Abstract
Background
Primaquine (PQ) prevents relapses of vivax malaria but may induce severe haemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficient patients. Data on the safety of primaquine in infants are limited.
Methods
A retrospective, hospital-based cohort study of infants aged 1–12 months with vivax malaria was carried out in Timika, Papua province, Indonesia. Risks of admission, death and severe haematological outcomes within 30 days of first presentation were compared between infants who did and did not receive primaquine. Infants were not tested routinely for G6PD deficiency as per local guidelines.
Results
Between 2004 and 2013, 4078 infants presented to the hospital for the first time with vivax malaria, of whom 3681 (90.3%) had data available for analysis. In total 1228 (33.4%) infants were aged between 1 and 6 months and 2453 (66.6%) between 6 and 12 months of age. Thirty-three (0.9%) patients received low-dose primaquine (LDP), 174 (4.7%) received high-dose primaquine (HDP), 3432 (93.2%) received no primaquine (NPQ) and 42 patients received either a single dose or an unknown dose of primaquine. The risk of the Hb concentration falling by > 25% to less than 5 g/dL was similar in the LDP or HDP groups (4.3%, 1/23) versus the NPQ group (3.5%, 16/461). Three infants (1.4%) died following receipt of PQ, all of whom had major comorbidities. Seventeen patients (0.5%) died in the NPQ group. None of the infants had documented massive haemolysis or renal impairment.
Conclusions
Severe clinical outcomes amongst infants treated with primaquine in Papua were rare. The risks of using primaquine in infancy must be weighed against the risks of recurrent vivax malaria in early life.
Keywords: Plasmodium vivax, Infants, Primaquine, Safety, Evaluation
Background
There are almost 12 million cases of Plasmodium vivax malaria each year [1]. The greatest burden of these infections is in poorly resourced communities where the disease is associated with severe anaemia and other adverse health and developmental effects in young children [2–6]. Much of the morbidity and transmission potential of P. vivax infections is attributable to relapsing disease arising from dormant liver stages of the parasite (hypnozoites) [7, 8].
The only widely available hypnozoitocidal agent for radical cure of P. vivax infection is primaquine (PQ), an 8-aminoquinoline anti-malarial drug [9, 10] which has potential to reduce the adverse consequences of severe anaemia due to recurrent episodes of parasite induced haemolysis [11]. This is particularly important in equatorial regions where the risk of P. vivax relapse is high and the interval between relapses is short [12, 13].
Primaquine results in oxidant stress which can cause severe and potentially fatal, drug-induced haemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficient individuals [14, 15]. G6PD deficiency is common in malaria endemic populations, reaching a prevalence of 40% in some regions [16]. The overall risk–benefit of primaquine therapy for vivax malaria, therefore, hinges upon the dangers of drug induced haemolysis weighed against the dangers of recurrent parasite induced haemolysis and other malaria-related morbidities.
In 2015, the World Health Organization (WHO) anti-malarial guidelines reduced the lower age threshold for recommending the use of primaquine from 4 years of age to those over 6 months old, with close monitoring for signs of haemolysis [10]. Although the WHO recommends that patients be tested for G6PD deficiency prior to administration of primaquine, in reality, G6PD testing is rarely available in poorly resourced endemic regions [17–19].
This study examined the safety outcomes of pragmatic and discretionary use of primaquine in infants at Mitra Masyarakat Hospital in southern Papua, an area where the risk of severe anaemia associated with P. vivax relapse is high and the prevalence of G6PD deficiency is low [2].
Methods
Study site
Timika (Papua, Indonesia) is an area of unstable malaria transmission, with little seasonal variation in the climate (Mimika Regency Statistics, 2013). The population in 2012 was estimated to be 202,350 with the majority living in the lowland regions. The annual estimated incidence of malaria in the area has declined from 876 per 1000 population in 2004 [20] to 450 per 1000 in 2013 (Annual Health Report, Mimika District-2013) with approximately equal proportions of P. vivax and Plasmodium falciparum infections. The local P. vivax strains are highly resistant to chloroquine and have a relapse interval of 3–4 weeks [12, 13]. About half of the population own insecticide-treated bed nets [21].
Mitra Masyarakat Hospital (RSMM) was the only hospital in the region until November 2008, when the second hospital started to operate. Since 2009 RSMM has received about 80% of patients presenting to hospital with malaria [22]. RSMM has a functioning high care unit for critically ill infants and children.
Study population
The ethnic groups in Timika are categorized into highland and lowland Papuans and non-Papuan Indonesians. Diarrhoea, lower respiratory tract infections and malaria are the predominant causes of morbidity and mortality in young children. The infant mortality rate is approximately 54 per 1000 live births [23] and the prevalence of G6PD deficiency is 1.3% (Pava et al. pers. commun.).
Study design and data sources
This was a retrospective cohort study using routinely collected surveillance data gathered at RSMM hospital between April 2004 and December 2013. Clinical and demographic details (including the date of presentation, age, sex, ethnicity, and clinical diagnoses [International Classification of Diseases-ICD 10]) assigned by the attending physician) of every patient presentation to hospital were recorded in a Q-Pro™ database. Each patient was identified by a unique hospital record number (HRN). Electronic data were also available from laboratory and pharmacy records and were merged with the demographic and clinical dataset using the HRN and date. For the purposes of this analysis, the merged database was limited to infants aged between 1 and 12 months presenting for the first time with P. vivax mono- or mixed Plasmodium species infection. Healthcare at the RSMM hospital is free to the majority of the local population and since the hospital has better facilities and clinicians are available 24 h a day, most patients prefer to attend the hospital to primary care facilities.
