Abstract
Rationale: Neutrophils are recruited to the airways of individuals with cystic fibrosis (CF). In adolescents and adults with CF, airway neutrophils actively exocytose the primary granule protease elastase (NE), whose extracellular activity correlates with lung damage. During childhood, free extracellular NE activity is measurable only in a subset of patients, and the exocytic function of airway neutrophils is unknown.
Objectives: To measure NE exocytosis by airway neutrophils in relation to free extracellular NE activity and lung damage in children with CF.
Methods: We measured lung damage using chest computed tomography coupled with the Perth-Rotterdam Annotated Grid Morphometric Analysis for Cystic Fibrosis scoring system. Concomitantly, we phenotyped blood and BAL fluid leukocytes by flow and image cytometry, and measured free extracellular NE activity using spectrophotometric and Förster resonance energy transfer assays. Children with airway inflammation linked to aerodigestive disorder were enrolled as control subjects.
Measurements and Main Results: Children with CF but not disease control children harbored BAL fluid neutrophils with high exocytosis of primary granules, before the detection of bronchiectasis. This measure of NE exocytosis correlated with lung damage (R = 0.55; P = 0.0008), whereas the molecular measure of free extracellular NE activity did not. This discrepancy may be caused by the inhibition of extracellular NE by BAL fluid antiproteases and its binding to leukocytes.
Conclusions: NE exocytosis by airway neutrophils occurs in all children with CF, and its cellular measure correlates with early lung damage. These findings implicate live airway neutrophils in early CF pathogenesis, which should instruct biomarker development and antiinflammatory therapy in children with CF.
Keywords: air trapping, degranulation, mucus plugging, proteolysis, scavenging
At a Glance Commentary
Scientific Knowledge on the Subject
Neutrophils are recruited into the airways of individuals with cystic fibrosis (CF), and actively exocytose the primary granule protease elastase, whose free extracellular activity correlates with CF lung damage, notably bronchiectasis. During childhood, free extracellular elastase activity is only measurable in a subset of patients, and the exocytic function of airway neutrophils is unknown.
What This Study Adds to the Field
Using flow cytometry, we show that neutrophils actively release elastase in the airway lumen of children with CF, before the detection of bronchiectasis. In a cross-sectional two-center study, this measure of elastase exocytosis correlated with early structural lung damage measured by chest computed tomography. These findings implicate live airway neutrophils in early CF airway pathogenesis, which should instruct biomarker development and antiinflammatory therapy in early CF.
Cystic fibrosis (CF) is a multiorgan disease caused by recessive mutations of the CFTR gene (1). Morbidity and mortality in CF relate primarily to progressive lung damage, leading eventually to respiratory failure. Although some studies point to anomalies in prenatal development of large airways in CF (2), the first overt symptoms may be detected months to years after birth (3–5), primarily because of involvement of the small airways (6). Thanks to newborn screening, CF infants can be diagnosed within a few weeks of birth and monitored closely from a very young age, which has led to improved patient care and outcomes (7). Among methods for monitoring in early CF are chest computed tomography (CT) scans (8), to assess structural lung damage, and BAL fluid (BALF) collection (9), to investigate infection status, as well as cellular and molecular biomarkers (10).
Nearly 20 years have revealed that children with CF develop signs of lung disease early in life (5, 11, 12). Inflammation as evidenced by increased levels of proinflammatory mediators and downstream recruitment of blood neutrophils into the airway lumen occurs within a few weeks after birth (13). It is still unclear whether early CF airway inflammation is triggered solely by cellular stress linked to abnormal CFTR expression (14), as suggested by several CF models in which infection can be excluded experimentally (15–18). Early infection is also believed to play a role in this process, possibly as a trigger, and most definitely as an accelerator, of inflammation (19). Still to be better defined are the roles played in early disease of typical CF-associated proinflammatory microorganisms, such as Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, and Aspergillus spp. (20–23), and of oral microflora that may also spread to the airways (24).
Critically, recruitment of blood neutrophils to the airways in early CF disease is marked by the appearance of neutrophil-derived metabolites in BALF (25, 26), and release of the protease neutrophil elastase (NE). A cross-sectional study close to a decade ago showed that free extracellular NE activity in BALF (measured with a conventional spectrophotometric assay) correlated with structural lung damage as assessed with a three-slice CT method (27). A follow-up longitudinal study showed that 3-month-old infants with CF whose BALF was positive for free extracellular NE activity had increased odds of developing bronchiectasis at 12 and 36 months than those for which BALF was negative (28).
Another important issue relates to the mode of release of NE by airway neutrophils, which is normally contained in primary granules in the cytosol (29). We showed in prior studies that live neutrophils recruited to the airway lumen in adolescents and adults with CF are characterized by hyperactive NE exocytosis and the loss of phagocytic receptors, among other anomalies (30–33). However, it remains unknown whether similar active dysfunction occurs in neutrophils present in the airway lumen of children with CF, and how this dysfunction may relate to free extracellular NE activity and structural lung damage. This question is particularly important at a time when novel therapies are slowing the appearance of symptoms in patients with CF (34).
