Abstract
Background
Chronic low-grade inflammation and oxidative stress play important roles in the development of obesity-induced cardiac hypertrophy. Here, we investigated the role of Fibronectin type III domain containing 5 (FNDC5) in cardiac inflammation and oxidative stress in obesity-induced cardiac hypertrophy.
Methods
Male wild-type and FNDC5−/− mice were fed normal chow or high fat diet (HFD) for 20 weeks to induce obesity, and primary cardiomyocytes and H9c2 cells treated with palmitate (PA) were used as in vitro model. The therapeutic effects of lentiviral vector-mediated FNDC5 overexpression were also examined in HFD-induced cardiac hypertrophy.
Results
High fat diet manifested significant increases in body weight and cardiac hypertrophy marker genes expression, while FNDC5 deficiency aggravated cardiac hypertrophy evidenced by increased Nppa, Nppb and Myh7 mRNA level and cardiomyocytes area, in association with enhanced cardiac inflammatory cytokines expression, oxidative stress level and JAK2/STAT3 activation in HFD-fed mice. FNDC5 deficiency in primary cardiomyocytes or FNDC5 knockdown in H9c2 cells enhanced PA-induced inflammatory responses and NOX4 expression. Exogenous FNDC5 pretreatment attenuated PA-induced cardiomyocytes hypertrophy, inflammatory cytokines up-regulation and oxidative stress in primary cardiomyocytes and H9c2 cells. FNDC5 overexpression attenuated cardiac hypertrophy as well as cardiac inflammation and oxidative stress in HFD-fed mice.
Conclusions
FNDC5 attenuates obesity-induced cardiac hypertrophy by inactivating JAK2/STAT3 associated-cardiac inflammation and oxidative stress. The cardio-protective role of FNDC5 shed light on future therapeutic interventions in obesity and related cardiovascular complications.
Electronic supplementary material
The online version of this article (10.1186/s12967-019-1857-8) contains supplementary material, which is available to authorized users.
Keywords: FNDC5, Cardiac hypertrophy, Obesity, Inflammation, Oxidative stress
Background
Cardiac hypertrophy has become an independent and predictive risk factor for adverse cardiovascular events [1]. Despite an increasing understanding toward the pathophysiological process of cardiac hypertrophy, progress in mechanism remains sluggish in recent years. Many factors are involved in the pathophysiological process of cardiac hypertrophy, of which inflammation and oxidative stress play crucial mediating role [2, 3]. Among cardiovascular diseases, obesity has a great impact on the cardiac remodeling and function in terms of hemodynamic load, myocardial fibrosis and impaired ventricular contractility, which leads to the progression of heart failure [4, 5]. Obesity has also been characterized as an inflammatory state [6, 7], and chronic low-grade inflammation and oxidative stress play important roles in the pathogenesis of obesity-related complications. It has been demonstrated both in vitro and in vivo that the increased free fatty acids in obesity can activate the nuclear factor-kappaB (NF-kB) pathway, subsequently increasing the expression of several pro-inflammatory cytokines and inducing cellular oxidative stress [8]. However, the precise molecular mechanisms involved in obesity-induced cardiac hypertrophy process still need further elucidation.
It is well established that fibronectin type III domain containing 5 (FNDC5) and its cleaved and secreted fragment, irisin, are beneficial to improve metabolic diseases in both humans and mice [9]. Recent studies in our lab have shown that FNDC5 overexpression ameliorates hyperlipemia and enhances lipolysis in adipose tissues of mice [10], and prevents high fat diet (HFD)-induced hyperlipemia, hepatic lipid accumulation and impaired fatty acid oxidation in liver [11]. Irisin improves glucose homoeostasis by reducing gluconeogenesis and increasing glycogenesis via PI3K/Akt pathway in HepG2 cells [12]. These findings of our previous studies confirmed the beneficial effects of FNDC5/irisin in metabolic diseases. Many studies have highlighted the role of exercise in the heart, of which serves as an important organ regulating metabolism. Exercise training has various physiologic effects on the cardiovascular system, most notably multiple metabolic changes in the myocardium resulting in improved tolerance for ischemia and reperfusion injury, a reduction of resting heart rate attributed to increased parasympathetic tone and an improved vascular endothelial function with augmented flow-mediated vasodilation during exercise [13–15]. Nevertheless, as an up-regulated myokine after exercise, the exact role of FNDC5 in cardiomyocytes remains unclear.
Growing evidence points to the regulatory capacity of JAK2/STAT3 pathway on inflammation-related diseases and cardiac hypertrophy [16–19]. STAT3 is the most important effector of the JAK2 in the initiation and development of cardiac hypertrophy. After phosphorylation, STAT3 translocates from the cytoplasm to the nucleus and promotes the transcription of multiple hypertrophic factors including natriuretic peptide type A and natriuretic peptide type B [20]. Therefore, it is worthy to examine whether JAK2/STAT3 pathway is involved in molecular mechanisms of obesity-induced cardiac hypertrophy. We recently reported the anti-inflammatory effects of FNDC5 in adipose tissue of mice [21]. Here, the aim of this study is to evaluate the role and molecular mechanism of FNDC5 on cardiac function, inflammatory and oxidative stress responses in HFD-induced obese mice, which would be used for future therapeutic interventions in obesity-related complications.
Methods
Animals
Male wild-type (WT) mice and FNDC5−/− mice on a C57BL/6 background (Nanjing BioMedical Research Institute of Nanjing University, Nanjing, China) were used in the study as previously reported [11]. Mice were housed at 22–26 °C temperature, 40% ± 5% humidity and 12-h light/dark cycle. At the age of 6 weeks either WT or FNDC5−/− mice were randomly divided to fed a normal chow (Ctrl, 14.7 kJ/g, 13% of energy as fat) or a HFD (21.8 kJ/g, 60% of energy as fat) for 20 weeks, respectively. The lard-based HFD were obtained from Nanjing Junke Biotechnology Corporation, Ltd (Nanjing, China).
FNDC5 overexpression in mice
As we previously described, WT and FNDC5−/− mice at the age of 6 weeks were fed with HFD for 20 weeks to induce obesity. FNDC5 overexpression were induced by lentivirus (1 × 108 TU/ml, 100 μl) intravenously injection [10]. The recombinant lentivirus expressing FNDC5 or vector were injected into the mice at the end of the 14th week after HFD. Acute experiments were carried out 6 weeks after the lentivirus injection.
Echocardiographic measurements
Cardiac function was measured by echocardiography (Vevo 2100, VisualSonic, Canada). WT and FNDC5−/− mice were anesthetized using 2.0% isoflurane and then two-dimensional echocardiographic views of the mid-ventricular short axis were obtained at the level of the papillary muscle tips below the mitral valve. Interventricular septal thickness (IVS), left ventricular internal dimensions (LVID) and left ventricular posterior wall dimensions (LVPW) were measured at the end of diastole and systole [22].
Western blot analysis
Tissues or cardiomyocytes were sonicated in RIPA lysis buffer containing 1% NonidetP-40, 0.1% SDS, protease inhibitor, while adding phosphatase inhibitors cocktail (Bimake, Houston, USA), and then homogenized. The nuclear and cytosolic protein were extracted by commercially available kit (Beyotime Biotechnology, Shanghai, China). Briefly, the cells expanded sufficiently under low osmotic pressure, then the cell membrane ruptured, releasing cytoplasmic proteins. Nucleus precipitation could be centrifuged to obtain after then. Finally, the nuclear protein was extracted by high salt nucleoprotein extraction reagent. Equal quantities of tissues or cardiomyocytes protein lysates were subjected to SDS-PAGE (Bio-Rad), and transferred onto PVDF membrane. Blocking was made at room temperature with 5% nonfat milk powder prepared in Tris-buffered saline containing 0.1% Tween 20. Then, membranes were incubated overnight at 4 °C with corresponding antibodies, and followed by incubation with appropriate secondary HRP-conjugated antibodies. Antibodies against p38 (#8690), P-p38 (#4511), NFκB-p65 (#8242), ERK (#4695), P-ERK (#4370), P-Stat3 (#9145), Stat3 (#4904) and GAPDH (#2118) were obtained from Cell signaling Technology (Danvers, USA). Antibodies against FNDC5 (ab174833), P-JAK2 (ab195055), JAK2 (ab108596), Nox2 (ab129068), Nox4 (ab154244) were obtained from Abcam (Cambridge, UK).
