Abstract
Rationale: Obstructive sleep apnea is a risk factor for mortality, but its diagnostic metric—the apnea–hypopnea index—is a poor risk predictor. The apnea–hypopnea index does not capture the range of physiological variability within and between patients, such as degree of hypoxemia and sleep fragmentation, that reflect differences in pathophysiological contributions of airway collapsibility, chemoreceptive negative feedback loop gain, and arousal threshold.
Objectives: To test whether respiratory event duration, a heritable sleep apnea trait reflective of arousal threshold, predicts all-cause mortality.
Methods: Mortality risk as a function of event duration was estimated by Cox proportional hazards in the Sleep Heart Health Study, a prospective community-based cohort. Gender-specific hazard ratios were also calculated.
Measurements and Main Results: Among 5,712 participants, 1,290 deaths occurred over 11 years of follow-up. After adjusting for demographic factors (mean age, 63 yr; 52% female), apnea–hypopnea index (mean, 13.8; SD, 15.0), smoking, and prevalent cardiometabolic disease, individuals with the shortest-duration events had a significant hazard ratio for all-cause mortality of 1.31 (95% confidence interval, 1.11–1.54). This relationship was observed in both men and women and was strongest in those with moderate sleep apnea (hazard ratio, 1.59; 95% confidence interval, 1.11–2.28).
Conclusions: Short respiratory event duration, a marker for low arousal threshold, predicts mortality in men and women. Individuals with shorter respiratory events may be predisposed to increased ventilatory instability and/or have augmented autonomic nervous system responses that increase the likelihood of adverse health outcomes, underscoring the importance of assessing physiological variation in obstructive sleep apnea.
Keywords: obstructive sleep apnea, sleep, epidemiology, mortality, prospective
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea (OSA) is a disorder characterized by repetitive cycles of upper airway collapse during sleep and subsequent arousals from sleep. The most common OSA-defining metric, the apnea–hypopnea index (AHI), has been shown to predict risk for mortality in some, but not all, studies, with differences in associations observed by age and gender. Emerging data indicate that there is considerable physiological variability across and between nights within patients with OSA that is not captured by the AHI. Studies using additional features of OSA may shed light on OSA-related morbidity and mortality in the population and provide insights into underlying pathophysiological differences that influence health outcomes.
What This Study Adds to the Field
We characterized respiratory event duration, a heritable measure that reflects physiological features of OSA (arousal threshold) in a large prospective cohort. Adjusted analyses showed that short event duration predicts all-cause mortality over and beyond that predicted by the AHI. Moreover, associations between short event duration and mortality were observed in both men and women. This measure, readily available from routine polysomnography records, may help improve phenotyping of OSA-associated risk in the population, including helping to identify subgroups with low arousal threshold at risk for adverse outcomes.
Obstructive sleep apnea (OSA) is a disorder characterized by repeated airway collapse during sleep. The occlusion of the airway causes a cascade of physiological responses, including hypoxemia, hypercapnia, intrathoracic pressure swings due to inspiratory effort, activation of the sympathetic nervous system, and arousal from sleep (1). These acute consequences of each apnea are presumed to play a role in the long-term comorbidities associated with OSA that include cardiovascular disease and mortality (1–4). Clinical practice characterizes OSA severity using the apnea–hypopnea index (AHI), defined as the average number of respiratory events experienced per hour of sleep, calculated across the total sleep time. Although easy to calculate, this measure ignores other parameters of the apneas that may be informative, including associated hypoxemia and sleep fragmentation, as well as the duration of each event and the distribution of the events in each lying posture, within the night and within sleep stages (5–7).
Recent data suggest that OSA may be characterized by clusters of polysomnographic features that differ in their predictive associations with cerebrovascular and cardiovascular outcomes (8), supporting the likelihood of significant physiological variability among patients with similar AHI levels. Although this report included measurements of sleep fragmentation, it did not include direct measurements of arousal threshold or indirect measurements, such as duration of respiratory events. Event duration is readily calculated, but it is an inadequately studied trait that will partly determine the extent of hypoxemia, hypercapnia, and end-inspiratory effort—all key physiological stressors. Event duration is a heritable phenotype (9), with shorter events reflecting greater arousability due to lower arousal threshold or increased sensitivity of the respiratory chemical control system (10–12). Therefore, short-duration events may indicate a phenotype of hypersensitivity and hyperarousability―expected to exhibit sympathoexcitation, sleep fragmentation, and insomnia rather than sleepiness―that may respond to interventions that help consolidate sleep (13–17). The recent discovery of novel genetic loci that associate with event duration further supports the importance of this phenotype (18).
Given the heritability of event duration and its association with physiologically important traits, we analyzed data from the prospective Sleep Heart Health Study (SHHS) to test the hypothesis that respiratory event duration is associated with all-cause mortality. Given the inconsistencies of the literature regarding the association between AHI and mortality and cardiovascular risk in women (2–4, 19, 20), we also explored gender-specific effects. Some preliminary results of these studies have been previously reported in an abstract (21).
