Abstract
Background:To examine the effects of a short-term weight reducing program on body composition, eating behaviors, and health-related quality of life (HRQL) of sedentary obese women characterized by different obesity degrees. Methods:44 women with a BMI under 34.9 kg/m2 and 39 women with a BMI above 35 kg/m2 were studied. Fat mass and lean mass (electrical bioimpedance), eating behaviors (Three-Factor Eating Questionnaire), and HRQL (36-item short form, SF-36, questionnaire) were determined before and after weight loss. Results:Disinhibition and hunger scores and their subscales decreased after weight loss in both groups (0.0001 < p < 0.04). Restriction increased after weight reduction in all women (p = 0.02). Among the five restriction subscales, flexible restriction increased in women with a BMI above 35 kg/m2 (p = 0.008), whereas rigid restraint and avoidance of fattening foods increased in both groups (0.006 < p < 0.02). SF-36 Mental Component Score increased after weight loss in all women (p < 0.0001). Conclusion:A 3week weight reducing program changes selected eating behaviors and components of HRQL, irrespective of women's obesity degree. Data suggest that women with a BMI above 35 kg/m2 could have a better weight control in the long term because of their higher flexible restriction after weight loss when compared to those whose BMI was under 34.9 kg/m2.
Key Words: Health related quality of life, Obesity, Weight loss, Eating behaviors, Women
Introduction
In 2006, the prevalence of obesity reached 12.4% in France, whereas in 1997 and 2003 it was only 8.2 and 11.3%, respectively. This increase from 1997 to 2006 seems to be, however, more important in women (64%) than in men (40%) [1]. Obesity is also well-known to have genetic and environmental causes [2]. Considering environmental risk factors, obesity may result from energy imbalance due to insufficient physical activity [3] and/or hypercaloric diet [4]. Previous studies have reported that changes in both eating and exercise behaviors are more effective at reducing weight than an approach focusing on either behavior alone [5,6]. The relationships of eating behaviors with obesity are well recognized [7,8]. Indeed, obese subjects generally display higher scores for disinhibition [8–10] and susceptibility to hunger [8,11] than normal-weight individuals. Moreover, these eating behaviors were already found to be positively associated with women's adiposity, irrespective of their age, menopausal status and energy intake [8], whereas cognitive dietary restraint was negatively correlated with energy intake [12]. Longitudinal experiments have already investigated the impact of very low-calorie diet (medically defined as being 800 kcal/day or less [7,11]), behavioral changes [13,14], surgery [15], or aerobic exercise combined with caloric restriction [16] on eating behaviors in women. However, with the exception of one study performed on obese men who were subjected to a progressive weight reducing program [17], none of them has examined yet the effect of weight loss on eating behavior subscales in individuals with different obesity degrees.
On the other hand, obesity is known to deteriorate health related quality of life (HRQL) [18,19]. Although there is no standard definition of HRQL, it is well accepted as being a subjective, multidimensional assessment of the physical, psychological, and social domains of health [20,21]. Indeed, Doll et al. [22] and Kolotkin et al. [23], examining the relationship between obesity and quality of life assessed by the SF-36 questionnaire, found that increasing obesity degrees were associated with decreasing physical well-being, regardless of the presence or absence of other chronic medical conditions. Fontaine et al. [24] also suggested that long-term treatment induced weight loss among mildly to moderately overweight individuals improved HRQL and, particularly, physical functioning as well as role-physical referring to problems with work or other daily activities as a result of physical health, general health, vitality, and mental health domains. More recently, Lemoine et al. [16] reported improvements in mental well-being after a weight loss intervention in moderately obese women, irrespective of their menopausal status. However, to the best of our knowledge, no study has explored the impact of obesity degree on HRQL and its different dimensions in response to a short-term weight reducing program.
Aims of this study were thus i) to determine the effect of obesity degree in response to a 3-week weight reducing program on eating behaviors and HRQL in women and ii) to verify whether improvements in eating behaviors or HRQL could be related to body weight and/or fat mass losses.
Material and Methods
Subjects and Experimental Design
Participants recruited from the suburbs of Toulouse (in the southwest of France) were referred by their personal physician to a specialized institution located in the greater Toulouse area (Clinique du Château de Vernhes, Bondigoux, France) to participate in a weight reducing program. Among these participants, 83 healthy Caucasian pre- and postmenopausal women who were sedentary (exercising less than 30 min/week) and obese (BMI ranging from 30 to 42.4 kg/m2) were included in our study. In order to examine the impact of obesity on eating behaviors and HRQL after weight loss, three groups of women were formed according to the WHO obesity definition [25]. It is important to note that menopausal status did not influence eating behaviors and HRQL in our study (not shown). However, as only 10 women showed class III obesity (BMI above 40 kg/m2), but 29 women presented with class II obesity (BMI ranging from 35 to 39.9 kg/m2) and 44 women with class I obesity (BMI values of 30 to 34.9 kg/ m2), class II and III obesity groups were thus combined in the context of the present investigation. Therefore, 44 women aged 52 ± 10 years (mean ± standard deviation;, SD) whose BMI ranged from 30 to 34.9 kg/m2 and 39 women aged 53 ± 11 years with a BMI from 35 to 50.4 kg/m2 participated in our study. None of the women had identified disease, e.g. cardiomyopathy, endocrine disorders which may cause irregular menstrual cycles, or orthopedic limitations that could affect physical activity. Women on medication that may potentially influence the outcomes of this study (mainly β-blockers, sympathomimetics, cholesterol lowering drugs including statins, antihypertensive drugs, and thyroxine for which a dosage change occurred during the last 6 months or is expected during the course of the study) were excluded from the present study. Finally, premenopausal women using oral contraceptives and postmenopausal women on hormone therapy were also excluded. The experimental design was approved by the University Ethical Committee on Human Research for Medical Sciences, and all subjects gave their written informed consent to participate in this study conformed to the Helsinki Declaration before the beginning of the weight loss program.