Quantitative real-time PCR analysis
Total RNA was separated using a Trizol reagent (Life Technologies, USA) according to the manufacturer’s protocols. RNA concentrations and purity were assessed by the measurement of optical density at 260 and 280 nm. The purified total-RNA was reverse transcribed with reverse transcription reagent kit (Takara, Japan) and quantitative real-time PCR was performed on Stepone Plus system using the SYBR Green Master Mix (Takara, Japan). The fold changes in the genes were analyzed based on comparative ΔΔCt method. The sequences of primers were listed in Additional file 1: Table S1.
Tissue embedding and H&E staining
The mice were anesthetized and the heart were quickly removed. Heart tissues were rinsed in ice-cold PBS, and incubated overnight in formalin. After washed and dehydrated by successive incubation in 70 to 100% ethanol solutions, heart tissues were fixed and embedded in paraffin, and slices were cut every 6 μm. The sections were stained with hematoxylin–eosin (H&E) and used for histopathological analysis. The degree of pathological changes was evaluated microscopically by measuring the cardiomyocyte area.
Cell culture and treatment
Neonatal primary cardiomyocytes (CMs) from 1 to 3-day-old WT or FNDC5−/− mice were prepared as reported [23]. Briefly, neonatal hearts were dissociated in cell suspension using the Neonatal Heart Dissociation Kit (MACS, Bergisch Gladbach, Germany). Dissociated cells were neutralized with DMEM and 4.5 g/l glucose supplemented with 7.5% FBS and passed through a 70-µm cell strainer. Then the dissociated cells were preplated at 37 °C for 1 h to remove fibroblasts and endothelial cells, and nonadhered cells were collected as CMs. CMs were plated in DMEM supplemented with 15% FBS, 1% penicillin, and 1% streptomycin in a humidified atmosphere of 5% CO2 at 37 °C. Embryonic rat heart-derived cell line H9c2 were obtained from Costar Corning Inc. (Corning, CA, USA). H9c2 was cultured in DMEM/F12 medium containing 2.25 g/l glucose medium supplemented with 10% FBS, 1% of penicillin, and 1% of streptomycin. CMs and H9c2 cells were treated with palmitate (PA, obtained from Sigma Aldrich) at 400 μM (bound to BSA at a ratio of 5:1) for 24 h to mimic high lipid stimulation and to induce inflammation. PA was administrated 4 h after exogenous FNDC5 (200 nM, Sigma Aldrich) pretreatment. For WP1066 (5 μM, Med Chem Express) treatment experiments in H9c2 cells, WP1066 was added 2 h before FNDC5 pretreatment, while PA was added 4 h after FNDC5 pretreatment. The measurement for phosphorylated protein level was made 12 h after PA administration, while for mRNA levels or protein levels were made 24 h after PA administration.
Small interfering RNA transfection
H9c2 cells were pre-treated with specific small interfering RNA (siRNA) against FNDC5 (FNDC5-siRNA). The FNDC5 specific siRNA (sense sequence: 5′GAUGGCCUCUAAGAACAAA3′; antisense sequence: 3′CUACCGGAGAUUCUUGUUU5′) was purchased from RiboBio (Guangzhou, China). Negative siRNAs (NA-siRNA) with no sequence homology to any known rat genes were used as a control. H9c2 cells were transfected with 50 nM siRNA using lipofectamine 3000 reagent (Invitrogen). Cells were lysed, 48 h after transfection, to analyze FNDC5 protein expression to examine knock-down efficiencies.
Oxidative stress detection
The heart tissues or cells were washed and added to phosphate-buffered saline, ground into homogenates, and centrifuged to collect the supernatant. The activities of superoxide dismutase 1 (SOD) and the content of malondialdehyde (MDA) were detected by commercially available kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Griess Reagent Kit (Thermo Fisher Scientific, Waltham, USA) was used to determine the estimation level of Nitric Oxide (NO) production in cell culture medium of CMs according to the manufacturer’s protocol.
ELISA
TNF-α, IL-1β levels in heart tissues were measured with mouse TNF-α and IL-1β ELISA Kits (Excell Bio, Shanghai, China). IL-6 levels in heart tissues were measured with mouse IL-6 ELISA Kit (Beyotime Biotechnology, Shanghai, China). ELISA kit for FNDC5 was obtained from Phoenix pharmaceuticals (EK-067-19, Burlingame, USA).
Statistical analysis
All values were presented as mean ± SEM. Student’s t-test was used for 2-group comparisons. Experiments with > 2 groups were compared by 1-way or 2-way ANOVA followed by Bonferroni’s multiple comparisons test. Statistical analyses were performed with SPSS software. A value of P < 0.05 was considered statistically significant.
Results
FNDC5 deficiency worsened HFD-induced cardiac hypertrophy in mice
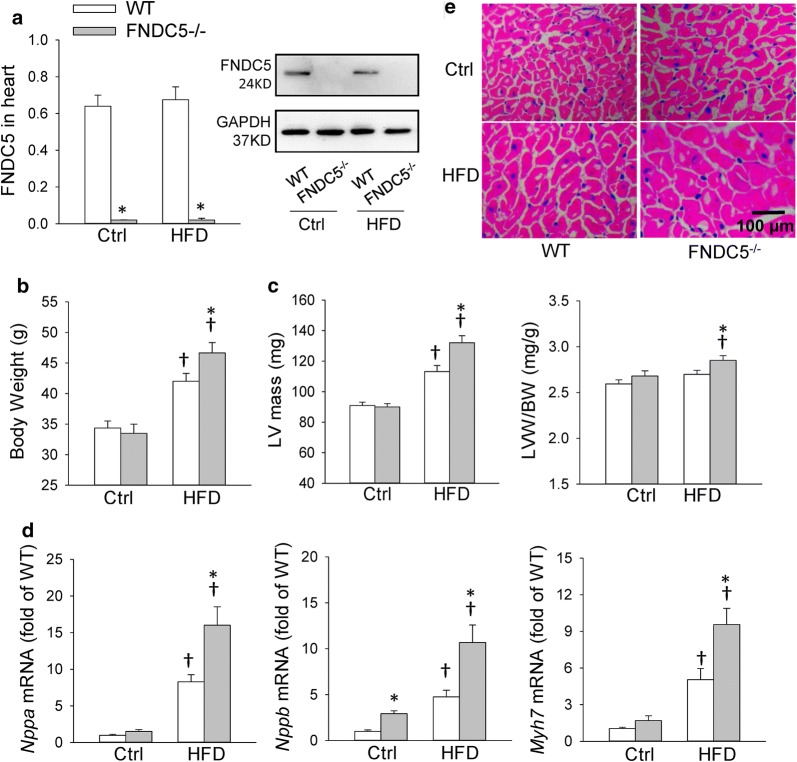
FNDC5−/− mice were used to determine the roles of FNDC5 in HFD-induced myocardial hypertrophy in the present study. We have identified the efficiency of FNDC5 deficiency in our recent study, and we further confirmed that FNDC5 was deleted in heart of mice in the present study (Fig. 1a). However, HFD feeding did not significantly change FNDC5 protein expression in heart. FNDC5 gene deletion aggravated HFD-induced obesity, which was consistent with our recent study [21] (Fig. 1b). Echocardiographic analysis showed that, despite lack of an obvious difference in left ventricular ejection fraction (LVEF) and fractional shortening (LVFS) among HFD-fed WT and FNDC5−/− mice, FNDC5 deficiency significantly augmented left ventricular hypertrophy evidenced by increased IVSd and LVPWd and cardiac hypertrophy markers (Nppa, Nppb, Myh7) mRNA expression as well as LVW/BW (Table 1, Fig. 1c, d) after HFD treatment. Moreover, HFD feeding significantly increased cardiomyocyte diameter as demonstrated by H&E staining, and FNDC5 gene deletion further aggravated it in HFD-fed mice (Fig. 1e, Additional file 1: Figure S1A). Taken together, these results indicate that FNDC5 deficiency was associated with deterioration in myocardial hypertrophy in response to HFD.
