To the Editor:
Adaptive servo ventilation (ASV) increased the risk for mortality in the SERVE-HF (Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure) trial, but the underlying mechanisms are unclear (1–3). Conceivably, device algorithms controlling respiratory rate and pressure support may have led to high e that caused hypocapnia and consequent arrhythmias (2, 4, 5). Whether such findings are a result of a device algorithm–based effect (“device-effect”) or apply to all servo-algorithm devices (“class-effect”) is uncertain (2, 6). We compared the performance of various ASV devices on measures of respiration and electrocardiography. Some of the results of these studies have been previously reported in the form of an abstract (7).
Methods
We performed a randomized controlled crossover physiological experiment of patients with complex sleep apnea with preserved cardiac contractility (left ventricular ejection fraction >45% by echocardiography) who were adherent to ASV therapy. Patients with untreated sleep disorders such as insomnia, periodic limb movement syndrome (leg movement index >10/h in prior laboratory-based polysomnography [PSG]), or restless legs syndrome were excluded. Patients were randomly assigned to 4 nights of PSG while receiving the device used in the SERVE-HF trial (ResMed S7 VPAP Adapt [ResMed]; hereafter, “S7 device”), a later version of the S7 device (ResMed S9 VPAP Adapt [ResMed]; hereafter, “S9 device”), a Philips ASV device (System One; Philips-Respironics, Inc.), and a later version of Philips ASV device (Dreamstation; Philips-Respironics, Inc.). For all devices, the expiratory positive airway pressure level was set from 4 to 15 cm H2O; the minimum pressure support was set at the lowest level possible (3 cm H2O for the S7 device and 0 cm H2O for all other devices); and maximum pressure support was 15 cm H2O, with maximum total pressure of 25 cm H2O with automatic back-up rate, whereas patients used the same mask interface on all nights. Conventional PSG with two electroencephalography leads each for frontal, occipital, and temporal; right and left electro-oculography; chin electromyography; lead II electrocardiography; finger pulse-oximetry; and respiratory signals derived from the device pneumotachograph output (airflow, Vt, and their derivatives: instantaneous respiratory rate [respiratory rate = 1/total respiratory cycle time] and e [product of Vt and respiratory rate]) were collected. Electrocardiography signals (200 Hz sampling rate) were analyzed for heart rate and QTc interval (MATLAB software). Patients were blinded to the device, and blinded observers scored PSG, respiratory, and electrocardiographic signals (8). Statistical analysis was performed by individuals blinded to study condition through numerical coding of the device using ANOVA or generalized linear model with repeated measures with adjustment for multiple comparisons (generalized linear model with Holm-Bonferroni correction that adjusts for control of family-wise error rate; IBM SPSS v25.0; IBM Corp.).
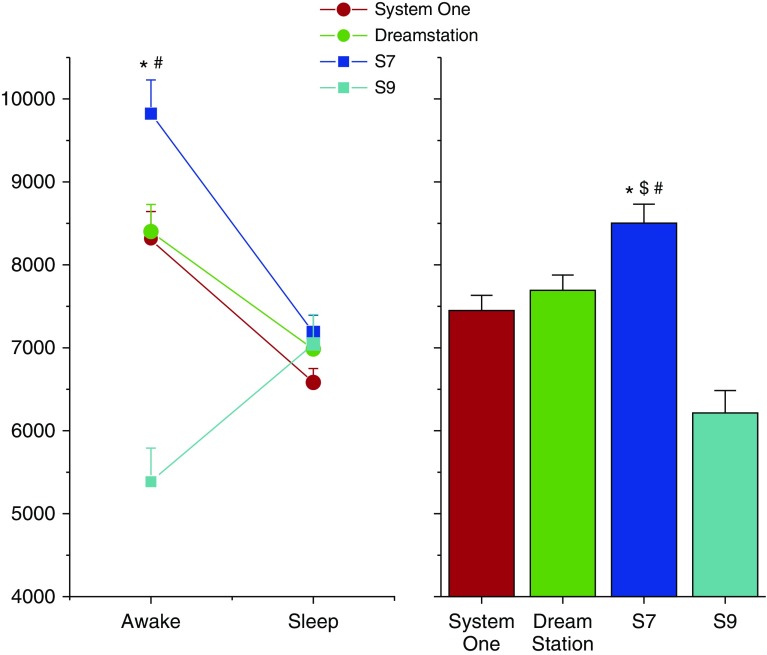
Fourteen patients underwent PSG on 4 nights while receiving treatment from four different devices. e was greater during treatment with the S7 device when compared with all other devices during wakefulness (P < 0.0001; Figure 1). The e for the entire night when receiving therapy with the S7 device was greater than the e for the entire night when receiving therapy with any of the other devices (P < 0.02; right upper panel of figure). Respiratory rate was greater with the S7 device when compared with the S9 device for the entire night (P < 0.0001; Table 1). During wakefulness, pressure support level was greater during S7 device therapy when compared with S9 device (P = 0.002; Table 1) and tended to be greater than pressure support level administered by the S9 device during sleep (P = 0.085). QT interval corrected for heart rate (QTc) during S7 therapy was not different than that during any of the other therapy nights (P = 0.24). The tendency for greater frequency of premature ventricular beats during S7 nights when compared with any other night was observed but did not reach statistical significance (P = 0.20). There were three episodes of nonsustained ventricular tachycardia during the entire study: two episodes during the S7 nights and one during a System One night. The total apnea–hypopnea index and apnea index were not different across the different devices (P > 0.6). The central apnea index tended to be lower in the S7 and S9 devices when compared with the System One and Dreamstation devices (ANOVA; P = 0.08).