To address this question, we performed phenotyping of airway leukocytes from children with CF using flow and image cytometry (35). Concomitantly, we used chest CT scans scored with the sensitive Perth-Rotterdam Annotated Grid Morphometric Analysis for Cystic Fibrosis (PRAGMA-CF) tool recently developed for early disease monitoring (36), and free extracellular NE activity measurement by a Förster resonance energy transfer (FRET)-based method (37–39). Our findings support the notion that NE exocytosis by airway neutrophils occurs in children with CF, and that its cellular measure correlates with early lung damage. Some of the results of these studies have been previously reported in the form of abstracts (40–43).
Methods
Human Subjects and Samples
Data were collected prospectively from 42 children with CF (age, 3–62 mo) enrolled in disease surveillance programs at Telethon Kids Institute, Perth, Australia and Erasmus Medical Center/Sophia Children's Hospital, Rotterdam, the Netherlands. Measures of NE exocytosis by freshly collected blood and BAL leukocytes, free extracellular NE activity in BALF, inflammatory mediator levels in BALF, and chest CT scans analyzed with the PRAGMA-CF scoring method were sought at the same visit.
Control subjects (n = 10) were enrolled among children without CF less than 6 years of age who were undergoing a bronchoscopy procedure as part of their diagnostic work-up in the Aerodigestive Clinic at Emory University. This clinic follows patients with airway disorders (laryngomalacia, subglottic stenosis, tracheomalacia, bronchomalacia), dysphagia, and chronic aspiration, and patients with complex medical conditions and genetic disorders associated with various malformations. We excluded patients with a tracheostomy, those with significant airway reconstruction (slide tracheoplasty), significant prematurity (<28 wk gestational age), or those with evidence of diffuse lung disease (childhood interstitial lung disorders)
Demographic data for the primary prospective cohorts of CF and control children are summarized in Table 1. Further details on human subjects and sample collection appear in the Methods section of the online supplement. Because samples from children were sometimes limiting in terms of cell yield, we list in Table E1 in the online supplement all CF and control subjects and the assays that were effectively implemented on their samples. In addition, fixed BALF leukocytes were available to conduct confirmatory image cytometry measurement of NE content from another prospective cohort of 10 children with CF enrolled in 2015 at Erasmus Medical Center/Sophia Children's Hospital (age, 13–37 mo).
Table 1.
Demographics of the Primary CF and Control Cohorts
| CF | Control | |
|---|---|---|
| Sex, n (%) | ||
| Males | 20 (47.6) | 5 (50.0) |
| Females | 22 (52.4) | 5 (50.0) |
| Age, mo, mean (SD) | 30.82 (23.0) | 21.4 (7.5) |
| Genotype, n (%) | ||
| F508Del homozygous | 18 (42.9) | NA |
| F508Del heterozygous | 19 (45.2) | NA |
| Other | 5 (11.9) | NA |
| Infection status (pathogens detected), n (%) | ||
| None | 32 (76.2) | 6 (60.0) |
| One | 7 (16.7) | 2 (20.0) |
| Two or more | 3 (7.1) | 2 (20.0) |
Definition of abbreviations: CF = cystic fibrosis; NA = not applicable.
Chest CT Imaging
Volumetric chest CT scans obtained under general anesthesia at Princess Margaret Hospital for Children (8) and free-breathing chest CT scans obtained without anesthesia at Erasmus Medical Center/Sophia Children's Hospital were scored using the PRAGMA-CF method (36). Total disease (PRAGMA-Dis%), bronchiectasis (PRAGMA-Bx%), and air trapping (PRAGMA-TA%) were reported.
Flow and Image Cytometry
Multiparametric flow cytometry was performed on cells obtained from blood and BALF, as detailed previously (33, 35), with some modifications detailed in the Methods section of the online supplement. Critical to the implementation of this study was the implementation of cell staining, and data acquisition protocols for flow cytometry that enabled cross-center standardization and downstream centralized analysis, as explained previously (35). Image cytometry data acquisition and analysis was performed as described previously (31). Gating of neutrophils and macrophages followed the strategy illustrated in Figure E2A.
BALF Assays for Inflammatory Mediators and Free Extracellular NE Activity and Inhibition
Levels of 20 inflammatory mediators were measured in BALF using a multiplexed high-sensitivity chemiluminescent assay (U-plex; Meso Scale Diagnostics), per the manufacturer's protocol. Free extracellular NE activity was measured using a conventional spectrophotometric assay (28) based on the chromogenic substrate N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (working range, 0.02–12.0 μg/ml), and the FRET-based NEmo-1 probe (Sirius Fine Chemicals SiChem GmbH), as previously described (37–39). The NEmo-1 probe was also used to assess the potential of native BALF from children with CF to inhibit NE activity, as before (38). Samples were measured in duplicate with repeated measures for values outside the working range.
Statistical Analysis
Data were tabulated in Excel (Microsoft) and transferred to JMP13 (SAS Institute) and Prism v7 (GraphPad) for statistical analysis. The potential effect of study site was evaluated for all measured variables based on linear models adjusting for age and site, with or without their interactions. Data for those variables found to be significantly affected were corrected for site effects while adjusting for age using a well-established nonparametric empirical Bayesian method (44) typically used for multisite analysis of data. Downstream statistical analyses were performed on the combined data with the site effect corrected. Data were analyzed using nonparametric statistics, including the Mann-Whitney test for unpaired samples, Wilcoxon signed rank test for paired outcomes, and Spearman test for correlations.