Fig. 1.
FNDC5 deficiency worsened HFD-induced cardiac hypertrophy in mice. WT and FNDC5−/− mice were fed with mouse chow diet (Ctrl) and high fat diet (HFD) for 20 weeks. The measurements were carried out at the end of 20 weeks after Ctrl or HFD feeding. a Expression of FNDC5 protein in heart determined with Western blot. b, c Body weight (BW), left ventricular (LV) mass and left ventricular weight/body weight (LVW/BW). d The mRNA expression of cardiac hypertrophy markers in heart. e Representative sections of H&E staining show the cardiomyocyte area in heart. Values are mean ± SEM. *P < 0.05 vs. WT. †P < 0.05 vs. Ctrl. n = 6
Table 1.
Echocardiographic assessment of left ventricle functions in mice
| Ctrl-WT | Ctrl-FNDC5−/− | HFD-WT | HFD-FNDC5−/− | |
|---|---|---|---|---|
| IVSd (mm) | 0.7 ± 0.02 | 0.74 ± 0.02 | 0.75 ± 0.01† | 0.82 ± 0.02*† |
| IVSs (mm) | 1.07 ± 0.02 | 1.1 ± 0.03 | 1.19 ± 0.02† | 1.20 ± 0.02† |
| LVIDd (mm) | 4.05 ± 0.11 | 4.0 ± 0.11 | 3.98 ± 0.14 | 4.01 ± 0.1 |
| LVIDs (mm) | 3.16 ± 0.13 | 2.98 ± 0.1 | 2.73 ± 0.12† | 2.84 ± 0.08 |
| LVPWd (mm) | 0.72 ± 0.02 | 0.74 ± 0.03 | 0.77 ± 0.02 | 0.85 ± 0.02*† |
| LVPWs (mm) | 1.17 ± 0.02 | 1.18 ± 0.03 | 1.25 ± 0.02† | 1.24 ± 0.03 |
| LVEF (%) | 59.4 ± 2 | 57.3 ± 1.6 | 48.1 ± 1.7† | 49.7 ± 1.7† |
| LVFS (%) | 32.2 ± 1.3 | 30.3 ± 1.2 | 25.2 ± 1.2† | 24.7 ± 1.5† |
IVSs and IVSd interventricular septum at end systole and diastole, LVIDs and LVIDd left ventricular internal dimensions at end systole and diastole, LVPWs and LVPWd left ventricular posterior wall dimensions at end systole and diastole, LVEF left ventricular ejection fraction, LVFS left ventricular fractional shorting
Values are mean ± SEM. *P < 0.05 vs. WT. †P < 0.05 vs. Ctrl. n = 6
FNDC5 deficiency aggravated cardiac inflammation in HFD-induced obesity
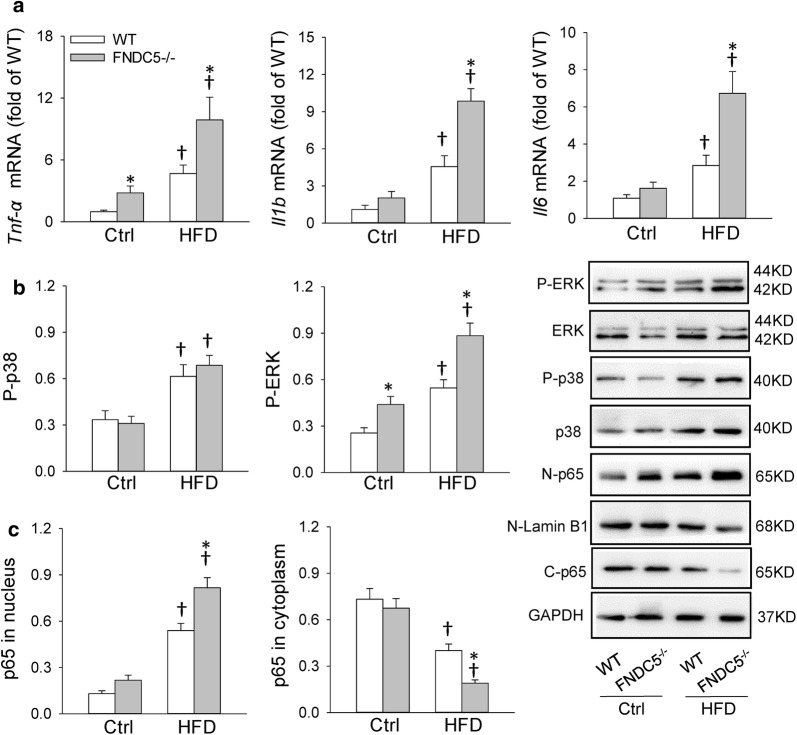
As we known, HFD causes substantial inflammatory responses in the heart, which mediates the pathogenesis of cardiac injury and remodeling. We firstly examined the expression of pro-inflammatory cytokines in heart. Tnf-α, Il1b and Il6 mRNA levels were upregulated after HFD, while FNDC5 gene deletion exacerbated the enhancement of them (Fig. 2a). However, FNDC5 deficiency had no significant effects in mice with control diet. The expression of cardiac inflammatory cytokines were also determined with ELISA (Additional file 1: Figure S1B–D). It is established that NLR family pyrin domain containing 3 (NLRP3) inflammasome activation were associated with increased inflammatory mediators and profibrotic factor production, resulting in myocardial fibrosis, cardiomyocyte hypertrophy and impaired cardiac function [24]. Here, we also examined the gene expression of NLRP3 inflammasome and downstream inflammatory mediators IL-18 in heart. As shown in Additional file 1: Figure S2, HFD caused an up-regulated levels of Nlrp3 and Il18 mRNA, while FNDC5 deficiency further aggravated the enhancement of mRNA levels.
Fig. 2.
FNDC5 deficiency aggravated HFD-induced inflammation in heart. a Cardiac Tnf-α, Il1b and Il6 mRNA levels determined with qPCR. b Phosphorylation levels of p38 and ERK in heart determined with Western blot. c NFκB activation in heart. N-p65: p65 in nucleus; C-p65: p65 in cytoplasm. Values are mean ± SEM. *P < 0.05 vs. WT. †P < 0.05 vs. Ctrl. n = 3
On the other hand, NFκB is a primary regulator of inflammatory responses, which induces the expression of pro-inflammatory cytokines in the heart [25]. NFκB activation and inflammation signals included phosphorylation of p38 mitogen-activated protein kinase and p44/42 mitogen-activated protein kinase (ERK) were also examined herein. The NFκB activation indicated by increased p65 in nucleus and reduced p65 in cytoplasm, and the enhanced phosphorylation level of ERK suggested that FNDC5 deficiency aggravated cardiac inflammation in obesity. However, there was no significant change in phosphorylation level of p38 between WT and FNDC5−/− mice fed with control diet or HFD (Fig. 2b, c).