Figure 1.
e by sleep–wakefulness state (left panel) and e for the entire night (right panel) are plotted for nights when undergoing laboratory-based polysomnography performed while receiving four different ASV devices: ResMed S7 VPAP Adapt (S7 device), a later version of the S7 device (ResMed S9 VPAP Adapt; S9 device), a Philips ASV device (System One), and an updated version of the Philips ASV device (Dreamstation). Mean and SE bars are plotted. Note that e for most devices was greater during wakefulness when compared with sleep. Also, the greatest e is during wakefulness while receiving therapy via the S7 device. There was no order effect for randomization sequence with regard to the e findings. *P < 0.05 when compared with Dreamstation device; #P < 0.05 when compared with S9 device; and $P < 0.05 when compared with System One. ASV = adaptive servo ventilation.
Table 1.
Breath Components and Pressure Assist Levels
| Device and Sleep Stage | Vt (ml) | Respiratory Rate (bpm) | PS Level (cm H2O) | Sleep Stage (%) | QTc Interval (ms) | CAI (Events/h) | |
|---|---|---|---|---|---|---|---|
| System One | |||||||
| Awake | 570 ± 33 | 14.9 ± 0.6 | 2.0 ± 0.4 | 22.8 ± 4.2 | 396.1 ± 6.2 | 6.3 ± 2.6 | |
| Sleep | 462 ± 17 | 14.7 ± 0.3 | 3.7 ± 0.2 | 14.9 ± 1.8 (N1) | 397.2 ± 5.4 | ||
| 41.9 ± 3.7 (N2) | |||||||
| 9.7 ± 1.4 (REM) | |||||||
| 10.6 ± 2.1 (SWS) | |||||||
| Dreamstation | |||||||
| Awake | 568 ± 33 | 15.3 ± 0.6 | 2.3 ± 0.4 | 29.0 ± 4.2 | 391.5 ± 6.4 | 4.2 ± 1.3 | |
| Sleep | 512 ± 17 | 14.2 ± 0.3 | 4 ± 0.2 | 16.0 ± 1.8 (N1) | 401.5 ± 5.4 | ||
| 38.8 ± 3.7 (N2) | |||||||
| 7.9 ± 1.4 (REM) | |||||||
| 8.1 ± 2.1 (SWS) | |||||||
| S7 | |||||||
| Awake | 624 ± 41 | 15.8 ± 0.8 | 2.6 ± 0.5 | 19.2 ± 5.2 | 400.9 ± 6.0 | 2.2 ± 1.1* | |
| Sleep | 460 ± 21 | 16 ± 0.4 | 2.6 ± 0.3 | 12.0 ± 2.2 (N1) | 403.5 ± 5.1 | ||
| 45.0 ± 4.5 (N2) | |||||||
| 9.2 ± 1.7 (REM) | |||||||
| 14.6 ± 2.6 (SWS) | |||||||
| S9 | |||||||
| Awake | 459 ± 41 | 12.0 ± 0.8 | 0.0.1 ± 0.5 | 49.8 ± 5.2† | 393.1 ± 6.2 | 2.2 ± 1.0* | |
| Sleep | 415 ± 24 | 11.1 ± 0.7 | 0.9 ± 0.4 | 5.1 ± 2.2 (N1) | 395.9 ± 5.5 | ||
| 28.5 ± 4.5 (N2) | |||||||
| 7.9 ± 1.7 (REM) | |||||||
| 8.6 ± 2.6 (SWS) |
Definition of abbreviations: bpm = breaths/min; CAI = central apnea index; N1 = non-REM sleep, stage 1; N2 = non-REM sleep, stage 2; PS = pressure support; SWS = slow wave sleep.
Data are shown as mean ± SE.
P = 0.08 when compared to other devices.
P < 0.05 when compared with other devices for wakefulness state.
Discussion
There were significant differences in e delivered by various ASV devices. During wakefulness, e was 15–40% greater during S7 night than with other devices. Moreover, the difference in e during wakefulness versus sleep states was greater for the S7 device (+2.64 ± 0.46) than any other device (System One [+1.73 ± 0.37]; Dreamstation [+1.42 ± 0.37]; and S9 device [−1.6 ± 0.1 L/min]; P < 0.0001). Such amplification factors of the wakefulness drive to breathe can create respiratory instability and potentiate central apneas, which, in turn, would require greater pressure support and respiratory rate by the servo mechanism and/or respiratory drive (9). Such increases in e during wakefulness cause hypocapnia (respiratory alkalosis), which, in turn, could cause hypokalemia (10). Hypokalemia resulting from nighttime intracellular shifts in potassium ions can prolong QT interval and lead to potentially life-threatening cardiac arrhythmias (10). Conceivably, nighttime alkalosis resulting from excessive ventilation may lead to daytime hypokalemia and QTc prolongation through renal loss of potassium at night, with consequent arrhythmogenic effects during the daytime. Although the observed QTc prolongation during S7 therapy was small in magnitude and not statistically significant, such effects may be magnified in patients with heart failure who develop metabolic alkalosis resulting from loop diuretics. We did not, however, measure CO2 levels or potassium levels, which is a study limitation. Future research needs to be performed with adequate sample size to distinguish such differences.