Methods
Sample Characteristics
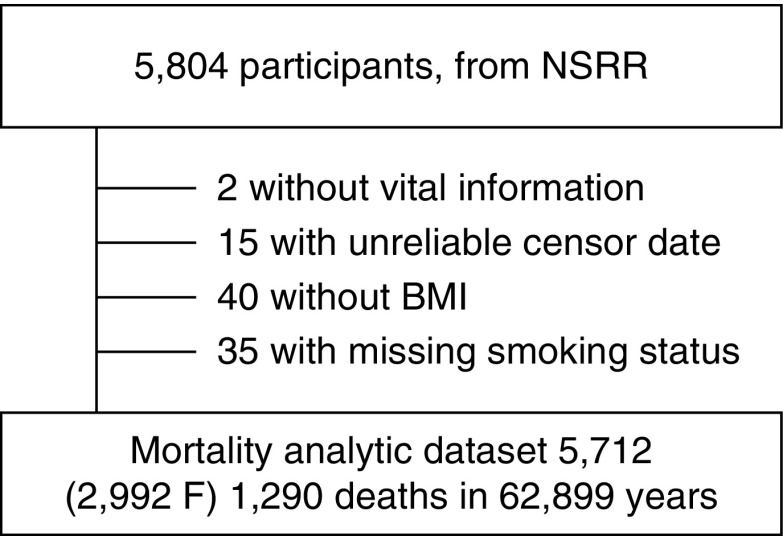
The sample is a subset of 5,804 subjects from the SHHS, publically available through the National Sleep Research Resource (https://sleepdata.org/). The SHHS is a community-based, prospective cohort study, designed to assess the cardiovascular consequences of sleep apnea (22). At baseline, participants completed questionnaires; had height, weight, and blood pressure measured; and underwent overnight unattended polysomnography. Additional covariate data were provided by the parent cohorts. Cardiovascular outcomes and mortality data through 2011 were analyzed (mean follow-up of 11 yr). The construction of the analytical sample is shown in Figure 1. The SHHS protocol was approved by the institutional review board of each participating center, and signed informed consent was provided by each subject. The dataset has been pseudonymized by removing all protected health information identifiers and assigning each participant a new randomly generated code. The Institutional Review Board of Oregon Health & Science University determined that the current study of pseudonymized data was not human subjects research (IRB ID 00017510). Complete methods are included in the online supplement and Tables E1 and E2 therein.
Figure 1.
Subject selection. BMI = body mass index; F = female; NSRR = National Sleep Research Resource.
Independent Variables, Covariates, and Endpoints
The primary exposure variable was the mean duration of all apneas and hypopneas during any stage of sleep and in any body position (details in the online supplement). Respiratory events (>10 s) were scored as apneas or hypopneas on the basis of oronasal airflow from thermistry. Obstructive apneas and hypopneas with and without desaturation criteria of 2%, 3%, or 4% were separately analyzed. Central apneas were not included in the analysis.
Other variables considered were age, gender, body mass index (BMI), race, smoking status, and prevalent hypertension, diabetes, coronary heart disease, stroke, and heart failure. Variables derived from polysomnography included: AHI (respiratory events with ≥3% desaturation per hour of sleep), arousal index, minimum oxygen saturation, percentage of sleep with oxygen saturation <90%, time in slow-wave sleep (stages 3 and 4), percentage of captured sleep time in REM, duration of the nighttime spent awake after the initial sleep onset (WASO), total sleep time, and sleep efficiency. Data for the last two measures were only available for 59–68% of the population (see online supplement).
The endpoint of all-cause mortality was defined as any confirmed death between the time of enrollment (1995–1998) and the end date of the study (2011). Mortality was ascertained from hospital records, obituaries, contact with next-of-kin, and the Social Security Administration, as described previously (2).
Statistical Analyses
Associations between respiratory event duration and mortality were assessed with Cox proportional hazard models. All models satisfied the proportional hazard assumption according to the Grambsch and Therneau test (23): all models had P > 0.12 for the global test. Individual plots of Schoenfeld residuals for each predictor against time to event showed no overt trends. Event duration was categorized into quartiles, with quartile 1 (Q1: longest event durations) serving as a reference category for quartiles 2 to 4 (Q2–Q4, in order of decreasing event duration). Three models were constructed. The base model included event duration and covariates of age, gender, race, smoking status, and BMI. Model 2 included the variables in model 1 plus AHI. Model 3 included the variables in model 2 plus prevalent hypertension, diabetes, stroke, coronary heart disease, and heart failure. Several other variables were added to model 3 in sensitivity analyses, as described in the Results.
Adjusted survival plots were constructed from the model 3 Cox regression coefficients. The reference survival curve represents the median risk on the basis of the distribution of risk scores (coefficients × predictors) for the full population, and then incremented by the risk associated with each quartile of apnea duration. Plots were also constructed for the gender-stratified Cox models.
In cross-sectional analyses, the correlations among event duration and other continuous variables were assessed by Spearman’s rho or χ2 likelihood ratio tests. Differences among quartiles were assessed by Wilcoxon/Kruskal-Wallis or one-way ANOVA. All statistical tests were conducted in R (Rstudio 1.1.383 running R version 3.3.2) or JMP Pro 11.0.0 (SAS Institute).
Results
The sample of 5,712 participants (Table 1) had a mean age of 63.3 years and mean BMI of 28 at baseline and were followed for an average of 11.0 years (median, 11.8 yr; range, 0.01–15.9 yr). The sample was 52% women, 85% white, and 9% African American (because of small sample sizes, other racial groups were combined: 6% of the sample). The sample included people across the range of OSA severity (12% severe, 20% moderate, 35% mild, 33% none). Prevalent hypertension and diabetes were 43% and 8%, respectively. During follow-up, 1,290 deaths were recorded over 62,899 person-years. Adjudicated cardiovascular outcomes were available from the parent cohorts for 1,166 deaths (other causes of death were not recorded). Within this set, 357 (30.6%) died of cardiovascular causes. This group included 233 deaths (20.0%) attributable to coronary heart disease and 35 deaths (3.0%) due to stroke. Two more people died from stroke but were not coded as dying from cardiovascular disease.
Table 1.