Hospital protocol dictates that any patient presenting with fever or history of fever or any patient who is severely ill should be checked for malaria by microscopy using Giemsa stained thick blood smears. Thin blood smears were performed if the parasitaemia was too high to count by thick film examination. The hospital microscopists received refresher training annually. Testing for G6PD deficiency was not available during the study period. Clinicians requested a complete blood count only when clinically indicated and this was done using a Coulter counter.
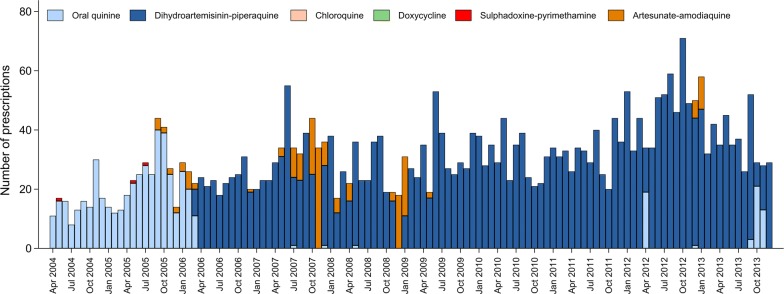
Prior to 2006, the first-line schizontocidal treatment for uncomplicated malaria in infants due to any Plasmodium species was 7 days of quinine. After March 2006, infants weighing more than 5 kgs received dihydroartemisinin-piperaquine (DHA + PIP) [24].
Definitions and outcomes of interest
Infants were defined as children aged between 1 and 12 months of age. Children less than 1 month old were excluded to avoid confounding from perinatal mortality. For infants with multiple presentations with P. vivax infection during the first year of life, only the first episode was included in this analysis. The total primaquine dose used was classified as no primaquine (NPQ), high dose primaquine (HDP; ≥ 5 mg/kg), low dose (LDP; ≥ 1.5 to < 5 mg/kg), unknown dose (matched primaquine prescription record but unable to determine dose) or single dose (< 1.5 mg/kg) [25].
The outcomes of interest were requirement for admission to hospital, all-cause death, severe anaemia (Haemoglobin concentration [Hb] < 5 g/dL) at any point during follow-up and a clinically significant drop in haemoglobin (reduction in Hb from baseline of > 25% to an absolute Hb concentration < 5 g/dL). Except in severe G6PD deficiency variants (such as “Mediterranean”), daily treatment with primaquine typically results in a nadir in haemoglobin around day 7 [17]. By this point, the rate of oxidative haemolysis has decreased due to the higher proportion of relatively G6PD replete young red blood cells and red cell production has increased to compensate sufficiently for the rate of loss. Outcomes, including death, occurring within the first 48 h were excluded as it was felt that these could not be caused plausibly by primaquine treatment. The timeline for possible primaquine associated haematological outcomes in this study was restricted to 3 to 30 days following initial presentation with vivax malaria.
Statistical analysis
All statistical analyses were done in STATA® version 15.1 (College Station, Texas). The risks of admission to hospital and death were presented visually using Kaplan–Meier failure curves. Normally distributed haemoglobin data were presented as mean and standard deviation. The risks of the binary safety outcomes of interest were presented as frequencies (percent) stratified by age group (1–6 months, 6–12 months), primaquine dose (NPQ, LDP, HDP) and, in the case of admission and death, period of follow-up (day 3–30, day 3–90). Comparisons of the frequency of safety outcomes were compared between those receiving no primaquine and those receiving either low dose or high dose primaquine and presented as odds ratios with 95% confidence intervals. Due to the very small numbers of events and multiple comparisons, p values are not presented.
Ethical approval
Ethical approval for this study was obtained from the Medical and Health Research Ethics Committee, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia (KE/FK/544/EC) and the ethics committee of Menzies School of Health Research, Darwin, Australia (HREC 10.1397).
Results
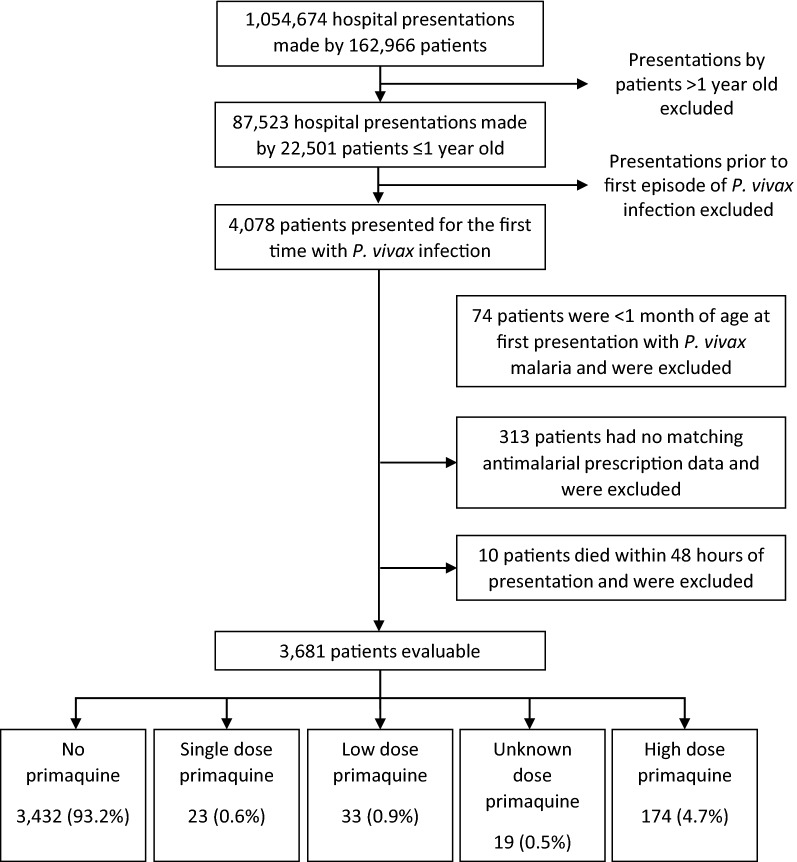
Between April 2004 and December 2013, 4078 infants presented to RSMM for the first time with P. vivax infection of whom 74 (1.8%) were under 1 month of age and excluded from subsequent analyses. Antimalarial prescription data were available for 3691 (92.2%) of the remaining infants. Ten (0.27%) infants died within the first 48 h of presentation and were censored prior to any of the clinical outcomes.
