcinoma of the urinary bladder did not reduce the incidence of intravesical recurrence. Interestingly, despite the small number of UC-UUT patients we studied, those with LVI who had received adjuvant 5-FU did not develop visceral metastases, in contrast to those who had not received it. These results suggest that adjuvant 5-FU chemotherapy might reduce the incidence of visceral metastasis, and thus improve prognosis.
We also investigated biomarkers that could be applicable for predicting the efficacy of adjuvant 5-FU chemotherapy. It is well known that the antitumor effect of 5-FU is influenced by the activities of enzymes related to its metabolism, such as DPD, OPRT and TS. In urothelial carcinoma, underexpression of OPRT, overexpression of TS and overexpression of DPD are reported to be associated with 5-FU resistance in vitro and in vivo (19–21). To our knowledge, no previous studies have investigated the correlation between the efficacy of adjuvant 5-FU chemotherapy and the levels of expression of 5-FU metabolism-related enzymes in patients with UC-UUT. In this study, when we focused on the relationship between 5-FU administration and DPD expression, we found that both the OS and PFS rates for patients with lower DPD expression were improved by adjuvant 5-FU therapy to a greater degree than in patients who did not receive 5-FU. Taking into consideration that all of the patients who received 5-FU chemotherapy were LVI-positive and thus at risk of a poor outcome, these results suggest that the level of DPD expression had a major influence on the efficacy of adjuvant 5-FU chemotherapy for UC-UUT, and that such chemotherapy would be highly effective for patients with lower expression of DPD. On the other hand, the patients with higher expression of DPD showed poorer OS and PFS rates, despite administration of adjuvant 5-FU. This might have been due to immediate breakdown of 5-FU by DPD, and the fact that the adjuvant 5-FU group included patients with LVI. In addition, among the patients who received 5-FU, the OS and PFS rates for those with lower expression of DPD were better than those for patients with higher expression. These results support the contention that the level of DPD expression influences the efficacy of adjuvant 5-FU chemotherapy for UC-UUT. Although DPD deficiency has been reported to increase the toxicity of 5-FU (18), none of the present patients developed any severe adverse events, irrespective of DPD expression. This may have been attributable to the low dose of 5-FU we administered.
In association with pathological factors, some groups have reported that DPD, OPRT and TS are associated with a high stage and high grade of urothelial carcinoma (19–22), and that OPRT and TS expression are associated with poor outcome (19,21,22), although DPD was not reported to be associated with outcome (20,21). Despite an apparent association of OPRT and TS expression with tumor cell proliferation, their role in carcinogenesis has not yet been confirmed (19,22). The associations among pathological factors, outcomes, 5-FU sensitivity and expression of some enzymes demonstrated in the present study were not concordant with previous reports, possibly because we analyzed samples of mRNA rather than protein expression or enzyme activity. Furthermore, biological differences in the cell lines and patients studied might have affected 5-FU sensitivity. In particular, TS and DPD expression might have had a stronger influence on outcome and 5-FU sensitivity than other factors. However, no definitive conclusion can be drawn at this stage.
Our present findings suggest that the level of DPD expression in patients undergoing surgical resection for UC-UUT might be a useful biomarker for predicting the efficacy of adjuvant 5-FU chemotherapy. Although this study was designed to investigate the level of DPD mRNA expression in tumor specimens, it has been reported that the levels of mRNA expression and protein activity of DPD are not necessarily correlated (18). Because the present data were limited in view of our retrospective design and the small numbers of patients studied, it will be necessary to conduct a large-scale randomized controlled trial to investigate the activity of DPD protein, interactions among enzymes related to 5-FU metabolism, and the metabolic pathways associated with the efficacy of 5-FU for treatment of UC-UUT. In addition, an oral 5-FU agent including a stronger DPD inhibitor, such as tegafur/gimeracil/oteracil (S-1™) (Taiho Pharmaceutical Co., Ltd.), might be more effective for patients with higher expression of DPD who do not show a good response to the UFT™ that was employed in the present study (32).
In conclusion, adjuvant 5-FU chemotherapy can improve the outcome of patients with lymphovascular-invasive UC-UUT and low expression of DPD. Assessment of the DPD expression level in UC-UUT might therefore be helpful for predicting the effectiveness of adjuvant 5-FU chemotherapy.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Kyoko Arai and Mrs. Hitomi Yamazaki (Department of Urology, Dokkyo Medical University, Tochigi, Japan) for their technical assistance.
Glossary
Abbreviations
- UC-UUT
urothelial carcinoma of the upper urinary tract
- LVI
lymphovascular invasion
- 5-FU
5-fluorouracil
- DPD
dihydropyrimidine dehydrogenase
- OPRT
orotatephosphoribosyltransferase
- TS
thymidylate synthase
- FdUMP
5-fluoro-2′-deoxyuridine 5′-monophosphate
- FFPE
formalin-fixed, paraffin-embedded
- LDA
low-density array
- Cq
quantitative cycle
- ACTB
β-actin
- OS
overall survival
- PFS
progression-free survival
Funding
This work was supported in part by Taiho Pharmaceutical Co., Ltd.