Results
Macrophages and Neutrophils Coexist in BAL from Children with CF and Disease Control Children
CT scans were successfully conducted on 36 out of 42 children with CF and analyzed using the PRAGMA-CF scoring method (36). This analysis yielded a PRAGMA-%Dis of 2.97 (interquartile range, 2.2–3.85), PRAGMA-%TA of 2.28 (0.48–5.06), and PRAGMA-%Bx of 0.0 (interquartile range, 0.0–0.26). The fact that bronchiectasis was not detectable or below 1% in most children in this study illustrates their early stage of disease. Airway leukocyte counts by the clinical laboratory showed a predominance of macrophages (median, 83%) over neutrophils (median, 14.5%) and lymphocytes (median, 3.0%). In disease control children, BAL counts showed a predominance of macrophages (median, 83.6%) over lymphocytes (median, 9.0%) and neutrophils (median, 4.35%). These data are consistent with BAL counts assessed in a cohort of 48 normal children aged 3–16 years (45), with macrophages predominant (median, 84%) over lymphocytes (median, 12.5%), and very few neutrophils (median, 0.9%). Absolute BAL counts presented in Table E2 show that children with CF had higher numbers of total leukocytes, macrophages, and neutrophils than disease control children enrolled in this study. However, disease control children still presented with significant numbers of BAL neutrophils, consistent with airway inflammation (albeit lower than in infants with CF).
Airway Neutrophils in Children with CF Show Distinct Changes Consistent with Hyperexocytosis
Flow cytometry was used to gain further insight into airway leukocytes from CF and control children. Matched blood samples collected at the same visit as the BAL procedure were also available for CF and control children (34 out of 42, and 9 out of 10, respectively). Our gating strategy discriminated major leukocyte subsets from blood and BAL (Figures 1A and E1), and yielded airway macrophage and neutrophil percentages that were highly correlated with those reported by the clinical laboratory for both CF and control cohorts (R > 0.7 for all), thus validating our analytical approach.
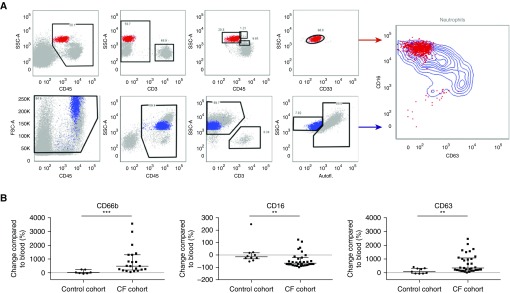
Figure 1.
Flow cytometry gating strategy and neutrophil phenotype. (A) After definition of singlets (not shown), blood (red, top left) and airway (blue, bottom left) neutrophils were gated in four sequential steps, as shown. Autofluorescence in the violet laser-excited 450/50 nm channel (Autofl.) was low in airway neutrophils and high in airway macrophages (see Figure E1). All children with cystic fibrosis (CF) showed a typical pattern in airway neutrophils with increased surface CD63 (primary granule exocytosis) and decreased surface CD16 (phagocytic receptor) expression (right panel). (B) Surface expression of CD66b (left; control cohort, n = 9; CF cohort, n = 22), CD16 (middle; control cohort n = 9; CF cohort n = 33), and CD63 (right; control cohort n = 9; CF cohort n = 33) on airway neutrophils are shown in % change compared with matched blood neutrophils. Significant differences in between-group analyses are indicated as **P < 0.01 and ***P < 0.0001. FSC = forward scatter; SSC = side scatter.
Next, we assessed surface marker expression on airway neutrophils. We found that all children with CF enrolled (age range, 3–62 mo) harbored a distinct population of live airway neutrophils that we previously found to be typical of CF airways in adolescents and adults (33), with increased CD63 expression (reflecting exocytosis of NE-rich primary granules), and decreased CD16 expression (reflecting phagocytic receptor loss) compared with blood neutrophils (Figure 1A). Upregulation of CD66b, reflecting exocytosis of secondary granules (Figure 1B) was also consistent with prior data in adolescents and adults with CF (32, 46). Because CD16 was also significantly downregulated on disease control airway neutrophils compared with blood (albeit to a lesser extent than CF airway neutrophils), this change may not be viewed as specific to CF. By contrast, neither surface CD66b nor surface CD63 were significantly upregulated on airway neutrophils from disease control children, which suggests that hyperactive exocytosis of secondary granules and, most importantly, of NE-rich primary granules may be distinguishing features of CF pathogenesis.
Cell-based Measure of NE Exocytosis by Airway Neutrophils, but Not Inflammatory Mediators or Free Extracellular NE Activity, Correlates Cross-Sectionally with Structural Lung Damage in Children with CF
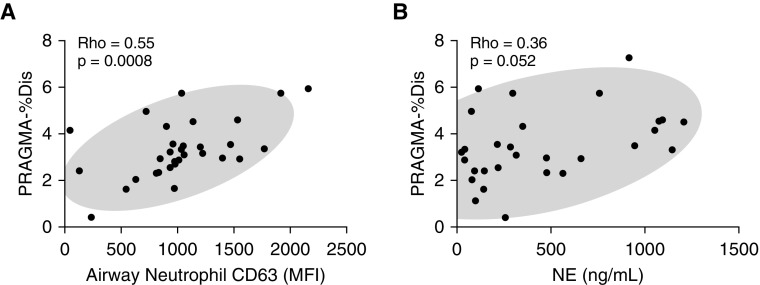
The exocytosis of NE-rich primary granules, reflected by airway neutrophil CD63 expression measured by flow cytometry, showed a significant positive correlation with structural lung damage (R = 0.55; P = 0.0008), as measured by PRAGMA-%Dis (Figure 2A). By contrast, PRAGMA-%Dis correlates neither with typical measures of inflammation, such as BAL neutrophil % or count (not shown), nor the levels of 20 inflammatory mediators (see Table E3), although we observed a trend toward a positive correlation for IL-8.