Of the 3432 infants in the analysis, 1208 (35.3%) were less than 6 months of age and 2224 (64.8%) between 6 and 12 months of age. Overall, 93.2% (3432/3681) of infants received no primaquine, 0.9% (33) received LDP, 4.7% (174) received HDP and 1.1% (42) received either a single dose or an unknown dose of primaquine (Table 1 and Fig. 1). The proportion of infants treated with primaquine was similar in outpatients compared with inpatients and those with mixed infections compared to those with P. vivax mono-infections. Infants aged 9–12 months were more likely to receive HDP (n = 137 (10.7%)) than younger babies (n = 37, 1.5%) (Table 1 and Fig. 2). Prior to the change of treatment policy in March 2006, oral quinine was the most commonly prescribed oral schizontocidal agent (n = 484, 89.5%) (Fig. 3). Thereafter, 91.9% (n = 2887) of infants received DHA + PIP.
Table 1.
Demographic and clinical features of the infants included in the analyses
| Primaquine dose | ||||||
|---|---|---|---|---|---|---|
| No PQ | Single dose PQ | Low dose PQ | High dose PQ | Unknown dose | Total | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Age category | ||||||
| 0–3 m | 464 (98.3) | 2 (0.4) | 0 (0) | 6 (1.3) | 0 (0) | 472 (100) |
| 3–6 m | 744 (98.4) | 1 (0.1) | 2 (0.3) | 7 (0.9) | 2 (0.3) | 756 (100) |
| 6–9 m | 1137 (97.3) | 4 (0.3) | 3 (0.3) | 24 (2.1) | 0 (0) | 1168 (100) |
| 9 m–1 yr | 1087 (84.6) | 16 (1.2) | 28 (2.2) | 137 (10.7) | 17 (1.3) | 1285 (100) |
| Gender | ||||||
| Male | 1831 (92.9) | 12 (0.6) | 16 (0.8) | 100 (5.1) | 11 (0.6) | 1970 (100) |
| Female | 1601 (93.6) | 11 (0.6) | 17 (1.0) | 74 (4.3) | 8 (0.5) | 1711 (100) |
| Ethnicity | ||||||
| Non Papuan | 100 (90.1) | 3 (2.7) | 0 (0) | 6 (5.4) | 2 (1.8) | 111 (100) |
| Highland Papuan | 2804 (93.4) | 17 (0.6) | 27 (0.9) | 142 (4.7) | 13 (0.4) | 3003 (100) |
| Lowland Papuan | 528 (93.1) | 3 (0.5) | 6 (1.1) | 26 (4.6) | 4 (0.7) | 567 (100) |
| Year | ||||||
| 2004 | 134 (82.7) | 4 (2.5) | 5 (3.1) | 18 (11.1) | 1 (0.6) | 162 (100) |
| 2005 | 265 (88.3) | 10 (3.3) | 15 (5.0) | 8 (2.7) | 2 (0.7) | 300 (100) |
| 2006 | 265 (90.1) | 2 (0.7) | 5 (1.7) | 22 (7.5) | 0 (0) | 294 (100) |
| 2007 | 393 (96.6) | 1 (0.2) | 1 (0.2) | 11 (2.7) | 1 (0.2) | 407 (100) |
| 2008 | 290 (90.1) | 4 (1.2) | 3 (0.9) | 25 (7.8) | 0 (0) | 322 (100) |
| 2009 | 358 (93.0) | 1 (0.3) | 1 (0.3) | 23 (6.0) | 2 (0.5) | 385 (100) |
| 2010 | 345 (91.5) | 1 (0.3) | 2 (0.5) | 26 (6.9) | 3 (0.8) | 377 (100) |
| 2011 | 377 (95.4) | 0 (0) | 1 (0.3) | 10 (2.5) | 7 (1.8) | 395 (100) |
| 2012 | 562 (95.6) | 0 (0) | 0 (0) | 23 (3.9) | 3 (0.5) | 588 (100) |
| 2013 | 443 (98.2) | 0 (0) | 0 (0) | 8 (1.8) | 0 (0) | 451 (100) |
| Species | ||||||
| Pv | 2847 (93.3) | 18 (0.6) | 28 (0.9) | 142 (4.7) | 17 (0.6) | 3052 (100) |
| Mix | 585 (93.0) | 5 (0.8) | 5 (0.8) | 32 (5.1) | 2 (0.3) | 629 (100) |
| Admission | ||||||
| Outpatient | 2598 (93.5) | 10 (0.4) | 19 (0.7) | 140 (5.0) | 13 (0.5) | 2780 (100) |
| Inpatient | 834 (92.6) | 13 (1.4) | 14 (1.6) | 34 (3.8) | 6 (0.7) | 901 (100) |
| Baseline Hb | 1939 | 17 | 23 | 87 | 7 | 2073 |
| Mean (SD) | 8.0 (2.2) | 5.4 (2.1) | 7.0 (2.1) | 7.7 (2.2) | 7.7 (3.4) | 8.0 (2.2) |
| Total | 3432 (93.2) | 23 (0.6) | 33 (0.9) | 174 (4.7) | 19 (0.5) | 3681 (100) |
m months, yr year, Pv Plasmodium vivax, Mix mixed Plasmodium species infection, Hb haemoglobin
Fig. 1.