FNDC5 deficiency aggravated cardiac oxidative stress in HFD-induced obesity
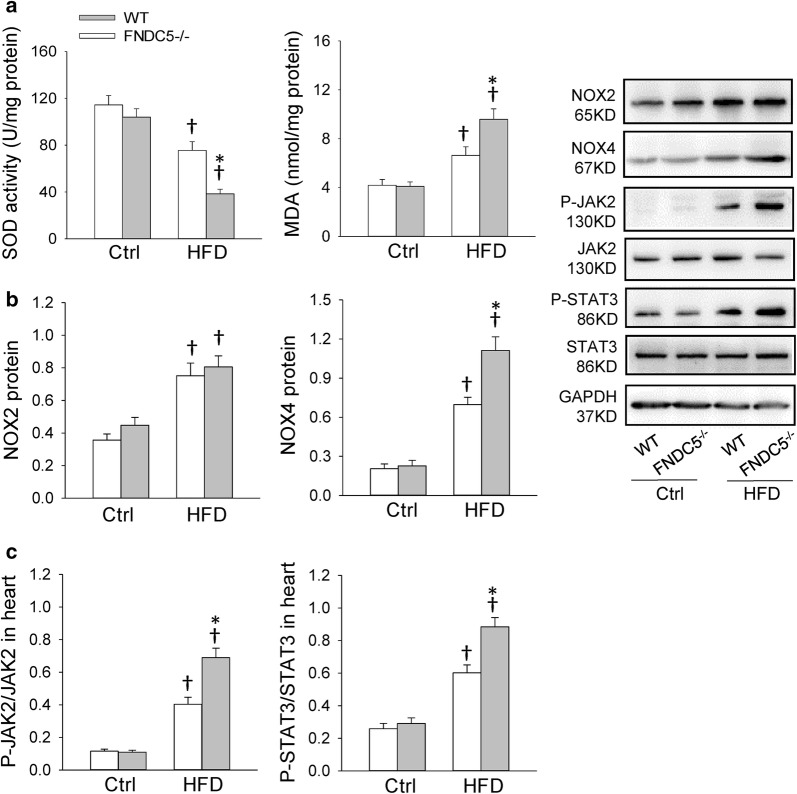
Studies suggested that hyperlipidemia can hyperpolarize mitochondria causing electron slippage and enhanced ROS production, leading to cardiac injury and obesity-related complications [26]. The imbalance between ROS generation and the antioxidant system occurs due to a change in the overall redox balance and in the modification of target molecules, resulting in inflammation [27]. Free radical scavenging enzymes, SOD, can eliminate excessive ROS during the cardiac injury. MDA is a biological marker of oxidative stress to determine the degree of the peroxidation of membrane lipid. The decreased SOD activity and increased MDA level caused by HFD were further exacerbated by FNDC5 gene deletion (Fig. 3a). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase are dominant mechanisms of ROS production in heart. Among the NADPH oxidase isoforms, NOX2 and NOX4 are abundantly expressed in cardiomyocytes [28]. As shown in Fig. 3b, HFD led to increased expression of NOX2 and NOX4, while FNDC5 deficiency further enhanced NOX4 level but not NOX2. These results suggest that FNDC5 is involved in obesity-induced cardiac oxidative stress.
Fig. 3.
FNDC5 deficiency enhanced HFD-induced oxidative stress and phosphorylated JAK2 and STAT3 level in heart. a Superoxide dismutase (SOD) activity and malondialdehyde (MDA) level in heart. b Expression of NAPDH oxidases (NOX2 and NOX4) protein in heart. c Phosphorylated JAK2 and STAT3 level in heart. Values are mean ± SEM. *P < 0.05 vs. WT. †P < 0.05 vs. Ctrl. n = 6
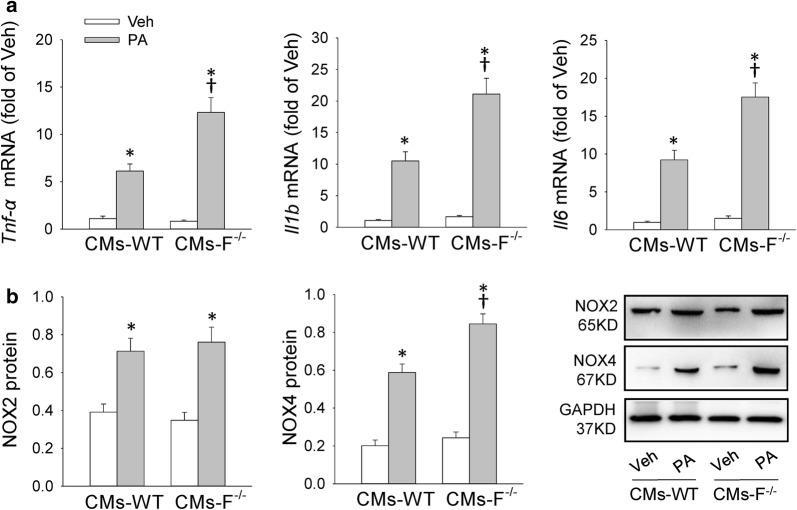
FNDC5 deficiency/knockdown aggravated palmitate-induced inflammation in primary cardiomyocytes and in H9c2 cells
The in vivo findings urged us to further determine the effects and mechanisms of FNDC5 on inflammation and oxidative stress in vitro. We used neonatal primary cardiomyocytes (CMs) and H9c2 cells treated with palmitate (PA) to mimic fatty acid stimulation. CMs derived from WT and FNDC5−/− mice and H9c2 cells with FNDC5 siRNA silencing were used to determine the role of FNDC5 in hyperlipidemia-induced cardiomyocytes damage. We observed that FNDC5 deficiency in CMs enhanced PA-induced Tnf-α, Il1b and Il6 mRNA levels augmentation as well as upregulated expression of NOX4 but not NOX2 (Fig. 4a, b). Also, transfection of si-FNDC5 significantly reduced FNDC5 protein expression in H9c2 cells, which further led to an increased gene expression of Tnf-α, Il1b and Il6 as well as NOX4 protein in H9c2 cells (Additional file 1: Figure S3). These results confirmed the important role of FNDC5 in high lipid induced cardiomyocyte hypertrophy in vitro.
Fig. 4.