Availability of data and materials
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
TN and TaK conceived and designed the study. TN, TsK, HA, TU, YT, IS, KS, KT, DN, GN, HK, HY, HB and TaK acquired the clinical data and samples. TN and TaK performed the experiments and statistical analyses, and interpreted the data. TN wrote the manuscript. TaK critically revised the manuscript for important intellectual content and supervised the project. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Ethics Review Board of Dokkyo Medical University Hospital (Tochigi, Japan) and was conducted in accordance with the Helsinki Declaration. Each patient signed an informed consent form that had been approved by our Institutional Committee on Human Rights in Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kamai T, Shirataki H, Nakanishi K, Furuya N, Kambara T, Abe H, Oyama T, Yoshida K. Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer. 2010;10:164. doi: 10.1186/1471-2407-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: A 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/S0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, Shirotake S, et al. Patient characteristics and outcomes in metastatic upper tract urothelial carcinoma after radical nephroureterectomy: The experience of Japanese multi-institutions. BJU Int. 2013;112:E28–E34. doi: 10.1111/bju.12133. [DOI] [PubMed] [Google Scholar]
- 4.Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, Lotan Y, Remzi M, Bolenz C, Langner C, Weizer A, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27:612–618. doi: 10.1200/JCO.2008.17.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuchi E, Horiguchi Y, Nakashima J, Hatakeyama N, Matsumoto M, Nishiyama T, Murai M. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol. 2005;174:2120–2124. doi: 10.1097/01.ju.0000181801.22474.8b. [DOI] [PubMed] [Google Scholar]
- 6.Ozsahin M, Zouhair A, Villà S, Storme G, Chauvet B, Taussky D, Gouders D, Ries G, Bontemps P, Coucke PA, Mirimanoff RO. Prognostic factors in urothelial renal pelvis and ureter tumours: A multicentre Rare Cancer Network study. Eur J Cancer. 1999;35:738–743. doi: 10.1016/S0959-8049(99)00012-X. [DOI] [PubMed] [Google Scholar]
- 7.Fujita K, Inamoto T, Yamamoto Y, Tanigawa G, Nakayama M, Mori N, Tsujihata M, Azuma H, Nonomura N, Uemura M. Role of adjuvant chemotherapy for lymph node-positive upper tract urothelial carcinoma and the prognostic significance of C-reactive protein: A multi-institutional, retrospective study. Int J Urol. 2015;22:1006–1012. doi: 10.1111/iju.12868. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Lee JS, Jeong CW, Kwak C, Kim HH, Ku JH. Adjuvant chemotherapy for locally advanced upper tract urothelial carcinoma: Updated results of the Seoul National University Hospital experience. Int Braz J Urol. 2015;41:1067–1079. doi: 10.1590/S1677-5538.IBJU.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaag MG, O'Malley RL, O'Malley P, Godoy G, Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58:581–587. doi: 10.1016/j.eururo.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane BR, Smith AK, Larson BT, Gong MC, Campbell SC, Raghavan D, Dreicer R, Hansel DE, Stephenson AJ. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer. 2010;116:2967–2973. doi: 10.1002/cncr.25043. [DOI] [PubMed] [Google Scholar]
- 11.Ho DH, Pazdur R, Covington W, Brown N, Huo YY, Lassere Y, Kuritani J. Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2′-tetrahydrofuryl)-5-fluorouracil. Clin Cancer Res. 1998;4:2085–2088. [PubMed] [Google Scholar]
- 12.Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, Greim G, Peters GJ, van der Born K, Wanders J, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: A randomised crossover trial in advanced colorectal cancer. Eur J Cancer. 2002;38:349–358. doi: 10.1016/S0959-8049(01)00371-9. [DOI] [PubMed] [Google Scholar]
- 13.Hamada C, Tanaka F, Ohta M, Fujimura S, Kodama K, Imaizumi M, Wada H. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol. 2005;23:4999–5006. doi: 10.1200/JCO.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Hamada C, Tsuboi M, Ohta M, Fujimura S, Kodama K, Imaizumi M, Wada H. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: An exploratory analysis from a meta-analysis of six randomized controlled trials. J Thorac Oncol. 2009;4:1511–1516. doi: 10.1097/JTO.0b013e3181bbf1f2. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Ohashi Y, Nakazato H, Koike A, Saji S, Suzuki H, Takagi H, Nimura Y, Hasumi A, Baba S, et al. Efficacy of oral UFT as adjuvant chemotherapy to curative resection of colorectal cancer: Multicenter prospective randomized trial. Langenbecks Arch Surg. 2002;386:575–581. doi: 10.1007/s00423-002-0278-x. [DOI] [PubMed] [Google Scholar]