Weight Reducing Program
Women were subjected to a multidisciplinary weight reducing program which combined dietary and physical activity instructions as well as psychological counseling 6 days/week during 3 weeks. During this period and regardless of their obesity degree, all women had four meals per day: a breakfast, a lunch, a snack and a dinner, and received a dietary plan (1,400 ± 200 kcal/day) which was composed of 20–25% proteins, 25–30% lipids, and 50–55% carbohydrates; no alcohol was allowed. Participants also followed a personalized physical training program with cycle ergometer and/or walking through 1 h/day, 6 days a week, for 3 weeks. Subjects’ heart rate (HR) was continuously monitored using a cardiofrequencemeter (Polar, FS1 type, Kempele, Finland) and intensity of exercise was set at 50% of their hypothetical maximal HR calculated from the following equation: 220 – age, where age is expressed in years.
Body composition, eating behaviors (restriction, disinhibition, and hunger scores as well as their corresponding subscales assessed by the Three-Factor Eating Questionnaire (TFEQ)) and HRQL (reflected by the SF-36 Physical Component and Mental Component Scores) were determined before and after the 3-week weight reducing program.
Body Composition
Body weight was measured to the nearest 0.1 kg using an electronic scale. Height was determined to the nearest 0.5 cm at head level, using a tape measure fixed to the wall, while subjects, in light clothing without shoes and in standing position, were looking straight ahead, their shoulders and buttocks against the wall, as well as joined feet and arms hanging on both sides. BMI was calculated as the ratio of weight (kg) to square height (m2). Fat mass and lean mass were measured by a standard electric bioimpedance technique (Bodystat 1500, Isle of Man, UK). Waist girth, obtained using a graduated flexible tape when subjects were in standing position, was measured at the narrowest circumference of the trunk. Waist circumference was chosen as a crude index of abdominal fat accumulation as it shows a high correlation with visceral adipose tissue accumulation assessed by computed tomography [26]. Anthropometric and body composition measures were repeated twice and then averaged [16].
Eating Behaviors
A French version of the TFEQ was completed by our participants before and after the weight reducing program. The TFEQ is a 51-item questionnaire initially developed by Stunkard and Messick [27] and validated for the French population [28]. Table 1 describes the three eating behaviors and their corresponding subscales with their respective range score. This questionnaire has already been validated in large cohorts which included middle-aged non-obese and obese men and women [8], and its three scales have been reported to show good test-retest reliability [27,28]. In addition, two of five subscales of TFEQ restriction, i.e. flexible and rigid controls, have been validated by Westenhoefer [29] and Westenhoefer et al. [30], and the eight other TFEQ subscales have been largely described by Bond et al. [31]. Although several studies have already used the TFEQ to examine eating behaviors after weight loss [14,16,17,32, 33], only one of them [17] has considered the different subscales in this context.
Health Related Quality of Life
HRQL was measured using the French version of the Medical Outcomes Study SF-36 Health Survey questionnaire [34] which is composed of 36 items that assess the following eight HRQL dimensions or scales: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. For each of the eight domains, scores were transformed linearly to a scale ranging from 0 (maximal impairment) to 100 (no impairment). Physical functioning, role physical, and bodily pain reflect the physical component score (PCS), whereas general health, vitality, social functioning, role emotional, and mental health comprise the mental component score (MCS). The eight dimensions were transformed to a scale of 0–100, and the two summary scales were aggregated from z-score transformations of the eight dimensions and then transformed to a mean of 50 and a standard deviation of 10 [35].
Calculation of PCS and MCS was performed using the following equations [35]:
PCS z-score = (physical functioning score × 0.42402) + (role physical score × 0.35119) + (bodily pain score × 0.31754) + (general health score × 0.24954) + (vitality score × 0.02877) + (social functioning score × −0.00753) + (role emotional score × −0.19206) + (emotional well-being score × −0.22069) (1).
MCS z-score = (physical functioning score × −0.22999) + (role physical score × −0.12329) + (bodily pain score × −0.09731) + (general health score × −0.01571) + (vitality score × 0.23534) + (social functioning score × 0.26876) + (role emotional score × 0.43407) + (emotional well-being score × 0.48581) (2).