Selected Baseline Characteristics
| Sample Characteristic | Data |
|---|---|
| N | 5,712 |
| Age, yr | 63.3 (11.1) [39–90] |
| BMI, kg/m2 | 28.1 (5.1) [18–50] |
| Gender, % female | 52 |
| AHI, events/h* | 13.8 (15.0) [0–157] |
| Sleep time, min | 360 (64) [35–519] |
| Sleep efficiency, % | 81.5 (10) [11.3–98.5] |
| % time in REM | 19.3 (6.9) [0–43] |
| WASO, min | 61.6 (44) [0–368] |
| Arousal index, h−1 | 19.2 (10.7) [0–110] |
| % sleep < 90% saturation | 3.5 (10.3) [0–100] |
| AHI, events/h | 13.8 (15.0) [0–157] |
| OSA severity, % | |
| None | 33 |
| Mild | 35 |
| Moderate | 20 |
| Severe | 12 |
| Race, % | |
| African American | 9 |
| White | 85 |
| Other | 6 |
| Smoking status, % | |
| Current | 10 |
| Former | 47 |
| Never | 43 |
| Prevalent disease, % | |
| Hypertension | 43 |
| Diabetes | 8 |
| CHD | 8 |
| Stroke | 3 |
| HF | 2 |
| Any CVD† | 11 |
| Outcomes | |
| Deaths | 1,290 |
| Follow-up time, person-years | 62,899 |
| Mortality rate per 1,000 person-years | 20.5 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CHD = coronary heart disease; CVD = cardiovascular disease; HF = heart failure; OSA = obstructive sleep apnea; WASO = wake after sleep onset.
Data are presented as mean (SD) [range] unless otherwise noted.
AHI defined by the respiratory disturbance index with a 3% desaturation threshold.
Any prevalent CHD, stroke, or HF.
Relationship of Event Duration to Demographics, Prevalent Health Conditions, and Sleep-disordered Breathing Severity
Event duration varied from 11.2 to 57.6 seconds (mean, 21.3 s; median, 20.6 s) and varied significantly with several participant characteristics (Table 2). Participants with shorter event durations were more likely to be younger, female, African American, and current smokers. Shorter event durations were also associated with higher BMI, lower AHI, and higher minimum blood oxygen saturation (trend test, all P < 0.001; Table 2 and Figure E1). The same pattern of significance was obtained when treating event duration as a continuous variable; event duration measured continuously was also significantly correlated with percentage of sleep below 90% saturation (Table 2; Spearman’s correlation). Absolute mortality rates declined from the longest event quartile to the shortest event quartile (trend test, z = 4.3; P < 0.001), but this association appeared to be confounded by age and gender (see below).
Table 2.
Sample Characteristics by Quartile of Respiratory Event Duration
| Quartiles | Statistical Tests | ||||||
|---|---|---|---|---|---|---|---|
| Q1 (Longest) | Q2 | Q3 | Q4 (Shortest) | Quartile Difference(P Value) | Spearman’s ρ | Trend Test(P Value) | |
| Apnea duration, s | |||||||
| Range | (24.1, 57.6] | (20.6, 24.1] | (17.7, 20.6] | [11.2, 17.7] | NA | NA | NA |
| Mean (SD) | 27.80 (3.72) | 22.17 (0.98) | 19.18 (0.81) | 15.91 (1.25) | NA | NA | NA |
| n | 1,428 | 1,428 | 1,428 | 1,428 | NA | NA | NA |
| Age, yr* | 66 (11) | 63 (11) | 63 (11) | 61 (11) | <0.001 | +0.17 (P < 0.001) | <0.001 |
| BMI, kg/m2† | 27.4 (4.8) | 28.1 (4.9) | 28.5 (5.1) | 28.5 (5.4) | <0.001 | −0.09 (P < 0.001) | <0.001 |
| Gender, % female‡ | 47 | 49 | 53 | 61 | <0.001 | NA | <0.001 |
| AHI, events/h† | 12.0 (14.7) | 8.5 (12.2) | 8.6 (11.9) | 5.6 (9.6) | <0.001 | +0.19 (P < 0.001) | <0.001 |
| Minimum SaO2, %† | 84.0 (8.4) | 85.9 (6.1) | 85.6 (6.2) | 86.5 (5.4) | <0.001 | −0.11 (P < 0.001) | <0.001 |
| Sleep time, h* | 6.0 (1.1) | 6.0 (1.1) | 6.0 (1.1) | 6.0 (1.1) | n.s. | −0.03 (n.s.) | <0.10 |
| Sleep efficiency, %* | 81.2 (10.7) | 81.5 (10.3) | 81.5 (10.3) | 81.9 (10.5) | n.s. | −0.02 (n.s.) | n.s. |
| % time in REM* | 19.1 (6.8) | 19.6 (6.8) | 19.5 (7.1) | 19.1 (6.9) | <0.05 | −0.00 (n.s.) | n.s. |
| WASO, min† | 65 (46) | 63 (45) | 60 (42) | 58 (43) | <0.001 | +0.07 (P < 0.10) | <0.001 |
| Arousal index, h−1† | 19.4 (11.5) | 18.7 (10.8) | 19.3 (10.1) | 19.3 (10.3) | <0.10 | −0.02 (n.s.) | n.s. |
| % sleep < 90% saturation† | 4.3 (10.8) | 2.9 (9.1) | 3.4 (9.8) | 3.4 (11.4) | <0.01 | +0.10 (P < 0.001) | <0.10 |
| Race, %‡ | <0.01 | NA | NA | ||||
| African American | 6 | 9 | 10 | 10 | |||
| White | 88 | 84 | 84 | 84 | |||
| Other | 6 | 7 | 6 | 6 | |||
| Smoking status, %‡ | <0.001 | NA | NA | ||||
| Current | 6 | 8 | 9 | 16 | |||
| Former | 45 | 44 | 45 | 39 | |||
| Never | 49 | 49 | 45 | 45 | |||
| Prevalent disease, %‡ | |||||||
| Hypertension | 50 | 42 | 43 | 39 | <0.001 | NA | <0.001 |
| Diabetes | 8 | 8 | 8 | 8 | n.s. | NA | n.s. |
| CHD | 15 | 11 | 11 | 9 | <0.001 | NA | <0.001 |
| Stroke | 5 | 4 | 3 | 2 | <0.01 | NA | <0.001 |
| HF | 6 | 3 | 3 | 2 | <0.001 | NA | <0.001 |
| Any CVD§ | 20 | 14 | 14 | 11 | <0.001 | NA | <0.001 |
| Outcomes | |||||||
| Deaths | 385 | 301 | 315 | 286 | <0.001 | NA | <0.001 |
| Follow-up time, person-years | 14,171 | 14,461 | 14,534 | 14,659 | NA | NA | NA |
| Mortality rate per 1,000 person-years | 27.2 | 20.8 | 21.7 | 19.5 | NA | NA | <0.001 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CHD = coronary heart disease; CVD = cardiovascular disease; HF = heart failure; NA = no analysis; n.s. = not significant; Q = quartile; WASO = wake after sleep onset.