FNDC5 deficiency enhanced palmitate-induced inflammation and NOX4 expression in primary cardiomyocytes (CMs). a Effects of palmitate (PA, 400 μM) treatment on Tnf-α, Il1b and Il6 mRNA levels in CMs of WT and FNDC5−/− mice. b Effects of PA treatment on NOX2 and NOX4 protein level in CMs of WT and FNDC5−/− mice. The measurement was 24 h after PA treatment for measuring the mRNA levels or protein levels. PA: Palmitate; Veh: Vehicle; F−/−: FNDC5−/−. Values are mean ± SEM. *P < 0.05 vs. Veh. †P < 0.05 vs. CMs-WT. n = 3
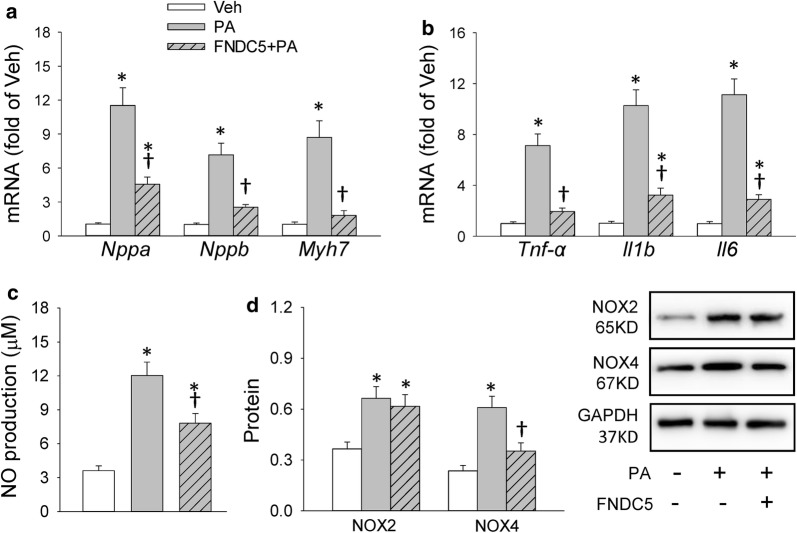
Exogenous FNDC5 pretreatment attenuated palmitate-induced cardiomyocyte hypertrophy, inflammation and oxidative stress in primary cardiomyocytes and in H9c2 cells
Since FNDC5 deficiency/knockdown aggravated inflammation in vitro, it is interesting to determine whether exogenous FNDC5 would play beneficial roles in attenuating PA-induced inflammation and oxidative stress in CMs derived from WT mice and in H9c2 cells. As shown in Fig. 5a, b, the up-regulated mRNA level of Nppa, Nppb, Myh7, Tnf-α, Il1b and Il6 induced by PA were significantly decreased after exogenous FNDC5 pretreatment in CMs, indicating the cardiomyocyte hypertrophy-inhibitory effects and anti-inflammatory effects of FNDC5 in vitro. Also, the reduced NO production and NOX4 expression by exogenous FNDC5 pretreatment in PA-stimulated CMs showed anti-oxidative effects of FNDC5 (Fig. 5c, d). The similar effects of FNDC5 were observed in H9c2 cells (Additional file 1: Figure S4).
Fig. 5.
Exogenous FNDC5 pretreatment attenuated palmitate-induced inflammation and oxidative stress in CMs. a, b Effects of exogenous FNDC5 pretreatment on cardiac hypertrophy markers (Nppa, Nppb and Myh7) and inflammation markers (Tnf-α, Il1b and Il6) mRNA levels in PA-stimulated CMs. c Effects of exogenous FNDC5 pretreatment on NO production in cell culture supernatant of PA-stimulated CMs. d Effects of exogenous FNDC5 pretreatment on NOX expression in PA-stimulated CMs. PA: Palmitate; Veh: Vehicle. Values are mean ± SEM. *P < 0.05 vs. Veh. †P < 0.05 vs. PA. n = 6
FNDC5 attenuated cardiac hypertrophy, inflammation and oxidative stress in association with JAK2/STAT3 inhibition
Increasing evidence suggests that the JAK2/STAT3 pathway is involved in the development of cardiac hypertrophy [29, 30]. We firstly examined whether the phosphorylation of JAK2/STAT3 is disrupted by absence of FNDC5. As shown in Fig. 3c, HFD caused significant increases in phosphorylated JAK2 and STAT3 level in heart, and the enhancement were further improved in HFD-fed FNDC5−/− mice. Similarly, we observed an increased phosphorylated JAK2 and STAT3 level in PA-stimulated H9c2 cells after knockdown of FNDC5 (Additional file 1: Figure S3C). Then we found WP1066, an inhibitor of JAK2 and STAT3, reversed exogenous FNDC5-induced cardiomyocyte hypertrophy-inhibitory effects, anti-inflammatory and anti-oxidative effects in PA-stimulated H9c2 cells (Additional file 1: Figure S4). Since both FNDC5 and WP1066 can inhibit JAK2/STAT3 signaling and thereby downregulate inflammation and oxidative stress, the reversed effects by WP1066 seems inconsistent. Moreover, we observed an increased phosphorylated JAK2/STAT3 level in WP + FNDC5 + PA group (Additional file 1: Figure S5). We speculated that maybe crosstalk between WP-related STAT3 signal and FNDC5/receptor downstream signaling antagonized the inhibited effects of WP and FNDC5 on JAK2/STAT3 signal, which leads to the augment of JAK2/STAT3 phosphorylation in WP + F+PA group. The augmented JAK2/STAT3 phosphorylation further contributes to the reversed effects by WP1066. Taken together, these results indicate that the effects of FNDC5 on cardiomyocytes are associated with JAK2/STAT3 inactivation.
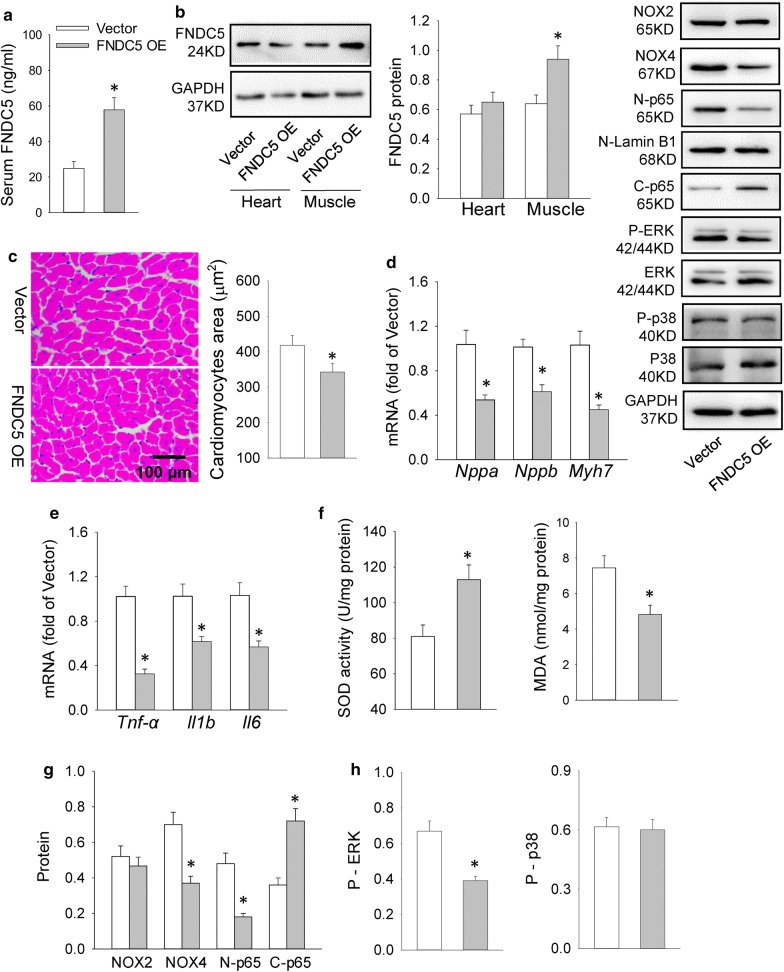
FNDC5 overexpression alleviated HFD-induced cardiac hypertrophy, inflammation and oxidative stress in mice
The effects of FNDC5 absence on cardiac inflammation further urged us to determine the possible therapeutic roles of FNDC5 in HFD-induced cardiac hypertrophy. Effectiveness of lentiviral vector-mediated FNDC5 overexpression was confirmed by the increased serum and muscle FNDC5 levels in mice (Fig. 6a, b), even it failed to increase FNDC5 expression in the heart (Fig. 6b). FNDC5 overexpression alleviated HFD-induced cardiac hypertrophy evidenced by reduced Nppa, Nppb, Myh7 mRNA level and cardiomyocytes area (Fig. 6c, d). Tnf-α, Il1b and Il6 mRNA level, NFκB activation, inflammatory signal (p-ERK) and oxidative stress markers (SOD activity and MDA level) as well as NOX4 expression were also decreased in FNDC5 overexpressed mice (Fig. 6e–h). As shown in Additional file 1: Figure S6, phospho-JAK2/STAT3 level were downregulated in FNDC5 OE mice, suggesting that the protective role of FNDC5 in cardiac inflammation are associated with inhibition of JAK2/STAT3 pathway.