Study profile
Fig. 2.
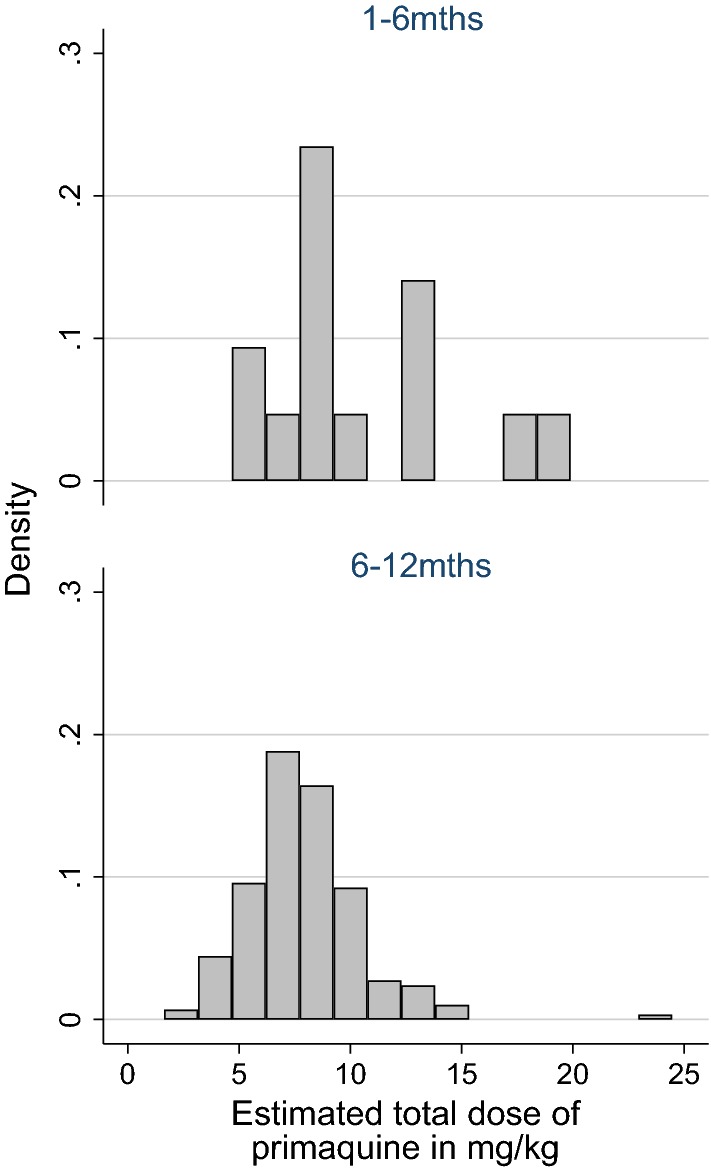
Histogram of estimated total primaquine dose in mg/kg by age category (excludes outlier values)
Fig. 3.
Oral schizontocidal prescriptions by month in patients ≤ 1 year old presenting with P. vivax monoinfection or mixed species infection
Haematological outcomes
At presentation haemoglobin concentration was available in 56.3% (2073/3681) of infants and at some point during the subsequent 3 to 30 days in 21.2% (782/3681) of infants. The mean haemoglobin concentration at presentation was 8.0 g/dL (SD = 2.2) in those not treated with primaquine, 7.0 g/dL (SD = 2.1) in those treated with LDP and 7.7 g/dL (SD = 2.2) in those treated with HDP. After excluding 234 infants with severe anaemia on presentation, a new diagnosis of severe anaemia occurred during follow-up in 4.4% (31/697) of patients who did not receive primaquine, 0% (0/6) of patients receiving LDP and 18.5% (5/27) of patients receiving HDP.
In 489 infants, a haemoglobin concentration was available both at presentation and between days 3 and 30. A clinically significant reduction in haemoglobin (reduction in Hb from baseline of > 25% to an absolute Hb concentration < 5 g/dL) was observed in 3.5% (16/461) of patients not treated with primaquine, 0% (0/4) of patients following LDP and 5.3% (1/19) of patients following HDP.
In total, 78.8% (2899/3681) of patients did not have a haemoglobin measurement between day 3 and 30 of follow-up. Since patients presenting with severe anaemia would most likely have clinical signs that would prompt a haemoglobin measurement, a sensitivity analysis was done in which patients without a follow-up haemoglobin measurement were assumed to not have severe anaemia. In this analysis the proportions of patients with new onset of severe anaemia during follow-up were 1.0% (31/3221) after NPQ, 0% (0/30) after LDP and 3% (5/165) after HDP and the proportions experiencing a clinically significant drop in haemoglobin were 0.5% (16/3432), 0% (0/33) and 0.6% (1/174), respectively.
