Globally, cystic fibrosis (CF) affects nearly 80,000 children and adults (1). This autosomal-recessive disorder includes multiple gene alterations of the chloride transporter CF transmembrane conductance regulator (CFTR), preventing its processing, translocation to cell membranes, and activity. CF presents as a progressive multiorgan disease with debilitating effects on the lung, including significant airflow resistance and airway obstruction that is associated with compromised mucus hydration, chronic bacterial and viral infections, and lingering inflammation. For these reasons, the airway epithelium has been a focus for understanding disease pathophysiology and developing new therapeutics. Indeed, very promising recent clinical trials using triple CFTR-targeting therapy involving a potentiator (to increase channel opening) and two small-molecule correctors (to promote CFTR translocation to the membrane) were born from preclinical in vitro studies with primary cultured airway epithelial cells (2, 3).
In this issue of the Journal, Matusovsky and colleagues (pp. 434–444) look beyond the airway epithelium, setting their sights on airway smooth muscle that encircles intrapulmonary airways, to determine whether intrinsic changes in muscle contractility further underpin airway obstruction in CF (4). There is a compelling rationale for addressing this issue. The term “CF-asthma” was coined because more than 50% of patients with CF exhibit hallmark symptoms of asthma, such as cough, wheeze, and airway hyperresponsiveness, and 80% exhibit bronchodilator-reversible airway obstruction (5). This accounts for the overlap in standard clinical therapies for managing asthma and CF, with inhaled corticosteroids being used to control lung inflammation, and long- or short-acting β-agonists being used to dampen and reverse recurrent airway smooth muscle bronchoconstriction. Airway remodeling that includes enlargement of airway smooth muscle mass is a pathologic hallmark of asthma (6) and CF (7), an observation that was reconfirmed by Matusovsky and colleagues in airways from patients with severe CF. Interestingly, the carrier frequency of CFTR mutations is anomalously high in patients with asthma (8). Moreover, in a porcine model, Cook and colleagues (9) showed that the absence of CFTR leads to airway smooth muscle hypercontractility due to increased muscle tone, prolonged actomyosin activation, and compromised reuptake of Ca2+ to the sarcoplasmic reticulum after bronchoconstrictor stimulation. This is associated with the absence of CFTR in the sarcoplasmic reticulum membrane, resulting in loss of Cl− current that would otherwise contribute to the driving potential for inward Ca2+ flow (9). It is important to note that this work was completed using neonatal porcine lung material devoid of inflammation, and therefore it may not wholly represent the degree of real-life airway smooth muscle dysfunction experienced by adult patients with CF who have an airway phenotype that has been molded by ongoing inflammation and clinical intervention.
Matusovsky and colleagues meet these questions and knowledge gaps head on, using freshly isolated airway smooth muscle preparations microdissected from intrapulmonary airways of human lung transplant donors to evaluate intrinsic contractile properties and the relaxation response to β2-receptor activation, and how these are affected by preexposure to IL-13. They used airways from eight adult CF transplant lungs with severe disease for contractile function studies. The donors included five individuals homozygous for the ΔF508 mutation and one individual with the ΔF508/R334W genotype. CFTR protein deficiency was confirmed by IB. All of the CF donor lungs were positive for bacterial infection. For comparison, control airways were obtained from bacteria-free lungs of donors who died of acute causes unrelated to resident lung disease. Surprisingly, despite the use of elegant and sensitive methods to investigate baseline contractility using servo-electric muscle lever systems, there were no differences in methacholine-mediated maximum force, stress, shortening velocity, or isoproterenol-induced relaxation of precontracted airway muscle strips. At first glance, these findings do not appear to support a paradigm in which CFTR defects significantly alter human airway smooth muscle contractility. However, this is where the story really gets interesting!
The majority of CF lungs used in this study were obtained from donors who were being managed clinically with oral prednisone and inhaled bronchodilator. Lung tissue was acquired 24 hours before airway dissection in physiologic buffers, which likely eliminated and/or compromised the viability of resident inflammatory cells, and diluted local mediators. Chronic and acute exacerbation-associated lung inflammation in CF prominently includes T-helper cell type 2 (Th2) and Th17 cytokines, including IL-13, IL-8, and IFN-γ (10). IL-13 is particularly germane because it directly promotes airway smooth muscle hypercontraction in response to histamine and acetylcholine (11, 12). IL-13 reportedly decreases the abundance of sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA) in human airway smooth muscle, an effect that leads to prolonged and elevated myoplasmic Ca2+ (13). An additional mechanism for this effect involves dimeric IL-13 receptors that mediate increased expression of CD38, a membrane-associated enzyme that synthesizes cyclic adenine diphosphonucleotide ribose (cADPR) (11). cADPR is an activating ligand for ryanodine receptors that serve as ports for additional Ca2+ entry into the myoplasm from the sarcoplasmic reticulum, which increases agonist-induced contractile force.