Fig. 6.
FNDC5 overexpression (OE) attenuated cardiac hypertrophy, inflammation and oxidative stress in HFD-fed mice. a, b Serum and heart/muscle FNDC5 levels after lentiviral vector-mediated FNDC5 overexpression. c Cardiomyocyte area in heart determined by H&E staining. d, e mRNA levels of the cardiac hypertrophy markers (Nppa, Nppb and Myh7) and inflammation markers (Tnf-α, Il1b and Il6). f SOD activity and MDA level in heart. g NOX2, NOX4 level and NFκB inactivation in heart. h Phosphorylation levels of p38 and ERK in heart. Values are mean ± SEM. *P < 0.05 vs. vector. n = 6
Discussion
Obesity has been recognized as the cause of various forms of cardiac abnormalities and development of heart failure [31, 32]. The primary novel findings in our study are that FNDC5 deficiency deteriorates, while FNDC5 overexpression attenuates HFD-induced cardiac hypertrophy, cardiac inflammation and oxidative stress. Supporting these in vivo observations, our in vitro studies also demonstrated that FNDC5 inhibits PA-induced cardiomyocytes hypertrophy via inactivated JAK2/STAT3 signaling-related inflammation and oxidative stress. Our results identify the protective role of FNDC5 in obesity-induced cardiac hypertrophy, which shed additional light on future FNDC5-based therapeutic interventions in obesity-related cardiac abnormalities.
Low grade inflammation serves as a proximate trigger, as well as an ultimate modulator of cardiac dysfunction in obesity and cardiovascular disease [33, 34]. Increased oxidative stress coupled with activation of down-stream pro-inflammatory cytokines have pivotal roles in the development of obesity-related complications [35, 36]. As we known, lipid overload is often associated with increased production and release of pro-inflammatory cytokines, and these cytokines activate a series of intracellular signaling pathways including NFκB, which up-regulate the transcription of more cytokines and exaggerate the inflammatory response. These events further trigger macrophage activation and tissue filtration, leading to tissue injury [37]. According to our data, FNDC5 gene deletion not only exacerbated HFD-induced enhancement of the pro-inflammatory cytokines levels, oxidative stress and NOX4 expression in heart, but also promoted NFκB activation and cardiac inflammatory signaling activation. The anti-inflammatory effects by FNDC5 were not only demonstrated in heart of HFD-fed mice, but also in PA-stimulated CMs and H9c2 cells. These data confirm the strong anti-inflammatory effects of FNDC5 on cardiomyocytes, which may contribute to its cardio-protective effects in the present study.
To support the direct role of fatty acid (FA) in cardiomyocyte hypertrophy, in vitro exposure of cardiomyocytes to PA was investigated in our study. Both CMs and H9c2 cells under high lipid treatment showed upregulated inflammatory responses as well as oxidative stress levels, which were also attenuated by exogenous FNDC5 pretreatment, indicating that FNDC5 is involved in the cardio-protective effects against PA-induced inflammation and oxidative stress in vitro. It has been shown that FNDC5 overexpression facilitates the process of cardiogenesis [38]. A recently study found that irisin, the secreted protein derived from FNDC5, protects heart against ischemia–reperfusion injury through a mitochondria mechanism [39]. Although previous studies reported some evidences of the positive role of FNDC5 in heart, the exact role in inflammation and oxidative stress on cardiomyocytes were first investigated in our present study.
According to our in vitro and in vivo data, FNDC5 absence/knockdown led to an enhanced phosphorylation level of JAK2 and STAT3 under high lipid treatment (HFD/PA) accompanied by aggravated inflammation, oxidative stress and cardiac hypertrophy. Phospho-JAK2/STAT3 level were downregulated in FNDC5 OE mice, suggesting that the protective role of FNDC5 in cardiac inflammation are associated with JAK2/STAT3 pathway inhibition. As we known, obesity results in marked alterations in cardiac energy metabolism, with a prominent effect being an increase in fatty acid uptake and oxidation by the heart [40]. Peroxisome proliferator activator receptor (PPAR)-α is expressed in abundant levels in the heart and enhanced PPAR-α signaling is especially associated with increased fatty acid oxidation in cardiomyocytes. Interestingly, PPAR-α acts downstream of FNDC5 [9]. We recently found that FNDC5 deficiency causes impairment in AMPK/PPAR-α-mediated fatty acid oxidation in hepatocytes [11]. However, it is unclear whether FNDC5 is involved in cardiac fatty acid oxidation during obesity, and the crosstalk between PPAR-α and JAK2/STAT3 signaling or other signal transduction molecules needs further investigation. Elucidation of the precise molecular mechanisms underpinning FNDC5 signaling involved in the cardiac hypertrophy process may prove useful in understanding the role of FNDC5 in metabolic-related cardiovascular diseases.
Adipose tissue inflammation induce systemic inflammation and insulin resistance, resulting in subcellular low-grade inflammation, and in turn, subcellular component abnormalities such as oxidative stress, mitochondrial dysfunction in the heart. The vicious cycle of increased subcellular component abnormalities, inflammatory cell infiltration, neurohumoral activation induce the adverse cardiac remodeling in metabolic-induced cardiomyopathy. Taken together the findings of our present and previous studies, FNDC5 not only ameliorated systemic inflammation, but also had direct effects on cardiomyocytes, which may both contribute to the cardio-protective actions. Although it is still difficult to unmask the probable effectors (receptors) of endogenous FNDC5 and its cleaved fragment, irisin, we considered that FNDC5 may exert its protective function through both direct and indirect pathways. Our data show that HFD did not lead to any significant change in FNDC5 expression level in heart. Similarly, FNDC5 overexpression only increased circulating and muscle expression but not FNDC5 in heart. It is possible that, circulating but not cardiac-derived FNDC5 contributes to the beneficial and cardio-protective effects in vivo.
Skeletal muscle can act as an endocrine organ, secreting diverse myokines in a context-dependent manner [41, 42]. Such myokines may exert specific endocrine effects on visceral fat, or work locally within the muscle and exert their effects on signaling pathways involved in fat oxidation and glucose uptake, contributing to its beneficial effects on obesity and related diseases. Sundarrajan et al. showed that exogenous irisin, the cleaved fragment of FNDC5, increased diastolic volume, heart rate and cardiac output in zebrafish by regulating cardiovascular physiology-associated modulators such as myostatin a/b, troponin C expression [43]. Also, it has been reported that irisin plays a protective role against ischemia–reperfusion injury to heart through a SOD2-dependent mitochondria mechanism [39]. These studies indicate significant translational value of myokines for therapeutic approaches in cardiovascular diseases. Our study highlights the important myokines as novel targets of FNDC5/irisin. Future studies will be required to elucidate the secretory and metabolic pathways for regulation of FNDC5/irisin levels in circulation, which may present significant translational value for clinical implications.
Conclusions
FNDC5 ameliorated HFD-induced cardiac hypertrophy, via the effects on inflammation, oxidative stress and NFκB activation, in association with JAK2/STAT3 signaling inhibition (Fig. 7). The cardio-protective role of FNDC5 servers for future therapeutic interventions in obesity-related cardiac complications.
Fig. 7.