Readmission
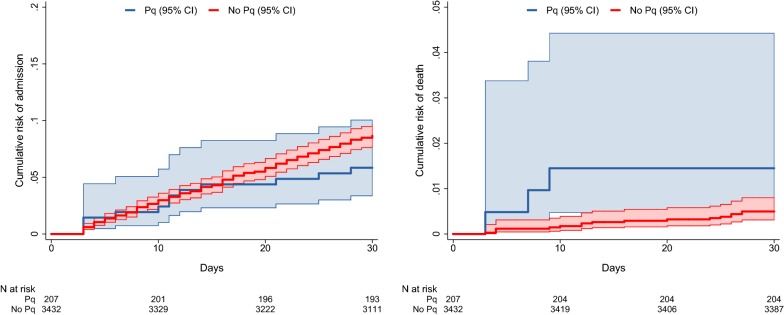
A total of 1642 (44.6%) infants represented to hospital between 3 and 30 days following their initial presentation; 45.7% (1569/3432) of those not treated with primaquine, 36.4% (12/33) of those treated with LDP and 35.1% (61/174) of those treated with HDP. The risk of admission between day 3 and 30 in infants treated with either LDP or HDP was 5.8% (12/207) compared to 8.6% (296/3432) in those not treated with primaquine; Odds ratio (OR) = 0.65 (95% confidence interval [95% CI] 0.36–1.18) (Table 2 and Fig. 4).
Table 2.
Frequency of outcomes of interest by primaquine dose category
| No PQ | LDP | HDP | LDP or HDP versus no PQ | |
|---|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | OR (95%CI) | |
| 1–6 m | ||||
| Severe anaemiaa | 10/246 (4.1) | 0/0 (–) | 0/0 (–) | – |
| Hb fall > 25% to < 5 g/dL | 4/183 (2.2) | 0/0 (–) | 0/0 (–) | – |
| Admission 3–30 d | 118/1208 (9.8) | 0/2 (0) | 0/13 (0) | – |
| Death 3–30 d | 11/1208 (0.91) | 0/2 (0) | 0/13 (0) | – |
| 6–12 m | ||||
| Severe anaemiaa | 21/451 (4.7) | 0/6 (0) | 5/27 (18.5) | 3.66 (1.28–10.4) |
| Hb fall > 25% to < 5 g/dL | 12/278 (4.3) | 0/4 (0) | 1/19 (5.3) | 1.01 (0.13–8.11) |
| Admission 3–30 d | 178/2224 (8.0) | 3/31 (9.7) | 9/161 (5.6) | 0.77 (0.42–1.40) |
| Death 3–30 d | 6/2224 (0.27) | 1/31 (3.2) | 2/161 (1.2) | 5.87 (1.46–23.6) |
| 1–12 m | ||||
| Severe anaemiaa | 31/697 (4.5) | 0/6 (0) | 5/27 (18.5) | 3.84 (1.39–10.6) |
| Hb fall > 25% to < 5 g/dL | 16/461 (3.5) | 0/4 (0) | 1/19 (5.3) | 1.26 (0.16–9.97) |
| Admission 3–30 d | 296/3432 (8.6) | 3/33 (9.1) | 9/174 (5.2) | 0.65 (0.36–1.18) |
| Death 3–30 d | 17/3432 (0.50) | 1/33 (3.0) | 2/174 (1.2) | 2.95 (0.86–10.2) |
PQ primaquine, LDP low dose primaquine, HDP high dose primaquine, n number with outcome, N denominator, OR odds ratio, 95% CI 95% confidence interval, Hb haemoglobin, m months, d day
aLimited to infants who were not severely anaemic at baseline
Fig. 4.
Cumulative risk of readmission and death by primaquine treatment category
Mortality
Twenty infants died between day 3 and day 30. The risk of death was 0.5% (17/3432) in infants not treated with primaquine, compared to 3.0% (1/33) in those treated with LDP and 1.1% (2/174) in those treated with HDP. Treatment with either LDP or HDP was associated with an OR of 2.95 (95% CI 0.86–10.2) for death. The clinical and haematological details of the patients who died are presented in Table 3. All infants had significant comorbidities other than P. vivax infection: 12 (60%) children had bacterial infection (bronchopneumonia or meningitis), 3 (15%) bronchopneumonia and gastroenteritis, 4 (20%) had tuberculosis, and 1 (5%) had gastroenteritis and malnutrition (Table 3). Four out of 17 infants who died having not been prescribed primaquine had their haemoglobin measured at both baseline and representation, two (50%) of whom had a clinically significant reduction. A 12-month old female died following HDP and had a clinically significant fall in haemoglobin from 6.4 to 4.6 g/dL, and an associated diagnosis of bronchopneumonia. A 12-month old male treated with HDP was diagnosed with chronic nephritic syndrome, tuberculosis and malnutrition and a 12-month old female treated with LDP had a diagnosis of malnutrition, dehydration and acidosis. None of the infants who died had a recorded diagnosis of blackwater fever or renal failure.
Table 3.