Data presented as mean (SD) or %, unless otherwise noted. Bivariate correlation coefficients (Spearman’s rho) against event duration were calculated for continuous measures. Trends across quartiles were assessed by the Cochran-Armitage trend test or by linear regression against quartile number.
Differences among quartiles were assessed by one-way ANOVA when variances were equal.
Differences among quartiles were assessed by Wilcoxon/Kruskal-Wallis test when variances were unequal (Bartlett test).
Differences among quartiles in proportions were assessed by χ2 test.
Any prevalent CHD, stroke, or HF.
Adjusted Associations between Event Duration and Other Measurements
Because anthropometric data varied with quartile, partial correlations between event duration and sleep measures were calculated after adjusting for age, gender, BMI, and AHI. Longer events were associated with a lower arousal index (partial correlation r = −0.12; P < 0.001), lower minimum nocturnal saturation (r = −0.09; P < 0.001), and slightly greater percentage of sleep time in REM (r = +0.04; P = 0.0035). After adjusting for covariates, event duration was not significantly correlated with total sleep time, sleep efficiency, WASO, or percentage time with saturation less than 90%.
Proportional Hazard Modeling for All-Cause Mortality
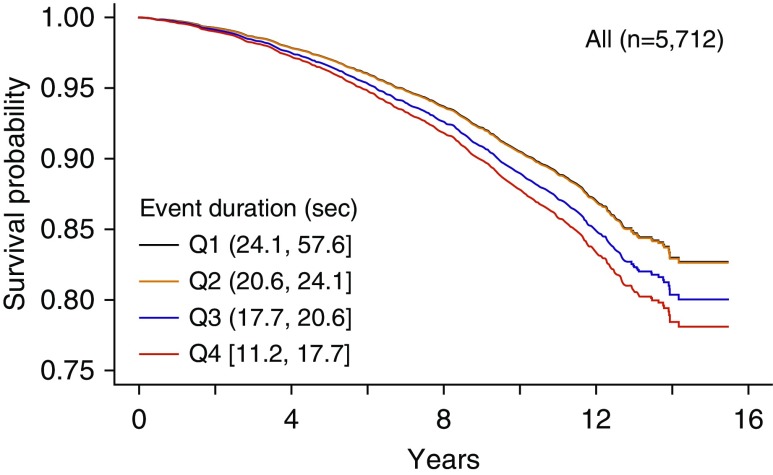
Associations of all-cause mortality with event duration quartile were assessed after adjusting for age, gender, BMI, race, and smoking status (model 1). Individuals with the shortest-duration events (Q4) experienced a 20% increased mortality rate compared with those with the longest-duration events (hazard ratio [HR], 1.20; 95% confidence interval [CI], 1.02–1.40; P = 0.024; Table 3). This relationship strengthened after adjusting for AHI (model 2; 26% increased risk; P = 0.0043). Adjusting for prevalent hypertension, diabetes, and cardiovascular disease also strengthened the results (model 3; 31% increased risk; P = 0.0013); the fully adjusted survival plot is shown in Figure 2. Replacing event duration quartiles with continuously measured duration did not alter the conclusion, showing that the results are not a function of quartile assignment. The HR per 10-second decrease in event duration was 1.15, indicating that shorter events conferred additional risk (95% CI, 1.03–1.30; P = 0.016). Finally, we added arousal index, because this is correlated to event duration and may be a similar physiological measure of arousal threshold. Including the arousal index in model 3 slightly strengthened the association (34% increased risk; P < 0.001).
Table 3.
Cox Proportional Hazard Ratios (95% Confidence Interval): Association of Event Duration (Quartile) with All-Cause Mortality
| Dataset | Model | Quartile |
AHI (Additional Hazard/5 Units) | |||
|---|---|---|---|---|---|---|
| Q1 (Longest) | Q2 | Q3 | Q4 (Shortest) | |||
| All subjects | 1 | Ref | 0.94 (0.81–1.09) | 1.12 (0.96–1.30) | 1.20 (1.02–1.40)* | NA |
| 2 | Ref | 0.96 (0.83–1.12) | 1.14 (0.98–1.33) | 1.26 (1.08–1.49)† | 1.03 (1.01–1.05)‡ | |
| 3 | Ref | 1.01 (0.86–1.17) | 1.18 (1.01–1.37)* | 1.31 (1.11–1.54)† | 1.02 (1.004–1.04)* | |
| All apneas | 3 | Ref | 0.98 (0.83–1.15) | 0.96 (0.80–1.15) | 1.21 (1.00–1.46)* | 1.03 (1.01–1.05)* |
| Hypopneas, ≥3% desaturation | 3 | Ref | 1.03 (0.88–1.19) | 1.07 (0.92–1.25) | 1.30 (1.11–1.53)† | 1.02 (0.999–1.04) |
| Non-REM | 3 | Ref | 1.04 (0.89–1.21) | 1.11 (0.95–1.30) | 1.28 (1.08–1.50)† | 1.02 (1.004–1.04)* |
| REM | 3 | Ref | 1.02 (0.87–1.20) | 0.96 (0.82–1.13) | 1.30 (1.11–1.52)* | 1.02 (0.998–1.04) |
| Women | 3 | Ref | 1.00 (0.79–1.26) | 1.10 (0.87–1.39) | 1.32 (1.05–1.66)* | 1.01 (0.98–1.05) |
| Men | 3 | Ref | 1.01 (0.83–1.23) | 1.23 (1.00–1.51) | 1.26 (1.00–1.59) | 1.03 (1.001–1.05)* |
Definition of abbreviations: AHI = apnea–hypopnea index; NA = no analysis; Q = quartile; Ref = reference.