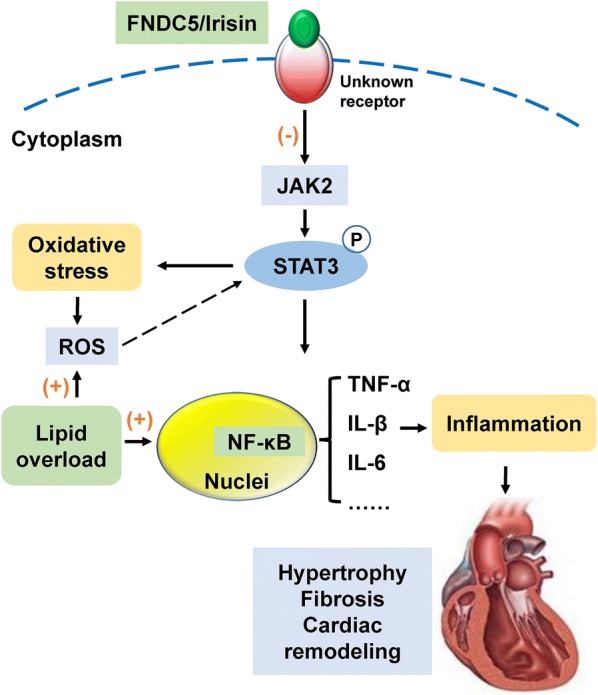
Schematic illustrates a possible mechanism of FNDC5 in obesity-induced cardiac hypertrophy. Obesity-induced lipid overload has a great impact on the production of reactive oxygen species (ROS) and upregulation of TNF-α, IL-1β and IL-6 levels, which leads to cardiac inflammation and oxidative stress. The enhanced cardiac inflammation and oxidative stress are involved in pathogenesis of cardiac injury and remodeling. FNDC5 may exert its protective function on the enhanced inflammation and oxidative stress via JAK2/STAT3 pathway through an unknown receptor
Additional file
Additional file 1: Table S1. Primers used for real-time PCR. Figure S1. FNDC5 deficiency aggravated HFD-induced cardiac hypertrophy and enhanced cardiac TNF-α, IL-1β and IL-6 levels in mice. Figure S2. FNDC5 deficiency enhanced NLRP3 and IL18 mRNA levels in heart of mice. Figure S3. Knockdown of FNDC5 enhanced palmitate-induced inflammation and NOX4 expression in H9c2 cells. Figure S4. Effects of exogenous FNDC5 and JAK2/STAT3 inhibitor pretreatment on inflammation and oxidative stress in H9c2 cells. Figure S5. Effects of exogenous FNDC5 and JAK2/STAT3 inhibitor pretreatment on phosphorylated JAK2 and STAT3 level in H9c2 cells. Figure S6. FNDC5 overexpression attenuated phosphorylated JAK2 and STAT3 level in heart of HFD-fed mice.
Authors’ contributions
ZG and XQX planned the experiments. ZG, WYF and YT performed the experiments. BZ and CY analyzed the data. XQX and YBZ wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the generous support of Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed in this study will be made available by the authors on reasonable request.
Consent for publication
This study consists of animal data and is devoid of any human data.
Ethics approval and consent to participate
This study was carried out in accordance with the principles of the Basel Declaration and recommendations of the Experimental Animal Care and Use Committee of Nanjing Medical University, and conformed to the Guide for the Care and Use of Laboratory Animal published by the US National Institutes of Health (NIH publication, 8th edition, 2011). The protocol was approved by Experimental Animal Care and Use Committee of Nanjing Medical University (Approval No. 1601246-1).
Funding
This work was supported by National Nature Science Foundation of China (81600323 and 81470539), Jiangsu Province University Natural Science Research Program (15KJB180010) and National Nature Science Foundation of Jiangsu (BK20150989).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- FNDC5
fibronectin type III domain containing 5
- HFD
high fat diet
- Nppa
natriuretic peptide type A
- Nppb
natriuretic peptide type B
- Myh7
myosin, heavy polypeptide 7, cardiac muscle, beta
- Tnfɑ
tumor necrosis factor ɑ
- Il1b
interleukin 1 beta
- Il6
interleukin 6
- Il18
interleukin 18
- Nlrp3
NLR family, pyrin domain containing 3
- PA
palmitate
- JAK2
Janus kinase 2
- STAT3
signal transducer and activator of transcription 3
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- SOD
superoxide dismutase
- MDA
malondialdehyde
Contributor Information
Zhi Geng, Email: gengzhino.1@163.com.
Wen-Yong Fan, Email: wyfan1603@163.com.
Bing Zhou, Email: zhoubing03@njmu.edu.cn.
Chao Ye, Email: chaoye@njmu.edu.cn.
Ying Tong, Email: tongy0617hg@njmu.edu.cn.
Ye-Bo Zhou, Email: zhouyebo666@njmu.edu.cn.
Xiao-Qing Xiong, Phone: +86-25-86869351, Email: xqxiong@njmu.edu.cn.
References
- 1.Zhao L, Wu D, Sang M, Xu Y, Liu Z, Wu Q. Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-kappaB and JAK/STAT signaling pathways in rats. Int Immunopharmacol. 2017;48:102–109. doi: 10.1016/j.intimp.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Medenwald D, Dietz S, Tiller D, Kluttig A, Greiser K, Loppnow H, Thiery J, Nuding S, Russ M, Fahrig A, et al. Inflammation and echocardiographic parameters of ventricular hypertrophy in a cohort with preserved cardiac function. Open Heart. 2014;1:e000004. doi: 10.1136/openhrt-2013-000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Wang F, Zhang Y, Kang Y, Wang H, Si M, Su L, Xin X, Xue F, Hao F, et al. Celecoxib prevents pressure overload-induced cardiac hypertrophy and dysfunction by inhibiting inflammation, apoptosis and oxidative stress. J Cell Mol Med. 2016;20:116–127. doi: 10.1111/jcmm.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Li L, Zhao H, Peng S, Zuo Z. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism. 2015;64:917–925. doi: 10.1016/j.metabol.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 6.Jin X, Liu L, Zhou Z, Ge J, Yao T, Shen C. Pioglitazone alleviates inflammation in diabetic mice fed a high-fat diet via inhibiting advanced glycation end-product-induced classical macrophage activation. FEBS J. 2016;283:2295–2308. doi: 10.1111/febs.13735. [DOI] [PubMed] [Google Scholar]
- 7.Wang HT, Liu CF, Tsai TH, Chen YL, Chang HW, Tsai CY, Leu S, Zhen YY, Chai HT, Chung SY, et al. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J Transl Med. 2012;10:145. doi: 10.1186/1479-5876-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, Khan ZA, Chakrabarti S, Wu L, Wang J, Liang G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-kappaB both in vitro and in vivo. J Mol Cell Cardiol. 2015;79:1–12. doi: 10.1016/j.yjmcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, Li YH, Zhou YB, Han Y, Zhang F, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta. 2015;1852:1867–1875. doi: 10.1016/j.bbadis.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Liu TY, Xiong XQ, Ren XS, Zhao MX, Shi CX, Wang JJ, Zhou YB, Zhang F, Han Y, Gao XY, et al. FNDC5 alleviates hepatosteatosis by restoring AMPK/mTOR-mediated autophagy, fatty acid oxidation, and lipogenesis in mice. Diabetes. 2016;65:3262–3275. doi: 10.2337/db16-0356. [DOI] [PubMed] [Google Scholar]
- 12.Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, Li YH, Wang JJ, Kang YM, Zhu GQ. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci. 2015;129:839–850. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 13.Cheng XW, Kuzuya M, Kim W, Song H, Hu L, Inoue A, Nakamura K, Di Q, Sasaki T, Tsuzuki M, et al. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation. 2010;122:707–716. doi: 10.1161/CIRCULATIONAHA.109.909218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:1221–1238. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- 15.Gielen S, Laughlin MH, O’Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis. 2015;57:347–355. doi: 10.1016/j.pcad.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Gao X, Du B, Zhao F, Feng X, Zhang H, Zhu Z, Xing J, Han Z, Tu P, Chai X. Ilex asprella aqueous extracts exert in vivo anti-inflammatory effects by regulating the NF-kappaB, JAK2/STAT3, and MAPK signaling pathways. J Ethnopharmacol. 2018;225:234–243. doi: 10.1016/j.jep.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Zhang W, Xu Q, Zhang J, Chen W, Xu Z, Li C, Wang Z, Zhang Y, Zhen Y, et al. Ang-(1-7) protects HUVECs from high glucose-induced injury and inflammation via inhibition of the JAK2/STAT3 pathway. Int J Mol Med. 2018;41:2865–2878. doi: 10.3892/ijmm.2018.3507. [DOI] [PubMed] [Google Scholar]