Clinical Data of infants who died between day 3 and 30 of initial presentation
| Patient number | Age | Sex | Ethnicity | Species | Initial Hb (g/dL) | Lowest F/U Hb (g/dL) | Days between presentation and lowest F/U Hb | Days between presentation and death | Schizontocidal drug used | Primaquine dose | Other clinical diagnoses assigned between presentation and death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 m | M | HP | Pv | 7.1 | 3.4 | 14 | 20 | IV artesunate/DHA + PIP | Nil | Gastroenteritis Malnutrition Dehydration Pulmonary tuberculosis |
| 2 | 1 m | M | HP | Mix | 2.3 | – | – | 4 | IV artesunate | Nil | Bacterial sepsis of newborn Neonatal jaundice Late metabolic acidosis of newborn Cerebral malaria |
| 3 | 1 m | F | HP | Pv | – | 9.3 | 27 | 27 | DHA + PIP | Nil | Oral candidiasis Staphylococcal scalded skin syndrome Stevens-Johnsons syndrome Hypoglycaemia |
| 4 | 2 m | M | HP | Pv | 6.3 | – | – | 13 | IV artesunate/DHA + PIP | Nil | Septic shock Respiratory distress syndrome of newborn Hypoalbuminaemia |
| 5 | 2 m | F | HP | Mix | – | – | – | 27 | DHA + PIP | Nil | Bacterial meningitis Acute upper respiratory tract infection |
| 6 | 2 m | M | HP | Pv | – | 9.8 | 20 | 25 | IV artesunate/DHA + PIP | Nil | Bronchopneumonia |
| 7 | 4 m | M | HP | Pv | 8.3 | 6.8 | 24 | 26 | PO quinine | Nil | Gastroenteritis Acidosis Meningitis |
| 8 | 4 m | F | HP | Pv | 11.1 | 11.2 | 6 | 16 | DHA + PIP | Nil | HIV Pulmonary tuberculosis Marasmus Candidiasis |
| 9 | 4 m | M | HP | Pv | – | 9.7 | 3 | 4 | DHA + PIP | Nil | Bacterial meningitis |
| 10 | 4 m | F | HP | Pv | – | 8 | 20 | 26 | DHA + PIP | Nil | Bronchopneumonia |
| 11 | 5 m | M | HP | Pv | 13 | – | – | 12 | IV artesunate/DHA + PIP | Nil | Bronchopneumonia Septic shock |
| 12 | 6 m | F | HP | Pv | 3.5 | – | – | 4 | IV/PO quinine | Nil | Bronchopneumonia Exfoliative dermatitis |
| 13 | 6 m | F | HP | Mix | 2.1 | – | – | 3 | IV artesunate/DHA + PIP | Nil | Bronchopneumonia |
| 14 | 8 m | F | HP | Pv | 9.6 | – | – | 12 | IV artesunate/DHA + PIP | Nil | Tuberculous meningitis Septic shock Conjunctivitis |
| 15 | 10 m | M | NP | Pv | 7.2 | – | – | 3 | IV artesunate/DHA + PIP | High dose | Tuberculosis of skin and subcutaneous tissue |
| 16 | 10 m | F | LP | Pv | – | – | – | 10 | DHA + PIP | Nil | Measles Gastroenteritis and colitis Bronchopneumonia |
| 17 | 12 m | F | HP | Pv | 6.4 | 4.6 | 3 | 9 | PO quinine | High dose | Bronchopneumonia |
| 18 | 12 m | M | HP | Pv | 8.7 | 4.3 | 23 | 24 | DHA + PIP | Nil | Gastroenteritis Dehydration Septic shock Acidosis Respiratory failure Febrile convulsion |
| 19 | 12 m | F | HP | Pv | 8.9 | – | – | 9 | DHA + PIP | Nil | Pneumonia Septic shock |
| 20 | 12 m | M | HP | Pv | 7.9 | – | – | 7 | DHA + PIP | Low dose | Gastroenteritis Malnutrition Dehydration Acidosis |
m months, M male, F female, HP Highland Papuan, LP Lowland Papuan, NP non-Papuan, Pv Plasmodium vivax, Mix mixed Plasmodium species infection, Hb haemoglobin, F/U follow-up, PO oral, IV intravenous, DHA + PIP dihydroartemisinin-piperaquine
Discussion
In view of the risk of haemolysis, limited capacity of cardiorespiratory compensatory mechanisms and the difficulty diagnosing G6PD deficiency in young infants, the WHO does not recommend primaquine for infants under the age of 6 months [10, 26, 27]. However the risks of primaquine use in this age group need to be balanced against the significant risks of recurrent P. vivax infections in the first year of life, which in Papua-Indonesia, also includes severe anaemia and both direct and indirect mortality [2, 28]. Approximately 65% of children presenting for the first time with P. vivax infection to hospital in Timika will represent to hospital within 12 months, 50% will represent with malaria within that time period and 25% will require admission (about 40% of these admissions are attributable to malaria) (Patriani et al., pers.commun.).
At the local hospital in Timika, primaquine was prescribed in 6.8% (249/3681) of infants presenting with P. vivax infection. The proportion of infants requiring admission to the hospital within 3 to 30 days was lower in those treated with HDP (5.7%) compared to infants who did not receive primaquine (8.6%). A plausible explanation for this is that primaquine prevented vivax relapse and thus morbidity associated with recurrent malaria. Previous study has shown that the overall effectiveness of unsupervised primaquine at RSMM is poor, but that there is some benefit in young children, potentially because of parental influence increasing adherence to treatment [25]. Alternatively, these observations could reflect bias caused by purposeful selection of less vulnerable infants for primaquine treatment. However, given the similar proportions of inpatients and outpatients treated with primaquine the latter explanation seems unlikely.
Quantifying the acute hemolytic effect of primaquine in infants is confounded by the haemolytic effects of malaria itself along with any other concomitant morbidities [15, 28]. The most appropriate metric for clinically relevant haemolysis is disputed. Since the fractional fall in hemoglobin is correlated positively with the starting Hb, the metric chosen were both development of severe anaemia (Hb < 5 g/dL) and a composite measure of a high fractional fall (> 25%) to an absolute Hb below 5 g/dL, at which level the risk of mortality rises significantly [28].
The mean Hb concentration in infants at baseline was similar (7–8 g/dL) between treatment groups. The crude frequency of severe anaemia and a clinically significant drop in haemoglobin was higher amongst patients who received HDP (18.5% and 5.3% respectively) compared to those who did not receive primaquine (4.5% and 3.5% respectively) whereas neither of these adverse haematological outcomes occurred in patients treated with low dose primaquine. One infant who had a clinically significant drop in haemoglobin after HDP had an initial Hb level of 6.4 g/dL falling to 4.6 g/dL by day 3, and died 9 days after presentation with bronchopneumonia (Table 3). Since biochemical laboratory tests were not available in this infant, severe hemolysis with acute renal failure and associated pulmonary oedema could not be ruled out.