PCS score = (PCS z-score × 10) + 50 (3).
MCS score = (MCS z-score × 10) + 50 (4).
However, among the 83 women selected for the purpose of our study, only 54 (25 women with a BMI under 34.9 kg/m2 and 29 women with a BMI above 35 kg/m2) agreed to complete this questionnaire before and after the intervention planned.
Statistical Analysis
Results are given as means ± standard error (SE) in figures and as means ± standard deviation (SD) in tables. The primary outcome variables (eating behaviors and HRQL) assessed before and after weight reduction were compared between two groups of women according to their obesity degree. A two-way analysis of variance (ANOVA) with repeated measures on one factor (time) was performed to compare women with a BMI lower than 34.9 kg/m2 with those whose BMI was greater than 35 kg/m2, for eating behaviors and HRQL as well as body composition before and after weight loss. When ANOVA was significant, simple test effects were performed to detect which conditions were statistically different from each other. All analyses were conducted with the SAS statistical computer program package (SAS Institute, Carry, NC, USA), and statistical significance was set at p < 0.05.
Results
Physical characteristics at baseline of both groups of women are presented in table 2. As expected, body weight and fat mass were higher in women characterized by a BMI greater than 35 kg/m2 than in women with a BMI lower than 34.9 kg/m2. However, age and lean mass were not significantly different between groups.
Effect of the Weight Reducing Program on Body Composition in Women according to Their Obesity Degree
Body weight and thus BMI decreased in both groups in response to weight reduction (p < 0.0001). Body weight loss and BMI decrease were similar when comparing women with the lowest versus the highest obesity degree. Not surprisingly, fat mass reduction was more important in women with a BMI above 35 kg/m2 than in those whose BMI was lower than 34.9 kg/m2 (–2.5 ± 1.9 kg vs. −1.8 ± 1.0 kg; p < 0.0001) (mean ± SD). Lean mass decrease (p < 0.02) was, however, comparable in both groups (table 2).
Effect of the Weight Reducing Program on Eating Behaviors (TFEQ) in Women according to Their Obesity Degree
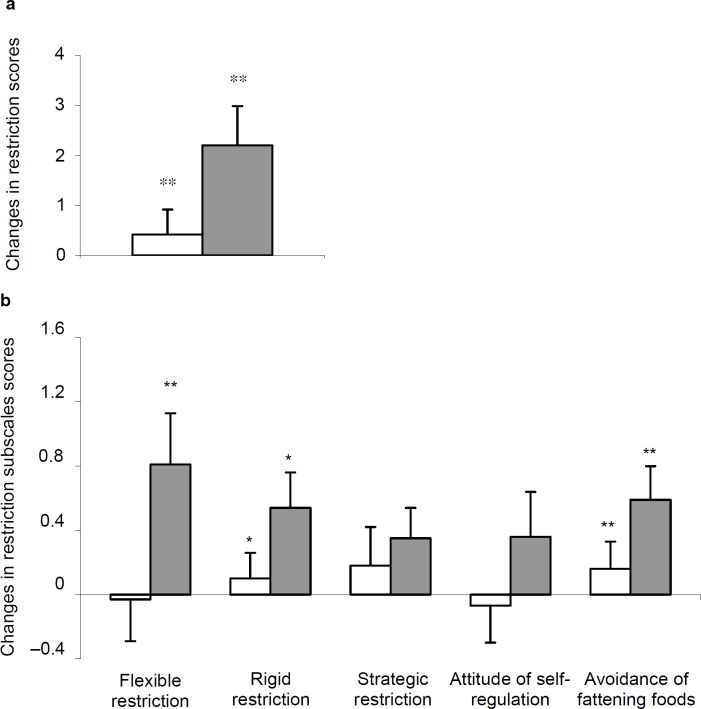
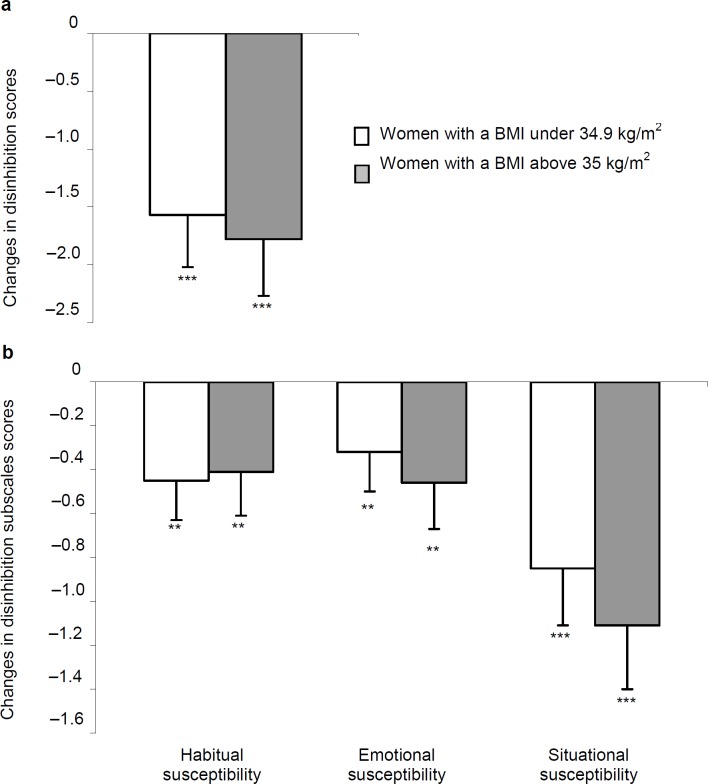
Before and after weight loss, women had similar eating behaviors (restriction, disinhibition, and hunger as well as their corresponding subscales), irrespective of their obesity degree. Although, the restriction score (fig. 1a) increased (p = 0.004), both disinhibition (fig. 2a) and hunger (fig. 3a) scores decreased in both groups (p < 0.0001), after weight loss. Changes in eating behaviors were similar, irrespective of women's obesity (p values ranging from 0.14 to 0.79).