Figure 2.
Correlation of neutrophil elastase (NE) exocytosis by airway neutrophils, and free extracellular NE activity with structural lung damage. (A) Correlation of the PRAGMA-%Dis with airway neutrophil CD63 levels (n = 33). (B) Correlation of the PRAGMA-%Dis score with free extracellular NE activity (interpolated to a concentration of recombinant NE standard expressed in ng/ml; n = 29). MFI = median fluorescence intensity; PRAGMA-%Dis = Perth-Rotterdam Annotated Grid Morphometric Analysis for Total Disease.
Because primary granule exocytosis by neutrophils results in extracellular NE release, we next assessed NE activity in BAL from this cohort. Of 42 patients, 35 had enough BALF for NE measurement. Of those, the conventional spectrophotometric NE activity assay (28) yielded measurable levels in 27 samples. To achieve higher sensitivity, we used a newly introduced FRET method based on the NEmo-1 probe (37–39). This method yielded measurable NE activity in 33 out of 35 samples. Despite its increased sensitivity, the FRET-based NE activity measure trended but did not correlate significantly with PRAGMA-%Dis (Figure 2B).
Cell-based Measure of NE Exocytosis by Airway Neutrophils and Free Extracellular NE Activity Are Not Impacted by Infection Status in Children with CF
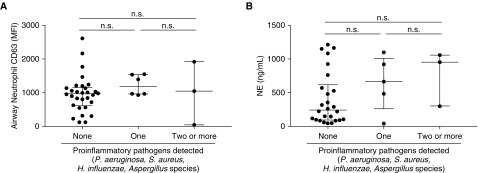
We investigated whether airway neutrophil CD63 expression and free extracellular NE activity differed based on infection, as determined by clinical microbiology. Infection status was classified based on the presence of 0, 1, or 2 or more proinflammatory pathogens, which were previously identified in studies of children with CF to encompass P. aeruginosa, S. aureus, H. influenzae, and Aspergillus spp. (21, 22). Among the 42 children with CF included in the present study, both airway neutrophil CD63 (Figure 3A) and free extracellular NE activity (Figure 3B) were not associated with infection status among children with CF. Similar results were observed in disease control subjects (not shown).
Figure 3.
Neutrophil elastase (NE) exocytosis by airway neutrophils and free extracellular NE activity are not impacted by infection status. (A) Airway neutrophil CD63, as a measure of NE exocytosis (in median fluorescence intensity; n = 39) is compared between children with cystic fibrosis based on infection status (0, 1, or 2 or more proinflammatory pathogens detected in BAL fluid). (B) Free extracellular NE activity (interpolated to a concentration of recombinant NE standard expressed in ng/ml; n = 34) is also compared between children with cystic fibrosis based on infection status. MFI = median fluorescence intensity; n.s. = not significant.
Extracellular NE Is Counteracted by Antiproteases and Compartmentalizes in Airway Leukocytes in BALF of Children with CF
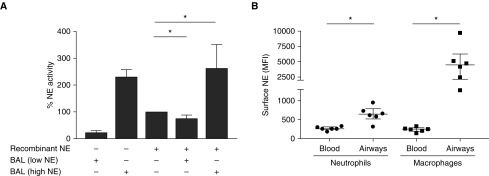
To explain the discrepancy between airway neutrophil CD63 expression and FRET-based free extracellular NE activity data, we investigated whether the activity of NE released by neutrophils could be counteracted by antiproteases present in the BALF of children with CF, and/or compartmentalize in airway leukocytes. The presence of a functional antiprotease shield was assessed using BALF from children with CF with high or low level of free extracellular NE (Figure 4A). We observed that the BAL from children with CF with low NE status was still able to inhibit the recombinant NE activity, whereas those with high NE were unable to inhibit recombinant NE, suggesting that the antiprotease shield in children with CF is still active at early stages of disease. Using flow cytometry, we found significant surface NE expression in children with CF on airway neutrophils and macrophages, with higher levels on the latter (Figure 4B).
Figure 4.
Free extracellular neutrophil elastase (NE) activity in children with cystic fibrosis is limited by BAL fluid antiprotease activity and by compartmentalization on airway leukocytes. (A) Activity of recombinant NE was measured in vitro by Förster resonance energy transfer in the absence or presence of BAL fluid from children with cystic fibrosis with either low or high intrinsic NE activity. Results were compared with the unopposed activity of recombinant NE in the absence of BAL fluid (100%). (B) Surface NE antigen was measured by flow cytometry in cystic fibrosis blood and airway neutrophils and macrophages (n = 6). *P < 0.05. MFI = median fluorescence intensity.
Finally, to assess whether NE could be found inside of airway leukocytes, we used fixed and permeabilized BAL cells from another prospectively enrolled CF cohort (n = 10; age, 13–37 mo), and used image cytometry to quantify NE burden (see Figure E2). As expected, airway neutrophils contained large amounts of total NE, although sizeable amounts were also detectable in airway macrophages. Because NE biosynthesis is confined to neutrophils, these data suggest that airway macrophages in children with CF can bind and internalize extracellular NE released by airway neutrophils.