In our study, we found lower sleep efficiency (greater awake time) during S9 therapy nights when compared with other devices, including the S7 device night (Table 1). Specifically, the proportion of wakefulness time during S7 device therapy nights was better than that during S9 device nights (generalized linear model, P < 0.0001; Holm-Bonferroni correction, P = 0.0002). In fact, the proportion of time during wakefulness during S9 therapy was worse than that during any other device night. In contrast to our study finding, Teschler and colleagues (11) performed an elegant study in which improvements in central apnea index and sleep architecture (notably greater REM sleep and slow wave sleep) was observed when performing a randomized crossover trial of ASV, continuous positive airway pressure, bilevel positive airway pressure, and oxygen treatment for central sleep apnea. In our study, we compared four different types of ASV devices and noticed differences in e and sleep architecture across such devices, although there were no appreciable differences in central apnea index. Interestingly, we found that the magnitude of e was inversely related to proportion of time spent awake (Pearson R2 = −0.93; P = 0.031). Conceivably, hypocapnia-induced cerebral vasoconstriction and reduced arousability may have played a role in such a finding (12, 13).
The internal validity of the study is reflected by the expected greater e during wakefulness than during sleep, regardless of device. Moreover, the observation that the S7 device delivers greater ventilation is similar to that observed in the SERVE-HF trial (1, 4), whereby device algorithms that control rate and pressure support were compounded by a minimum default pressure support of 3 cm H2O that provided high levels of e (external validity) (4).
In conclusion, there were significant differences in e and sleep architecture while receiving ASV therapy from various available devices, and higher e was associated with small but statistically nonsignificant QTc prolongation. We speculate that the mechanisms underlying the adverse effects of ASV may be secondary to excessive ventilation resulting from a device-based effect rather than a class effect.
Supplementary Material
Footnotes
Funding support was provided by Philips-Respironics, Inc. The funding institution did not have any role in the design, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The statements in this manuscript are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. S.P. was also supported by NIH grants (HL138377 and MD011600) and PCORI (EAIN #3394-UoA and PPRND-1507-31666) during the writing of this manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201807-1303LE on January 3, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magalang UJ, Pack AI. Adaptive servo-ventilation for central sleep apnea in heart failure. N Engl J Med. 2016;374:691. doi: 10.1056/NEJMc1515007. [DOI] [PubMed] [Google Scholar]
- 3.Peker Y, Strollo PJ., Jr. A meta-analysis of positive airway pressure treatment for cardiovascular prevention: why mix apples and pears? Evid Based Med. 2017;22:218–219. doi: 10.1136/ebmed-2017-110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamauchi M, Combs D, Parthasarathy S. Adaptive servo-ventilation for central sleep apnea in heart failure. N Engl J Med. 2016;374:689. doi: 10.1056/NEJMc1515007. [DOI] [PubMed] [Google Scholar]
- 5.Naughton MT, Kee K. Sleep apnoea in heart failure: to treat or not to treat? Respirology. 2017;22:217–229. doi: 10.1111/resp.12964. [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Brown LK, Randerath WJ. Positive airway pressure therapy with adaptive servoventilation: part 1. Operational algorithms. Chest. 2014;146:514–523. doi: 10.1378/chest.13-1776. [DOI] [PubMed] [Google Scholar]
- 7.Knitter J, Patel SN, Bailey O, Poongkunran C, Flores A, Martinez L, et al. Comparison of performance of four adaptive servo ventilation devices in patients with complex sleep apnea [abstract] Sleep. 2018;41:203–204. doi: 10.1164/rccm.201807-1303LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol (1985) 1998;85:1929–1940. doi: 10.1152/jappl.1998.85.5.1929. [DOI] [PubMed] [Google Scholar]
- 10.Urso C, Brucculeri S, Caimi G. Acid-base and electrolyte abnormalities in heart failure: pathophysiology and implications. Heart Fail Rev. 2015;20:493–503. doi: 10.1007/s10741-015-9482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–619. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 12.Vinayak AG, Gehlbach B, Pohlman AS, Hall JB, Kress JP. The relationship between sedative infusion requirements and permissive hypercapnia in critically ill, mechanically ventilated patients. Crit Care Med. 2006;34:1668–1673. doi: 10.1097/01.CCM.0000218412.86977.40. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Kanno I, Ibaraki M, Hatazawa J, Miura S. Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2003;23:665–670. doi: 10.1097/01.WCB.0000067721.64998.F5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.