Analysis of the TFEQ subscales scores revealed a lack of between-group differences before and after weight loss. As shown in figure 1b, flexible restriction increased in women with a BMI above 35 kg/m2 only (p < 0.02), whereas rigid restraint and avoidance of fattening food scores increased similarly in both groups (p values ranging from 0.005 to 0.01). The three disinhibition and two hunger subscales scores were also decreased after weight loss in women (p values ranging from 0.0001 to 0.03), irrespective of their obesity degree (fig. 2b, 3b).
As illustrated in figure 4, SF-36 PCS remained unchanged in both groups after weight loss (–0.9 ± 5.1 vs. 1.1 ± 6.5; p = 0.21), whereas SF-36 MCS increased by 10.1 ± 11.5 in all women after weight loss (p < 0.0001).
Relationships between Body Composition Assessed at Baseline and after Weight Loss and Changes in Eating Behaviors or HRQL
Correlational analyses were performed to verify whether changes in eating behaviors and HRQL could be related to women's body composition at baseline or after weight reduction according to their obesity degree. In the 83 women considered, positive relationships were observed between basal BMI and changes in both restriction (r = 0.27; p < 0.05) and attitude of self-regulation (r = 0.22; p = 0.05) scores. However, BMI reductions or fat mass losses were not related to changes in eating behaviors or HRQL scores (–0.15 < r < 0.24; 0.06 < p < 0.98). Finally, high levels of restriction at baseline were associated with fat mass reductions (r = −0.36; p < 0.05) exclusively in women with a BMI above 35 kg/m2.
Discussion
The present study was conducted to determine the impact of a 3-week weight reducing program on eating behaviors and HRQL in sedentary women taking into account their obesity degree. Whether improvements in eating behaviors and/or HRQL could be related to women's body composition at baseline or after weight loss was also examined.
Our data showed a similar decrease in body weight and fat mass in all women who participated to the short-term weight reducing program. The decrease in body weight (and thus BMI) and fat mass found in both groups of our study is in good agreement with the reduction in adiposity previously observed in obese women subjected to weight reducing programs combining aerobic exercise and dietary restriction [36–38]. The fact that our participants lost a similar fat mass as women subjected to long-term (3- to 6-month) weight reducing programs with comparable [38] or less rigid caloric restriction [36,37] indicates that short-term low-intensity physical activity associated with caloric restriction seems to be sufficient to promote body weight loss in women with different obesity degrees. Furthermore, the lean mass decrease of 13% seen after the short-term weight reducing program in all women is lower than that noted at the same time of long-term (lasting from 9 to 16 weeks) very-low- or low-caloric diet studies [39]. These results confirm that combining caloric restriction with physical activity is important to allow maintenance or limit lean mass reduction [39].
Our data revealed that all women have modified their eating behaviors after the 3-week weight reducing program, irrespective of their obesity degree. Indeed, the decreased disinhibition and hunger scores (20%) noted in both groups after weight reduction are concordant with previous observations in middle-aged obese women subjected to a long-term weight loss treatment [7]. However, the increased restriction score (11%) noted after 3 weeks of a long-term (24-week) caloric restriction in women with a BMI of 37.2 ± 5.6 kg/m2 [7] was lower than that of women whose BMI was above 35 kg/m2(21%) but higher than that of participants with a BMI lower than 34.9 kg/m2 (4%) in our 3 week-weight reducing program. Comparing our results with those of Foster et al. [7], it can thus be hypothesized that the duration of weight loss intervention may also have an impact on how eating behaviors are affected by the intervention. Indeed, restriction appears to be more dependent on the intervention length than disinhibition and hunger scores, especially in women with a BMI above 35 kg/m2. The positive relationship found between basal BMI and increased restriction suggests that high BMI at baseline was associated with elevated restriction after weight loss. In this regard, increased restriction was already shown to be one of the best predictors of weight maintenance after weight loss [40]. Although rigid restriction increased in all women after our 3-week weight reducing program, increased flexible restriction in women with a BMI above 35 kg/m2 suggested a more successful weight reduction and weight loss maintenance in the long term [14,32]. According to Westenhoefer et al. [30], rigid restriction actually seems to be associated with higher BMI as well as with more frequent and severe binge eating episodes. These results indicate that behavioral intervention is a potential factor involved in weight control which may be included in a weight reducing program in women, especially in those with a BMI under 34.9 kg/m2, in order to increase flexible and decrease rigid restriction. Although participants lost approximately 2.8% of their body weight after our short-term weight reducing program, we, in contrast to Provencher et al. [33], did not find any relationship between decreased body weight and habitual susceptibility to disinhibition and flexible restriction. However, the latter study [33] aimed at a lifestyle intervention, and not at a clinical weight reducing program as our study. The obesity degree seems to be an important factor involved in the increased restriction, even in a short-term weight reducing program. Our results emphasize the fact that women with a BMI above 35 kg/m2 have more favorable changes in selected eating behaviors than women with BMI values lower than 34.9 kg/m2. Again, restriction score appears to be the best predictor of fat mass decrease in women with a high BMI value. The lack of significant association between disinhibition or hunger scores at baseline and weight loss in our study was also concordant with previous findings reported by Foster et al. [7].