Discussion
Through cellular phenotyping, we demonstrate that children with CF at all stages of disease harbor a distinct population of airway neutrophils with high active exocytosis of NE-rich primary granules. Airway neutrophil CD63 expression correlated positively with structural lung damage (R = 0.55), and did not depend on infection status. In contrast, conventional biomarkers of neutrophilic inflammation used in individuals with CF to track the severity of lung damage (10), such as BAL neutrophil % and inflammatory mediator levels, did not correlate with lung damage. However, the correlations of IL-8 levels (measured by a multiplexed chemoluminescent assay) and free extracellular NE activity (measured by a sensitive FRET-based assay) with lung damage both trended toward significance.
CD66b, a marker of secondary granule exocytosis, was upregulated on airway neutrophils from CF but not children without CF, compared with blood as we previously observed on airway neutrophils from older patients with CF (32, 33). Also consistent with previous findings in older patients with CF, we found that airway neutrophils in children with CF downregulated the phagocytic receptor CD16 (33), although similar (albeit lesser) downregulation was seen in disease control children. Together, our findings suggest that neutrophils recruited to the airway lumen of children with CF are conditioned to adopt an activated state with distinguishing features (increased CD63 expression). This is consistent with the notion that the CF airway environment can trigger early inflammation, as previously proposed in select mouse models of CF (38, 47, 48), human CF fetal xenografts (15), CF ferrets (18, 49), and children with CF (13). Insights gained from this study of children with CF can help calibrate models (50).
Our study confirms that recruitment and dysfunction of neutrophils are key processes in early CF airway disease, whether triggered by infectious or sterile mechanisms (51). Our findings have important therapeutic implications, because they highlight the need for efficient leukocyte-targeted interventions (52) to help curb down the progression of structural lung damage in children with CF. A prime target for intervention is NE, for which inhibitors (53) should be tested for their ability to block not only extracellular, but also surface-associated forms. Alternative strategies include reducing the number of neutrophils recruited from blood (54, 48) and their ability to exocytose primary granules (55).
The apparent discrepancy between the cellular measure of NE release (airway neutrophil CD63 expression) and the molecular measure of free extracellular NE activity (FRET-based assay) is explained, at least in part, by our findings that NE is present at the surface and inside of airway neutrophils and macrophages, and that the airway antiprotease shield is still able to inhibit NE activity in children with CF, at least until its maximum buffering capacity is reached. This finding also suggests that assays for free extracellular NE activity do not reflect the overall burden of NE in CF airways.
The finding of high NE expression on airway macrophages also suggests a role for this subset in controlling the fate of NE. Therefore, it may also be beneficial to design targeted interventions aimed at optimizing airway macrophage function in children with CF (56). Understanding the fate of extracellular NE is critical to correct the protease-antiprotease imbalance that characterizes CF airways (57). However, targeting NE may not suffice, because similar complex activation and compartmentalization may occur with other proteases, such as matrix metalloproteinases 2, 9, and 12, which originate from neutrophils and macrophages (46, 58–60). Future work should aim to identify the mutual impacts that these proteases have on each other, and points for intervention at molecular and/or cellular levels.
The present study extends prior data (27, 28) gained with a less sensitive method for CT scoring, and for free extracellular NE measurement, yielding categorical data (presence/absence) adequate only for odds ratio calculation over time, whereas the FRET method used here yields continuous data enabling correlation analysis. The recently developed PRAGMA-CF CT scoring method (36) was critical in detecting early disease events in our cohort of patients with CF. Indeed, very few children showed evidence of bronchiectasis, including among 5 year olds. This is in contrast with studies conducted in the past decade, and may reflect improved abilities to preserve the integrity of lung architecture in children with CF. This emphasizes the need for sensitive methods able to detect pathologic processes occurring in CF airways before the detection of bronchiectasis.
The present study also presents several limitations. First, disease control subjects included in this study had higher numbers of neutrophils in their BAL than healthy children from a prior study (45), but did not include patients with primary ciliary dyskinesia or idiopathic bronchiectasis, who may undergo very pronounced neutrophil recruitment to the airways and hence may have represented more appropriate control subjects. With regards to primary ciliary dyskinesia itself, the onset of neutrophilic inflammation is not known and prior pediatric studies have enrolled patients older than 6 years of age (61–63), which exceeds the age range of children with CF (5 yr and under) enrolled in this study. Further studies investigating BAL of infants with early onset primary ciliary dyskinesia or idiopathic bronchiectasis are needed to shed light on whether these conditions show similar neutrophil exocytosis profiles as infants with CF.
Second, it is important to note that the infection status of children with CF in our study used routine clinical microbiologic methods, rather than highly sensitive genomics-based methods (23). Therefore, although we can safely state that the presence of typical CF proinflammatory pathogens (P. aeruginosa, S. aureus, H. influenzae, and Aspergillus spp.) does not impact airway neutrophil CD63 expression at the time of the measurement, we cannot exclude potential impact of prior infections, or low-level presence of atypical organisms (24). Viral infections may also be at play, although a recent study suggested that children with CF do not suffer more frequent viral infections or increased inflammatory responses on viral infections than children without CF (64).