- 16.Akasu T, Moriya Y, Ohashi Y, Yoshida S, Shirao K, Kodaira S, National Surgical Adjuvant Study of Colorectal Cancer Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: A multicenter randomized controlled trial. Jpn J Clin Oncol. 2006;36:237–244. doi: 10.1093/jjco/hyl014. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto J, Hamada C, Yoshida S, Kodaira S, Yasutomi M, Kato T, Oba K, Nakazato H, Saji S, Ohashi Y. An individual patient data meta-analysis of adjuvant therapy with uracil-tegafur (UFT) in patients with curatively resected rectal cancer. Br J Cancer. 2007;96:1170–1177. doi: 10.1038/sj.bjc.6603686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maring JG, Groen HJ, Wachters FM, Uges DR, de Vries EG. Genetic factors influencing pyrimidine-antagonist chemotherapy. Pharmacogenomics J. 2005;5:226–243. doi: 10.1038/sj.tpj.6500320. [DOI] [PubMed] [Google Scholar]
- 19.Mizutani Y, Wada H, Fukushima M, Yoshida O, Nakanishi H, Li YN, Miki T. Prognostic significance of orotate phosphoribosyltransferase activity in bladder carcinoma. Cancer. 2004;100:723–731. doi: 10.1002/cncr.11955. [DOI] [PubMed] [Google Scholar]
- 20.Mizutani Y, Wada H, Fukushima M, Yoshida O, Ukimura O, Kawauchi A, Miki T. The significance of dihydropyrimidine dehydrogenase (DPD) activity in bladder cancer. Eur J Cancer. 2001;37:569–575. doi: 10.1016/S0959-8049(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 21.Ide H, Kikuchi E, Hasegawa M, Kozakai N, Kosaka T, Miyajima A, Oya M. Prognostic significance of 5-fluorouracil metabolism-relating enzymes and enhanced chemosensitivity to 5-fluorouracil by 5-chloro 2,4-dihydroxy-pyridine in urothelial carcinoma. BMC Cancer. 2012;12:420. doi: 10.1186/1471-2407-12-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizutani Y, Wada H, Ogawa O, Yoshida O, Fukushima M, Nonomura N, Miki T. Prognostic significance of thymidylate synthase activity in bladder carcinoma. Cancer. 2001;92:510–518. doi: 10.1002/1097-0142(20010801)92:3<510::AID-CNCR1349>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Sobin LH, Gospodarowicz MK, Wittekind C. Wiley-Blackwell; New Jersey: 2009. TNM classification of malignant tumours. [Google Scholar]
- 24.Kimura M, Imamura F, Inoue T, Nishino K, Uchida J, Kumagai T, Okami J, Higasiyama M, Kamoshida S. Protein and mRNA expression of folic acid-associated enzymes as biomarkers for the cytotoxicity of the thymidylate synthase-targeted drugs, pemetrexed and S-1, in non-small cell lung cancer. Mol Clin Oncol. 2017;7:15–23. doi: 10.3892/mco.2017.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa W, Terashima M, Ochiai A, Kitada K, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M. Impact of insulin-like growth factor-1 receptor and amphiregulin expression on survival in patients with stage II/III gastric cancer enrolled in the adjuvant chemotherapy trial of S-1 for gastric cancer. Gastric Cancer. 2017;20:263–273. doi: 10.1007/s10120-016-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Abe T, Kitamura H, Obara W, Matsumura N, Tsukamoto T, Fujioka T, Hara I, Murai S, Shinohara N, Nonomura K. Outcome of metastasectomy for urothelial carcinoma: A multi-institutional retrospective study in Japan. J Urol. 2014;191:932–936. doi: 10.1016/j.juro.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Tanji N, Ozawa A, Miura N, Yanagihara Y, Sasaki T, Nishida T, Kikugawa T, Ikeda T, Ochi T, Shimamoto K, et al. Long-term results of combined chemotherapy with gemcitabine and cisplatin for metastatic urothelial carcinomas. Int J Clin Oncol. 2010;15:369–375. doi: 10.1007/s10147-010-0069-2. [DOI] [PubMed] [Google Scholar]
- 29.Kubota Y, Noguchi S, Hosaka M. UFT in bladder cancer. Oncology (Williston Park) 1999;13(Suppl 3):S112–S115. [PubMed] [Google Scholar]
- 30.Kubota Y, Hosaka M, Fukushima S, Kondo I. Prophylactic oral UFT therapy for superficial bladder cancer. Cancer. 1993;71:1842–1845. doi: 10.1002/1097-0142(19930301)71:5<1842::AID-CNCR2820710520>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Harada K, Miyake H, Terakawa T, Fujisawa M. Significance of uracil/tegafur for preventing intravesical recurrence of non-muscle invasive urothelial carcinoma of the bladder. Curr Urol. 2012;6:27–32. doi: 10.1159/000338866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takechi T, Fujioka A, Matsushima E, Fukushima M. Enhancement of the antitumour activity of 5-fluorouracil (5-FU) by inhibiting dihydropyrimidine dehydrogenase activity (DPD) using 5-chloro-2,4-dihydroxypyridine (CDHP) in human tumour cells. Eur J Cancer. 2002;38:1271–1277. doi: 10.1016/S0959-8049(02)00048-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the present study are available from the corresponding author on reasonable request.