Profound haemolysis leading to severe anaemia, is likely to prompt representation to hospital and be detected clinically by the attending physician. Hence the incidence of severe anaemia amongst those who did not have a repeat haemoglobin was probably low. If this assumption is true, the true frequencies of new onset severe anaemia or a clinically significant reduction in haemoglobin following high dose primaquine are likely to be substantially lower than those observed (as demonstrated in the sensitivity analyses). Nevertheless, the frequency of severe haematological events at a population level needs to be considered in the context of the prevalence of the susceptible phenotype, namely severe G6PD deficiency, which in Timika is estimated to be about 1.3%.
Three infants treated with LDP or HDP died following treatment with primaquine. All three had significant comorbidity (tuberculosis in one case, severe malnutrition, gastroenteritis, dehydration and acidosis in another case and bronchopneumonia). The initial haemoglobin was less than 8 g/dL in all three infants, and thus a small fall in haemoglobin attributable to either acute malaria or drug, associated with significant co-morbidities, could have contributed to their clinical decline [29].
The relevance of these findings to other endemic locations needs to be considered with caution. In the study population the prevalence of G6PD deficiency (< 30% activity) is low and absent in those of highland ethnicity, who make up more than 70% of patients presenting to the hospital (Pava et al., pers.commun.). Elsewhere in Indonesia, the G6PD deficiency prevalence is much higher, the dominant variants being Vanua Lava, Viangchan and Chatam which are associated with enzyme activity of less than 10% [16, 19]. Use of primaquine without prior G6PD testing in those regions, particularly in infants, would carry significantly greater risk and G6PD testing would be mandatory prior to primaquine administration
This study has several important limitations. Firstly infants who died at home or visited other health facilities following their initial presentation to RSMM with vivax malaria will not have been detected. In a treatment seeking survey conducted in 2013, family members from households in which a member had died within the preceding year, reported that the death had occurred at the RSMM hospital in 30% of cases [30]. Hence whilst many deaths will have been identified by our surveillance, some would have been missed. This attrition bias is likely be similar for infants treated with or without primaquine. Secondly it was not possible to confirm that infants completed a full course of primaquine after discharge from the hospital, and a previous study showed poor effectiveness, which was assumed to be secondary to poor adherence to the 14-day primaquine regimen. Thus, incomplete treatment will have resulted in underestimation of the frequency of adverse events that would be observed in a fully supervised regimen [25]. Finally haemoglobin measures were not undertaken routinely. Most patients had their haemoglobin measured only once and this was generally on the day of admission or representation. Thereafter haemoglobin was only measured when there was clinical concern. In view of this, a composite measure of significant haemolysis was chosen, which comprised a high fractional fall to below an absolute concentration of 5 g/dL—a point at which anaemia would presumably have been apparent clinically, triggering laboratory investigation. The true minimum haemoglobin concentration for each individual may have been lower than the lowest measured concentration recorded in the hospital database.
Conclusion
The clinical decision to give primaquine as radical treatment for P. vivax malaria and mixed infections in this vulnerable group without G6PD testing should be made with great caution, particularly in areas with a higher prevalence of G6PD deficiency. If primaquine is to be prescribed, parents and guardians must be informed of the early signs of haemolysis and advised to seek immediate medical attention should haemolysis occur [15, 31]. Considering the potential life-threatening consequences of haemolysis from either recurrent P. vivax malaria or antirelapse treatment in G6PD deficient infants, there is an urgent need for a highly sensitive point of care G6PD test to ensure that radical cure can be deployed safely to those who need it most.
Authors’ contributions
AS, EA, RNP and JRP designed the study. AS, JRP, EK, DAL, NMD and RNP collected and extracted the data. AS, EA, JRP, NMD, RNP and JAS analysed the data. AS and JRP wrote the first draft of the manuscript. EK, AH, DAL, KT, NMA, PS, JAS, NMD, AS, EA, RNP and JRP reviewed and revised the final manuscript draft. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Lembaga Pengembangan Masyarakat Amungme Kamoro and Mitra Masyarakat Hospital (RSMM) in Timika, Papua, Indonesia. We thank Professor Yati Soenarto and Dr. Yodi Mahendradhata from the Faculty of Medicine Universitas Gadjah Mada, Yogyakarta for their excellent support to the study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study used deidentified, secondary electronic data from the hospital’s routine surveillance system. Individual consent was not obtained. Ethical approval for this study was obtained from the Medical and Health Research Ethics Committee, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia (KE/FK/544/EC) and the ethics committee of Menzies School of Health Research, Darwin, Australia (HREC 10.1397).
Funding
The study was funded by the Wellcome Trust (Training Fellowship in Tropical Medicine awarded to JRP 099875), and the Australian National Health and Medical Research Council (Senior Research Fellowship to JAS (1104975) and Senior Principal Research Fellowship to NMA (1135820). The Timika Research Facility and Papuan Health and Community Development Foundation were supported by the Department of Foreign Affairs and Trade and the National Health and Medical Research Council of Australia (Program Grant 1037304). This work was supported by the Australian Centre for Research Excellence on Malaria Elimination, funded by the NHMRC (1134989).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- PQ
primaquine
- G6PD
glucose-6-phosphate dehydrogenase
- RSMM
Mitra Masyarakat Hospital
- HDP
high dose primaquine
- LDP
low dose primaquine
- NPQ
no primaquine
- ICD
International Classification of Diseases
- Hb
Haemoglobin
- DHA + PIP
dihydroartemisinin-piperaquine
- SD
standard deviation
- STATA
statistics and data science
- n
number with outcome
- N
denominator
- OR
odds ratio
- 95% CI
95% confidence interval
- m
months
- yr
year
- Pv
Plasmodium vivax
- Mix
mixed Plasmodium species infection
- M
male
- F
female
- HP
Highland Papuan
- LP
Lowland Papuan
- NP
non-Papuan
- F/U
follow-up
- PO
oral
- IV
intravenous
References
- 1.WHO. World malaria report 2017. Geneva: World Health Organization; 2017. http://www.who.int/malaria/publications/world-malaria-report-2017/report/en/. Accessed 12 Dec 2018.