Third, it is important to acknowledge that this study is cross-sectional in nature, and although it shows a significant correlation between NE exocytosis, as indicated by NE CD63 surface expression, and early lung damage, which excludes bronchiectasis for all but a handful of patients, it thus does not provide any information on its predictive value for future damage. A follow-up longitudinal study is a clear future direction for our multisite research effort.
Finally, our attempt to generate CT, BALF, and BAL leukocyte analyses at the same visit from children with CF younger than 5 years of age sometimes faced limitations of volumes that prevented some downstream analyses. In that context, it is critical in follow-up studies to choose surrogate endpoints wisely, and airway neutrophil CD63 emerges as a worthwhile candidate in that regard.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all children and parents willing to enroll in the study cohorts in Perth, Rotterdam, and Atlanta. They also thank the Telethon Kids Institute flow cytometry core, Erasmus Medical Center Department of Cell Biology flow cytometry core, and Children’s Healthcare of Atlanta and Emory University pediatric flow cytometry core for their cooperation. They also thank Dr. J. Chandler for access to instrumentation and helpful discussions, and A. Portelli, A. Taurone, L. Silva, L. Berry, C. Mok, and Dr. A. Mohandas for help with data collection.
Footnotes
Supported by the NIH (R01HL126603, H.H., M.R.B., B.J.S., H.M.J., L.P., and R.T.), CF@LANTA Research Development Program Fellowship (C.M.) as funded by the U.S. Cystic Fibrosis Foundation (MCCART15R0), National Health and Medical Research Council (NHMRC; Australia) (111142505, L.W.G., R.T., A.K., and S.M.S.), Peter Doherty Fellowship (1141479, L.W.G.), Western Australian Department of Health Merit Award (L.W.G.), the German Ministry for Education and Research (FKZ 82DZL00401 and FKZ 82DZL004A1, M.A.M.), the German Cystic Fibrosis Association Mukoviszidose e. V. (Project number 1605, A.S.D.), and the Heidelberg Research Center for Molecular Medicine Career Development Fellowship (A.S.D.). AREST CF is supported by several sources, including the NHMRC, NIH, U.S. Cystic Fibrosis Foundation Therapeutics, and Cystic Fibrosis Australia. IMPEDE-CF is supported by the CF@LANTA Research Development Program Pilot Fund (R.T. and L.G.) as funded by the U.S. Cystic Fibrosis Foundation (MCCART15R0).
Author Contributions: Study conception, R.T., S.M.S., M.A.M., and H.M.J. Study implementation, C.M., L.W.G., A.S.D., S.T.M., and H.H. Critical technical and/or logistical support, D.L.F., M.R.B., T.R., C.S., B.J.S., L.G., A.K., and L.P. Manuscript writing, C.M. and R.T. Manuscript review, L.W.G., B.J.S., L.G., L.P., H.M.J., M.A.M., and S.M.S.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201803-0442OC on October 3, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of AREST-CF and IMPEDE-CF
References
- 1.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasemann H, Ratjen F. Early lung disease in cystic fibrosis. Lancet Respir Med. 2013;1:148–157. doi: 10.1016/S2213-2600(13)70026-2. [DOI] [PubMed] [Google Scholar]
- 4.VanDevanter DR, Kahle JS, O'Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros. 2016;15:147–157. doi: 10.1016/j.jcf.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Ranganathan SC, Hall GL, Sly PD, Stick SM, Douglas TA Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Early lung disease in infants and preschool children with cystic fibrosis. What have we learned and what should we do about it? Am J Respir Crit Care Med. 2017;195:1567–1575. doi: 10.1164/rccm.201606-1107CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiddens HA, Donaldson SH, Rosenfeld M, Paré PD. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol. 2010;45:107–117. doi: 10.1002/ppul.21154. [DOI] [PubMed] [Google Scholar]
- 7.Coffey MJ, Whitaker V, Gentin N, Junek R, Shalhoub C, Nightingale S, et al. Differences in outcomes between early and late diagnosis of cystic fibrosis in the newborn screening era. J Pediatr. 2017;181:137–145. doi: 10.1016/j.jpeds.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, et al. AREST CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67:509–516. doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 9.Fayon M, Kent L, Bui S, Dupont L, Sermet I European Cystic Fibrosis Society Clinical Trial Network Standardisation Committee. Clinimetric properties of bronchoalveolar lavage inflammatory markers in cystic fibrosis. Eur Respir J. 2014;43:610–626. doi: 10.1183/09031936.00017713. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey KA, Schultz A, Stick SM. Biomarkers in paediatric cystic fibrosis lung disease. Paediatr Respir Rev. 2015;16:213–218. doi: 10.1016/j.prrv.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 12.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013;68:1157–1162. doi: 10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- 15.Tirouvanziam R, Khazaal I, Péault B. Primary inflammation in human cystic fibrosis small airways. Am J Physiol Lung Cell Mol Physiol. 2002;283:L445–L451. doi: 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 16.Fritzsching B, Zhou-Suckow Z, Trojanek JB, Schubert SC, Schatterny J, Hirtz S, et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015;191:902–913. doi: 10.1164/rccm.201409-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paemka L, McCullagh BN, Abou Alaiwa MH, Stoltz DA, Dong Q, Randak CO, et al. Monocyte derived macrophages from CF pigs exhibit increased inflammatory responses at birth. J Cyst Fibros. 