Concerning HRQL, we found similar SF-36 PCS and MCS at baseline, irrespective of women's obesity degree. However, previous studies showed high SF-36 scores among individuals with BMI levels ranging from underweight to morbid obesity, even after statistical adjustment for age, gender, and frequency of health service utilization [18]. Overweight and obesity (reflected by BMI values of 30–40 kg/m2 or more) have also been associated with low levels of subjective health status, especially physical well-being. Physical, but not emotional well-being was markedly deteriorated with increasing degrees of overweight and was reduced in obese subjects who did not show any other chronic condition [22]. Indeed, Kolotkin et al. [23,41] reported that increased BMI was associated with impaired HRQL, and more particularly with physical function. However, all women recruited in our study should be devoid of any physical problem in order to perform regular aerobic exercise during the 3-week intervention. This criterion may partly explain the unchanged SF-36 PCS observed after weight reduction, although its already high value at baseline may also be involved. In this regard, SF-36 PCS of our participants are higher than the norm population values (being approximately 50 ± 0.2, mean ± SE) [35]. Even if adults with BMI values above 40 kg/m2 showed a lower general health state and greater physical complications compared to individuals with a lower BMI threshold [42,43], our participants did not show any differences in SF-36 MCS at baseline. SF-36 MCS increased in all women, irrespective of their obesity degree. The increased SF-36 MCS noted after weight reduction is also in agreement with studies in which women participated to a program similar to ours [20,44]. However, the lack of relationships between weight reduction and changes in eating behaviors and/or in HRQL could be due to the magnitude of weight loss achieved in our study, as it is lower than the one reported after bariatric surgery [15] or in response to longer weight reducing interventions using strategies similar to ours [45,46]. Nevertheless, correlational analyses revealed that increases in the Physical and Mental Component Scores were related to decreased disinhibition, independently of body weight loss. A short-term weight reducing program seems thus to be sufficient to improve selected eating behaviors and components of HRQL of obese women with different obesity degrees.
Some limitations may, however, deserve further attention. As the weight reducing program of the Clinique du Château de Vernhes included dietary restriction and aerobic exercise, women recruited for the purpose of our study needed to be in good physical condition to perform regular aerobic exercise 1 h/day, 6 days a week, during 3 weeks. Therefore, women with cardiomyopathy, type 2 diabetes, endocrine disorders and/or orthopedic limitations that would have affected physical activity were excluded from our study. Therefore, the fact that obese women selected for the purpose of our study were thus rather healthy, probably more health conscious, and motivated in losing weight and improving their quality of life could also be questioned. Although consequences of possible or even likely sampling bias of the results obtained are difficult to estimate, it seems realistic to consider that our data could not be extended to the overall population. On the other hand, taking into account the short duration of our experiment (3 weeks) and the exclusion criteria listed in ‘Methods’, the number of women recruited for our study was unfortunately too low to consider the three different classes of obesity in our statistical analyses. Finally, although all participants received a caloric restriction of 1,400 ± 200 kcal/day (mean ± SD), irrespective of their obesity degree, this energy deficit was probably different among women with a BMI value above 35 kg/m2 and those whose BMI ranging between 30 and 34.9 kg/m2. We suggest that this issue should be better addressed in future experiments conducted on individuals with different obesity degrees.
Conclusion
Despite its short duration (3 weeks), our weight reducing program which combines aerobic physical activity and caloric restriction has an impact on selected eating behaviors and some components of HRQL of women who displayed different obesity degrees. Nevertheless, changes in restriction (flexible restriction and attitude of self-regulation) assessed by the TFEQ seem to be more important in women with a BMI above 35 kg/m2. Our results suggest that basal BMI of the women has to be taken into account when aiming to improve their eating behaviors. Moderate exercise combined with dieting, even in a short term, should thus be considered as a promising option to improve women's HRQL, irrespective of their obesity degree. Finally, in order to optimize clinical weight loss prescription, behavioral approaches specifically designed to increase flexible and to decrease rigid restriction scores are clearly warranted especially in women whose BMI is under 34.9 kg/m2.