In this context, Matusovsky and colleagues specifically interrogated how IL-13 preexposure affects airway smooth muscle contraction and relaxation. In so doing, they uncovered two significant disease effects that could contribute markedly to excessive and difficult-to-reverse bronchoconstriction (summarized in Figure 1).
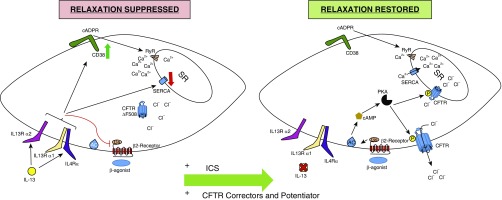
Figure 1.
Mechanisms contributing to altered airway smooth muscle contraction and relaxation in cystic fibrosis (CF). Left panel: chronic airway inflammation in CF includes IL-13, which mediates effects via dimeric receptors (IL13Rα1 with the IL4Rα or IL13R2A subunit). IL-13 induces expression of CD38, which catalyzes cyclic adenine diphosphonucleotide ribose (cADPR), which opens ryanodine receptors (RyR) to release Ca2+ from the sarcoplasmic reticulum (SR). IL-13 also reduces sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA), which facilitates Ca2+ reuptake. Reduced and improperly processed CF transmembrane conductance regulator (CFTR) further limits the driving force for Ca2+ reuptake. Collectively, this leads to prolonged elevation of myoplasmic Ca2+, and increased maximum force production in response to contractile agonist stimulation. IL-13 inhibits β2-receptor-Gs–mediated adenylate cyclase activation and cAMP synthesis, which markedly reduces muscle relaxation. Right panel: effective antiinflammatory therapy (shown here as inhaled corticosteroids [ICS]) can eliminate disease-specific effects of IL-13 that inhibit bronchodilator signaling, and renew cAMP synthesis and protein kinase A (PKA) mechanisms for smooth muscle relaxation, including phosphorylation and activation of CFTR. Additionally, well-controlled inflammation prevents IL-13 effects on CD38-RyR and SERCA, resulting in reduced cytoplasmic Ca2+ and mitigation of smooth muscle hypercontractility. New triple CFTR therapy could also restore CFTR function, better enabling Ca2+ reuptake to the SR and reducing Ca2+ influx via store- and voltage-operated channels in the plasma membrane. AC = adenylyl cyclase.
First, IL-13 exposure uniquely and significantly increased methacholine-stimulated maximum force by ∼90% in airway smooth muscle from donors with CF. An explanation for this finding in these tissues appears to lie in the parallel observation that IL-13 also significantly increased the abundance of myosin light chain kinase, the essential enzyme for actomyosin activation and contraction. Increased myosin light chain kinase content here mimics a fundamental phenotype described for airway smooth muscle in patients with asthma, suggesting a common pathway that contributes to airway hyperresponsiveness (14).
A second provocative finding is that IL-13 preexposure of CF airway smooth muscle resulted in a 50% decrease in relaxation caused by the β2 adrenergic receptor agonist isoproterenol. The mechanisms for this effect are not fully evident from the current study, but the investigators do confirm that β2-receptor expression is unchanged by IL-13. The capacity of IL-13 to decrease the β-agonist relaxation response of airway smooth muscle has actually been recognized for some time (15, 16). The current study’s demonstration that CF is a predisposing disease state for β-receptor hyporesponsiveness is an important and unique revelation with potentially significant implications for clinical management. Long-standing work shows that IL-13 can underpin β-adrenergic hyporesponsiveness in cultured human airway smooth muscle cells from healthy donors by diminishing generation of cAMP, but in the absence of direct effects on adenylate cyclase activity or the relaxing effects of exogenously added cAMP (16). How IL-13 can compromise activation of endogenous adenylate cyclase via the β2-receptor-Gs protein axis is not clear, although there is a requirement for p42/p44 mitogen-activated protein kinase activity, which can be involved with β-arrestin regulation in G-protein–coupled receptor signaling, internalization, and desensitization. The extent to which specific mechanisms may be operative and contribute to an asthma-like phenotype in patients with CF in whom CFTR is functionally absent, and who have ongoing airway inflammation and airway smooth muscle remodeling, is a matter that needs continued investigation.