2017;16:471–474. doi: 10.1016/j.jcf.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen BH, Evans TIA, Moll SR, Gray JS, Liang B, Sun X, et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med. 2018;197:1308–1318. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, et al. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. 2005;40:500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld M, Ramsey BW, Gibson RL. Pseudomonas acquisition in young patients with cystic fibrosis: pathophysiology, diagnosis, and management. Curr Opin Pulm Med. 2003;9:492–497. doi: 10.1097/00063198-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Sagel SD, Gibson RL, Emerson J, McNamara S, Burns JL, Wagener JS, et al. Inhaled Tobramycin in Young Children Study Group; Cystic Fibrosis Foundation Therapeutics Development Network. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J Pediatr. 2009;154:183–188. doi: 10.1016/j.jpeds.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangell C, Gard S, Douglas T, Park J, de Klerk N, Keil T, et al. AREST CF. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis. 2011;53:425–432. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 23.Pittman JE, Wylie KM, Akers K, Storch GA, Hatch J, Quante J, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis. Association of antibiotics, airway microbiome, and inflammation in infants with cystic fibrosis. Ann Am Thorac Soc. 2017;14:1548–1555. doi: 10.1513/AnnalsATS.201702-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhlebach MS, Zorn BT, Esther CR, Hatch JE, Murray CP, Turkovic L, et al. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 2018;14:e1006798. doi: 10.1371/journal.ppat.1006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esther CR, Jr, Turkovic L, Rosenow T, Muhlebach MS, Boucher RC, Ranganathan S, et al. AREST CF. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J. 2016;48:1612–1621. doi: 10.1183/13993003.00524-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandler JD, Margaroli C, Horati H, Kilgore MB, Veltman M, Liu HK, et al. Myeloperoxidase oxidation of methionine associates with early cystic fibrosis lung disease. Eur Respir J. 2018;52:1801118. doi: 10.1183/13993003.01118-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 28.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 29.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2:98–108. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingersoll SA, Laval J, Forrest OA, Preininger M, Brown MR, Arafat D, et al. Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death-ligand 1. J Immunol. 2015;194:5520–5528. doi: 10.4049/jimmunol.1500312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laval J, Touhami J, Herzenberg LA, Conrad C, Taylor N, Battini JL, et al. Metabolic adaptation of neutrophils in cystic fibrosis airways involves distinct shifts in nutrient transporter expression. J Immunol. 2013;190:6043–6050. doi: 10.4049/jimmunol.1201755. [DOI] [PubMed] [Google Scholar]
- 32.Makam M, Diaz D, Laval J, Gernez Y, Conrad CK, Dunn CE, et al. Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc Natl Acad Sci USA. 2009;106:5779–5783. doi: 10.1073/pnas.0813410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, et al. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc Natl Acad Sci USA. 2008;105:4335–4339. doi: 10.1073/pnas.0712386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heltshe SL, Cogen J, Ramos KJ, Goss CH. Cystic fibrosis: the dawn of a new therapeutic era. Am J Respir Crit Care Med. 2017;195:979–984. doi: 10.1164/rccm.201606-1250PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirouvanziam R, Diaz D, Gernez Y, Laval J, Crubezy M, Makam M. An integrative approach for immune monitoring of human health and disease by advanced flow cytometry methods. In: Tuchin VV, editor. Advanced optical flow cytometry. Wiley-VCH Verlag GmbH & Co. KGaA. Germany, Weinheim: 2011. pp. 333–362. [Google Scholar]
- 36.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med. 2015;191:1158–1165. doi: 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- 37.Gehrig S, Mall MA, Schultz C. Spatially resolved monitoring of neutrophil elastase activity with ratiometric fluorescent reporters. Angew Chem Int Ed Engl. 2012;51:6258–6261. doi: 10.1002/anie.201109226. [DOI] [PubMed] [Google Scholar]
- 38.Gehrig S, Duerr J, Weitnauer M, Wagner CJ, Graeber SY, Schatterny J, et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am J Respir Crit Care Med. 2014;189:1082–1092. doi: 10.1164/rccm.201311-1932OC. [DOI] [PubMed] [Google Scholar]
- 39.Dittrich AS, Kühbandner I, Gehrig S, Rickert-Zacharias V, Twigg M, Wege S, et al. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur Respir J. 2018In press:1701910. doi: 10.1183/13993003.01910-2017. [DOI] [PubMed] [Google Scholar]
- 40.Horati H, Margaroli C, Scholte BJ, Veltman M, Peng L, Tiddens HAWM, et al. Exocytosis of elastase-rich granules by live airway neutrophils correlates with PRAGMA-CF score in early CF lung disease. Presented at the 40th European CF Society Conference. June 7–10, 2017, Sevilla, Spain [Google Scholar]
- 41.Horati H, Margaroli C, Scholte BJ, Veltman M, Peng L, Tiddens HAWM, et al. Granule exocytosis by airway neutrophils correlates with PRAGMA-CF score of structural airway damage in early CF lung disease. Presented at the 31st Annual North American Cystic Fibrosis Conference. November 2–4, 2017, Indianapolis, IN [Google Scholar]