Disclosure
The authors declared no conflict of interest.
Fig. 1.
Changes in a the restriction score and b its 5 subscales after weight reduction in women according to their BMI at baseline. Significant change at *p < 0.05; **p < 0.01.
Fig. 2.
Changes in a the disinhibition score and b its 3 subscales after weight reduction in women according to their BMI at baseline. Significant change at **p < 0.01; ***p < 0.0001.
Fig. 3.
Changes in a the hunger score and b its 2 subscales after weight reduction in women according to their BMI at baseline. Significant change at *p < 0.05; **p < 0.01; ***p < 0.0001.
Fig. 4.
Fig. 4. Changes in the SF-36 Physical Component Score (PCS) and Mental Component Score (MCS) after weight reduction in women according to their BMI at baseline. Significant change at ***p < 0.0001.
Table 1.
The Three-Factor Eating Questionnaire's behaviors and their subscales.
| TFEQ behaviors (range score) | TFEQ behaviors subscales (range score) | Examples from TFEQ |
|---|---|---|
| Restriction (0–21): Represents the intent to restrict food intake to control weight. |
Flexible restriction (0–7) [29,30]: A more gradual approach to eating, dieting and weight. |
Ex: ‘When I have eaten my quota of calories, I am usually good about not eating any more.’ |
|
Rigid restriction (0–7) [29,30]: An all-or-nothing approach of eating, dieting and weight. |
Ex: ‘I count my calories as a conscious means of controlling my weight.’ | |
|
Strategic restriction (0–4) [31]: Measures the purposeful choices that restrained eaters make about food intake. |
Ex: ‘I consciously hold back at meals in order to not gain weight.’ | |
|
Attitude of self-regulation (0–5) [31]: A conscious and internally behavior for the promotion of health. |
Ex: ‘Life is too short to worry about dieting.’ | |
|
Avoidance of fattening foods (0–4) [31]: A behavioral construct that targets a specific method of weight control. |
Ex: ‘I do not eat some foods because they make me fat.’ | |
| Disinhibition (0–16): Measures how external factors (environmental events and emotional reactions) disinhibit the control of eating. |
Habitual susceptibility (0–7) [31]: When circumstances could predispose to recurrent disinhibition. Emotional susceptibility (0–3) [31]: Disinhibition associated with negative affective states. |
Ex: ‘Since my weight goes up and down, I have gone on reducing diets more than once.’ Ex: ‘When I feel anxious, I find myself eating.’ |
|
Situational susceptibility (0–7) [31]: A type of disinhibition initiated by specific environmental cues. |
Ex: ‘I usually eat too much at social occasions, like parties and picnics.’ | |
| Hunger (0–14): Refers to food intake in response to feelings and perceptions of hunger. |
Internal locus (0–6) [31]: A type of hunger interpreted and regulated internally. |
Ex: ‘Dieting is so hard for me because I just get too hungry.’ |
| External locus (0–6) [31]: Triggered by external cues. | Ex: ‘Being with someone who is eating often makes me hungry enough to eat also.’ | |
Table 2.
Physical characteristics of both groups of women at baseline and changes in response to the 3-week weight reducing programa
| BMI ≤ 34.9 kg/m2(n = 44) | BMI ≥ 35 kg/m2(n = 39) | |
|---|---|---|
| Age, years | 52 ± 10 | 53 ± 11 |
| Baseline | ||
| Body weight, kg | 85.7 ± 8.5 | 98.8 ± 10.6† |
| BMI, kg/m2 | 32.6 ± 1.4 | 38.6 ± 3.0† |
| Fat mass, kg | 37.8 ± 4.8 | 49.1 ± 7.3† |
| Lean mass, kg | 48.0 ± 5.7 | 49.7 ± 4.8 |
| Changes in | ||
| Body weight, kg | –2.4 ± 1.1*** | –2.7 ± 0.8*** |
| BMI, kg/m2 | –0.9 ± 0.3*** | –1.1 ± 0.3*** |
| Fat mass, kg | –1.8 ± 1.0*** | –2.5 ± 1.9*** + |
| Lean mass, kg | –0.6 ± 1.3* | –0.2 ± 1.7* |
Changes are calculated as differences between values before (e.g. baseline) and after weight loss.
p < 0.05
p < 0.0001: significant change after weight loss.
p < 0.05; †p < 0.0001: significant difference between groups.
Acknowledgments
This study was supported by the Conseil Régional Midi-Pyrénées and the Fédération Française d’Éducation Physique et de Gymnastique Volontaire (F.F.E.P.G.V.). Thanks are expressed to staff members of the Clinique du Château de Vernhes, Bondigoux (France). The cooperation of subjects who participated to this weight reducing program was greatly appreciated. We also gratefully thank Claude Leblanc for his statistical analyses.