The current study is timely and important in the context of recent advances in the clinical management of CF. Two clinical trials using combinations of a CFTR potentiator and correctors showed a significant sustained reversal of airway obstruction in stable patients with CF, with a 15% improvement in forced expiratory volume in 1 second (2, 3). The degree to which effects of CFTR therapies are manifested by reductions in airway smooth muscle tone and contractility has not been considered; rather, therapeutic effects are chiefly ascribed to effects on epithelial cell pathobiology. Interestingly, studies using ex vivo porcine lung slices have shown that CFTR potentiation with ivacaftor decreases airway reactivity (8). Given a role for CFTR in fostering Ca2+ reuptake and muscle relaxation, it is intriguing to speculate that triple CFTR therapy may well have significant relaxing effects on airway smooth muscle. Can the new insights into inflammation-associated bronchodilator hyporesponsiveness in CF airway smooth muscle described here by Matusovsky and colleagues provide an avenue to further enhance the success of triple CFTR therapy in the context of a CF-asthma phenotype? Can the well-managed use of inhaled or oral corticosteroids to limit airway inflammation significantly improve the potential to achieve maximum reversal of airway obstruction, and compound the benefits of triple CFTR therapy in reducing airflow limitation? These are exciting times, as the arsenal of informed strategies to improve lung function and control the common CF-asthma phenotype is growing.
Supplementary Material
Footnotes
Supported by the Canada Research Chairs Program (A.J.H.) and by a Canadian Institutes of Health Research Banting Fellowship (C.D.P).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Elborn JS. Cystic fibrosis. Lancet. 2016;388:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 2.Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, et al. VX16-659-101 Study Group. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1599–1611. doi: 10.1056/NEJMoa1807119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX16-445-001 Study Group. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matusovsky OS, Kachmar L, Ijpma G, Panariti A, Benedetti A, Martin JG, et al. Contractile properties of intrapulmonary airway smooth muscle in cystic fibrosis. Am J Respir Cell Mol Biol. 2019;60:434–444. doi: 10.1165/rcmb.2018-0005OC. [DOI] [PubMed] [Google Scholar]
- 5.Colombo JL. Long-acting bronchodilators in cystic fibrosis. Curr Opin Pulm Med. 2003;9:504–508. doi: 10.1097/00063198-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 6.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, et al. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185:1058–1064. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 7.Hays SR, Ferrando RE, Carter R, Wong HH, Woodruff PG. Structural changes to airway smooth muscle in cystic fibrosis. Thorax. 2005;60:226–228. doi: 10.1136/thx.2004.028340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzetis M, Efthymiadou A, Strofalis S, Psychou P, Dimakou A, Pouliou E, et al. CFTR gene mutations—including three novel nucleotide substitutions—and haplotype background in patients with asthma, disseminated bronchiectasis and chronic obstructive pulmonary disease. Hum Genet. 2001;108:216–221. doi: 10.1007/s004390100467. [DOI] [PubMed] [Google Scholar]
- 9.Cook DP, Rector MV, Bouzek DC, Michalski AS, Gansemer ND, Reznikov LR, et al. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle. Implications for airway contractility. Am J Respir Crit Care Med. 2016;193:417–426. doi: 10.1164/rccm.201508-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, et al. Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2013;187:621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, et al. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol. 2004;31:36–42. doi: 10.1165/rcmb.2003-0313OC. [DOI] [PubMed] [Google Scholar]
- 12.Risse PA, Jo T, Suarez F, Hirota N, Tolloczko B, Ferraro P, et al. Interleukin-13 inhibits proliferation and enhances contractility of human airway smooth muscle cells without change in contractile phenotype. Am J Physiol Lung Cell Mol Physiol. 2011;300:L958–L966. doi: 10.1152/ajplung.00247.2010. [DOI] [PubMed] [Google Scholar]
- 13.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L26–L34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. Am J Respir Crit Care Med. 2000;161:257–263. doi: 10.1164/ajrccm.161.1.9901005. [DOI] [PubMed] [Google Scholar]
- 15.Townley RG. Interleukin 13 and the beta-adrenergic blockade theory of asthma revisited 40 years later. Ann Allergy Asthma Immunol. 2007;99:215–224. doi: 10.1016/S1081-1206(10)60656-4. [DOI] [PubMed] [Google Scholar]
- 16.Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, et al. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001;164:141–148. doi: 10.1164/ajrccm.164.1.2008060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.