Quartile 1 is the reference quartile. Other covariates: model 1: age, gender, body mass index, race, and smoking status (gender not included in gender-stratified models); model 2: model 1 plus AHI; model 3: model 2 plus prevalent hypertension, diabetes, coronary heart disease, stroke, and heart failure.
P < 0.05 compared to Q1.
P < 0.01 compared to Q1.
P < 0.001 compared to Q1.
Figure 2.
Differential survival from the fully adjusted Cox proportional hazard model 3. The reference survival curve represents the median risk over all predictors. Because the hazard ratio for quartile 2 (Q2) is 1.01, the curves for Q1 (reference) and Q2 are almost superimposable.
Other sensitivity analyses were conducted to test the potential role of OSA severity and continuous positive airway pressure (CPAP) treatment in the fully adjusted model 3. Adjusting for OSA severity as defined by clinical categories of none/mild/moderate/severe rather than continuous AHI did not change the results (e.g., Q4 relative to Q1: HR, 1.28; 95% CI, 1.09–1.51; P = 0.0030). Removing 120 subjects who either reported CPAP treatment or did not know at the interim SHHS2 follow-up likewise did not alter the results (Q4 relative to Q1: HR, 1.31; 95% CI, 1.11–1.54; P = 0.0014).
Effect of Event Subtype
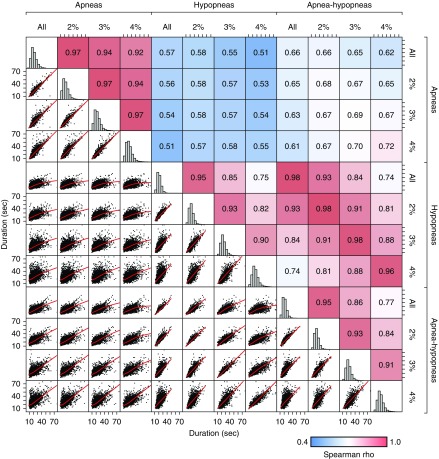
The magnitude of airflow limitation and the associated desaturation are factors used to classify apnea and hypopnea subtypes and characterize severity of each event. Therefore, we tested whether these factors would affect analyses. As expected, event duration was highly correlated across different event subtypes (Figure 3; all P < 0.001). The duration of all events (apneas plus hypopneas) was more strongly correlated with hypopnea duration (ρ, 0.74–0.98) than with apnea duration (ρ, 0.51–0.72), reflecting the predominance of hypopneas. The choice of event duration definition was further explored by separately considering obstructive apnea duration versus hypopnea duration in the Cox hazard models, each with and without a 3% desaturation criteria. Commonly used clinical definitions (all apneas without a desaturation threshold and hypopneas with ≥3% desaturation) are included in Table 3. HRs for the shortest events (Q4 vs. Q1) were similar when assessing hypopneas only (all hypopneas: HR, 1.27; 95% CI, 1.08–1.49; P = 0.0035; and hypopneas with ≥3% desaturation: HR, 1.30; 95% CI, 1.11–1.53; P = 0.0012; Table E3). HRs (Q4 vs. Q1) were slightly lower when events were restricted to obstructive apneas (all apneas: HR, 1.21; 95% CI, 1.00–1.46; P = 0.046). The sample size was smaller when only apneas with ≥3% desaturation were included, and this association was not significant but in the same direction (HR, 1.17; 95% CI, 0.96–1.43; P = 0.12).
Figure 3.
Respiratory event subtype correlation matrix. The matrix shows the relationship across different definitions of event type (apnea, hypopnea, and desaturation criteria). The combined apnea–hypopnea measure is more closely correlated with hypopnea duration than apnea duration. All correlations are significant but vary from 0.51 to 0.98 (blue to red). The highest correlations are between hypopnea duration and apnea–hypopnea duration for a given desaturation criteria (ρ, 0.96–0.98). Red lines are locally estimated scatterplot smoothing fits.
Non-REM versus REM Events
Respiratory events in REM sleep tend to be longer than in non-REM (NREM) sleep, but separate analysis of events in NREM versus REM had a minimal effect on results (NREM only: Q4/Q1 HR, 1.28; 95% CI, 1.08–1.50; P = 0.0036; REM only: Q4/Q1 HR, 1.30; 95% CI, 1.11–1.52; P = 0.0014). Likewise, adjusting further for the percentage of events that were in REM did not account for the association of event duration with mortality (HR, 1.27; 95% CI, 1.08–1.50; P = 0.0038). Finally, the amount of time in slow-wave sleep (N3/4) was added to model 3; it was not significant (P = 0.68) and did not affect the risk attributable to event duration.
Gender and Mortality Risk
Gender significantly predicted mortality in the fully adjusted model but did not modify the effect of duration on mortality: a gender-by–event duration (continuous) interaction term was added, but this was not significant (P = 0.88). Cox proportional hazard models were constructed for men and women alone to determine if HRs were similar in both groups. In the fully adjusted model 3, women and men with the shortest event duration had a 32% and 26% increased HR for death, respectively, compared with their counterparts with the longest event duration (Table 3 and Figure E2). This association was significant in women (P = 0.019) and approached significance in men (P = 0.050). For comparison, in alternative analyses similar to a prior report (2), the mortality risk associated with AHI differed between genders. Each additional 5 units of AHI increased the mortality risk in men (+3% risk; P = 0.043) but not in women (+1% risk; P = 0.48; Table 3, last column). Substituting categorical OSA severity for AHI or restratifying the quartiles within gender did not affect the conclusions (data not shown).