- 2.Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Hasanuddin A, Warikar N, et al. Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis. 2009;48:1704–1712. doi: 10.1086/599041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenangalem E, Karyana M, Burdarm L, Yeung S, Simpson JA, Tjitra E, et al. Plasmodium vivax infection: a major determinant of severe anaemia in infancy. Malar J. 2016;15:321. doi: 10.1186/s12936-016-1373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith T, Charlwood JD, Kitua AY, Masanja H, Mwankusye S, Alonso PL, et al. Relationships of malaria morbidity with exposure to Plasmodium falciparum in young children in a highly endemic area. Am J Trop Med Hyg. 1998;59:252–257. doi: 10.4269/ajtmh.1998.59.252. [DOI] [PubMed] [Google Scholar]
- 5.Maitland K, Williams TN, Bennett S, Newbold CI, Peto TE, Viji J, et al. The interaction between Plasmodium falciparum and P. vivax in children on Espiritu Santo island, Vanuatu. Trans R Soc Trop Med Hyg. 1996;90:614–620. doi: 10.1016/S0035-9203(96)90406-X. [DOI] [PubMed] [Google Scholar]
- 6.Vitor-Silva S, Reyes-Lecca RC, Pinheiro TR, Lacerda MV. Malaria is associated with poor school performance in an endemic area of the Brazilian Amazon. Malar J. 2009;8:230. doi: 10.1186/1475-2875-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12:e1001891. doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–435. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- 9.John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11:280. doi: 10.1186/1475-2875-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . Guidelines for the treatment of malaria. 3. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 11.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–227. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi, et al. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997;56:621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- 13.Ratcliff A, Siswantoro H, Kenangalem E, Wuwung M, Brockman A, Edstein MD, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101:351–359. doi: 10.1016/j.trstmh.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Working Group Glucose-6-phosphate dehydrogenase deficiency. Bull World Health Organ. 1989;67:601–611. [PMC free article] [PubMed] [Google Scholar]
- 15.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 16.Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9:e1001339. doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashley EA, Recht J, White NJ. Primaquine: the risks and the benefits. Malar J. 2014;13:418. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol. 2013;81:133–201. doi: 10.1016/B978-0-12-407826-0.00004-7. [DOI] [PubMed] [Google Scholar]
- 19.Satyagraha AW, Sadhewa A, Baramuli V, Elvira R, Ridenour C, Elyazar I, et al. G6PD deficiency at Sumba in Eastern Indonesia is prevalent, diverse and severe: implications for primaquine therapy against relapsing Vivax malaria. PLoS Negl Trop Dis. 2015;9:e0003602. doi: 10.1371/journal.pntd.0003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karyana M, Burdarm L, Yeung S, Kenangalem E, Wariker N, Maristela R, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. doi: 10.1186/1475-2875-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thio F, Indrawanti R, Price R. The Timika Household Survey Dataset. Timika: Timika Research Facility; 2013. [Google Scholar]
- 22.Devine A, Kenangalem E, Burdam FH, Anstey NM, Poespoprodjo JR, Price RN, et al. Treatment-seeking behavior after the implementation of a unified policy of dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria in Papua, Indonesia. Am J Trop Med Hyg. 2018;98:543–550. doi: 10.4269/ajtmh.17-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infant Mortality Rate in each Province in Indonesia. https://www.bps.go.id/linkTabelStatis/view/id/1270. Accessed 2 May 2017.
- 24.Indonesian Ministry of Health . The national guidelines for malaria treatment in Indonesia. Jakarta: Indonesian Ministry of Health; 2006. [Google Scholar]
- 25.Douglas NM, Poespoprodjo JR, Patriani D, Malloy MJ, Kenangalem E, Sugiarto P, et al. Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: a hospital-based cohort study. PLoS Med. 2017;14:e1002379. doi: 10.1371/journal.pmed.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathers LH, Frankel LR, Kache S. Pediatric emergencies and resuscitation. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics-18th edition. Philadelphia: Saunders Elsevier; 2007. p. 389. [Google Scholar]
- 27.Frankel LR, Kache S. Shock. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics-18th edition. Philadelphia: Saunders Elsevier; 2007. pp. 413–416. [Google Scholar]
- 28.Douglas NM, Lampah DA, Kenangalem E, Simpson JA, Poespoprodjo JR, Sugiarto P, et al. Major burden of severe anemia from non-falciparum malaria species in Southern Papua: a hospital-based surveillance study. PLoS Med. 2013;10:e1001575. doi: 10.1371/journal.pmed.1001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas NM, Pontororing GJ, Lampah DA, Yeo TW, Kenangalem E, Poespoprodjo J, et al. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua, Indonesia. BMC Med. 2014;12:217. doi: 10.1186/s12916-014-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karyana M, Devine A, Kenangalem E, Burdarm L, Poespoprodjo JR, Vemuri R, et al. Treatment-seeking behaviour and associated costs for malaria in Papua, Indonesia. Malar J. 2016;15:536. doi: 10.1186/s12936-016-1588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization . Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale malaria: policy brief. Geneva: World Health Organization; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.