References
- 1.Enquête épidémiologique nationale sur le surpoids et l'obésité Neuilly-sur-Seine, France, Institut National de la Santé et de la Recherche Médicale (I.N.S.E.E.) Sociétés Françaises d'Enquêtes et de Sondages (S.O.F.R.E.S.) 2006 [Google Scholar]
- 2.Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med. 1997;337:396–407. doi: 10.1056/NEJM199708073370606. [DOI] [PubMed] [Google Scholar]
- 3.Prentice A, Jebb S. Physical activity level and weight control in adults. In: Bouchard C, Physical Activity and Obesity, editor. Champaign: Human, Kinetics; 2000. pp. pp 247–261. [Google Scholar]
- 4.Dionne I, Tremblay A. Human energy and nutrient balance. In: Bouchard C, Physical Activity and Obesity, editor. Champaign: Human Kinetics; 2000. pp. pp 151–179. [Google Scholar]
- 5.Dunn CL, Hannan PJ, Jeffery RW, Sherwood NE, Pronk NP, Boyle R. The comparative and cumulative effects of a dietary restriction and exercise on weight loss. Int J Obes (Lond) 2006;30:112–121. doi: 10.1038/sj.ijo.0803046. [DOI] [PubMed] [Google Scholar]
- 6.Jakicic JM, Wing RR, Winters-Hart C. Relationship of physical activity to eating behaviors and weight loss in women. Med Sci Sports Exerc. 2002;34:1653–1659. doi: 10.1097/00005768-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Foster GD, Wadden TA, Swain RM, Stunkard AJ, Platte P, Vogt RA. The Eating Inventory in obese women clinical correlates and relationship to weight loss. Int J Obes Relat Metab Disord. 1998;22:778–785. doi: 10.1038/sj.ijo.0800659. [DOI] [PubMed] [Google Scholar]
- 8.Provencher V, Drapeau V, Tremblay A, Després JP, Lemieux S. Eating behaviors and indexes of body composition in men and women from the Québec family study. Obes Res. 2003;11:783–792. doi: 10.1038/oby.2003.109. [DOI] [PubMed] [Google Scholar]
- 9.Hays NP, Bathalon GP, McCrory MA, Roubenoff R, Lipman R, Roberts SB. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. Am J Clin Nutr. 2002;75:476–483. doi: 10.1093/ajcn/75.3.476. [DOI] [PubMed] [Google Scholar]
- 10.Lawson OJ, Williamson DA, Champagne CM, DeLany JP, Brooks ER, Howat PM, Wozniak PJ, Bray GA, Ryan DH. The association of body weight, dietary intake and energy expenditure with dietary restraint and disinhibition. Obes Res. 1995;3:153–161. doi: 10.1002/j.1550-8528.1995.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson J, Hallgren P, Kral J, Lindroos AK, Sjöström L, Sullivan M. Predictors and effects of long-term dieting on mental well-being and weight loss in obese women. Appetite. 1994;23:15–26. doi: 10.1006/appe.1994.1031. [DOI] [PubMed] [Google Scholar]
- 12.Lindroos AK, Lissner L, Mathiassen ME, Karlsson J, Sullivan M, Bengtsson C, Sjostrom L. Dietary intake in relation to restrained eating, disinhibition and hunger in obese and nonobese Swedish women. Obes Res. 1997;5:175–182. doi: 10.1002/j.1550-8528.1997.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 13.Boschi V, Iorio D, Margiotta N, D'Orsi P, Falconi C. The three-factor eating questionnaire in the evaluation of eating behaviour in subjects seeking participation in a dietotherapy programme. Ann Nutr Metab. 2001;45:72–77. doi: 10.1159/000046709. [DOI] [PubMed] [Google Scholar]
- 14.Provencher V, Begin C, Tremblay A, Mongeau L, Boivin S, Lemieux S. Short-term effects of a ‘health-at-every-size’ approach on eating behaviors and appetite ratings. Obesity. 2007;15:957–966. doi: 10.1038/oby.2007.638. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS) – an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998;22:113–126. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 16.Lemoine S, Rossell N, Drapeau V, Poulain M, Garnier S, Sanguignol F, Mauriège P. Effect of weight reduction on quality of life and eating behaviors in obese women. Menopause. 2007;14:432–440. doi: 10.1097/gme.0b013e31802e46c2. [DOI] [PubMed] [Google Scholar]
- 17.Chaput JP, Drapeau V, Hetherington M, Lemieux S, Provencher V, Tremblay A. Psychobiological impact of a progressive weight loss program in obese men. Physiol Behav. 2005;86:224–232. doi: 10.1016/j.physbeh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 19.Hulens M, Vansant G, Claessens AL, Lysens R, Muls E, Rzewnicki R. Health-related quality of life in physically active and sedentary obese women. Am J Hum Biol. 2002;14:777–785. doi: 10.1002/ajhb.10095. [DOI] [PubMed] [Google Scholar]
- 20.Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults intervention and 24 month follow-up effects. Health Qual Life Outcomes. 2006;4:43–50. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 22.Doll HA, Petersen SE, Stewart-Brown SL. Obesity and physical and emotional well-being: associations between body mass index, chronic illness and the physical and mental components of the SF-36 questionnaire. Obes Res. 2000;8:160–170. doi: 10.1038/oby.2000.17. [DOI] [PubMed] [Google Scholar]