Interaction of Event Duration with AHI
Short event durations conferred mortality risk whether or not the model was adjusted for AHI. We next tested whether there was any interaction of AHI and event duration and if the risk associations held across all severities of OSA. The fully adjusted proportional hazard model was rerun adjusting for continuous AHI, event duration, and their interaction. Short event duration remained a significant predictor (HR per 10-s decrease in event duration was 1.20; 95% CI, 1.02–1.43; P = 0.029). In this model, there was no significant association for either AHI (HR, 0.99 per 5-unit increase; 95% CI, 0.92–1.07; P = 0.86) or the interaction of event duration and AHI (P = 0.45). After stratifying by clinical OSA severity categories, participants with the shortest events had a 15% to 59% increased risk for mortality, depending on their clinical OSA severity, with highest HRs in the group with an AHI of 15 to 30 (HR, 1.59; 95% CI, 1.11–2.28; P = 0.012; Table E4).
Discussion
In this study, we identify the novel association between average respiratory event duration during sleep and all-cause mortality in a large community sample. Even after adjusting for risk conferred by OSA severity (AHI) and key confounders, shorter event duration predicted higher 11-year mortality rates. Although the mechanisms that determine event durations are not fully understood, this measure is reflective of a low arousal threshold (24, 25), a phenotype that may associate with sleep fragmentation and elevated sympathetic tone. In contrast to the AHI, event duration predicted mortality in women, in whom OSA is often associated with insomnia symptoms and sleep fragmentation (26), but in whom long-term outcomes related to elevated AHI levels are poorly understood. Risk associations also were strongest in those with intermediate levels of AHI (15–30), a group in whom there are also varying data regarding OSA and mortality associations. Our findings, which held for men as well as women, suggest that consideration of this polysomnographic metric may help phenotype individuals who differ in OSA pathogenesis and outcomes and may particularly help identify individuals with intermediate AHI levels at increased risk for death.
Identifying prognostic features is of high priority for risk stratification and for focusing interventions at specific physiological perturbations (1, 27). OSA, as defined by AHI levels, has been shown to be a risk factor for mortality in some, but not all, studies (2, 4, 20, 28–31). Some studies show increased OSA-related mortality in younger compared with older individuals (32, 33) and in men compared with women (2, 20). Differences may relate to our ignorance of the most important features of sleep-disordered breathing that confer risk across the population. Other limitations of AHI-related prognosis relate to weaknesses of the AHI as a primary disease-defining metric, including its insensitivity to physiological disturbances of relevance to health outcomes (5–7, 34). This has led to efforts to identify better thresholds for risk on the basis of cohort outcomes (35, 36). It has also spurred efforts to test different aspects of the syndrome for risk (8). For example, relative to AHI, the extent of apnea-related desaturation and overnight hypoxemia appear to be better predictors of stroke and mortality (2, 37, 38). OSA fragments sleep and increases the arousal index; in one cohort study, arousal index was associated with prevalent subclinical cardiovascular disease (39). Zinchuk and colleagues (8) also showed that individuals characterized by “arousal and poor sleep” tended to have higher rates of myocardial infarction, atrial fibrillation, and chronic lung disease. Nevertheless, these and most other published studies have not analyzed the duration of respiratory events. In this large prospective sample, we showed that event duration predicted mortality over and beyond AHI and, furthermore, suggested that the predictive information associated with event duration was strongest in individuals with moderate to severe OSA, a group in whom there has been inconsistency in relationships with clinical outcomes.
Event duration is of particular interest because it may be a genetically encoded feature of OSA (9) that varies across population groups (e.g., shorter in women and African Americans) (40). We also discovered a novel genetic locus (rs35424364) that is associated with event duration in Latino/Hispanic Americans (18). In the current study of individuals with generally mild to moderately high AHI levels, we found that event duration had only weak associations with many typically used measures of sleep disturbance, including sleep time and efficiency, WASO, percentage of sleep in REM, and percentage of sleep with desaturation. This suggests that event duration contains unique information over and beyond other polysomnography measurements. These findings support the event duration as an independent, inherited trait that may help explain individual differences in OSA-related phenotypes.
Mechanistically, respiratory events will be terminated earlier (shorter duration) if, 1) an individual arouses from a lighter depth of sleep, 2) an individual arouses from the same stage of sleep but at a low level of chemical drive or respiratory effort (i.e., low arousal threshold), or 3) chemical drive builds up rapidly during an event (higher chemoreflex sensitivity to hypoxic hypercapnia or increased loop gain). All three mechanisms therefore reflect a state of increased “arousability” that could potentially promote mortality via greater sleep fragmentation, shorter sleep, and excess sympathetic tone. Indeed, we found that shorter events were associated with a greater arousal index (partial correlation: r = −0.12). Reduced sleepiness (consistent with greater arousability) is associated with increased sympathetic activity in heart failure (41). Hypersensitivity to chemical stimuli at the carotid bodies is also a mechanism of increased sympathoexcitation and attendant development of hypertension (42) and is a predictor of mortality in patients with heart failure (43).
Event duration varied significantly with several demographic and health-related factors. Individuals with shorter event duration were more likely to be younger, female, African American, and current smokers. The association of smoking with shorter events is especially interesting. Nicotine promotes alertness and lighter sleep, suggesting a lower arousal threshold. Smoking also disturbs EEG measures of sleep (44) and causes subjective complaints of disturbed sleep (45). Although nicotine can influence the release of central neurotransmitters associated with sleep–wake control (46), the mechanisms by which smoking may shorten apneas—for example, by reducing arousal threshold—requires further investigation.
In adjusted analyses, individuals with shorter events tended to have higher minimum SaO2 (reflecting shorter periods of obstructive breathing or reduced metabolic rate). However, after adjusting for the AHI, event duration was not associated with sleep time spent at low levels of oxygen saturation. Hypoxic burden derives from the combination of number of events, their duration, and their desaturation. A larger product of event duration and desaturation has been associated with long-term risk (5, 47). Our results—showing short rather than long events as predictors of risk—do not refute this. Rather, hypoxemia and event duration may represent different features of OSA-related stress, both of which may contribute to mortality through independent pathways. Our findings suggest a need to further identify the intermediate mechanisms that link event duration to long-term outcomes.