- 18.Yu Q, Zeng KW, Ma XL, Jiang Y, Tu PF, Wang XM. Ginsenoside Rk1 suppresses pro-inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells by inhibiting the Jak2/Stat3 pathway. Chin J Nat Med. 2017;15:751–757. doi: 10.1016/S1875-5364(17)30106-1. [DOI] [PubMed] [Google Scholar]
- 19.Gurzov EN, Stanley WJ, Pappas EG, Thomas HE, Gough DJ. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016;283:3002–3015. doi: 10.1111/febs.13709. [DOI] [PubMed] [Google Scholar]
- 20.Beckles DL, Mascareno E, Siddiqui MA. Inhibition of Jak2 phosphorylation attenuates pressure overload cardiac hypertrophy. Vasc Pharmacol. 2006;45:350–357. doi: 10.1016/j.vph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Xiong XQ, Geng Z, Zhou B, Zhang F, Han Y, Zhou YB, Wang JJ, Gao XY, Chen Q, Li YH, et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41. doi: 10.1016/j.metabol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YC, Xu LW, Ding S, Ji QQ, Lin N, He Q, Gao LC, Su YY, Pu J, He B. Nuclear receptor retinoid-related orphan receptor alpha deficiency exacerbates high-fat diet-induced cardiac dysfunction despite improving metabolic abnormality. Biochim Biophys Acta. 2017;1863:1991–2000. doi: 10.1016/j.bbadis.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Wodsedalek DJ, Paddock SJ, Wan TC, Auchampach JA, Kenarsary A, Tsaih SW, Flister MJ, O’Meara CC. IL-13 promotes in vivo neonatal cardiomyocyte cell cycle activity and heart regeneration. Am J Physiol Heart Circ Physiol. 2019;316:H24–H34. doi: 10.1152/ajpheart.00521.2018. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Lu K, Wang Y, Chen M, Zhang F, Shen H, Yao D, Gong K, Zhang Z. Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem Biophys Res Commun. 2017;485:69–75. doi: 10.1016/j.bbrc.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113:389–398. doi: 10.1093/cvr/cvx012. [DOI] [PubMed] [Google Scholar]
- 26.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Xie G, Xu Y, Ma L, Tong C, Fan D, Du F, Yu H. PEP-1-MsrA ameliorates inflammation and reduces atherosclerosis in apolipoprotein E deficient mice. J Transl Med. 2015;13:316. doi: 10.1186/s12967-015-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teshima Y, Takahashi N, Nishio S, Saito S, Kondo H, Fukui A, Aoki K, Yufu K, Nakagawa M, Saikawa T. Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Circ J. 2014;78:300–306. doi: 10.1253/circj.CJ-13-1187. [DOI] [PubMed] [Google Scholar]
- 29.Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.CIR.0000057545.82749.FF. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, Liu Y, Huang H, Tang Y, Liu W, Mei Y, Wan N, Liu X, Huang C. SH2B1 is critical for the regulation of cardiac remodelling in response to pressure overload. Cardiovasc Res. 2015;107:203–215. doi: 10.1093/cvr/cvv170. [DOI] [PubMed] [Google Scholar]
- 31.Graner M, Siren R, Nyman K, Lundbom J, Hakkarainen A, Pentikainen MO, Lauerma K, Lundbom N, Adiels M, Nieminen MS, Taskinen MR. Cardiac steatosis associates with visceral obesity in nondiabetic obese men. J Clin Endocrinol Metab. 2013;98:1189–1197. doi: 10.1210/jc.2012-3190. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 33.Huh JH, Kim HM, Lee ES, Kwon MH, Lee BR, Ko HJ, Chung CH. Dual CCR2/5 antagonist attenuates obesity-induced insulin resistance by regulating macrophage recruitment and M1/M2 status. Obesity. 2018;26:378–386. doi: 10.1002/oby.22103. [DOI] [PubMed] [Google Scholar]
- 34.Sell H, Poitou C, Habich C, Bouillot JL, Eckel J, Clement K. Heat shock protein 60 in obesity: effect of bariatric surgery and its relation to inflammation and cardiovascular risk. Obesity. 2017;25:2108–2114. doi: 10.1002/oby.22014. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Singh AP, Singh M, Krishan P. Impact of obesity on hypertension-induced cardiac remodeling: role of oxidative stress and its modulation by gemfibrozil treatment in rats. Free Radic Biol Med. 2011;50:363–370. doi: 10.1016/j.freeradbiomed.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Qian Y, Zhang Y, Zhong P, Peng K, Xu Z, Chen X, Lu K, Chen G, Li X, Liang G. Inhibition of inflammation and oxidative stress by an imidazopyridine derivative X22 prevents heart injury from obesity. J Cell Mol Med. 2016;20:1427–1442. doi: 10.1111/jcmm.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabiee F, Forouzanfar M, Ghazvini Zadegan F, Tanhaei S, Ghaedi K, Motovali Bashi M, Baharvand H, Nasr-Esfahani MH. Induced expression of Fndc5 significantly increased cardiomyocyte differentiation rate of mouse embryonic stem cells. Gene. 2014;551:127–137. doi: 10.1016/j.gene.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Chen K, Han Y, Zhu H, Zhou X, Tan T, Zeng J, Zhang J, Liu Y, Li Y, et al. Irisin protects heart against ischemia–reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72:259–269. doi: 10.1097/FJC.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–347. doi: 10.1161/CIRCRESAHA.107.150417. [DOI] [PubMed] [Google Scholar]
- 41.Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhan G, Huang N, Li S, Hua D, Zhang J, Fang X, Yang N, Luo A, Yang C. PGC-1alpha-FNDC5-BDNF signaling pathway in skeletal muscle confers resilience to stress in mice subjected to chronic social defeat. Psychopharmacology. 2018;235:3351–3358. doi: 10.1007/s00213-018-5041-2. [DOI] [PubMed] [Google Scholar]
- 43.Sundarrajan L, Yeung C, Hahn L, Weber LP, Unniappan S. Irisin regulates cardiac physiology in zebrafish. PLoS ONE. 2017;12:e0181461. doi: 10.1371/journal.pone.0181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primers used for real-time PCR. Figure S1. FNDC5 deficiency aggravated HFD-induced cardiac hypertrophy and enhanced cardiac TNF-α, IL-1β and IL-6 levels in mice. Figure S2. FNDC5 deficiency enhanced NLRP3 and IL18 mRNA levels in heart of mice. Figure S3. Knockdown of FNDC5 enhanced palmitate-induced inflammation and NOX4 expression in H9c2 cells. Figure S4. Effects of exogenous FNDC5 and JAK2/STAT3 inhibitor pretreatment on inflammation and oxidative stress in H9c2 cells. Figure S5. Effects of exogenous FNDC5 and JAK2/STAT3 inhibitor pretreatment on phosphorylated JAK2 and STAT3 level in H9c2 cells. Figure S6. FNDC5 overexpression attenuated phosphorylated JAK2 and STAT3 level in heart of HFD-fed mice.
Data Availability Statement
The datasets used and/or analyzed in this study will be made available by the authors on reasonable request.