- 23.Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res. 2002;10:748–756. doi: 10.1038/oby.2002.102. [DOI] [PubMed] [Google Scholar]
- 24.Fontaine KR, Barofsky I, Bartlett SJ, Franckowiak SC, Andersen RE. Weight loss and health-related quality of life: results at 1-year follow-up. Eat Behav. 2004;5:85–88. doi: 10.1016/S1471-0153(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 25.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults - The Evidence Report National Institutes of Health. Obes Res. 1998;6((suppl 2)):51S–209S. [PubMed] [Google Scholar]
- 26.Pouliot MC, Desprβs JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 27.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 28.Lluch A. Identification des conduites alimentaires par approches nutritionnelles et psychométriques: implications thérapeutiques et préventives dansl'obésité humaine. Ph.D. thesis, Nancy, Université Henri Poincaré. 1995 [Google Scholar]
- 29.Westenhoefer J. Dietary restraint and disinhibition is restraint a homogeneous construct? Appetite. 1991;16:45–55. doi: 10.1016/0195-6663(91)90110-e. [DOI] [PubMed] [Google Scholar]
- 30.Westenhoefer J, Stunkard AJ, Pudel V. Validation of the flexible and rigid control dimensions of dietary restraint. Int J Eat Disord. 1999;26:53–64. doi: 10.1002/(sici)1098-108x(199907)26:1<53::aid-eat7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Bond MJ, McDowell AJ, Wilkinson JY. The measurement of dietary restraint, disinhibition and hunger an examination of the factor structure of the Three Factor Eating Questionnaire (TFEQ) Int J Obes Relat Metab Disord. 2001;25:900–906. doi: 10.1038/sj.ijo.0801611. [DOI] [PubMed] [Google Scholar]
- 32.Drapeau V, Provencher V, Lemieux S, Després JP, Bouchard C, Tremblay A. Do 6-y changes in eating behaviors predict changes in body weight? Results from the Québec Family Study. Int J Obes Relat Metab Disord. 2003;27:808–814. doi: 10.1038/sj.ijo.0802303. [DOI] [PubMed] [Google Scholar]
- 33.Provencher V, Begin C, Piché ME, Bergeron J, Corneau L, Weisnagel SJ, Nadeau A, Lemieux S. Disinhibition, as assessed by the Three-Factor Eating Questionnaire, is inversely related to psychological well-being in postmenopausal women. Int J Obes (Lond) 2007;31:315–320. doi: 10.1038/sj.ijo.0803405. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Kosinski M. 2nd ed. Lincoln: QualityMetric; 2001. SF-36 Physical and Mental Health Summary Scales A Manual for Users of Version 1. [Google Scholar]
- 36.Carels RA, Darby LA, Cacciapaglia HM, Douglass OM. Reducing cardiovascular risk factors in postmenopausal women through a lifestyle change intervention. J Womens Health. 2004;13:412–426. doi: 10.1089/154099904323087105. [DOI] [PubMed] [Google Scholar]
- 37.Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol A Biol Sci Med Sci. 2003;58:181–189. doi: 10.1093/gerona/58.2.m181. [DOI] [PubMed] [Google Scholar]
- 38.Rippe JM, Price JM, Hess SA, Kline G, DeMers KA, Damitz S, Kreidieh I, Freedson P. Improved psychological well-being, quality of life, and health practices in moderately overweight women participating in a 12-week structured weight loss program. Obes Res. 1998;6:208–218. doi: 10.1002/j.1550-8528.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 39.Chaston TB, Dixon JB, OβBrien PE. Changes in fatfree mass during significant weight loss a systematic review. Int J Obes (Lond) 2007;31:743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 40.Vogels N, Diepvens K, Westerterp-Plantenga MS. Predictors of long-term weight maintenance. Obes Res. 2005;13:2162–2168. doi: 10.1038/oby.2005.268. [DOI] [PubMed] [Google Scholar]
- 41.Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11:157–171. doi: 10.1023/a:1015081805439. [DOI] [PubMed] [Google Scholar]
- 42.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 43.Owens TM. Morbid obesity the disease and comorbidities. Crit Care Nurs Q. 2003;26:162–165. doi: 10.1097/00002727-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Stewart KJ, Turner KL, Bacher AC, DeRegis JR, Sung J, Tayback M, Ouyang P. Are fitness, activity, and fatness associated with health-related quality of life and mood in older persons? J Cardiopulm Rehabil. 2003;23:115–121. doi: 10.1097/00008483-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Fontaine KR, Barofsky I, Andersen RE, Bartlett SJ, Wiersema L, Cheskin LJ, Franckowiak SC. Impact of weight loss on health-related quality of life. Qual Life Res. 1999;8:275–277. doi: 10.1023/a:1008835602894. [DOI] [PubMed] [Google Scholar]
- 46.Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001;9:564–571. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]