Event duration predicted mortality in both men and women, whereas AHI was only associated with mortality in men, as has been reported before (2). OSA is more likely to be associated with insomnia in women than in men (26, 48), and it is possible that, in women, OSA risk is less associated with the numbers of events during sleep and more associated with arousal-related responses to respiratory disturbances. Indeed, gender differences in OSA phenotypes are well described, with women generally showing greater airflow limitation, increased work of breathing, and sleep fragmentation (26, 49); recent studies also show gender-specific genetic variants for OSA (50). The current results show that mortality risk in women can be predicted from features of their sleep-disordered breathing, but the critical measure is likely not the AHI.
Strengths and Limitations of the Study
As a community-based prospective cohort, the study sample was not influenced by clinic or treatment selection biases. The long follow-up time, adjudicated outcomes, and rigorous collection of sleep data and other covariates allowed us to study the effects of event duration while also controlling for a number of confounders. Limitations include the single night of a sleep study which, although used for clinical decision making, may not fully characterize a person’s typical sleep. There is also no information about medications taken on the night of the sleep study. Some medications, especially psychoactive drugs, could alter arousability, although this community cohort is a general population sample that likely has limited psychoactive medication use. Future studies of event duration should explore the potential role of medications on arousability and event duration. We also investigated a number of potential causes for heterogeneity through sensitivity analyses to ensure that the main conclusions were not affected by statistical modeling or definitions of events. First, we found the same conclusions when the clinical OSA severity grouping (none/mild/moderate/severe) was substituted for AHI. Second, results were consistent whether quartiles of event duration were based on the entire sample or were gender specific. We also found generally consistent results when we only examined apneas and hypopneas, when we considered state-specific events, and when we included all events versus only those associated with desaturations. We focused on all-cause mortality to maximize statistical power. Future research is needed to discern associations with cause-specific mortality. Finally, results were not altered by the inclusion of subjects that reported CPAP use at the interim follow-up visit.
We cannot determine whether the short events reflect a direct causal mechanism for increased mortality or rather are markers for underlying autonomic or other physiological phenotypes that associated with unfavorable outcomes. Regardless, our data suggest that the occurrence of short event duration in individuals with mild to moderately elevated AHI levels may help identify individuals at risk for adverse health. Current clinical trial data are ambiguous regarding the role of OSA on mortality or cardiovascular incidence (SAVE [Sleep Apnea Cardiovascular Endpoints] study [51]). Reanalysis of those data considering variation in event duration may provide insights into possible heterogeneity of response to CPAP related to this phenotype.
The sample had few participants with very long events: only 5% had events that averaged longer than 30 seconds. Therefore, whether our results generalize to those with severe events is unclear. Further study, particularly in clinical populations, is warranted to understand the associations between very long event durations and hypoxemia to mortality.
In summary, this study shows that a relatively easily derived parameter from overnight polysomnography, respiratory event duration, predicts all-cause mortality over and beyond that predicted by the AHI. Moreover, event duration predicted mortality in both men and women independent of many potential confounders. These data provide new information to inform emerging initiatives to identify sleep apnea subphenotypes reflective of individuals with different underlying mechanisms who may experience different outcomes and respond differently to treatments. Event duration may measure a component of a phenotype characterized by low arousal threshold. Further research is needed to address whether these individuals are at increased risk for outcomes such as increased mortality due to elevated sympathetic tone or abnormalities in arousal-mediated stress responses.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Michael Lasarev for statistical advice, the Sleep Heart Health Study Reading Center team for expert sleep scoring, and the National Sleep Research Resource for providing the data.
Footnotes
Supported by NIH grants R21HL140377 (M.P.B., S.R., S. A. Shea, and A.W.), R01HL125893 (M.P.B. and S. A. Shea), R35HL135818 (S. A. Sands and S.R.), and R24HL114473 (S.R.); an American Sleep Medicine Foundation Focused Project Award (M.P.B.); American Heart Association grant 15SDG25890059 (S. A. Sands); and the American Thoracic Society Foundation (S. A. Sands).