- 42.Margaroli C, Horati H, Scholte BJ, Peng L, Tiddens HAWM, Janssens HM, et al. Cystic fibrosis infant airways harbor a pathogenic subset of live neutrophils. Presented at the 14th European CF Society Basic Science Conference. March 29–April 1, 2017, Albufeira, Portugal [Google Scholar]
- 43.Tirouvanziam R.Mechanisms of neutrophil-mediated inflammation in cystic fibrosis. Presented at the 15th European CF Society Basic Science Conference. March 21–24, 2018, Loutraki, Greece [Google Scholar]
- 44.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 45.Ratjen F, Bredendiek M, Brendel M, Meltzer J, Costabel U. Differential cytology of bronchoalveolar lavage fluid in normal children. Eur Respir J. 1994;7:1865–1870. doi: 10.1183/09031936.94.07101865. [DOI] [PubMed] [Google Scholar]
- 46.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, et al. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros. 2012;11:363–382. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Ng HP, Zhou Y, Song K, Hodges CA, Drumm ML, Wang G. Neutrophil-mediated phagocytic host defense defect in myeloid Cftr-inactivated mice. PLoS One. 2014;9:e106813. doi: 10.1371/journal.pone.0106813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veltman M, Stolarczyk M, Radzioch D, Wojewodka G, De Sanctis JB, Dik WA, et al. Correction of lung inflammation in a F508del CFTR murine cystic fibrosis model by the sphingosine-1-phosphate lyase inhibitor LX2931. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1000–L1014. doi: 10.1152/ajplung.00298.2016. [DOI] [PubMed] [Google Scholar]
- 49.Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol. 2015;52:683–694. doi: 10.1165/rcmb.2014-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stick SM, Kicic A, Ranganathan S. Of pigs, mice, and men: understanding early triggers of cystic fibrosis lung disease. Am J Respir Crit Care Med. 2016;194:784–785. doi: 10.1164/rccm.201605-1094ED. [DOI] [PubMed] [Google Scholar]
- 51.Montgomery ST, Mall MA, Kicic A, Stick SM AREST CF. Hypoxia and sterile inflammation in cystic fibrosis airways: mechanisms and potential therapies. Eur Respir J. 2017;49:1600903. doi: 10.1183/13993003.00903-2016. [DOI] [PubMed] [Google Scholar]
- 52.Gernez Y, Tirouvanziam R, Chanez P. Neutrophils in chronic inflammatory airway diseases: can we target them and how? Eur Respir J. 2010;35:467–469. doi: 10.1183/09031936.00186109. [DOI] [PubMed] [Google Scholar]
- 53.Polverino E, Rosales-Mayor E, Dale GE, Dembowsky K, Torres A. The role of neutrophil elastase inhibitors in lung diseases. Chest. 2017;152:249–262. doi: 10.1016/j.chest.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 54.Elborn JS, Horsley A, MacGregor G, Bilton D, Grosswald R, Ahuja S, et al. Phase I studies of acebilustat: biomarker response and safety in patients with cystic fibrosis. Clin Transl Sci. 2017;10:28–34. doi: 10.1111/cts.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson JL, Ramadass M, He J, Brown SJ, Zhang J, Abgaryan L, et al. Identification of neutrophil exocytosis inhibitors (nexinhibs), small molecule inhibitors of neutrophil exocytosis and inflammation: druggability of the small GTPase Rab27a. J Biol Chem. 2016;291:25965–25982. doi: 10.1074/jbc.M116.741884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, et al. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/- mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Twigg MS, Brockbank S, Lowry P, FitzGerald SP, Taggart C, Weldon S. The role of serine proteases and antiproteases in the cystic fibrosis lung. Mediators Inflamm. 2015;2015:293053. doi: 10.1155/2015/293053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garratt LW, Sutanto EN, Ling KM, Looi K, Iosifidis T, Martinovich KM, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur Respir J. 2015;46:384–394. doi: 10.1183/09031936.00212114. [DOI] [PubMed] [Google Scholar]
- 59.Trojanek JB, Cobos-Correa A, Diemer S, Kormann M, Schubert SC, Zhou-Suckow Z, et al. Airway mucus obstruction triggers macrophage activation and matrix metalloproteinase 12-dependent emphysema. Am J Respir Cell Mol Biol. 2014;51:709–720. doi: 10.1165/rcmb.2013-0407OC. [DOI] [PubMed] [Google Scholar]
- 60.Wagner CJ, Schultz C, Mall MA. Neutrophil elastase and matrix metalloproteinase 12 in cystic fibrosis lung disease. Mol Cell Pediatr. 2016;3:25. doi: 10.1186/s40348-016-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown DE, Pittman JE, Leigh MW, Fordham L, Davis SD. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol. 2008;43:514–516. doi: 10.1002/ppul.20792. [DOI] [PubMed] [Google Scholar]
- 62.Magnin ML, Cros P, Beydon N, Mahloul M, Tamalet A, Escudier E, et al. Longitudinal lung function and structural changes in children with primary ciliary dyskinesia. Pediatr Pulmonol. 2012;47:816–825. doi: 10.1002/ppul.22577. [DOI] [PubMed] [Google Scholar]
- 63.Ratjen F, Waters V, Klingel M, McDonald N, Dell S, Leahy TR, et al. Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur Respir J. 2016;47:829–836. doi: 10.1183/13993003.01390-2015. [DOI] [PubMed] [Google Scholar]
- 64.Korten I, Kieninger E, Klenja S, Mack I, Schläpfer N, Barbani MT, et al. SCILD and BILD study groups. Respiratory viruses in healthy infants and infants with cystic fibrosis: a prospective cohort study. Thorax. 2018;73:13–20. doi: 10.1136/thoraxjnl-2016-209553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.