Author Contributions: M.P.B., J.T.E., M.R., and S.R. designed the research and analyzed data. M.P.B., S.R., A.W., S. A. Shea, and S. A. Sands interpreted the data and wrote the manuscript. S.R. oversaw data collection and made it available via the National Sleep Research Resource.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0758OC on October 19, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput. 2013;51:697–708. doi: 10.1007/s11517-013-1039-4. [DOI] [PubMed] [Google Scholar]
- 6.Tam S, Woodson BT, Rotenberg B. Outcome measurements in obstructive sleep apnea: beyond the apnea-hypopnea index. Laryngoscope. 2014;124:337–343. doi: 10.1002/lary.24275. [DOI] [PubMed] [Google Scholar]
- 7.Shahar E. Apnea-hypopnea index: time to wake up. Nat Sci Sleep. 2014;6:51–56. doi: 10.2147/NSS.S61853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73:472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang J, Cade BE, Wang H, Chen H, Gleason KJ, Larkin EK, et al. Comparison of heritability estimation and linkage analysis for multiple traits using principal component analyses. Genet Epidemiol. 2016;40:222–232. doi: 10.1002/gepi.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 11.Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep. 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sforza E, Boudewijns A, Schnedecker B, Zamagni M, Krieger J. Role of chemosensitivity in intrathoracic pressure changes during obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:1741–1747. doi: 10.1164/ajrccm.154.6.8970364. [DOI] [PubMed] [Google Scholar]
- 13.Smales ET, Edwards BA, DeYoung PN, McSharry DG, Wellman A, Velasquez A, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12:758–764. doi: 10.1513/AnnalsATS.201408-399OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:1293–1300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards BA, Sands SA, Owens RL, White DP, Genta PR, Butler JP, et al. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol. 2014;592:4523–4535. doi: 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cade BE, Chen H, Stilp AM, Gleason KJ, Sofer T, Ancoli-Israel S, et al. Genetic associations with obstructive sleep apnea traits in Hispanic/Latino Americans. Am J Respir Crit Care Med. 2016;194:886–897. doi: 10.1164/rccm.201512-2431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 20.Yeboah J, Redline S, Johnson C, Tracy R, Ouyang P, Blumenthal RS, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis. 2011;219:963–968. doi: 10.1016/j.atherosclerosis.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler MP, Emch JT, Rueschman M, Lasarev M, Wellman A, Shea SA, et al. Apnea duration and inter-apnea interval as predictors of mortality in a prospective study [abstract] Sleep. 2015;38:A160. [Google Scholar]
- 22.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 23.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 24.Kimoff RJ, Cheong TH, Olha AE, Charbonneau M, Levy RD, Cosio MG, et al. Mechanisms of apnea termination in obstructive sleep apnea: role of chemoreceptor and mechanoreceptor stimuli. Am J Respir Crit Care Med. 1994;149:707–714. doi: 10.1164/ajrccm.149.3.8118640. [DOI] [PubMed] [Google Scholar]
- 25.Koo BB, Mansour A. Correlates of obstructive apnea duration. Lung. 2014;192:185–190. doi: 10.1007/s00408-013-9510-4. [DOI] [PubMed] [Google Scholar]
- 26.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30:312–319. doi: 10.1093/sleep/30.3.312. [DOI] [PubMed] [Google Scholar]
- 27.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 28.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JE, Lee CH, Lee SJ, Ryu Y, Lee WH, Yoon IY, et al. Mortality of patients with obstructive sleep apnea in Korea. J Clin Sleep Med. 2013;9:997–1002. doi: 10.5664/jcsm.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Kripke DF, Klauber MR, Fell R, Stepnowsky C, Estline E, et al. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep. 1996;19:277–282. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- 32.Rich J, Raviv A, Raviv N, Brietzke SE. All-cause mortality and obstructive sleep apnea severity revisited. Otolaryngol Head Neck Surg. 2012;147:583–587. doi: 10.1177/0194599812450256. [DOI] [PubMed] [Google Scholar]
- 33.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 34.Redline S, Sanders M. Hypopnea, a floating metric: implications for prevalence, morbidity estimates, and case finding. Sleep. 1997;20:1209–1217. doi: 10.1093/sleep/20.12.1209. [DOI] [PubMed] [Google Scholar]
- 35.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–1155. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campos-Rodriguez F, Martínez-García MA, Reyes-Nuñez N, Selma-Ferrer MJ, Punjabi NM, Farre R. Impact of different hypopnea definitions on obstructive sleep apnea severity and cardiovascular mortality risk in women and elderly individuals. Sleep Med. 2016;27–28:54–58. doi: 10.1016/j.sleep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone KL, Blackwell TL, Ancoli-Israel S, Barrett-Connor E, Bauer DC, Cauley JA, et al. Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39:531–540. doi: 10.5665/sleep.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutsey PL, McClelland RL, Duprez D, Shea S, Shahar E, Nagayoshi M, et al. Objectively measured sleep characteristics and prevalence of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis Sleep study. Thorax. 2015;70:880–887. doi: 10.1136/thoraxjnl-2015-206871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Cade BE, Stilp AM, Sofer T, Sands SA, Ancoli-Israel S, et al. Presented at the 67th Annual Meeting of The American Society of Human Genetics. Orlando, FL: 2017. Genome-wide trans-ethnic meta-analysis for a novel sleep apnea endophenotype [abstract, 2697F] pp. 17–21. [Google Scholar]
- 41.Taranto Montemurro L, Floras JS, Millar PJ, Kasai T, Gabriel JM, Spaak J, et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142:1222–1228. doi: 10.1378/chest.11-2963. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher EC, Lesske J, Behm R, Miller CC, III, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol (1985) 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 43.Giannoni A, Emdin M, Bramanti F, Iudice G, Francis DP, Barsotti A, et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol. 2009;53:1975–1980. doi: 10.1016/j.jacc.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Samet J, Caffo B, Bankman I, Punjabi NM. Power spectral analysis of EEG activity during sleep in cigarette smokers. Chest. 2008;133:427–432. doi: 10.1378/chest.07-1190. [DOI] [PubMed] [Google Scholar]
- 45.Phillips BA, Danner FJ. Cigarette smoking and sleep disturbance. Arch Intern Med. 1995;155:734–737. [PubMed] [Google Scholar]
- 46.Davila DG, Hurt RD, Offord KP, Harris CD, Shepard JW., Jr Acute effects of transdermal nicotine on sleep architecture, snoring, and sleep-disordered breathing in nonsmokers. Am J Respir Crit Care Med. 1994;150:469–474. doi: 10.1164/ajrccm.150.2.8049831. [DOI] [PubMed] [Google Scholar]
- 47.Muraja-Murro A, Kulkas A, Hiltunen M, Kupari S, Hukkanen T, Tiihonen P, et al. The severity of individual obstruction events is related to increased mortality rate in severe obstructive sleep apnea. J Sleep Res. 2013;22:663–669. doi: 10.1111/jsr.12070. [DOI] [PubMed] [Google Scholar]
- 48.Appleton SL, Gill TK, Lang CJ, Taylor AW, McEvoy RD, Stocks NP, et al. Prevalence and comorbidity of sleep conditions in Australian adults: 2016 Sleep Health Foundation national survey. Sleep Health. 2018;4:13–19. doi: 10.1016/j.sleh.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10:1075–1084. doi: 10.1016/j.sleep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Cade BE, Gleason KJ, Bjonnes AC, Stilp AM, Sofer T, et al. Multi-ethnic meta-analysis identifies RAI1 as a possible obstructive sleep apnea related quantitative trait locus in men Am J Respir Cell Mol Biol 201858:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.